n-3 Polyunsaturated Fatty Acids Impede the TCR Mobility and the TCR–pMHC Interaction of Anti-Viral CD8+ T Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Mice
2.2. Virus and Infection
2.3. Reagents and Antibodies
2.4. Isolation of CD8+ Cells
2.5. In Vitro Activation of CD8+ Cells
2.6. Generation of Bone Marrow-Derived Dendritic Cells
2.7. Trans-Well Chemotaxis Assay
2.8. pMHC-TCR Binding Assay
2.9. Highly Inclined and Laminated Optical Sheet (HILO) Microscopic Analysis
2.10. Statistical Analyses
3. Results
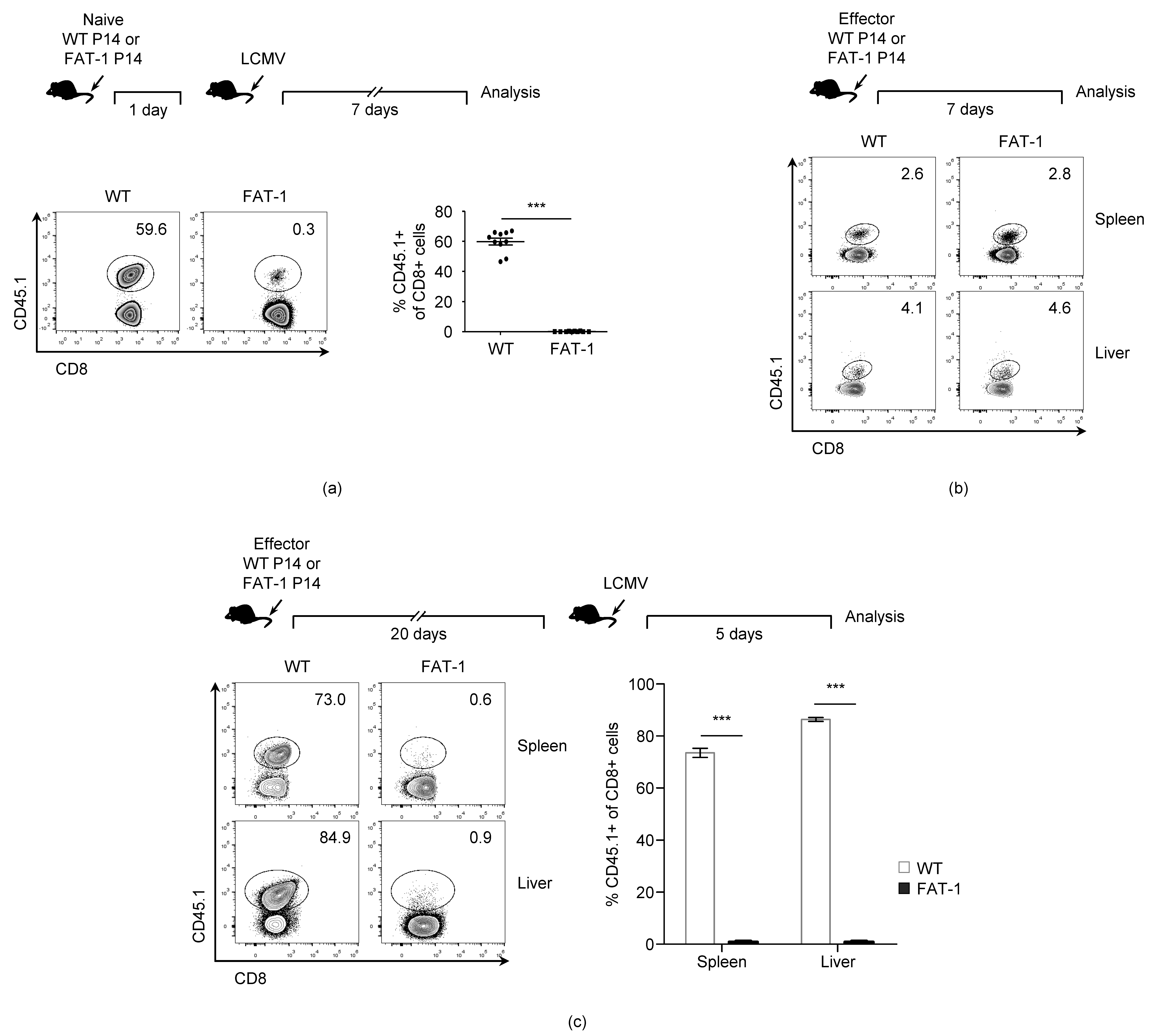
3.1. n-3 PUFAs Reduce in Vivo Expansion of Anti-Viral CD8+ T Cells
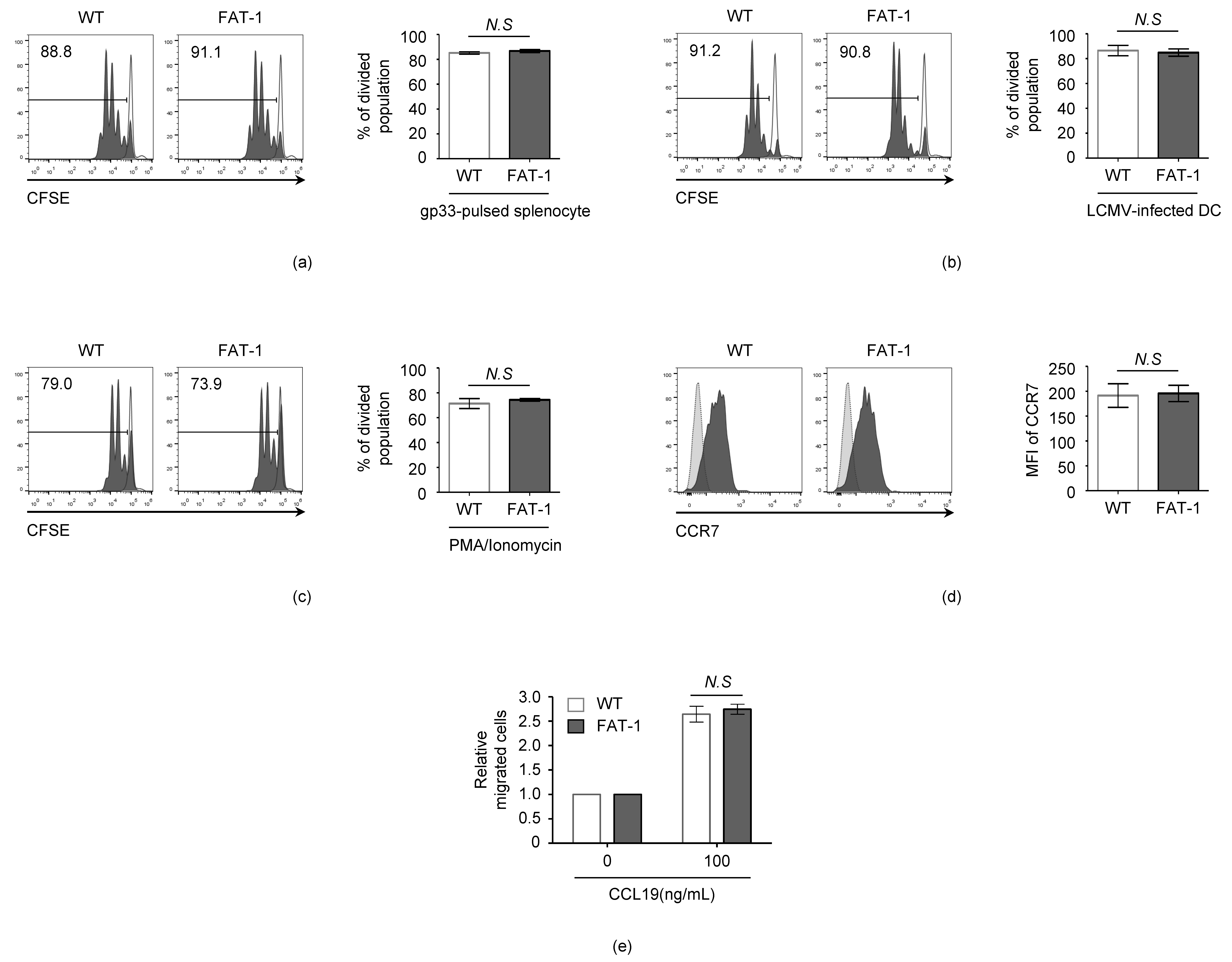
3.2. n-3 PUFAs Do Not Affect the Intrinsic Activation and Migration Potential of CD8+ T Cells
3.3. n-3 PUFAs Decrease TCR Mobility on CD8+ T Cell Membrane
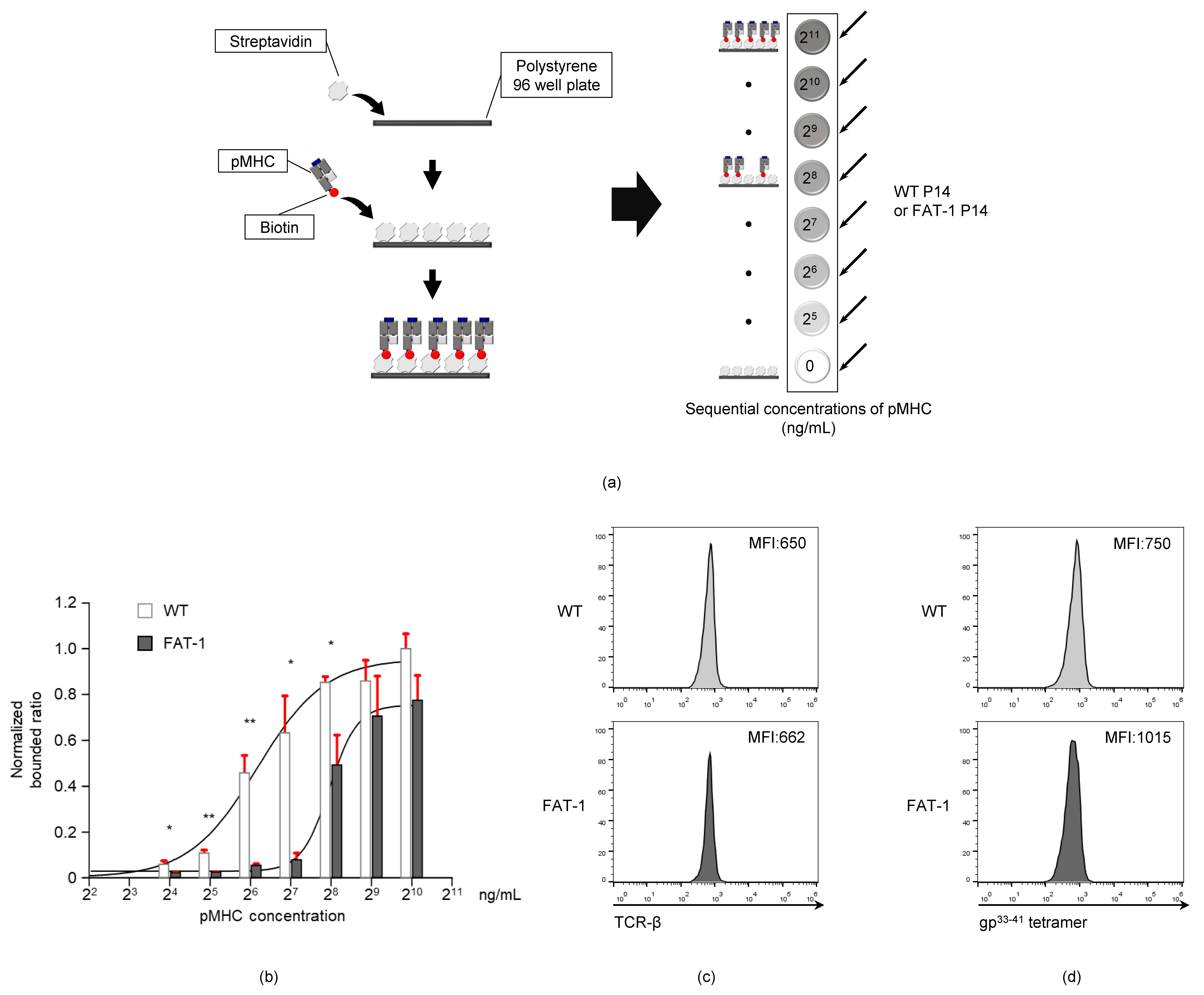
3.4. Endogenous n-3 PUFAs Interfere with TCR-pMHC Interactions
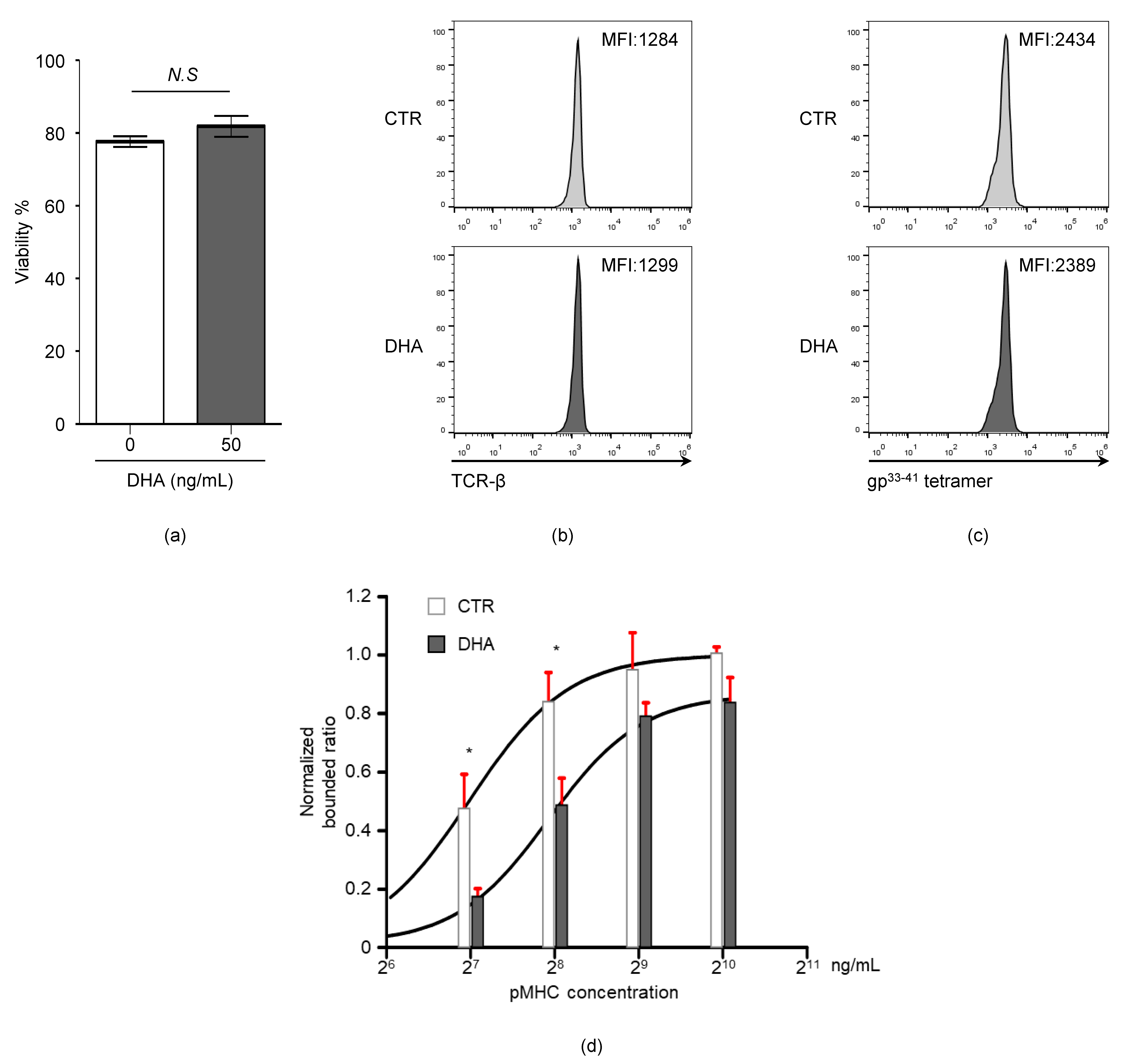
3.5. Docosahexaenoic Acid (DHA) Treatment Reduces CD8+ T Cell TCR-pMHC Bond Formation
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Grimm, H.; Mayer, K.; Mayser, P.; Eigenbrodt, E. Regulatory potential of n-3 fatty acids in immunological and inflammatory processes. Br. J. Nutr. 2002, 87, S59–S67. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, S.; Svahn, S.L.; Johansson, M.E. Effects of Omega-3 Fatty Acids on Immune Cells. Int. J. Mol. Sci 2019, 20, 5028. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.Y.; Lim, K.; Lee, S.M.; Park, B.H. Effects of n-3 PUFA on the CD4(+) type 2 helper T-cell-mediated immune responses in Fat-1 mice. Mol. Nutr. Food Res. 2014, 58, 365–375. [Google Scholar] [CrossRef]
- Mickleborough, T.D.; Ionescu, A.A.; Rundell, K.W. Omega-3 Fatty acids and airway hyperresponsiveness in asthma. J. Altern. Complement. Med. 2004, 10, 1067–1075. [Google Scholar] [CrossRef]
- Mozaffari, H.; Daneshzad, E.; Larijani, B.; Bellissimo, N.; Azadbakht, L. Dietary intake of fish, n-3 polyunsaturated fatty acids, and risk of inflammatory bowel disease: A systematic review and meta-analysis of observational studies. Eur. J. Nutr. 2020, 59, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Lewis, J.D.; Abreu, M.T. Diet as a Trigger or Therapy for Inflammatory Bowel Diseases. Gastroenterology 2017, 152, 398–414. [Google Scholar] [CrossRef]
- Ruggiero, C.; Lattanzio, F.; Lauretani, F.; Gasperini, B.; Andres-Lacueva, C.; Cherubini, A. Omega-3 Polyunsaturated Fatty Acids and Immune-Mediated Diseases: Inflammatory Bowel Disease and Rheumatoid Arthritis. Curr. Pharm. Design 2009, 15, 4135–4148. [Google Scholar] [CrossRef] [PubMed]
- Philippou, E.; Nikiphorou, E. Are we really what we eat? Nutrition and its role in the onset of rheumatoid arthritis. Autoimmun. Rev. 2018, 17, 1074–1077. [Google Scholar] [CrossRef]
- Bonilla, D.L.; Fan, Y.Y.; Chapkin, R.S.; McMurray, D.N. Transgenic mice enriched in omega-3 fatty acids are more susceptible to pulmonary tuberculosis: Impaired resistance to tuberculosis in fat-1 mice. J. Infect. Dis. 2010, 201, 399–408. [Google Scholar] [CrossRef]
- Pierre, M.; Husson, M.O.; Le Berre, R.; Desseyn, J.L.; Galabert, C.; Beghin, L.; Beermann, C.; Dagenais, A.; Berthiaume, Y.; Cardinaud, B.; et al. Omega-3 polyunsaturated fatty acids improve host response in chronic Pseudomonas aeruginosa lung infection in mice. Am. J. Physiol. Lung Cell Mol. Physiol. 2007, 292, L1422–L1431. [Google Scholar] [CrossRef][Green Version]
- Fritsche, K.L.; Shahbazian, L.M.; Feng, C.; Berg, J.N. Dietary fish oil reduces survival and impairs bacterial clearance in C3H/Hen mice challenged with Listeria monocytogenes. Clin. Sci. (Lond) 1997, 92, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Schwerbrock, N.M.; Karlsson, E.A.; Shi, Q.; Sheridan, P.A.; Beck, M.A. Fish oil-fed mice have impaired resistance to influenza infection. J. Nutr. 2009, 139, 1588–1594. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.W.; Kim, S.; Cho, Y.B.; Ryu, S.R.; Seo, Y.J.; Lee, S.M. Endogenous n-3 Polyunsaturated Fatty Acids Are Beneficial to Dampen CD8(+) T Cell-Mediated Inflammatory Response upon the Viral Infection in Mice. Int. J. Mol. Sci. 2019, 20, 4510. [Google Scholar] [CrossRef] [PubMed]
- Jones, G.J.B.; Roper, R.L. The effects of diets enriched in omega-3 polyunsaturated fatty acids on systemic vaccinia virus infection. Sci. Rep. 2017, 7, 15999. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.; Fan, Y.Y.; Barhoumi, R.; Smith, R.; McMurray, D.N.; Chapkin, R.S. n-3 polyunsaturated fatty acids suppress the localization and activation of signaling proteins at the immunological synapse in murine CD4+ T cells by affecting lipid raft formation. J. Immunol. 2008, 181, 6236–6243. [Google Scholar] [CrossRef] [PubMed]
- Arrington, J.L.; Chapkin, R.S.; Switzer, K.C.; Morris, J.S.; McMurray, D.N. Dietary n-3 polyunsaturated fatty acids modulate purified murine T-cell subset activation. Clin. Exp. Immunol. 2001, 125, 499–507. [Google Scholar] [CrossRef]
- Lian, M.; Luo, W.; Sui, Y.; Li, Z.; Hua, J. Dietary n-3 PUFA Protects Mice from Con A Induced Liver Injury by Modulating Regulatory T Cells and PPAR-gamma Expression. PLoS ONE 2015, 10, e0132741. [Google Scholar] [CrossRef]
- Bi, X.; Li, F.; Liu, S.; Jin, Y.; Zhang, X.; Yang, T.; Dai, Y.; Li, X.; Zhao, A.Z. omega-3 polyunsaturated fatty acids ameliorate type 1 diabetes and autoimmunity. J. Clin. Investig. 2017, 127, 1757–1771. [Google Scholar] [CrossRef] [PubMed]
- Monk, J.M.; Liddle, D.M.; Brown, M.J.; Zarepoor, L.; De Boer, A.A.; Ma, D.W.; Power, K.A.; Robinson, L.E. Anti-inflammatory and anti-chemotactic effects of dietary flaxseed oil on CD8(+) T cell/adipocyte-mediated cross-talk. Mol. Nutr. Food Res. 2016, 60, 621–630. [Google Scholar] [CrossRef]
- Liddle, D.M.; Monk, J.M.; Hutchinson, A.L.; Ma, D.W.L.; Robinson, L.E. CD8(+) T cell/adipocyte inflammatory cross talk and ensuing M1 macrophage polarization are reduced by fish-oil-derived n-3 polyunsaturated fatty acids, in part by a TNF-alpha-dependent mechanism. J. Nutr. Biochem. 2020, 76, 108243. [Google Scholar] [CrossRef]
- Hou, T.Y.; Barhoumi, R.; Fan, Y.Y.; Rivera, G.M.; Hannoush, R.N.; McMurray, D.N.; Chapkin, R.S. n-3 polyunsaturated fatty acids suppress CD4(+) T cell proliferation by altering phosphatidylinositol-(4,5)-bisphosphate [PI(4,5)P2] organization. Biochim. Biophys. Acta 2016, 1858, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.Y.; Fuentes, N.R.; Hou, T.Y.; Barhoumi, R.; Li, X.C.; Deutz, N.E.P.; Engelen, M.; McMurray, D.N.; Chapkin, R.S. Remodelling of primary human CD4+ T cell plasma membrane order by n-3 PUFA. Br. J. Nutr. 2018, 119, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Limozin, L.; Bridge, M.; Bongrand, P.; Dushek, O.; van der Merwe, P.A.; Robert, P. TCR-pMHC kinetics under force in a cell-free system show no intrinsic catch bond, but a minimal encounter duration before binding. Proc. Natl. Acad. Sci. USA 2019, 116, 16943–16948. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Zarnitsyna, V.I.; Liu, B.; Edwards, L.J.; Jiang, N.; Evavold, B.D.; Zhu, C. The kinetics of two-dimensional TCR and pMHC interactions determine T-cell responsiveness. Nature 2010, 464, 932–936. [Google Scholar] [CrossRef]
- Seo, Y.J.; Jothikumar, P.; Suthar, M.S.; Zhu, C.; Grakoui, A. Local Cellular and Cytokine Cues in the Spleen Regulate In Situ T Cell Receptor Affinity, Function, and Fate of CD8(+) T Cells. Immunity 2016, 45, 988–998. [Google Scholar] [CrossRef]
- Tagliapietra, V.; Rosa, R.; Hauffe, H.C.; Laakkonen, J.; Voutilainen, L.; Vapalahti, O.; Vaheri, A.; Henttonen, H.; Rizzoli, A. Spatial and temporal dynamics of lymphocytic choriomeningitis virus in wild rodents, northern Italy. Emerg. Infect. Dis. 2009, 15, 1019–1025. [Google Scholar] [CrossRef][Green Version]
- Wherry, E.J.; Blattman, J.N.; Murali-Krishna, K.; van der Most, R.; Ahmed, R. Viral persistence alters CD8 T-cell immunodominance and tissue distribution and results in distinct stages of functional impairment. J. Virol. 2003, 77, 4911–4927. [Google Scholar] [CrossRef]
- Sullivan, B.M.; Teijaro, J.R.; de la Torre, J.C.; Oldstone, M.B. Early virus-host interactions dictate the course of a persistent infection. PLoS Pathog. 2015, 11, e1004588. [Google Scholar] [CrossRef]
- Kang, J.X.; Wang, J.; Wu, L.; Kang, Z.B. Transgenic mice: Fat-1 mice convert n-6 to n-3 fatty acids. Nature 2004, 427, 504. [Google Scholar] [CrossRef]
- Welsh, R.M.; Seedhom, M.O. Lymphocytic choriomeningitis virus (LCMV): Propagation, quantitation, and storage. Curr. Protoc. Microbiol. 2008, 8, 1–11. [Google Scholar] [CrossRef]
- Tokunaga, M.; Imamoto, N.; Sakata-Sogawa, K. Highly inclined thin illumination enables clear single-molecule imaging in cells. Nat. Methods 2008, 5, 159–161. [Google Scholar] [CrossRef] [PubMed]
- Calviello, G.; Palozza, P.; Di Nicuolo, F.; Maggiano, N.; Bartoli, G.M. n-3 PUFA dietary supplementation inhibits proliferation and store-operated calcium influx in thymoma cells growing in Balb/c mice. J. Lipid Res. 2000, 41, 182–189. [Google Scholar] [PubMed]
- Johansson, I.; Monsen, V.T.; Pettersen, K.; Mildenberger, J.; Misund, K.; Kaarniranta, K.; Schonberg, S.; Bjorkoy, G. The marine n-3 PUFA DHA evokes cytoprotection against oxidative stress and protein misfolding by inducing autophagy and NFE2L2 in human retinal pigment epithelial cells. Autophagy 2015, 11, 1636–1651. [Google Scholar] [CrossRef] [PubMed]
- Serini, S.; Calviello, G. Modulation of Ras/ERK and Phosphoinositide Signaling by Long-Chain n-3 PUFA in Breast Cancer and Their Potential Complementary Role in Combination with Targeted Drugs. Nutrients 2017, 9, 185. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Rozell, M.; Verma, R.K.; Albu, D.I.; Califano, D.; VanValkenburgh, J.; Merchant, A.; Rangel-Moreno, J.; Randall, T.D.; Jenkins, N.A.; et al. Antigen-specific clonal expansion and cytolytic effector function of CD8+ T lymphocytes depend on the transcription factor Bcl11b. J. Exp. Med. 2010, 207, 1687–1699. [Google Scholar] [CrossRef]
- Mason, R.P.; Jacob, R.F.; Shrivastava, S.; Sherratt, S.C.R.; Chattopadhyay, A. Eicosapentaenoic acid reduces membrane fluidity, inhibits cholesterol domain formation, and normalizes bilayer width in atherosclerotic-like model membranes. Biochim. Biophys. Acta 2016, 1858, 3131–3140. [Google Scholar] [CrossRef]
- Yoshida, K.; Nagatoishi, S.; Kuroda, D.; Suzuki, N.; Murata, T.; Tsumoto, K. Phospholipid Membrane Fluidity Alters Ligand Binding Activity of a G Protein-Coupled Receptor by Shifting the Conformational Equilibrium. Biochemistry 2019, 58, 504–508. [Google Scholar] [CrossRef]
- Hishikawa, D.; Valentine, W.J.; Iizuka-Hishikawa, Y.; Shindou, H.; Shimizu, T. Metabolism and functions of docosahexaenoic acid-containing membrane glycerophospholipids. FEBS Lett. 2017, 591, 2730–2744. [Google Scholar] [CrossRef]
- Zynda, E.R.; Grimm, M.J.; Yuan, M.; Zhong, L.; Mace, T.A.; Capitano, M.; Ostberg, J.R.; Lee, K.P.; Pralle, A.; Repasky, E.A. A role for the thermal environment in defining co-stimulation requirements for CD4(+) T cell activation. Cell Cycle 2015, 14, 2340–2354. [Google Scholar] [CrossRef]
- Van Laethem, F.; Liang, X.; Andris, F.; Urbain, J.; Vandenbranden, M.; Ruysschaert, J.M.; Resh, M.D.; Stulnig, T.M.; Leo, O. Glucocorticoids alter the lipid and protein composition of membrane rafts of a murine T cell hybridoma. J. Immunol. 2003, 170, 2932–2939. [Google Scholar] [CrossRef]
- Stillwell, W.; Wassall, S.R. Docosahexaenoic acid: Membrane properties of a unique fatty acid. Chem. Phys. Lipids 2003, 126, 1–27. [Google Scholar] [CrossRef]
- Valentine, R.C.; Valentine, D.L. Omega-3 fatty acids in cellular membranes: A unified concept. Prog. Lipid Res. 2004, 43, 383–402. [Google Scholar] [CrossRef] [PubMed]
- Favier, B.; Burroughs, N.J.; Wedderburn, L.; Valitutti, S. TCR dynamics on the surface of living T cells. Int. Immunol. 2001, 13, 1525–1532. [Google Scholar] [CrossRef]
- Maulucci, G.; Cohen, O.; Daniel, B.; Sansone, A.; Petropoulou, P.I.; Filou, S.; Spyridonidis, A.; Pani, G.; De Spirito, M.; Chatgilialoglu, C.; et al. Fatty acid-related modulations of membrane fluidity in cells: Detection and implications. Free Radic. Res. 2016, 50, S40–S50. [Google Scholar] [CrossRef] [PubMed]
- Kamat, S.G.; Roy, R. Evaluation of the effect of n-3 PUFA-rich dietary fish oils on lipid profile and membrane fluidity in alloxan-induced diabetic mice (Mus musculus). Mol. Cell Biochem. 2016, 416, 117–129. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.; Barhoumi, R.; McMurray, D.N.; Chapkin, R.S. Dietary fish oil and DHA down-regulate antigen-activated CD4+ T-cells while promoting the formation of liquid-ordered mesodomains. Br. J. Nutr. 2014, 111, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Kim, W.; Zhou, L.; Wang, N.; Ly, L.H.; McMurray, D.N.; Chapkin, R.S. Dietary fish oil inhibits antigen-specific murine Th1 cell development by suppression of clonal expansion. J. Nutr. 2006, 136, 2391–2398. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lim, Y.; Kim, S.; Kim, S.; Kim, D.-I.; Kang, K.W.; Hong, S.-H.; Lee, S.-M.; Koh, H.R.; Seo, Y.-J. n-3 Polyunsaturated Fatty Acids Impede the TCR Mobility and the TCR–pMHC Interaction of Anti-Viral CD8+ T Cells. Viruses 2020, 12, 639. https://doi.org/10.3390/v12060639
Lim Y, Kim S, Kim S, Kim D-I, Kang KW, Hong S-H, Lee S-M, Koh HR, Seo Y-J. n-3 Polyunsaturated Fatty Acids Impede the TCR Mobility and the TCR–pMHC Interaction of Anti-Viral CD8+ T Cells. Viruses. 2020; 12(6):639. https://doi.org/10.3390/v12060639
Chicago/Turabian StyleLim, Younghyun, Seyoung Kim, Sehoon Kim, Dong-In Kim, Kyung Won Kang, So-Hee Hong, Sang-Myeong Lee, Hye Ran Koh, and Young-Jin Seo. 2020. "n-3 Polyunsaturated Fatty Acids Impede the TCR Mobility and the TCR–pMHC Interaction of Anti-Viral CD8+ T Cells" Viruses 12, no. 6: 639. https://doi.org/10.3390/v12060639
APA StyleLim, Y., Kim, S., Kim, S., Kim, D.-I., Kang, K. W., Hong, S.-H., Lee, S.-M., Koh, H. R., & Seo, Y.-J. (2020). n-3 Polyunsaturated Fatty Acids Impede the TCR Mobility and the TCR–pMHC Interaction of Anti-Viral CD8+ T Cells. Viruses, 12(6), 639. https://doi.org/10.3390/v12060639





