Cathepsin B Protease Facilitates Chikungunya Virus Envelope Protein-Mediated Infection Via Endocytosis or Macropinocytosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Cells
2.2. Plasmids
2.3. Pseudotyped MLV Vector
2.4. Cell Viability
2.5. Macropinocytosis Assay
2.6. Mouse Antiserum against CHIKV E2 Protein
2.7. Western Immunoblotting
2.8. RT-PCR
2.9. Statistical Analysis
3. Results
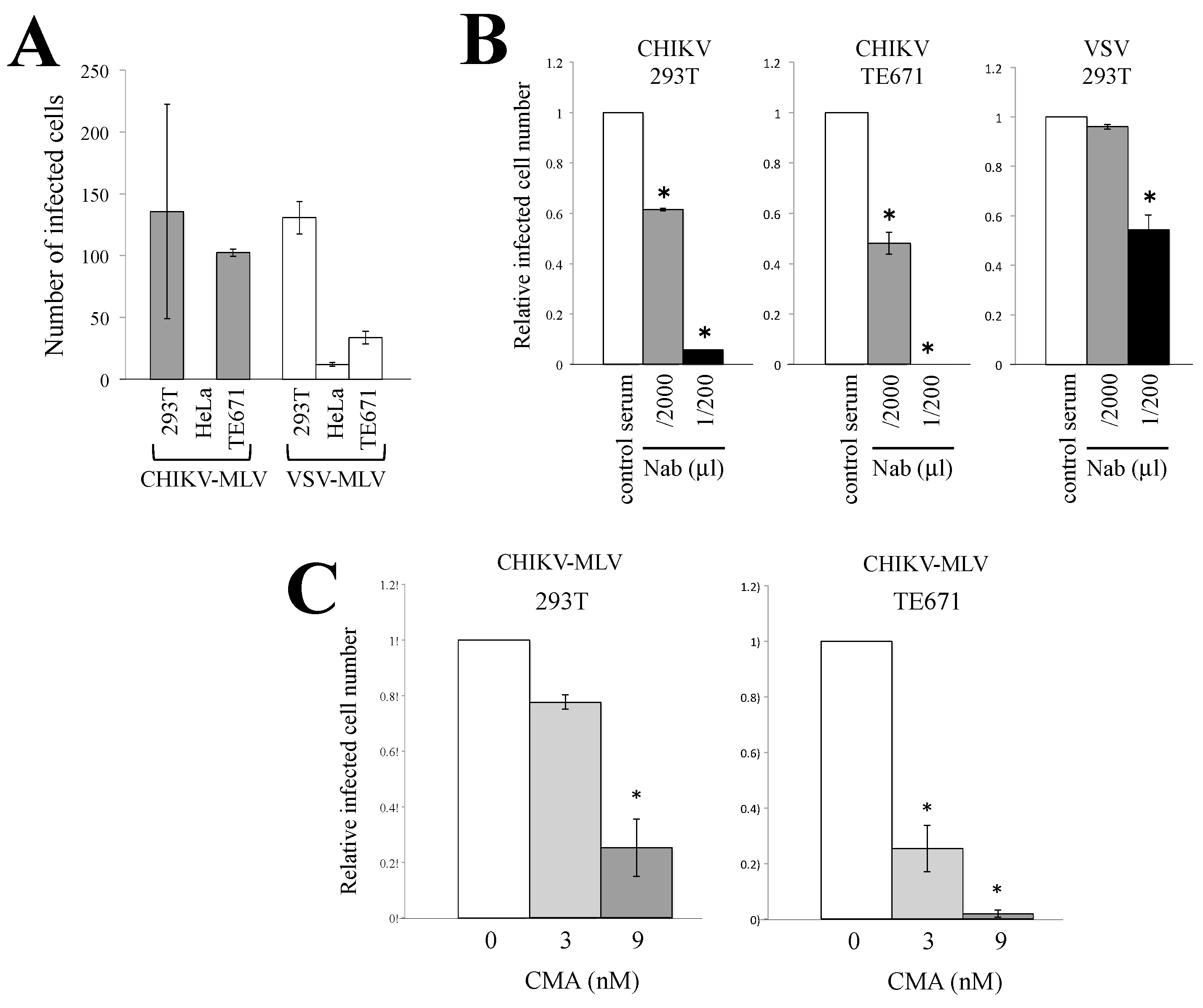
3.1. Characterization of CHIKV-Pseudotyped Retrovirus Vector
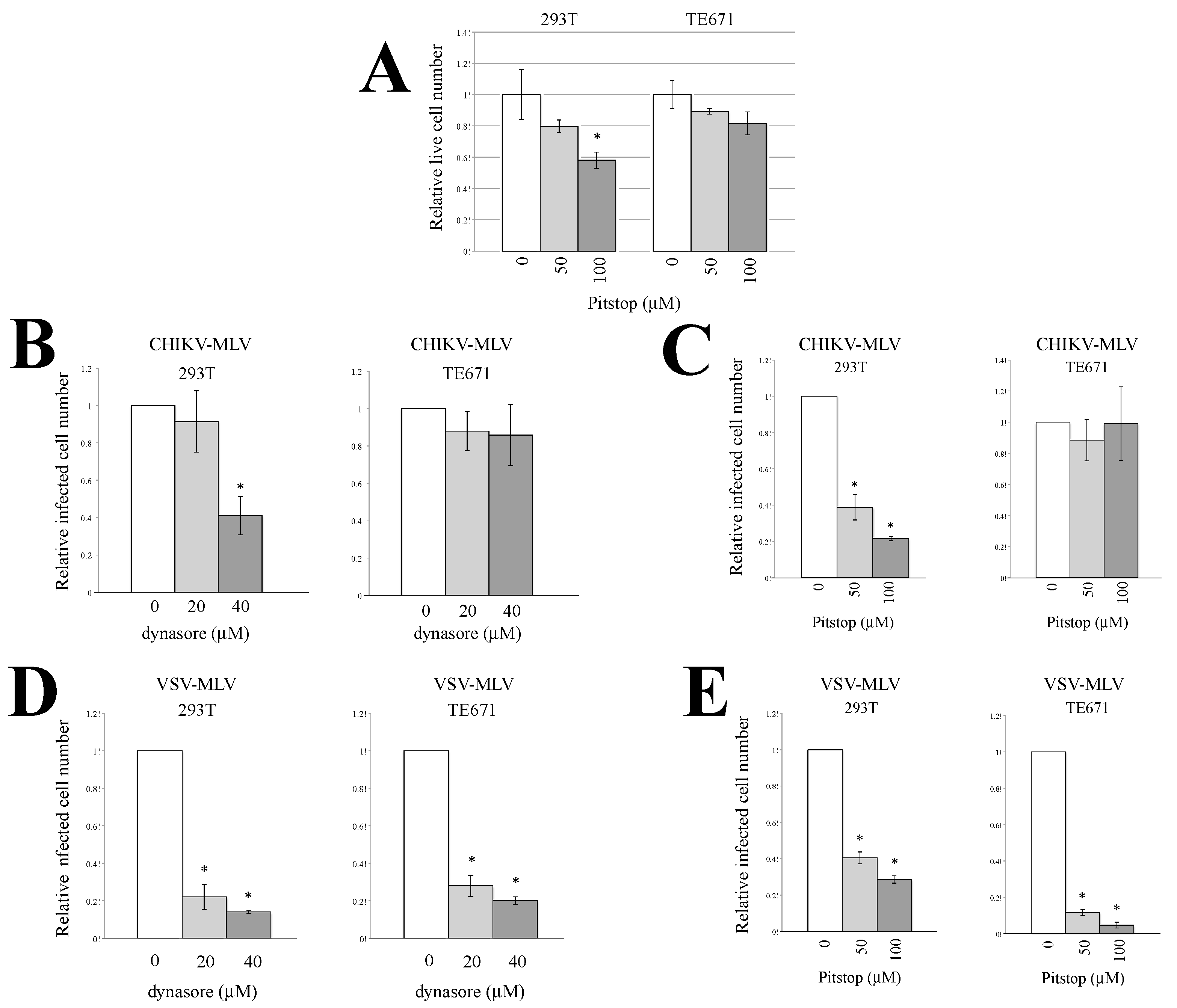
3.2. Endocytosis Is Required for CHIKV-Pseudotyped MLV Vector Infection in 293T but Not in TE671 Cells
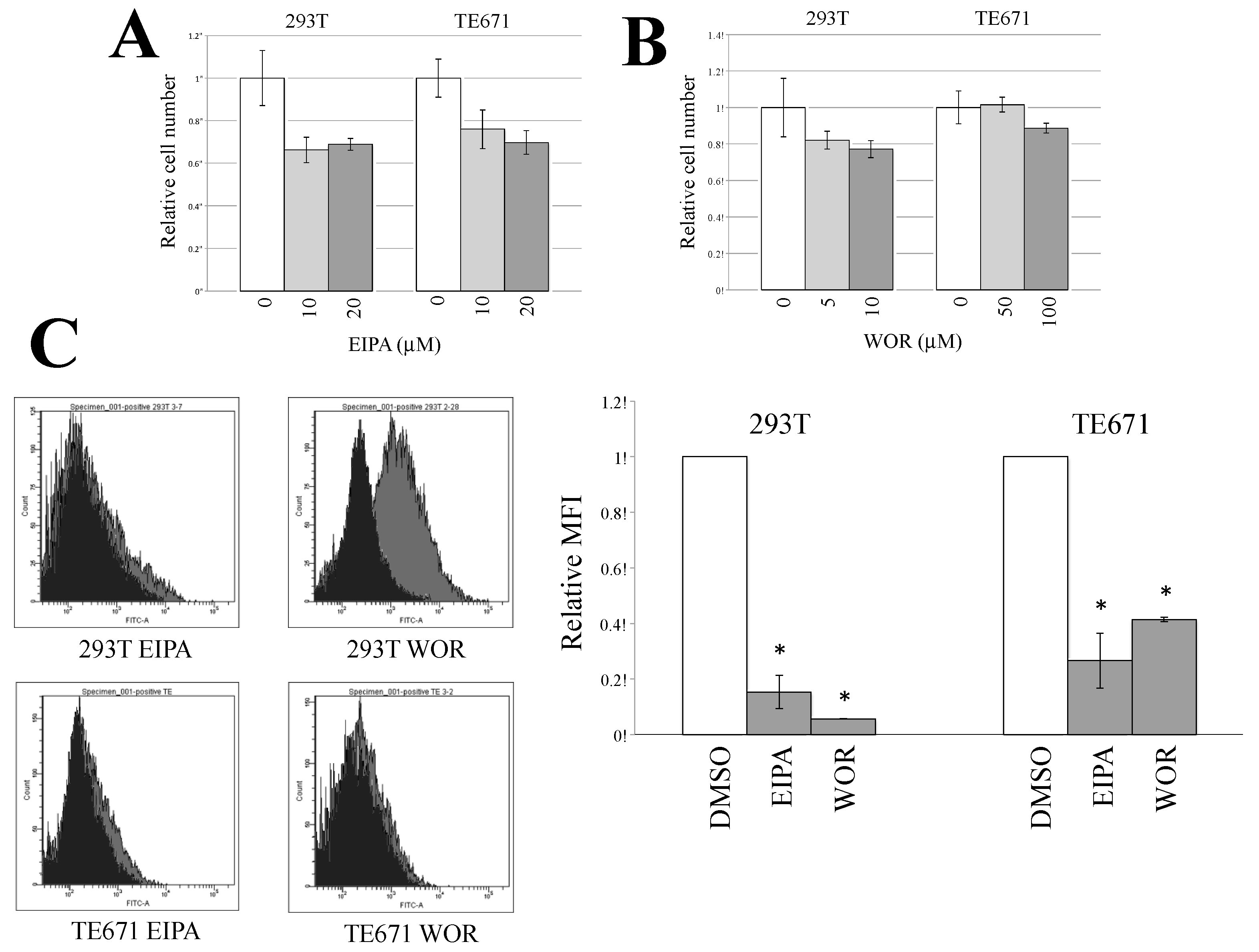
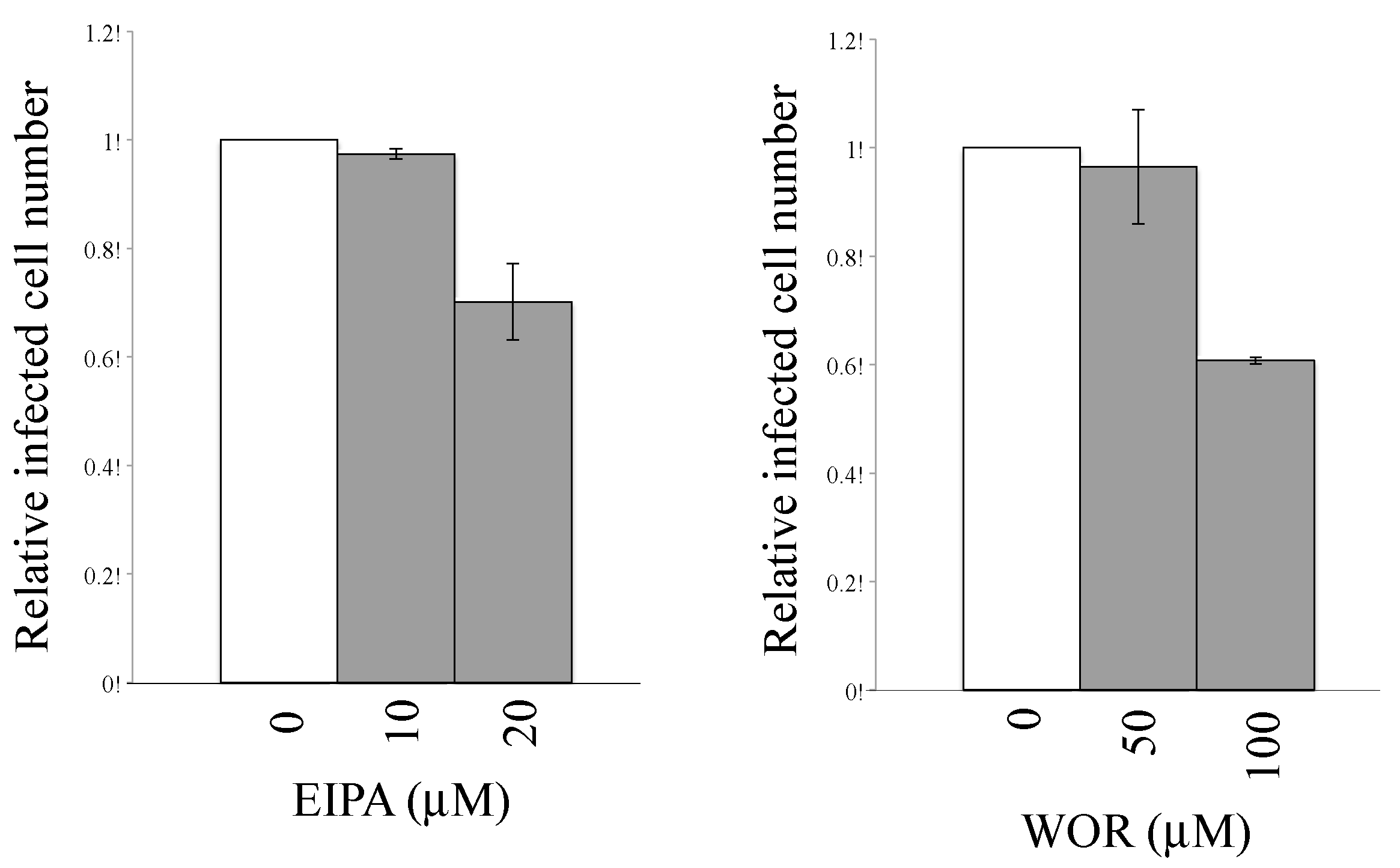
3.3. Macropinocytosis Is Required for CHIKV-Pseudotyped MLV Vector Infection in TE671 Cells but Not in 293T Cells
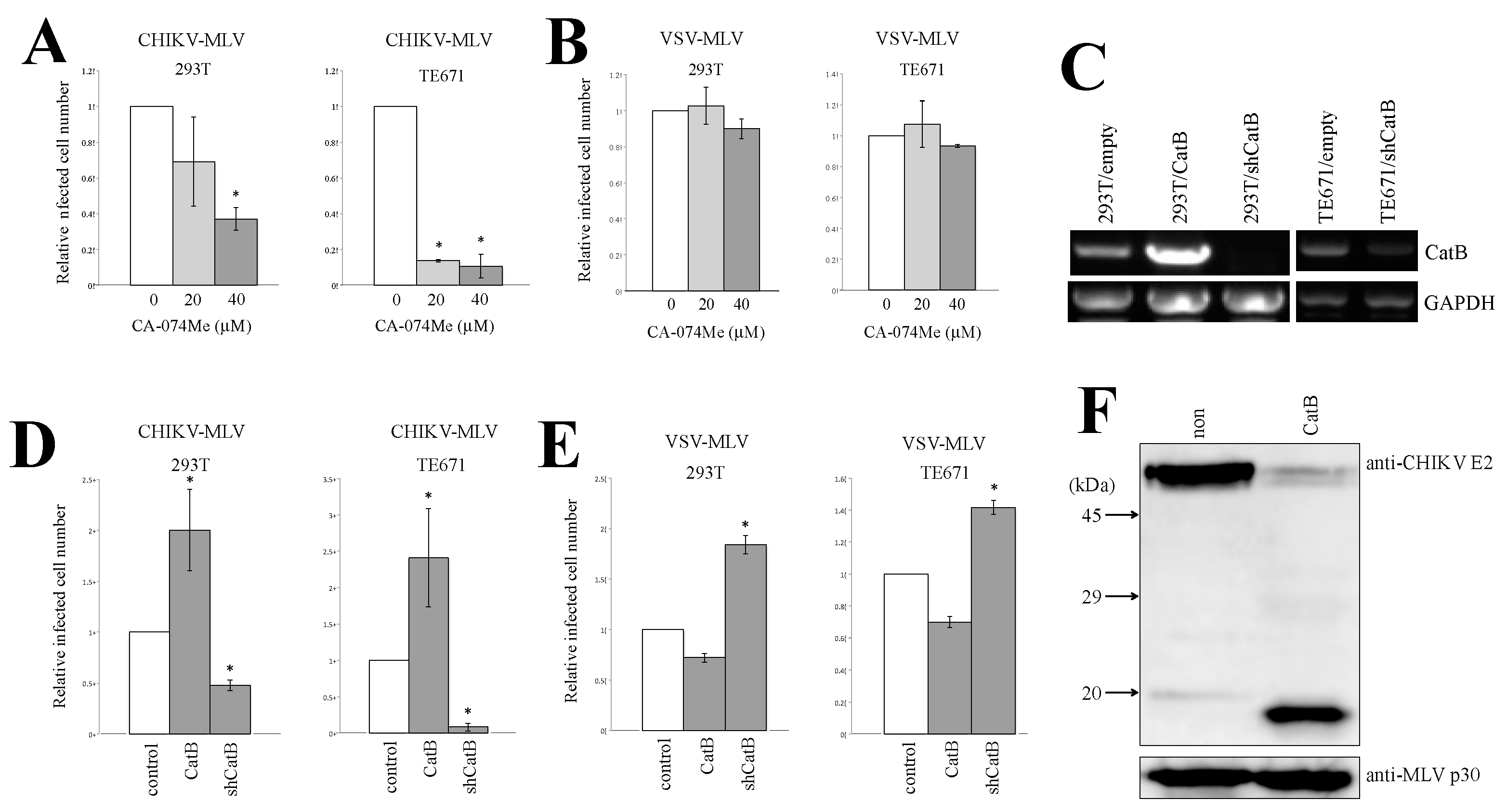
3.4. Cathepsin Protease Is Required for CHIKV-Pseudotyped MLV Vector Infection in 293T and TE671 Cells
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Vairo, F.; Haider, N.; Kock, R.; Ntoumi, F.; Ippolito, G.; Zumla, A. Chikungunya: Epidemiology, pathogenesis, clinical features, management, and prevention. Infect. Dis. Clin. N. Am. 2019, 33, 1003–1025. [Google Scholar] [CrossRef]
- Wintachai, P.; Wikan, N.; Kuadkitkan, A.; Jaimipuk, T.; Ubol, S.; Pulmanausahakul, R.; Auewarakul, P.; Kasinrerk, W.; Weng, W.Y.; Panyasrivanit, M.; et al. Identification of prohibitin as a Chikungunya virus receptor protein. J. Med. Virol. 2012, 84, 1757–1770. [Google Scholar] [CrossRef]
- Moller-Tank, S.; Kondratowicz, A.S.; Davey, R.A.; Rennert, P.D.; Maury, W. Role of the phosphatidylserine receptor TIM-1 in enveloped-cirus entry. J. Virol. 2013, 87, 8327–8341. [Google Scholar] [CrossRef]
- Silva, L.A.; Khomandiak, S.; Ashbrook, A.W.; Weller, R.; Heise, M.T.; Morrison, T.E.; Dermody, T.S. A single-amino-acid polymorphism in Chikungunya virus E2 glycoprotein influences glycosaminoglycan utilization. J. Virol. 2014, 88, 2385–2397. [Google Scholar] [CrossRef]
- Tanaka, A.; Tumkosit, U.; Nakamura, S.; Motooka, D.; Kishishita, N.; Priengprom, T.; Sa-Ngasang, A.; Kinoshita, T.; Takeda, N.; Maeda, Y. Genome-wide screening uncovers the significance of N-sulfation of heparan sulfate as a host cell factor for chikungunya virus infection. J. Virol. 2017, 91, e00432-17. [Google Scholar] [CrossRef]
- Fongsaran, C.; Jirakanwisal, K.; Kuadkitkan, A.; Wikan, N.; Wintachai, P.; Theppsrit, C.; Ubol, S.; Phaonakrop, N.; Roytrakul, S.; Smith, D.R. Involvement of ATP synthase β subunit in chikungunya virus entry into insect cells. Arch. Virol. 2014, 159, 3353–3364. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Kim, A.S.; Fox, J.M.; Nair, S.; Basore, K.; Klimstra, W.B.; Rimkunas, R.; Fong, R.H.; Lin, H.; Poddar, S.; et al. Mxra8 is a receptor for multiple arthritogenic alphaviruses. Nature 2018, 557, 570–574. [Google Scholar] [CrossRef] [PubMed]
- Sourisseau, M.; Schilte, C.; Casartelli, N.; Trouilliet, C.; Guivel-Benhassine, F.; Rudnicka, D.; Sol-Foulon, N.; Le, R.K.; Prevost, M.C.; Fsihi, H.; et al. Characterization of reemerging chigungunya virus. PLoS Pathog. 2007, 3, e89. [Google Scholar] [CrossRef] [PubMed]
- Bernard, E.; Solignat, M.; Gay, B.; Chazal, N.; Higgs, S.; Devaux, C.; Briant, L. Endocytosis of chikungunya virus into mammalian cells: Role of clathrin and early endosomal compartments. PLoS ONE 2010, 5, e11479. [Google Scholar] [CrossRef]
- Hoornweg, T.E.; van Duijl-Richter, M.K.S.; Ayala Nuñez, N.V.; Albulescu, I.C.; van Hemert, M.J.; Smit, J.M. Dynamics of chikungunya virus cell entry unraveled by single-virus tracking in living cells. J. Virol. 2016, 90, 4745–4756. [Google Scholar] [CrossRef]
- Lee, R.C.; Hapuarachchi, H.C.; Chen, K.C.; Hussain, K.M.; Chen, H.; Low, S.L.; Ng, L.C.; Lin, R.; Ng, M.M.; Chu, J.J. Mosquito cellular factors and functions in mediating the infectious entry of chikungunya virus. PLoS Negl. Trop. Dis. 2013, 7, e2050. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; Santhosh, S.R.; Tiwari, M.; Lakshmana Rao, P.V.; Parida, M. Assessment of in vitro prophylactic and therapeutic efficacy of chloroquine against chikungunya virus in vero cells. J. Med. Virol. 2010, 82, 817–824. [Google Scholar] [CrossRef] [PubMed]
- Gay, B.; Bernardm, E.; Solignat, M.; Chazal, N.; Devaux, C.; Briant, L. pH-dependent entry of chikungunya virus into Aedes albopictus cells. Infect. Genet. Evol. 2012, 12, 1275–1281. [Google Scholar] [CrossRef] [PubMed]
- Nuckols, J.T.; McAuley, A.J.; Huang, Y.J.; Horne, K.M.; Higgs, S.; Davey, R.A.; Vanlandingham, D.L. pH-Dependent entry of chikungunya virus fusion into mosquito cells. Virol. J. 2014, 11, 215. [Google Scholar] [CrossRef]
- Krejbich-Trotot, P.; Denizot, M.; Hoarau, J.J.; Jaffar-Bandjee, M.C.; Gasque, P. Chikungunya virus mobilizes the apoptotic machinery to invade host cell defenses. FASEB J. 2011, 25, 314–325. [Google Scholar] [CrossRef]
- Takada, A.; Kawaoka, Y. Antibody-dependent enhancement of viral infection: Molecular mechanisms and in vivo implications. Rew. Med. Virol. 2003, 13, 387–398. [Google Scholar] [CrossRef]
- Aleksandrowicz, P.; Marzi, A.; Biedenkopf, N.; Beimforde, N.; Becker, S.; Hoenen, T.; Feldmann, H.; Schnittler, H.J. Ebola virus enters host cells by macropinocytosis and clathrin-mediated endocytosis. J. Infect. Dis. 2011, 204, S957–S967. [Google Scholar] [CrossRef]
- Kamiyama, H.; Kakoki, K.; Yoshii, H.; Iwao, M.; Igawa, T.; Sakai, H.; Hayashi, H.; Matsuyama, T.; Yamamoto, N.; Kubo, Y. Infection of XC cells by MLVs and Ebola virus is endosome-dependent but acidification-independent. PLoS ONE 2011, 6, e26180. [Google Scholar] [CrossRef]
- Simmons, G.; Reeves, J.D.; Rennekamp, A.J.; Smberg, S.M.; Piefer, A.J.; Bates, P. Characterization of severe acute respiratory syndrome-associated coronavirus (SARS-CoV) spike glycoprotein-mediated viral entry. Proc. Natl. Acad. Sci. USA 2004, 101, 4240–4245. [Google Scholar] [CrossRef]
- Qu, X.; Liu, Y.; Lei, X.; Li, P.; Mi, D.; Ren, L.; Guo, L.; Guo, R.; Chen, T.; Hu, J.; et al. Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Nat. Commun. 2020, 11, 1620. [Google Scholar]
- Chandran, K.; Sullivan, N.J.; Felbor, U.; Whelan, S.P.; Cunningham, J.M. Endosomal proteolysis of the Ebola virus glycoprotein is necessary for infection. Science 2005, 308, 1643–1645. [Google Scholar] [CrossRef] [PubMed]
- Simmons, G.; Gosalia, D.N.; Rennekamp, A.J.; Reeves, J.D.; Diamond, S.L.; Bates, P. Inhibitors of cathepsin L prevent severe acute respiratory syndrome coronavirus entry. Proc. Natl. Acad. Sci. USA 2005, 102, 11876–11881. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Nachagari, D.; Fields, C.; Franks, J.; Albritton, L.M. Host cell cathepsins potentiate Moloney murine leukemia virus infection. J. Virol. 2007, 81, 10506–10514. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yoshii, H.; Kamiyama, H.; Minematsu, K.; Goto, K.; Mizota, T.; Oishi, K.; Katunuma, N.; Yamamoto, N.; Kubo, Y. Cathepsin L is required for ecotropic murine leukemia virus infection in NIH3T3 cells. Virology 2009, 394, 227–234. [Google Scholar] [CrossRef][Green Version]
- Fu, J.Y.L.; Chua, C.L.; Vythilingam, I.; Sulaiman, W.Y.W.; Wong, H.V.; Chan, Y.F.; Sam, I.C. An amino acid change in nsP4 of chikungunya virus confers fitness advantage in human cell lines rather than in Aedes albopictus. J. Gen. Virol. 2019, 100, 1541–1553. [Google Scholar] [CrossRef]
- Oo, A.; Rausalu, K.; Merits, A.; Higgs, S.; Vanlandingham, D.; Baker, S.A.; Zandi, K. Deciphering the potential of baicalin as an antiviral agent for Chikungnya virus infection. Antivir. Res. 2018, 150, 101–111. [Google Scholar] [CrossRef]
- Chalaem, P.; Chusri, S.; Fernandez, S.; Chotigeat, W.; Anguita, J.; Pal, U.; Promnares, K. Characterization of a Chikungunya virus strain isolated from banked patients’ sera. Virol. J. 2016, 13, 150. [Google Scholar] [CrossRef][Green Version]
- Paingankar, M.S.; Arankalle, A. Identification of chikungunya virus interacting proteins in mammalian cells. J. Biosci. 2014, 39, 389–399. [Google Scholar] [CrossRef]
- Matkovic, R.; Bernard, E.; Fontanel, S.; Eldin, P.; Chazal, N.; Hersi, D.H.; Merits, A.; Peloponese, J.M.J.; Briant, L. The host DHX9 DExH-box helicase is recuited to chikungnya virus replication complexes for optimal genomic RNA translation. J. Virol. 2019, 93, e01764-18. [Google Scholar]
- Zhang, N.; Zhao, H.; Zhang, L. Fatty acid syntase promotes the palmitoylation of chikungunya virus nsP1. J. Virol. 2019, 93, e01747-18. [Google Scholar]
- Roberts, G.C.; Zothner, C.; Remenyi, R.; Merits, A.; Stonehouse, N.J.; Harris, M. Evaluation of a range of mammalian and mosquito cell lines for use in Chikungunya virus research. Sci. Rep. 2017, 7, 14641. [Google Scholar] [CrossRef]
- Couderc, T.; Chretien, F.; Schilte, C.; Disson, O.; Brigitte, M.; Guivel-Benhassine, F.; Touret, Y.; Barau, G.; Cayet, N.; Schuffenecker, I.; et al. A mouse model for Chikungunya: Young age and inefficient type-I interferon signaling are risk factors for severe disease. PLoS Pathog. 2008, 4, e29. [Google Scholar] [CrossRef]
- Akache, B.; Grimm, D.; Shen, X.; Fuess, S.; Yant, S.R.; Glazer, D.S.; Park, J.; Kay, M.A. A two-hybrid screen identifies cathepsin B and L as uncoating factors for adeno-associated virus 2 and 8. Mol. Ther. 2007, 15, 330–339. [Google Scholar] [CrossRef] [PubMed]
- Maetzig, T.; Galla, M.; Baum, C.; Schambach, A. Gammaretroviral vectors: Biology, technology, and application. Viruses 2011, 3, 677–713. [Google Scholar] [CrossRef] [PubMed]
- Mann, R.; Mulligan, R.C.; Baltimore, D. Construction of a retrovirus packaging mutant and its use to produce helper-free defective retrovirus. Cell 1983, 33, 153–159. [Google Scholar] [CrossRef]
- Iwakuma, T.; Cui, Y.; Chang, L.J. Self-inactivating lentivirus vectors with U3 and U5 modifications. Virology 1999, 261, 120–132. [Google Scholar] [CrossRef] [PubMed]
- Pandey, B.D.; Neupane, B.; Pandey, K.; Tun, M.M.; Morita, K. Detection of chikungunya virus in Nepal. Am. J. Trop. Med. Hyg. 2015, 93, 697–700. [Google Scholar] [CrossRef]
- Pandey, K.; Pandey, B.D.; Chauraslya, R.R.; Thakur, M.; Neupane, B.; Shah, Y.; Tun, M.M.; Morita, K. Evidence of chikungunya virus circulation in the Terai region of Nepal in 2014 and 2015. Trans. R. Soc. Trop. Med. Hyg. 2017, 111, 294–299. [Google Scholar] [CrossRef]
- Ngwe Tun, M.M.; Inoue, S.; Thant, K.Z.; Talemaitoga, N.; Aryati, A.; Dimaano, E.M.; Matias, R.R.; Buerano, C.C.; Natividad, F.F.; Abeyewickremw, W.; et al. Retrospective seroepidemiological study of chikungunya infection in South Asia, Southeast Asia and the Pacific region. Epidemiol. Infect. 2016, 144, 2268–2275. [Google Scholar] [CrossRef]
- Guinea, R.; Carrasco, L. Concanamycin A: A powerful inhibitor of enveloped animal virus entry into cells. Biochem. Biophys. Res. Commun. 1994, 201, 1270–1278. [Google Scholar] [CrossRef]
- Macia, E.; Ehrlich, M.; Massol, R.; Boucrot, E.; Brunner, C.; Kirchhausen, T. Dynasore, a cell-permeable inhibitor of dynamin. Dev. Cell 2006, 10, 839–850. [Google Scholar] [CrossRef] [PubMed]
- Robertson, M.J.; Deane, F.M.; Stahlschmidt, W.; von Kleist, L.; Haucke, V.; Robinson, P.J.; McCluskey, A. Synthesis of the Pitstop family of clathrin inhibitors. Nat. Protoc. 2014, 9, 1592–1606. [Google Scholar] [CrossRef] [PubMed]
- Vigne, P.; Frelin, C.; Cragoe, E.J.J.; Lazdunski, M. Ethylisopropyl-amiloride: A new and highly potent derivative of amiloride for the inhibition of the Na+ H+ exchange system in various cell types. Biochem. Biophys. Res. Commun. 1983, 116, 86–90. [Google Scholar] [CrossRef]
- Wymann, M.P.; Bulgarelli-Leva, G.; Zvelebil, M.J.; Pirola, L.; Vanhaesebroeck, B.; Waterfield, M.D.; Panayotou, G. Wortmannin inactivates phosphoinositide 3-kinase by covalent modification of Lys-802, a residue involved in the phosphate transfer reaction. Mol. Cell. Biol. 1996, 16, 1722–1733. [Google Scholar] [CrossRef]
- Hua, C.; Lee, R.; Hussain, K.M.; Chu, J.J.H. Macropinocytosis dependent entry of Chikungunya virus into human muscle cells. PLoS Negl. Trop. Dis. 2019, 13, e0007610. [Google Scholar]
- Kubo, Y.; Hayashi, H.; Matsuyama, T.; Sato, H.; Yamamoto, N. Retroviral entry by endocytosis and cathepsin proteases. Adv. Virol. 2012, 2012, 640894. [Google Scholar] [CrossRef]
- Voss, J.E.; Vaney, M.C.; Duquerroy, S.; Vonrhein, C.; Girard-Blanc, C.; Crublet, E.; Thompson, A.; Bricogne, G.; Rey, F.A. Glycoprotein organization of Chikungunya virus particles revealed by X-ray crystallography. Nature 2010, 468, 709–712. [Google Scholar] [CrossRef]
- Yoshii, H.; Kamiyama, H.; Goto, K.; Oishi, K.; Katsunuma, N.; Tanaka, Y.; Hayashi, H.; Matsuyama, T.; Sato, H.; Yamamoto, N.; et al. CD4-independent human immunodeficiency virus infection involves participation of endocytosis and cathepsin B. PLoS ONE 2011, 6, e19352. [Google Scholar] [CrossRef]
- Judith, D.; Montowy, S.; Bourai, M.; Gangneux, N.; Lelek, M.; Lucas-Hourani, M.; Cayet, N.; Jacob, Y.; Prevost, M.C.; Pierre, P.; et al. Species-specific impact of the autophagy machinery on chikungunya virus infection. EMBO Rep. 2013, 14, 534–544. [Google Scholar] [CrossRef]
- Rubinsztein, D.C.; Codogno, P.; Levine, B. Autophagy modulation as a potential therapeutic target for diverse diseases. Nat. Rev. Drug Discov. 2012, 11, 709–730. [Google Scholar] [CrossRef]
- Qiao, S.; Tao, S.; de la Vega, M.R.; Park, S.L.; Vonderfecht, A.A.; Jacobs, S.L.; Zhang, D.D.; Wondrak, G.T. The antimalaria amodiaquine causes autophagic lysosomal and prokiferative blockade sensitization human melanoma cells to starvation, and chemothrapy-induced cell deth. Autophagy 2013, 9, 2087–2102. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Zhang, L. Key components of COPI and COPII machineries are required for chikungunya virus replication. Biochem. Biophys. Res. Commun. 2017, 493, 1190–1196. [Google Scholar] [CrossRef] [PubMed]
- Khongwichit, S.; Wikan, N.; Abere, B.; Thepparit, C.; Kuadkitkan, A.; Ubol, S.; Smith, D.R. Cell-type specific variation in the induction of ER stress and downstream events in chikungunya virus infection. Microb. Pathog. 2016, 101, 104–118. [Google Scholar] [CrossRef]
- Ying, Z.; Sandoval, M.; Beronja, S. Oncogenic activation of PI3K induces progenitor cell differentiation to suppress epidermal growth. Nat. Cell Biol. 2018, 20, 1256–1266. [Google Scholar] [CrossRef] [PubMed]
- Russell, D.W.; Berger, M.S.; Miller, A.D. The effects of human serum and cerebrospinal fluid on retroviral vectors and packaging cell lines. Hum. Gene Ther. 1995, 6, 635–641. [Google Scholar] [CrossRef] [PubMed]
- Guibinga, G.H.; Miyanohara, A.; Esko, J.D.; Friedmann, T. Cell surface heparan sulfate is a receptor for attachment of envelope protein-free retrovirus-like particles and VSV-G pseudotyped MLV-derived retrovirus vectors to target cells. Mol. Ther. 2002, 5, 538–546. [Google Scholar] [CrossRef] [PubMed]
- Batra, R.K.; Olsen, J.C.; Hoganson, D.K.; Caterson, B.; Boucher, R.C. Retroviral gene transfer is inhibited by chondroitin sulfate proteoglycans/glycosaminoglycans in malignant pleural effusions. J. Biol. Chem. 1997, 272, 11736–11743. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Izumida, M.; Hayashi, H.; Tanaka, A.; Kubo, Y. Cathepsin B Protease Facilitates Chikungunya Virus Envelope Protein-Mediated Infection Via Endocytosis or Macropinocytosis. Viruses 2020, 12, 722. https://doi.org/10.3390/v12070722
Izumida M, Hayashi H, Tanaka A, Kubo Y. Cathepsin B Protease Facilitates Chikungunya Virus Envelope Protein-Mediated Infection Via Endocytosis or Macropinocytosis. Viruses. 2020; 12(7):722. https://doi.org/10.3390/v12070722
Chicago/Turabian StyleIzumida, Mai, Hideki Hayashi, Atsushi Tanaka, and Yoshinao Kubo. 2020. "Cathepsin B Protease Facilitates Chikungunya Virus Envelope Protein-Mediated Infection Via Endocytosis or Macropinocytosis" Viruses 12, no. 7: 722. https://doi.org/10.3390/v12070722
APA StyleIzumida, M., Hayashi, H., Tanaka, A., & Kubo, Y. (2020). Cathepsin B Protease Facilitates Chikungunya Virus Envelope Protein-Mediated Infection Via Endocytosis or Macropinocytosis. Viruses, 12(7), 722. https://doi.org/10.3390/v12070722





