Flavivirus Infection Associated with Cerebrovascular Events
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Medical History and Sample Collection
2.3. Diagnostic Analyses
2.3.1. Virus RNA Extraction and Real Time Multiplex PCR
2.3.2. Enzyme-Linked Immunosorbent Assay (ELISA)
2.3.3. Rapid Immunochromatographic Assay (ICA)
3. Results
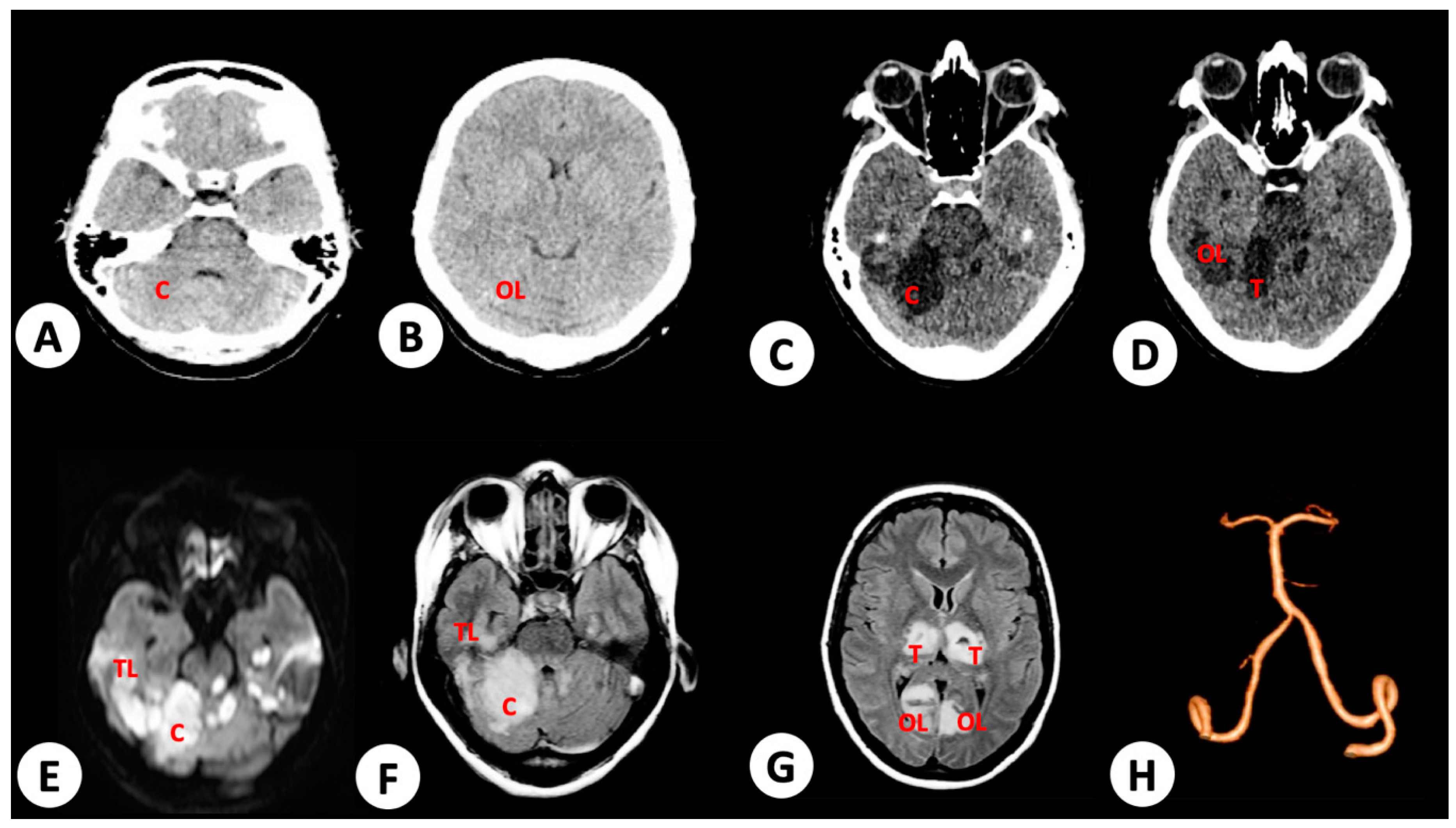
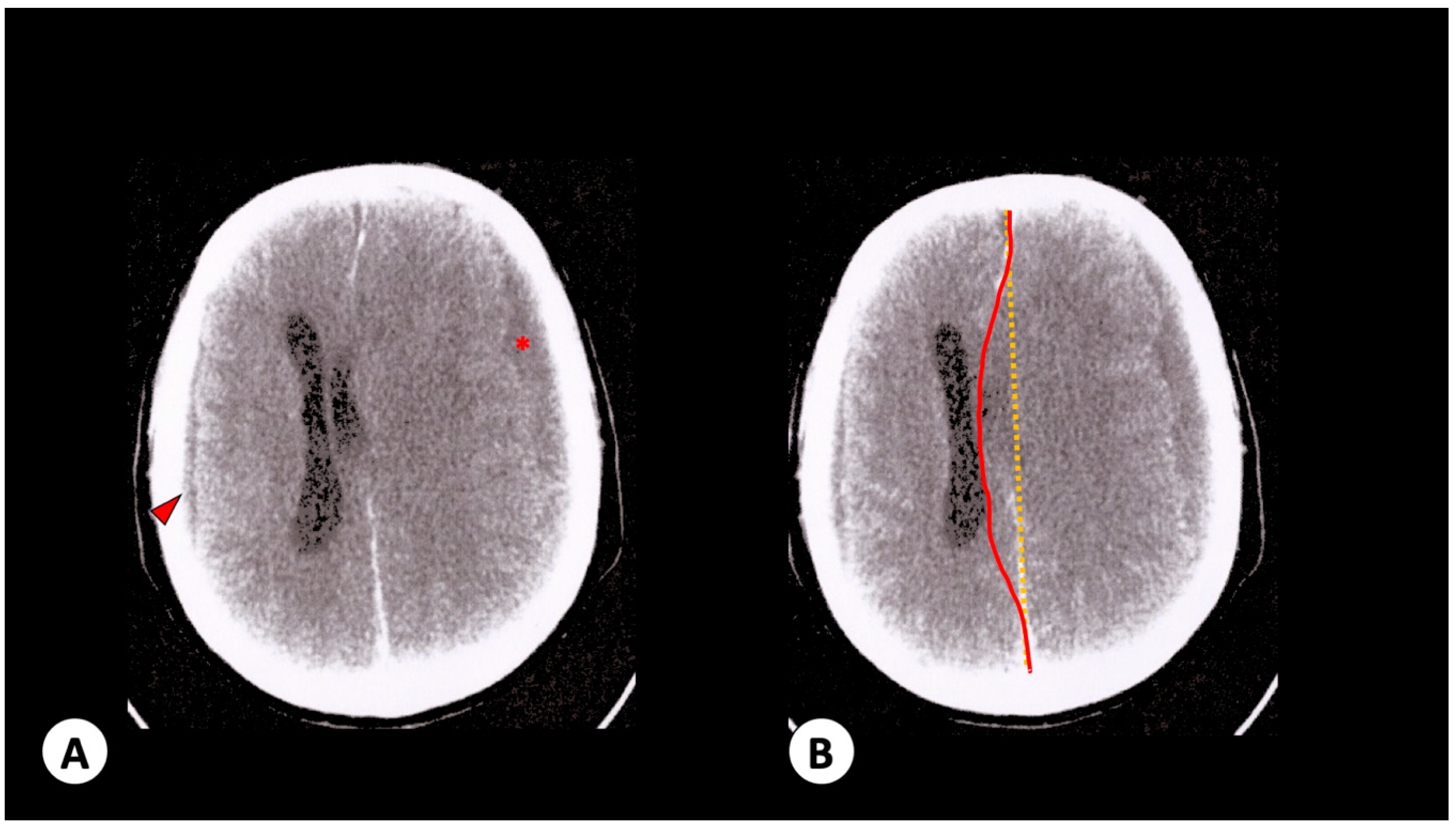
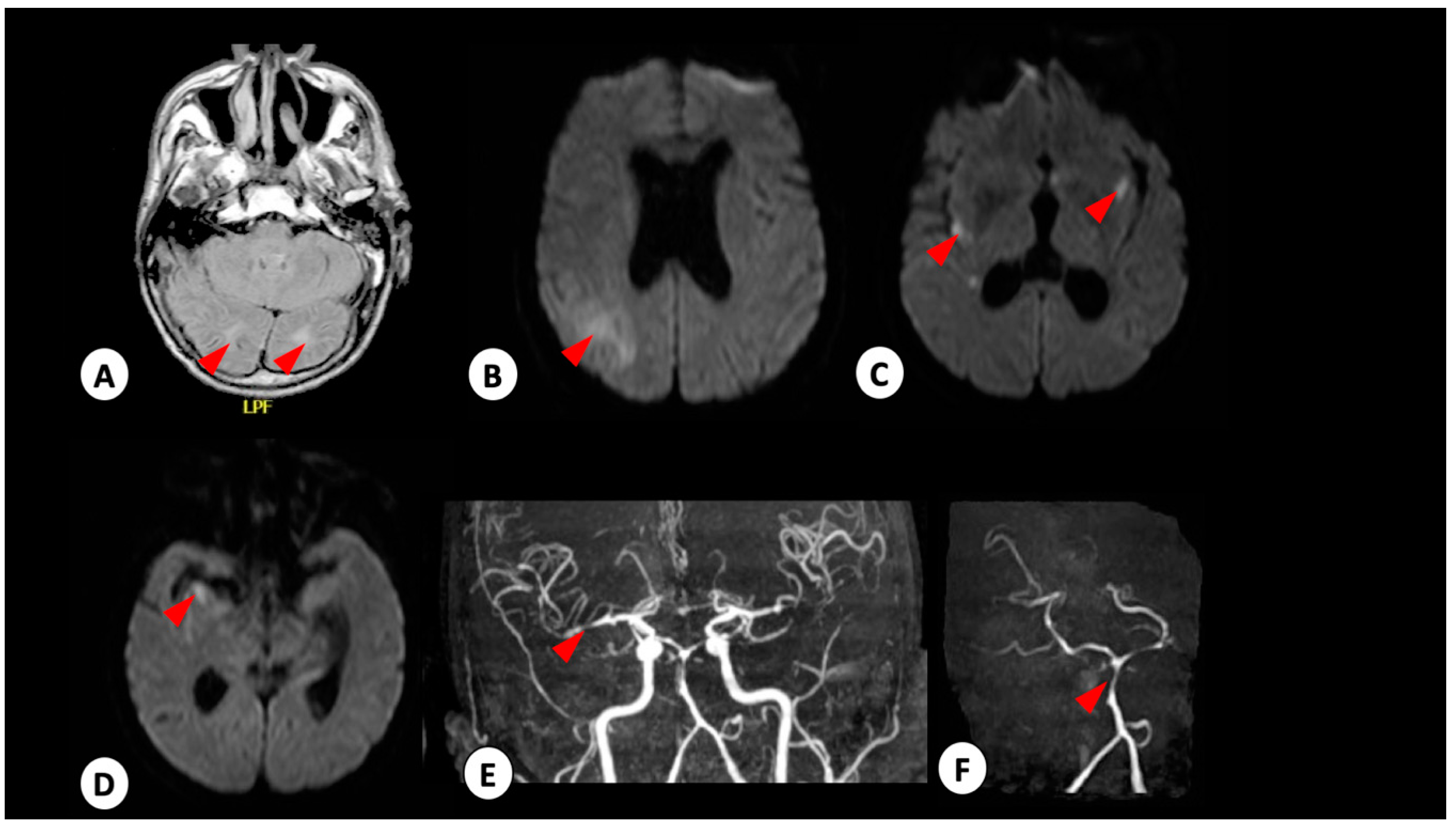
3.1. Case 1
3.2. Case 2
3.3. Case 3
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Wilder-Smith, A.; Gubler, D.J.; Weaver, S.C.; Monath, T.P.; Heymann, D.L.; Scott, T.W. Epidemic arboviral diseases: Priorities for research and public health. Lancet Infect. Dis. 2017, 17, e101–e106. [Google Scholar] [CrossRef]
- Bhatt, S.; Gething, P.W.; Brady, O.J.; Messina, J.P.; Farlow, A.W.; Moyes, C.L.; Drake, J.M.; Brownstein, J.S.; Hoen, A.G.; Sankoh, O.; et al. The global distribution and burden of dengue. Nature 2013, 496, 504–507. [Google Scholar] [CrossRef] [PubMed]
- Duffy, M.R.; Chen, T.H.; Hancock, W.T.; Powers, A.M.; Kool, J.L.; Lanciotti, R.S.; Pretrick, M.; Marfel, M.; Holzbauer, S.; Dubray, C.; et al. Zika virus outbreak on Yap Island, Federated States of Micronesia. N. Engl. J. Med. 2009, 360, 2536–2543. [Google Scholar] [CrossRef]
- Aliota, M.T.; Bassit, L.; Bradrick, S.S.; Cox, B.; Garcia-Blanco, M.A.; Gavegnano, C.; Friedrich, T.C.; Golos, T.G.; Griffin, D.E.; Haddow, A.D.; et al. Zika in the Americas, year 2: What have we learned? What gaps remain? A report from the Global Virus Network. Antivir. Res. 2017, 144, 223–246. [Google Scholar] [CrossRef] [PubMed]
- Araujo, F.M.; Araujo, M.S.; Nogueira, R.M.; Brilhante, R.S.; Oliveira, D.N.; Rocha, M.F.; Cordeiro, R.A.; Araujo, R.M.; Sidrim, J.J. Central nervous system involvement in dengue: A study in fatal cases from a dengue endemic area. Neurology 2012, 78, 736–742. [Google Scholar] [CrossRef] [PubMed]
- Weeratunga, P.N.; Caldera, M.C.; Gooneratne, I.K.; Gamage, R.; Perera, P. Neurological manifestations of dengue: A cross sectional study. Travel Med. Infect. Dis. 2014, 12, 189–193. [Google Scholar] [CrossRef]
- Goncalves, E. Acute inflammatory demyelinating polyradiculoneuropathy (Guillain-Barre syndrome) following dengue fever. Rev. Do Inst. De Med. Trop. De Sao Paulo 2011, 53, 223–225. [Google Scholar] [CrossRef][Green Version]
- Sharma, C.M.; Kumawat, B.L.; Ralot, T.; Tripathi, G.; Dixit, S. Guillain-Barre syndrome occurring during dengue fever. J. Indian Med. Assoc. 2011, 109, 682. [Google Scholar]
- Soares, C.N.; Cabral-Castro, M.; Oliveira, C.; Faria, L.C.; Peralta, J.M.; Freitas, M.R.; Puccioni-Sohler, M. Oligosymptomatic dengue infection: A potential cause of Guillain Barré syndrome. Arq. Neuro Psiquiatr. 2008, 66, 234–237. [Google Scholar] [CrossRef]
- Ralapanawa, D.M.; Kularatne, S.A.; Jayalath, W.A. Guillain-Barre syndrome following dengue fever and literature review. BMC Res. Notes 2015, 8, 729. [Google Scholar] [CrossRef]
- Gutch, M.; Agarwal, A.; Amar, A. Hypokalemic quadriparesis: An unusual manifestation of dengue fever. J. Nat. Sci. Biol. Med. 2012, 3, 81–83. [Google Scholar] [CrossRef] [PubMed]
- Puccioni-Sohler, M.; Rosadas, C.; Cabral-Castro, M.J. Neurological complications in dengue infection: A review for clinical practice. Arq. De Neuro Psiquiatr. 2013, 71, 667–671. [Google Scholar] [CrossRef] [PubMed]
- Jha, S.; Ansari, M. Dengue infection causing acute hypokalemic quadriparesis. Neurol. India 2010, 58, 592. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.; Lehl, S.; Singh, R.; Sachdev, A. Hypokalemic periodic paralysis: 2 novel causes. Internet J. Neurol. 2008, 12, 1–3. [Google Scholar]
- Soares, C.N.; Brasil, P.; Carrera, R.M.; Sequeira, P.; de Filippis, A.B.; Borges, V.A.; Theophilo, F.; Ellul, M.A.; Solomon, T. Fatal encephalitis associated with Zika virus infection in an adult. J. Clin. Virol. 2016, 83, 63–65. [Google Scholar] [CrossRef]
- Mécharles, S.; Herrmann, C.; Poullain, P.; Tran, T.H.; Deschamps, N.; Mathon, G.; Landais, A.; Breurec, S.; Lannuzel, A. Acute myelitis due to Zika virus infection. Lancet 2016, 387, 1481. [Google Scholar] [CrossRef]
- Estofolete, C.F.; de Oliveira Mota, M.T.; Bernardes Terzian, A.C.; de Aguiar Milhim, B.H.G.; Ribeiro, M.R.; Nunes, D.V.; Mourão, M.P.; Rossi, S.L.; Nogueira, M.L.; Vasilakis, N. Unusual clinical manifestations of dengue disease—Real or imagined? Acta Trop. 2019, 199, 105134. [Google Scholar] [CrossRef]
- Moore, C.A.; Staples, J.E.; Dobyns, W.B.; Pessoa, A.; Ventura, C.V.; Fonseca, E.B.; Ribeiro, E.M.; Ventura, L.O.; Neto, N.N.; Arena, J.F.; et al. Characterizing the Pattern of Anomalies in Congenital Zika Syndrome for Pediatric Clinicians. JAMA Pediatr. 2017, 171, 288–295. [Google Scholar] [CrossRef]
- Krauer, F.; Riesen, M.; Reveiz, L.; Oladapo, O.T.; Martínez-Vega, R.; Porgo, T.V.; Haefliger, A.; Broutet, N.J.; Low, N.; Group, W.Z.C.W. Zika Virus Infection as a Cause of Congenital Brain Abnormalities and Guillain-Barré Syndrome: Systematic Review. PLoS Med. 2017, 14, e1002203. [Google Scholar] [CrossRef]
- Oehler, E.; Watrin, L.; Larre, P.; Leparc-Goffart, I.; Lastere, S.; Valour, F.; Baudouin, L.; Mallet, H.; Musso, D.; Ghawche, F. Zika virus infection complicated by Guillain-Barre syndrome—Case report, French Polynesia, December 2013. Euro Surveill. 2014, 19, 20720. [Google Scholar] [CrossRef]
- Dirlikov, E.; Major, C.G.; Mayshack, M.; Medina, N.; Matos, D.; Ryff, K.R.; Torres-Aponte, J.; Alkis, R.; Munoz-Jordan, J.; Colon-Sanchez, C.; et al. Guillain-Barré Syndrome During Ongoing Zika Virus Transmission—Puerto Rico, January 1–July 31, 2016. Morb. Mortal. Wkly. Rep. 2016, 65, 910–914. [Google Scholar] [CrossRef]
- do Rosário, M.S.; de Jesus, P.A.; Vasilakis, N.; Farias, D.S.; Novaes, M.A.; Rodrigues, S.G.; Martins, L.C.; Vasconcelos, P.F.; Ko, A.I.; Alcântara, L.C.; et al. Guillain-Barré Syndrome After Zika Virus Infection in Brazil. Am. J. Trop. Med. Hyg. 2016, 95, 1157–1160. [Google Scholar] [CrossRef] [PubMed]
- Salimi, H.; Cain, M.D.; Klein, R.S. Encephalitic Arboviruses: Emergence, Clinical Presentation, and Neuropathogenesis. Neurotherapeutics 2016, 13, 514–534. [Google Scholar] [CrossRef] [PubMed]
- Chanthamat, N.; Sathirapanya, P. Acute transverse myelitis associated with dengue viral infection. J. Spinal Cord Med. 2010, 33, 425–427. [Google Scholar] [CrossRef] [PubMed]
- Mota, M.T.; Estofolete, C.F.; Zini, N.; Terzian, A.C.; Gongora, D.V.; Maia, I.L.; Nogueira, M.L. Transverse Myelitis as an Unusual Complication of Dengue Fever. Am. J. Trop. Med. Hyg. 2017, 96, 380–381. [Google Scholar] [CrossRef]
- Cam, B.V.; Fonsmark, L.; Hue, N.B.; Phuong, N.T.; Poulsen, A.; Heegaard, E.D. Prospective case-control study of encephalopathy in children with dengue hemorrhagic fever. Am. J. Trop. Med. Hyg. 2001, 65, 848–851. [Google Scholar] [CrossRef]
- Row, D.; Weinstein, P.; Murray-Smith, S. Dengue fever with encephalopathy in Australia. Am. J. Trop. Med. Hyg. 1996, 54, 253–255. [Google Scholar] [CrossRef]
- Hendarto, S.K.; Hadinegoro, S.R. Dengue encephalopathy. Acta Paediatr. Jpn. 1992, 34, 350–357. [Google Scholar] [CrossRef]
- Mehta, M.; Sharma, P.K.; Garg, R.K. An Uncommon Complication of Dengue. J. Stroke Cereb. Dis. 2018, 27, e46–e47. [Google Scholar] [CrossRef]
- Mathew, S.; Pandian, J.D. Stroke in patients with dengue. J. Stroke Cerebrovasc. Dis. 2010, 19, 253–256. [Google Scholar] [CrossRef]
- Liou, L.-M.; Lan, S.-H.; Lai, C.-L. Dengue fever with ischemic stroke: A case report. Neurologist 2008, 14, 40–42. [Google Scholar] [CrossRef] [PubMed]
- Nanda, S.K.; Jayalakshmi, S.; Mohandas, S. Pediatric ischemic stroke due to dengue vasculitis. Pediatr. Neurol. 2014, 51, 570–572. [Google Scholar] [CrossRef] [PubMed]
- Verma, R.; Sahu, R.; Singh, A.; Atam, V. Dengue infection presenting as ischemic stroke: An uncommon neurological manifestation. Neurol. India 2013, 61, 317. [Google Scholar] [CrossRef]
- Yoganathan, S.; Sudhakar, S.V.; Priyambada, L.; Thomas, M. Stroke in a Child with Dengue Encephalopathy. Ann. Indian Acad. Neurol. 2017, 20, 329–331. [Google Scholar]
- Landais, A.; Césaire, A.; Fernandez, M.; Breurec, S.; Herrmann, C.; Delion, F.; Desprez, P. ZIKA vasculitis: A new cause of stroke in children? J. Neurol. Sci. 2017, 383, 211–213. [Google Scholar] [CrossRef] [PubMed]
- Kamath, S.R.; Ranjit, S. Clinical features, complications and atypical manifestations of children with severe forms of dengue hemorrhagic fever in South India. Indian J. Pediatr. 2006, 73, 889–895. [Google Scholar] [CrossRef]
- De Souza, L.J.; de Oliveira Martins, A.L.; Paravidini, P.C.L.; Nogueira, R.M.R.; Gicovate Neto, C.; Bastos, D.A.; Siqueira, E.W.S.; Carneiro, R.C. Hemorrhagic encephalopathy in dengue shock syndrome: A case report. Braz. J. Infect. Dis. 2005, 9, 257–261. [Google Scholar] [CrossRef]
- Beatty, P.R.; Puerta-Guardo, H.; Killingbeck, S.S.; Glasner, D.R.; Hopkins, K.; Harris, E. Dengue virus NS1 triggers endothelial permeability and vascular leak that is prevented by NS1 vaccination. Sci. Transl. Med. 2015, 7, 304ra141. [Google Scholar] [CrossRef]
- Modhiran, N.; Watterson, D.; Muller, D.A.; Panetta, A.K.; Sester, D.P.; Liu, L.; Hume, D.A.; Stacey, K.J.; Young, P.R. Dengue virus NS1 protein activates cells via Toll-like receptor 4 and disrupts endothelial cell monolayer integrity. Sci. Transl. Med. 2015, 7, 304ra142. [Google Scholar] [CrossRef]
- Puerta-Guardo, H.; Glasner, D.R.; Espinosa, D.A.; Biering, S.B.; Patana, M.; Ratnasiri, K.; Wang, C.; Beatty, P.R.; Harris, E. Flavivirus NS1 Triggers Tissue-Specific Vascular Endothelial Dysfunction Reflecting Disease Tropism. Cell Rep. 2019, 26, 1598–1613.e8. [Google Scholar] [CrossRef]
- Colombo, T.E.; Vedovello, D.; Pacca-Mazaro, C.C.; Mondini, A.; Araújo, J.P.; Cabrera, E.; Lopes, J.C.; Penha Dos Santos, I.N.; Negri Reis, A.F.; Costa, F.R.; et al. Dengue virus surveillance: Detection of DENV-4 in the city of São José do Rio Preto, SP, Brazil. Acta Trop. 2016, 164, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Mondini, A.; de Moraes Bronzoni, R.V.; Nunes, S.H.; Chiaravalloti Neto, F.; Massad, E.; Alonso, W.J.; Lázzaro, E.S.; Ferraz, A.A.; de Andrade Zanotto, P.M.; Nogueira, M.L. Spatio-temporal tracking and phylodynamics of an urban dengue 3 outbreak in São Paulo, Brazil. PLoS Negl. Trop. Dis. 2009, 3, e448. [Google Scholar] [CrossRef]
- Estofolete, C.F.; Terzian, A.C.; Parreira, R.; Esteves, A.; Hardman, L.; Greque, G.V.; Rahal, P.; Nogueira, M.L. Clinical and laboratory profile of Zika virus infection in dengue suspected patients: A case series. J. Clin. Virol. 2016, 81, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Drumond, B.P.; Mondini, A.; Schmidt, D.J.; Bosch, I.; Nogueira, M.L. Population dynamics of DENV-1 genotype V in Brazil is characterized by co-circulation and strain/lineage replacement. Arch. Virol. 2012, 157, 2061–2073. [Google Scholar] [CrossRef] [PubMed]
- Drumond, B.P.; Mondini, A.; Schmidt, D.J.; de Morais Bronzoni, R.V.; Bosch, I.; Nogueira, M.L. Circulation of different lineages of Dengue virus 2, genotype American/Asian in Brazil: Dynamics and molecular and phylogenetic characterization. PLoS ONE 2013, 8, e59422. [Google Scholar] [CrossRef]
- Chiaravalloti-Neto, F.; Pereira, M.; Fávaro, E.A.; Dibo, M.R.; Mondini, A.; Rodrigues-Junior, A.L.; Chierotti, A.P.; Nogueira, M.L. Assessment of the relationship between entomologic indicators of Aedes aegypti and the epidemic occurrence of dengue virus 3 in a susceptible population, São José do Rio Preto, São Paulo, Brazil. Acta Trop. 2015, 142, 167–177. [Google Scholar] [CrossRef]
- Villabona-Arenas, C.J.; Mondini, A.; Bosch, I.; Schimdt, D.J.; Schimitt, D.; Calzavara-Silva, C.E.; Zanotto, P.M.; Nogueira, M.L. Dengue virus type 3 adaptive changes during epidemics in São Jose de Rio Preto, Brazil, 2006–2007. PLoS ONE 2013, 8, e63496. [Google Scholar] [CrossRef]
- Nogueira, M.L.; Nery Júnior, N.R.R.; Estofolete, C.F.; Bernardes Terzian, A.C.; Guimarães, G.F.; Zini, N.; Alves da Silva, R.; Dutra Silva, G.C.; Junqueira Franco, L.C.; Rahal, P.; et al. Adverse birth outcomes associated with Zika virus exposure during pregnancy in São José do Rio Preto, Brazil. Clin. Microbiol. Infect. 2018, 24, 646–652. [Google Scholar] [CrossRef]
- Terzian, A.C.B.; Schanoski, A.S.; Mota, M.T.O.; da Silva, R.A.; Estofolete, C.F.; Colombo, T.E.; Rahal, P.; Hanley, K.A.; Vasilakis, N.; Kalil, J.; et al. Viral Load and Cytokine Response Profile Does Not Support Antibody-Dependent Enhancement in Dengue-Primed Zika Virus-Infected Patients. Clin. Infect. Dis. 2017, 65, 1260–1265. [Google Scholar] [CrossRef]
- Nogueira, M.L.; Estofolete, C.F.; Terzian, A.C.; Mascarin do Vale, E.P.; da Silva, R.C.; da Silva, R.F.; Ramalho, H.J.; Fernandes Charpiot, I.M.; Vasilakis, N.; Abbud-Filho, M. Zika Virus Infection and Solid Organ Transplantation: A New Challenge. Am. J. Transpl. 2017, 17, 791–795. [Google Scholar] [CrossRef]
- Colombo, T.E.; Estofolete, C.F.; Reis, A.F.N.; da Silva, N.S.; Aguiar, M.L.; Cabrera, E.M.S.; Dos Santos, I.N.P.; Costa, F.R.; Cruz, L.E.A.A.; Rombola, P.L.; et al. Clinical, laboratory and virological data from suspected ZIKV patients in an endemic arbovirus area. J. Clin. Virol. 2017, 96, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Estofolete, C.F.; Terzian, A.C.B.; Colombo, T.E.; de Freitas Guimarães, G.; Ferraz, H.C.; da Silva, R.A.; Greque, G.V.; Nogueira, M.L. Co-infection between Zika and different Dengue serotypes during DENV outbreak in Brazil. J. Infect. Public Health 2018, 12, 178–181. [Google Scholar] [CrossRef] [PubMed]
- Bosch, I.; de Puig, H.; Hiley, M.; Carre-Camps, M.; Perdomo-Celis, F.; Narvaez, C.F.; Salgado, D.M.; Senthoor, D.; O’Grady, M.; Phillips, E.; et al. Rapid antigen tests for dengue virus serotypes and Zika virus in patient serum. Sci. Transl. Med. 2017, 9, eaan1589. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Barraquer, I.; Costa, F.; Nascimento, E.J.M.; Nery, N.; Castanha, P.M.S.; Sacramento, G.A.; Cruz, J.; Carvalho, M.; De Olivera, D.; Hagan, J.E.; et al. Impact of preexisting dengue immunity on Zika virus emergence in a dengue endemic region. Science 2019, 363, 607–610. [Google Scholar] [CrossRef]
- Terzian, A.C.; Mondini, A.; Bronzoni, R.V.; Drumond, B.P.; Ferro, B.P.; Cabrera, E.M.; Figueiredo, L.T.; Chiaravalloti-Neto, F.; Nogueira, M.L. Detection of Saint Louis encephalitis virus in dengue-suspected cases during a dengue 3 outbreak. Vector Borne Zoonotic Dis. 2011, 11, 291–300. [Google Scholar] [CrossRef]
- Mondini, A.; Bronzoni, R.V.; Cardeal, I.L.; dos Santos, T.M.; Lazaro, E.; Nunes, S.H.; Silva, G.C.; Madrid, M.C.; Rahal, P.; Figueiredo, L.T.; et al. Simultaneous infection by DENV-3 and SLEV in Brazil. J. Clin. Virol. 2007, 40, 84–86. [Google Scholar] [CrossRef]
- WHO. Dengue: Guidelines for diagnosis, treatment, prevention and control—New edition. In World Health Organization (WHO) and the Special Programme for Research and Training in Tropical Diseases (TDR); WHO: Geneva, Switzerland, 2009; p. 160. [Google Scholar]
- Ministério da Saúde. Brasil, Dengue: Diagnóstico e Manejo Clínico: Adulto e Criança [Recurso Eletrônico]; Ministério da Saúde: Brasília, Brazil, 2016; p. 58. [Google Scholar]
- Johnson, B.W.; Russell, B.J.; Lanciotti, R.S. Serotype-specific detection of dengue viruses in a fourplex real-time reverse transcriptase PCR assay. J. Clin. Microbiol. 2005, 43, 4977–4983. [Google Scholar] [CrossRef]
- Carod-Artal, F.J.; Wichmann, O.; Farrar, J.; Gascón, J. Neurological complications of dengue virus infection. Lancet Neurol. 2013, 12, 906–919. [Google Scholar] [CrossRef]
- Li, G.H.; Ning, Z.J.; Liu, Y.M.; Li, X.H. Neurological Manifestations of Dengue Infection. Front. Cell Infect. Microbiol. 2017, 7, 449. [Google Scholar] [CrossRef]
- Kanade, T.; Shah, I. Dengue encephalopathy. J. Vector Borne Dis. 2011, 48, 180–181. [Google Scholar]
- Stewart, F.H. Dengue: Analysis of the clinical syndrome at a South Pacific advance base. U.S. Nav. Med. Bull. 1944, 42, 1233–1240. [Google Scholar]
- Lum, L.C.; Lam, S.K.; Choy, Y.S.; George, R.; Harun, F. Dengue encephalitis: A true entity? Am. J. Trop. Med. Hyg. 1996, 54, 256–259. [Google Scholar] [CrossRef] [PubMed]
- Gulati, S.; Maheshwari, A. Atypical manifestations of dengue. Trop. Med. Int. Health 2007, 12, 1087–1095. [Google Scholar] [CrossRef] [PubMed]
- Miagostovich, M.P.; Ramos, R.G.; Nicol, A.F.; Nogueira, R.M.; Cuzzi-Maya, T.; Oliveira, A.V.; Marchevsky, R.S.; Mesquita, R.P.; Schatzmayr, H.G. Retrospective study on dengue fatal cases. Clin. Neuropathol. 1997, 16, 204–208. [Google Scholar]
- Ramos, C.; Sánchez, G.; Pando, R.H.; Baquera, J.; Hernández, D.; Mota, J.; Ramos, J.; Flores, A.; Llausás, E. Dengue virus in the brain of a fatal case of hemorrhagic dengue fever. J. Neurovirol. 1998, 4, 465–468. [Google Scholar] [CrossRef]
- Angibaud, G.; Luaute, J.; Laille, M.; Gaultier, C. Brain involvement in Dengue fever. J. Clin. Neurosci. 2001, 8, 63–65. [Google Scholar] [CrossRef]
- Domingues, R.B.; Kuster, G.W.; Onuki-Castro, F.L.; Souza, V.A.; Levi, J.E.; Pannuti, C.S. Involvement of the central nervous system in patients with dengue virus infection. J. Neurol. Sci. 2008, 267, 36–40. [Google Scholar] [CrossRef]
- Solomon, T.; Dung, N.M.; Vaughn, D.W.; Kneen, R.; Thao, L.T.; Raengsakulrach, B.; Loan, H.T.; Day, N.P.; Farrar, J.; Myint, K.S.; et al. Neurological manifestations of dengue infection. Lancet 2000, 355, 1053–1059. [Google Scholar] [CrossRef]
- Oehler, E.; Le Hénaff, O.; Ghawche, F. Manifestations neurologiques de la dengue. La Presse Médicale 2012, 41, e547–e552. [Google Scholar] [CrossRef]
- Varatharaj, A. Encephalitis in the clinical spectrum of dengue infection. Neurol. India 2010, 58, 585–591. [Google Scholar] [CrossRef]
- Thisyakorn, U.; Thisyakorn, C.; Limpitikul, W.; Nisalak, A. Dengue infection with central nervous system manifestations. Southeast. Asian J. Trop. Med. Public Health 1999, 30, 504–506. [Google Scholar] [PubMed]
- Soares, C.N.; Cabral-Castro, M.J.; Peralta, J.M.; Freitas, M.R.; Puccioni-Sohler, M. Meningitis determined by oligosymptomatic dengue virus type 3 infection: Report of a case. Int. J. Infect. Dis. 2010, 14, e150–e152. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, U.C.; Dhawan, R.; Khanna, M.; Mathur, A. Breakdown of the blood-brain barrier during dengue virus infection of mice. J. Gen. Virol. 1991, 72, 859–866. [Google Scholar] [CrossRef] [PubMed]
- Mustafá, Y.M.; Meuren, L.M.; Coelho, S.V.A.; de Arruda, L.B. Pathways Exploited by Flaviviruses to Counteract the Blood-Brain Barrier and Invade the Central Nervous System. Front. Microbiol. 2019, 10, 525. [Google Scholar] [CrossRef]
- Sips, G.J.; Wilschut, J.; Smit, J.M. Neuroinvasive flavivirus infections. Rev. Med. Virol. 2012, 22, 69–87. [Google Scholar] [CrossRef]
- Oishi, K.; Saito, M.; Mapua, C.A.; Natividad, F.F. Dengue illness: Clinical features and pathogenesis. J. Infect. Chemother. 2007, 13, 125–133. [Google Scholar] [CrossRef]
- Seet, R.C.; Lim, E.C. Dysarthria-clumsy hand syndrome associated with dengue type-2 infection. J. Neurol. 2007, 254, 1129–1130. [Google Scholar] [CrossRef]
- Basu, A.; Chaturvedi, U.C. Vascular endothelium: The battlefield of dengue viruses. FEMS Immunol. Med. Microbiol. 2008, 53, 287–299. [Google Scholar] [CrossRef]
- Carteaux, G.; Maquart, M.; Bedet, A.; Contou, D.; Brugieres, P.; Fourati, S.; Cleret de Langavant, L.; de Broucker, T.; Brun-Buisson, C.; Leparc-Goffart, I.; et al. Zika Virus Associated with Meningoencephalitis. N. Engl. J. Med. 2016, 374, 1595–1596. [Google Scholar] [CrossRef]
- Brasil, P.; Sequeira, P.C.; Freitas, A.D.; Zogbi, H.E.; Calvet, G.A.; de Souza, R.V.; Siqueira, A.M.; de Mendonca, M.C.; Nogueira, R.M.; de Filippis, A.M.; et al. Guillain-Barré syndrome associated with Zika virus infection. Lancet 2016, 387, 1482. [Google Scholar] [CrossRef]
- Ventura, C.V.; Maia, M.; Bravo-Filho, V.; Góis, A.L.; Belfort, R. Zika virus in Brazil and macular atrophy in a child with microcephaly. Lancet 2016, 387, 228. [Google Scholar] [CrossRef]
- Furtado, J.M.; Espósito, D.L.; Klein, T.M.; Teixeira-Pinto, T.; da Fonseca, B.A. Uveitis Associated with Zika Virus Infection. N. Engl. J. Med. 2016, 375, 394–396. [Google Scholar] [CrossRef] [PubMed]
- Parra, B.; Lizarazo, J.; Jiménez-Arango, J.A.; Zea-Vera, A.F.; González-Manrique, G.; Vargas, J.; Angarita, J.A.; Zuñiga, G.; Lopez-Gonzalez, R.; Beltran, C.L.; et al. Guillain-Barré Syndrome Associated with Zika Virus Infection in Colombia. N. Engl. J. Med. 2016, 375, 1513–1523. [Google Scholar] [CrossRef] [PubMed]
- Cao-Lormeau, V.M.; Blake, A.; Mons, S.; Lastere, S.; Roche, C.; Vanhomwegen, J.; Dub, T.; Baudouin, L.; Teissier, A.; Larre, P.; et al. Guillain-Barré Syndrome outbreak associated with Zika virus infection in French Polynesia: A case-control study. Lancet 2016, 387, 1531–1539. [Google Scholar] [CrossRef]
- Li, H.; Saucedo-Cuevas, L.; Shresta, S.; Gleeson, J.G. The Neurobiology of Zika Virus. Neuron 2016, 92, 949–958. [Google Scholar] [CrossRef] [PubMed]
- Shao, Q.; Herrlinger, S.; Yang, S.L.; Lai, F.; Moore, J.M.; Brindley, M.A.; Chen, J.F. Zika virus infection disrupts neurovascular development and results in postnatal microcephaly with brain damage. Development 2016, 143, 4127–4136. [Google Scholar] [CrossRef]
- Mladinich, M.C.; Schwedes, J.; Mackow, E.R. Zika Virus Persistently Infects and Is Basolaterally Released from Primary Human Brain Microvascular Endothelial Cells. MBio 2017, 8, e00952-17. [Google Scholar] [CrossRef]
- Libraty, D.H.; Young, P.R.; Pickering, D.; Endy, T.P.; Kalayanarooj, S.; Green, S.; Vaughn, D.W.; Nisalak, A.; Ennis, F.A.; Rothman, A.L. High circulating levels of the dengue virus nonstructural protein NS1 early in dengue illness correlate with the development of dengue hemorrhagic fever. J. Infect. Dis. 2002, 186, 1165–1168. [Google Scholar] [CrossRef]
- Zhang, H.; Li, W.; Wang, J.; Peng, H.; Che, X.; Chen, X.; Zhou, Y. NS1-based tests with diagnostic utility for confirming dengue infection: A meta-analysis. Int. J. Infect. Dis. 2014, 26, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Avirutnan, P.; Punyadee, N.; Noisakran, S.; Komoltri, C.; Thiemmeca, S.; Auethavornanan, K.; Jairungsri, A.; Kanlaya, R.; Tangthawornchaikul, N.; Puttikhunt, C.; et al. Vascular leakage in severe dengue virus infections: A potential role for the nonstructural viral protein NS1 and complement. J. Infect. Dis. 2006, 193, 1078–1088. [Google Scholar] [CrossRef]
- Thomas, L.; Najioullah, F.; Verlaeten, O.; Martial, J.; Brichler, S.; Kaidomar, S.; Moravie, V.; Cabié, A.; Césaire, R. Relationship between nonstructural protein 1 detection and plasma virus load in Dengue patients. Am. J. Trop. Med. Hyg. 2010, 83, 696–699. [Google Scholar] [CrossRef] [PubMed]
- Duong, V.; Ly, S.; Lorn Try, P.; Tuiskunen, A.; Ong, S.; Chroeung, N.; Lundkvist, A.; Leparc-Goffart, I.; Deubel, V.; Vong, S.; et al. Clinical and virological factors influencing the performance of a NS1 antigen-capture assay and potential use as a marker of dengue disease severity. PLoS Negl. Trop. Dis. 2011, 5, e1244. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, S.; Tan, K.H.; Rathore, A.P.; Rozen-Gagnon, K.; Shuai, W.; Ruedl, C.; Vasudevan, S.G. The magnitude of dengue virus NS1 protein secretion is strain dependent and does not correlate with severe pathologies in the mouse infection model. J. Virol. 2012, 86, 5508–5514. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Puerta-Guardo, H.; Biering, S.B.; Glasner, D.R.; Tran, E.B.; Patana, M.; Gomberg, T.A.; Malvar, C.; Lo, N.T.N.; Espinosa, D.A.; et al. Endocytosis of flavivirus NS1 is required for NS1-mediated endothelial hyperpermeability and is abolished by a single N-glycosylation site mutation. PLoS Pathog. 2019, 15, e1007938. [Google Scholar] [CrossRef] [PubMed]
- Vieira, M.; Costa, C.H.N.; Linhares, A.D.C.; Borba, A.S.; Henriques, D.F.; Silva, E.; Tavares, F.N.; Batista, F.M.A.; Guimaraes, H.C.L.; Martins, L.C.; et al. Potential role of dengue virus, chikungunya virus and Zika virus in neurological diseases. Mem. Do Inst. Oswaldo Cruz 2018, 113, e170538. [Google Scholar] [CrossRef] [PubMed]
- Hermann, L.L.; Thaisomboonsuk, B.; Poolpanichupatam, Y.; Jarman, R.G.; Kalayanarooj, S.; Nisalak, A.; Yoon, I.K.; Fernandez, S. Evaluation of a dengue NS1 antigen detection assay sensitivity and specificity for the diagnosis of acute dengue virus infection. PLoS Negl. Trop. Dis. 2014, 8, e3193. [Google Scholar] [CrossRef] [PubMed]
- Chiaravalloti-Neto, F.; da Silva, R.A.; Zini, N.; da Silva, G.C.D.; da Silva, N.S.; Parra, M.C.P.; Dibo, M.R.; Estofolete, C.F.; Fávaro, E.A.; Dutra, K.R.; et al. Seroprevalence for dengue virus in a hyperendemic area and associated socioeconomic and demographic factors using a cross-sectional design and a geostatistical approach, state of São Paulo, Brazil. BMC Infect. Dis. 2019, 19, 441. [Google Scholar] [CrossRef]
- Liu, S.; DeLalio, L.J.; Isakson, B.E.; Wang, T.T. AXL-Mediated Productive Infection of Human Endothelial Cells by Zika Virus. Circ. Res. 2016, 119, 1183–1189. [Google Scholar] [CrossRef]
- Cheng, F.; Ramos da Silva, S.; Huang, I.C.; Jung, J.U.; Gao, S.J. Suppression of Zika Virus Infection and Replication in Endothelial Cells and Astrocytes by PKA Inhibitor PKI 14-22. J. Virol. 2018, 92, e02019-17. [Google Scholar] [CrossRef]
- ICTV. ICTV Master Species List 2018 v.1.0.; International Committee on Taxonomy of Viruses: London, UK, 2018. [Google Scholar]
- Song, H.; Qi, J.; Haywood, J.; Shi, Y.; Gao, G.F. Zika virus NS1 structure reveals diversity of electrostatic surfaces among flaviviruses. Nat. Struct. Mol. Biol. 2016, 23, 456–458. [Google Scholar] [CrossRef]
- de Carvalho, G.C.; Borget, M.Y.; Bernier, S.; Garneau, D.; da Silva Duarte, A.J.; Dumais, N. RAGE and CCR7 mediate the transmigration of Zika-infected monocytes through the blood-brain barrier. Immunobiology 2019, 224, 792–803. [Google Scholar] [CrossRef] [PubMed]
- Andersson, U.; Wang, H.; Palmblad, K.; Aveberger, A.C.; Bloom, O.; Erlandsson-Harris, H.; Janson, A.; Kokkola, R.; Zhang, M.; Yang, H.; et al. High mobility group 1 protein (HMG-1) stimulates proinflammatory cytokine synthesis in human monocytes. J. Exp. Med. 2000, 192, 565–570. [Google Scholar] [CrossRef] [PubMed]
- Hueston, L.; Grau, G.E.; Mahalingam, S. Inhibition of Interleukin 1β Signaling by Anakinra Ameliorates Proinflammatory Cytokine Responses in Zika Virus-Infected Human Blood-Brain Barrier Endothelial Cells. J. Infect. Dis. 2019, 220, 1539–1540. [Google Scholar] [CrossRef] [PubMed]
- Khaiboullina, S.F.; Ribeiro, F.M.; Uppal, T.; Martynova, E.V.; Rizvanov, A.A.; Verma, S.C. Zika Virus Transmission Through Blood Tissue Barriers. Front. Microbiol. 2019, 10, 1465. [Google Scholar] [CrossRef] [PubMed]
- Jorgačevski, J.; Korva, M.; Potokar, M.; Lisjak, M.; Avšič-Županc, T.; Zorec, R. ZIKV Strains Differentially Affect Survival of Human Fetal Astrocytes versus Neurons and Traffic of ZIKV-Laden Endocytotic Compartments. Sci. Rep. 2019, 9, 8069. [Google Scholar] [CrossRef] [PubMed]
- Lindqvist, R.; Mundt, F.; Gilthorpe, J.D.; Wölfel, S.; Gekara, N.O.; Kröger, A.; Överby, A.K. Fast type I interferon response protects astrocytes from flavivirus infection and virus-induced cytopathic effects. J. Neuroinflammation 2016, 13, 277. [Google Scholar] [CrossRef]
- Ledur, P.F.; Karmirian, K.; Pedrosa, C.D.S.G.; Souza, L.R.Q.; Assis-de-Lemos, G.; Martins, T.M.; Ferreira, J.C.C.G.; de Azevedo Reis, G.F.; Silva, E.S.; Silva, D.; et al. Zika virus infection leads to mitochondrial failure, oxidative stress and DNA damage in human iPSC-derived astrocytes. Sci. Rep. 2020, 10, 1218. [Google Scholar] [CrossRef]
- Sher, A.A.; Glover, K.K.M.; Coombs, K.M. Zika Virus Infection Disrupts Astrocytic Proteins Involved in Synapse Control and Axon Guidance. Front. Microbiol. 2019, 10, 596. [Google Scholar] [CrossRef]
- Stefanik, M.; Formanova, P.; Bily, T.; Vancova, M.; Eyer, L.; Palus, M.; Salat, J.; Braconi, C.T.; Zanotto, P.M.A.; Gould, E.A.; et al. Characterization of Zika virus infection in primary human astrocytes. BMC Neurosci. 2018, 19, 5. [Google Scholar] [CrossRef]
- ECDC Rapid Risk Assessment: Zika Virus Epidemic in the Americas: Potential Association with Microcephaly and Guillain-Barré Syndrome. Available online: https://www.ecdc.europa.eu/en/publications-data/rapid-risk-assessment-zika-virus-epidemic-americas-potential-association (accessed on 10 January 2020).
- CDC. Fact Sheet for Healthcare Providers: Interpreting Zika MAC-ELISA Test Results; CDC: Atlanta, GA, USA, 2017.
- CDC Arboviral Diseases, Neuroinvasive and Non-neuroinvasive 2015 Case Definition. Available online: https://wwwn.cdc.gov/nndss/conditions/arboviral-diseases-neuroinvasive-and-non-neuroinvasive/case-definition/2015/ (accessed on 10 January 2020).
- Verma, S.; Kumar, M.; Gurjav, U.; Lum, S.; Nerurkar, V.R. Reversal of West Nile virus-induced blood-brain barrier disruption and tight junction proteins degradation by matrix metalloproteinases inhibitor. Virology 2010, 397, 130–138. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Estofolete, C.F.; Milhim, B.H.G.A.; Zini, N.; Scamardi, S.N.; Selvante, J.D.; Vasilakis, N.; Nogueira, M.L. Flavivirus Infection Associated with Cerebrovascular Events. Viruses 2020, 12, 671. https://doi.org/10.3390/v12060671
Estofolete CF, Milhim BHGA, Zini N, Scamardi SN, Selvante JD, Vasilakis N, Nogueira ML. Flavivirus Infection Associated with Cerebrovascular Events. Viruses. 2020; 12(6):671. https://doi.org/10.3390/v12060671
Chicago/Turabian StyleEstofolete, Cássia F., Bruno H. G. A. Milhim, Nathalia Zini, Samuel N. Scamardi, Joana D’Arc Selvante, Nikos Vasilakis, and Maurício L. Nogueira. 2020. "Flavivirus Infection Associated with Cerebrovascular Events" Viruses 12, no. 6: 671. https://doi.org/10.3390/v12060671
APA StyleEstofolete, C. F., Milhim, B. H. G. A., Zini, N., Scamardi, S. N., Selvante, J. D., Vasilakis, N., & Nogueira, M. L. (2020). Flavivirus Infection Associated with Cerebrovascular Events. Viruses, 12(6), 671. https://doi.org/10.3390/v12060671





