Identification and Functional Analysis of Temperate Siphoviridae Bacteriophages of Acinetobacter baumannii
Abstract
1. Introduction
- Virulent (lytic) phages follow the lytic infection cycle in which the phage overtakes the bacterial cell, reproduces itself at the expense of the host, and finally lyses and kills the host cell to release its progeny to the environment. This ability is the cornerstone of phage therapy [21].
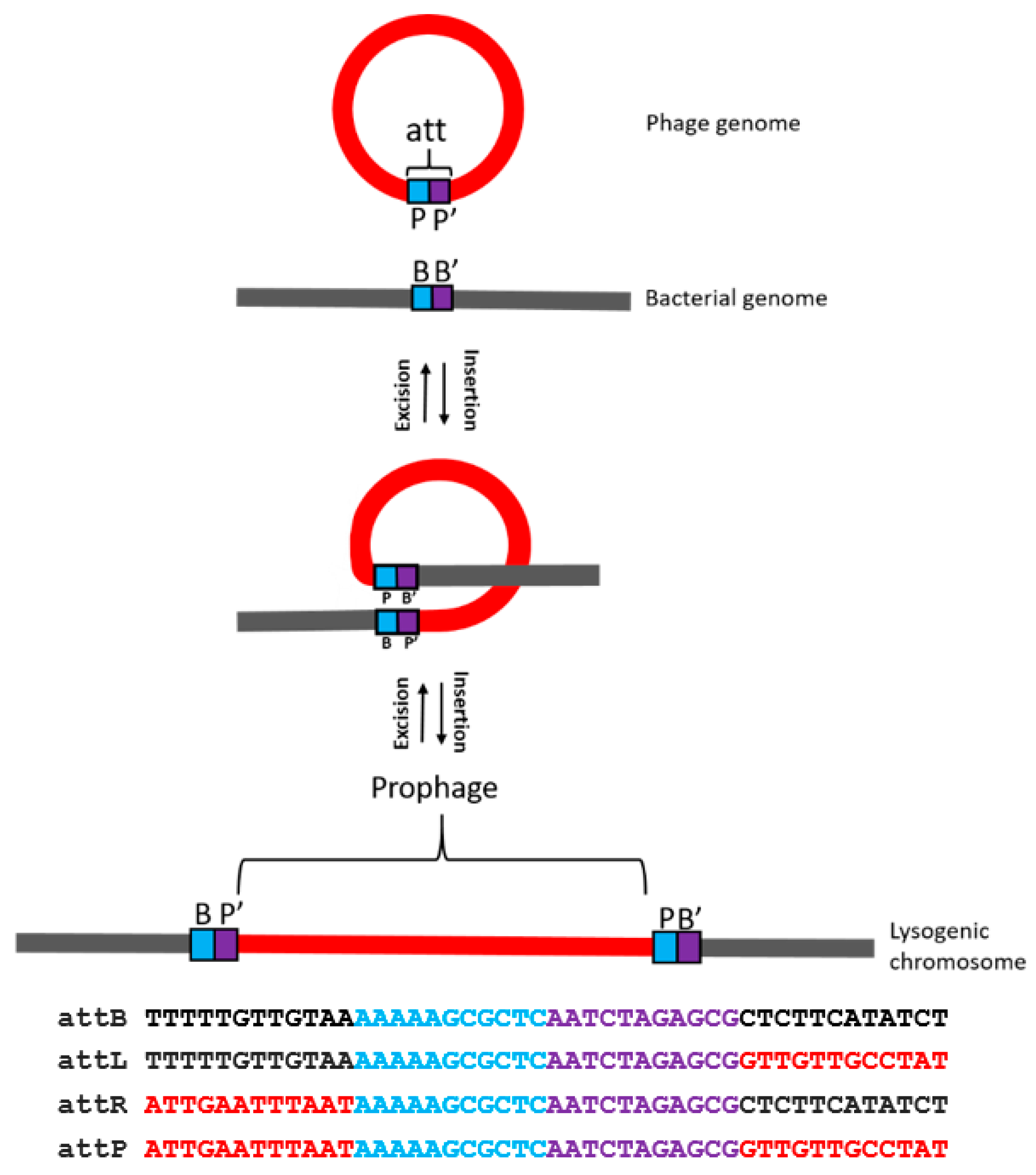
- Temperate phages may enter the lysogenic cycle in which the phage genome integrates into the host genome using the activity of phage-encoded integrase, and the resulting bacteria are called lysogens. The integrated phage is called a prophage, it replicates as part of the host genome, and stays dormant for extended periods of time, due to the activity of a specific repressor that prevents the expression of the phage lytic cycle genes. The prophage may become induced and turn on the lytic cycle genes when the lysogen encounters adverse environmental conditions. The stress triggers the SOS (emergency) response, and the overexpression of proteases causes the degradation of the phage repressor thereby turning on the lytic cycle. The archetype of temperate phages is phage lambda [22].
2. Materials and Methods
2.1. Bacterial Strains, Media and Growth Conditions
2.2. Bacteriophage Isolation and Propagation
2.3. Bacteriophage Titration by Droplet and Double-Agar Overlay Methods
2.4. Plaque Purification
2.5. Preparation of Phage Stocks
2.6. Phage Purification from Liquid Lysates
2.7. Transmission Electron Microscopy
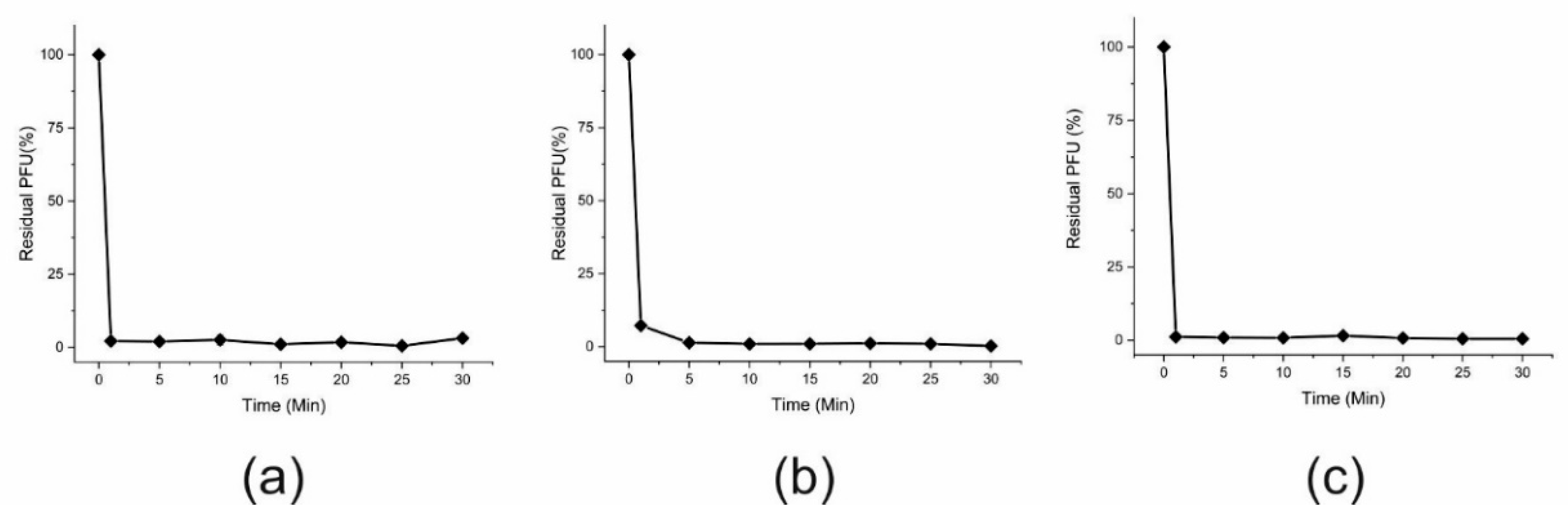
2.8. Phage Adsorption Assay
2.9. Phage Host Range Determination
2.10. Phage and Bacterial DNA Extraction
2.11. Genome Sequencing, Assembly and Bioinformatics Based on Sequence Analysis
2.12. PCR and Sanger Sequencing
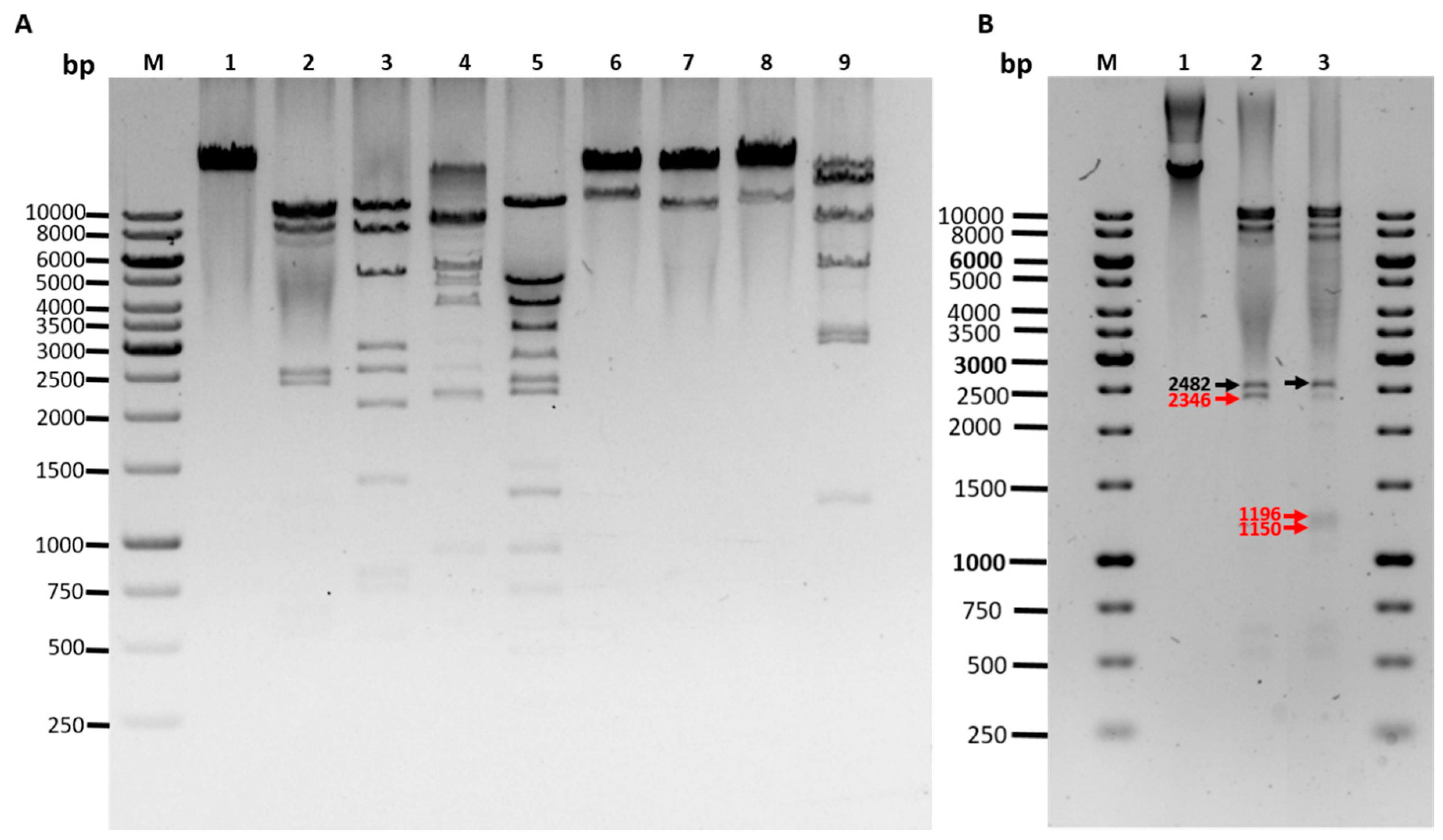
2.13. Restriction Endonuclease Analysis
2.14. Lysogenization Experiment
2.15. Prophage Induction Experiments
2.16. Nucleotide Sequence Accession Numbers
3. Results
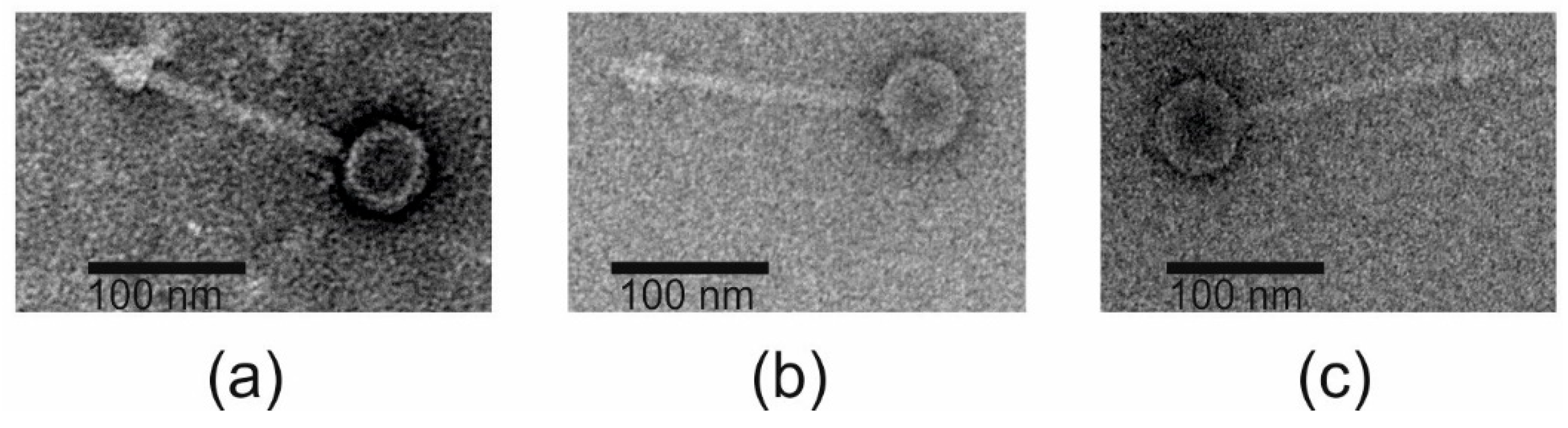
3.1. Phage Isolation and Phenotypic Characterization
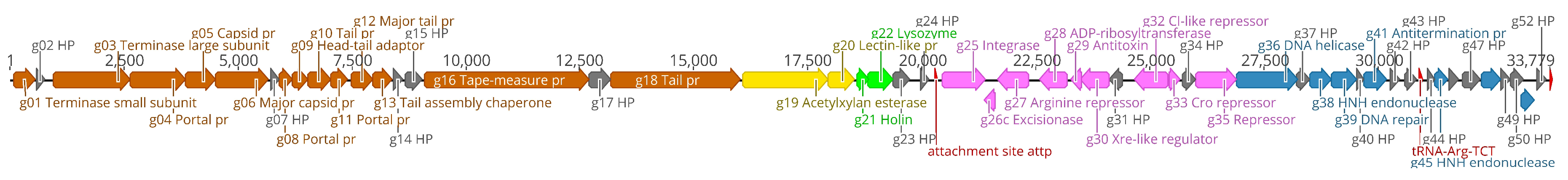
3.2. Phage Genomic Characterization, Annotation and Comparison to Other Phages
- EcoRI (10231, 8997, 8427, 2482, 1196 left end, 1150 right end, 604, 522, 170 bp)
- HincII (9559, 8170, 5179, 2713, 2546, 2074, 1228 right end, 767, 711, 496, 219, 117 left end)
- NsiI (9006, 8804, 6595, 3916, 2482 right end, 2104, left end, 872)
- PstI (11219, 5337, 4003, 3313, 2405, 2240, 1474 left end, 1295 right end, 912, 699, 444, 438)
- SaII (22168 right end, 11611 left end)
- ScaI (23619 right end, 10160 left end)
- SexAI (22976 right end, 10803 left end)
- SpeI (13517 left end, 7659, 5298, 2926, 2907, 1185, 287 right end)
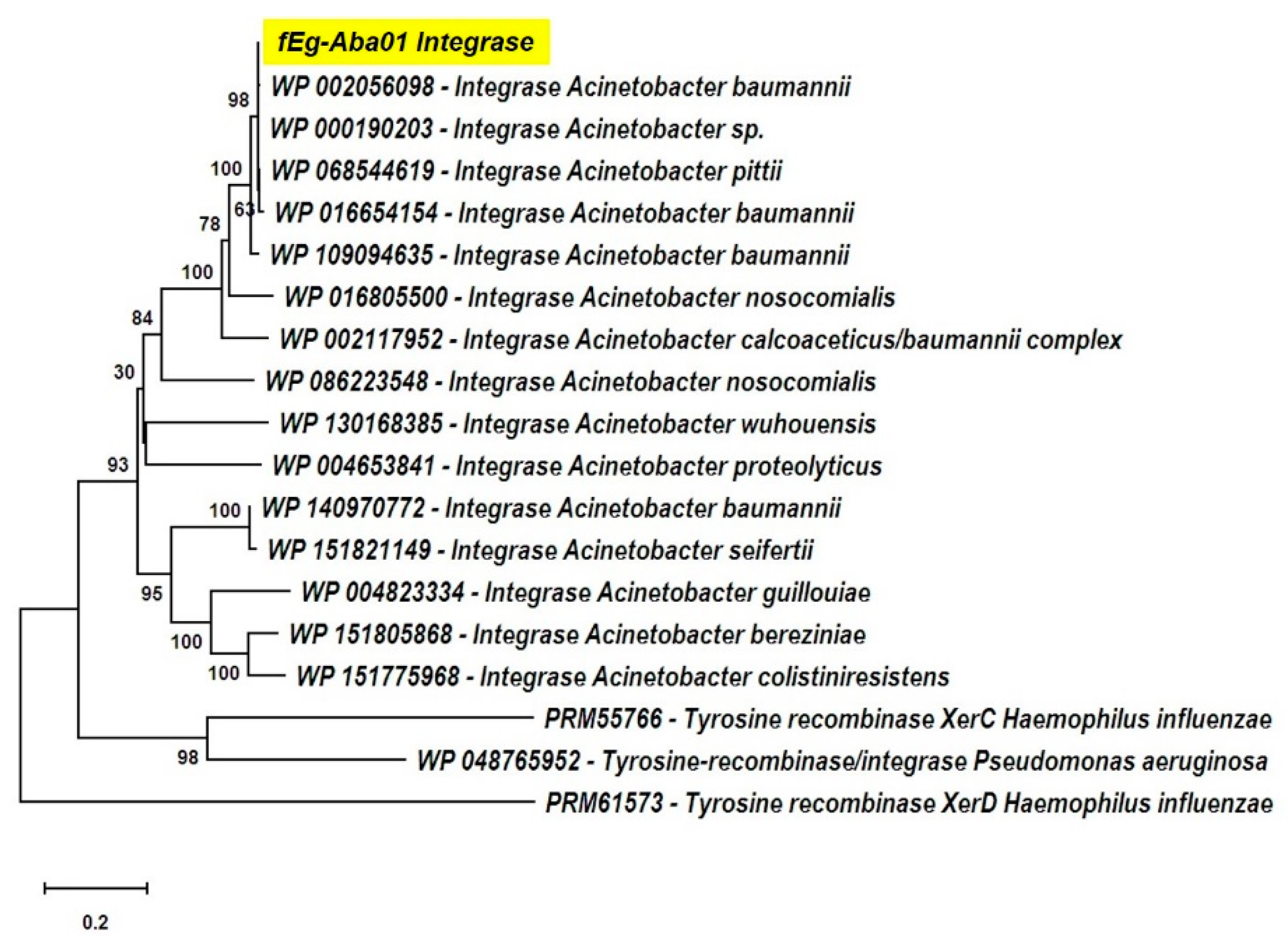
- The lysogen decision gene clusters of the three phages were identified and the gene products annotated based on similarity searches (Table 2). The integrase (Gp25 in fEg-Aba01; Gp27 in fLi-Aba02 and fLi-Aba03) is 100% identical in all three phages. It is the site-specific recombinase that by catalyzing recombination between the two DNA molecules results in either integration or excision of the prophage into and from the host chromosome [62]. The arginine (Gp27c; Gp29c) and the CI-like (Gp32c; Gp34c) repressors account for the establishment of lysogeny and the immunity of lysogens to superinfection. The production of CI represses the Cro (Gp33; Gp35) synthesis. The excisionase (Gp26c; Gp28c) binds to the integrase and enables it to reverse the integration process and liberate the prophage. The Xre family transcriptional regulator (Gp30c; Gp32c) and another repressor (Gp35; Gp37) may also be involved in the regulation of lysogeny/lysis decision. Traditionally, the lysogeny/lysis decision is regulated by the competition between CI repressor and Cro proteins for the occupancy of the operator (OR) region. The Cro protein binds to the OR and OL promotors and turns off the transcription of the CI repressor gene and thus allows transcription and translation of the lytic cycle and other late genes [63]. Some integrases are associated in the integration and excision of phage genomes, whilst others are essential for the maintenance of plasmid copy number or elimination of chromosomal dimers [64,65,66]. A phylogenetic tree of Acinetobacter site-specific integrase sequences was constructed (Figure 5). The results demonstrate that Gp25 integrase clusters among the bacteriophage Hp1-like tyrosine integrases in the XerC superfamily. The ADP-ribosyltransferase (Gp28c; Gp30c) and the antitoxin (Gp29c; Gp31c) may be a toxin-antitoxin pair stabilizing the lysogen [67]. The hypothetical protein (Gp31c; Gp33c) is similar to a A. baumannii protein that shares a fold with a cell division regulator protein of Streptococcus pneumonia GbsP [68] and may interact with the bacterial cell wall synthesis.
- The DNA synthesis, regulation and replication gene products include the Xre family transcriptional regulator (Gp30c; Gp32c), the DNA helicase (Gp36; Gp38) that unwinds the DNA to create template for DNA replication [69], and another repressor (Gp35; Gp37) likely involved in gene regulation. The putative HNH endonucleases (Gp38, Gp45 and Gp51; Gp40, Gp47 and Gp53) may play a variety of roles in replication, recombination, and repair pathways [70]. Finally, the antiterminator protein (Gp41; Gp43) renders RNA polymerase resistant to termination signals [71].
- The DNA packaging into the phage head is carried out by the terminase complex containing the small and large terminase subunits (Gp01 and Gp03, respectively). It recognizes the cos site, introduces there the nicks to generate the cohesive ends to the genome and separates the cohesive ends in a reaction requiring ATP hydrolysis [72]. The terminase and the phage portal proteins (Gp04, Gp08 and Gp11) are believed to be the initiators of the head assembly. The NinB homolog (Gp48; Gp50) may be associated in recombination.
- The phage structural proteins are encoded by the genes encoded in the left half of the genome (Figure 4), and include Gp05 and Gp06 that are annotated as capsid and major capsid proteins, respectively, that initiate formation of the procapsid [73], Gp09 is the putative phage head-tail adaptor, Gp16, the tail length tape-measure protein, and Gp12, the major tail protein. Gp8 and Gp10 are also structural peorteins, and Gp18 (Gp20 in fLi-Aba02 and fLi-Aba03) is also predicted to be a tail component (Table S3). Gp13 is a putative tail assembly chaperone. The predicted tail length based on the tape measure protein of 1195 amino acid residues, 1.5 Å per residue [74], would be ca 180 nm, that is 20–30 nm longer than the measured tail lengths (Table 1). Perhaps the tape measure protein extends to the tip of the bulky tail baseplate omitted from the measures.
- The host lysis protein holin is predicted to be Gp21 (Gp23 in fLi-Aba02 and fLi-Aba03), and Gp22 (Gp24 in fLi-Aba02 and fLi-Aba03), the lysozyme, involved in the bacterial lysis and release of the phage progeny [75]. The predicted peptidoglycan hydrolase (Gp19 in fLi-Aba02 and fLi-Aba03) encoding gene is not present in fEg-Aba01, actually, it is deleted and the g18 in fEg-Aba01 is a fusion product containing 5’-end of gene g18 and 3’-end of gene g20 of fLi-Aba02 (Table S3). The acetylxylan esterase and lectin-like proteins (Gp19;Gp21 and Gp20;Gp21, respectively) are both carbohydrate-associated proteins and might have a function in degrading the bacterial capsule polysaccharides.
3.3. Prophage Lysogenic Characteristics
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bergogne-Berezin, E.; Towner, K.J. Acinetobacter spp. as nosocomial pathogens: Microbiological, clinical, and epidemiological features. Clin. Microb. Rev. 1996, 9, 148–165. [Google Scholar] [CrossRef]
- Peleg, A.Y.; Seifert, H.; Paterson, D.L. Acinetobacter baumannii: Emergence of a successful pathogen. Clin. Microbiol. Rev. 2008, 21, 538–582. [Google Scholar] [CrossRef] [PubMed]
- Davis, K.A.; Moran, K.A.; McAllister, C.K.; Gray, P.J. Multidrug-resistant Acinetobacter extremity infections in soldiers. Emerg. Infect. Dis. 2005, 11, 1218–1224. [Google Scholar] [CrossRef] [PubMed]
- Abbott, I.; Cerqueira, G.M.; Bhuiyan, S.; Peleg, A.Y. Carbapenem resistance in Acinetobacter baumannii: Laboratory challenges, mechanistic insights and therapeutic strategies. Expert Rev. Anti-Infect. Ther. 2013, 11, 395–409. [Google Scholar] [CrossRef] [PubMed]
- Dijkshoorn, L.; Nemec, A.; Seifert, H. An increasing threat in hospitals: Multidrug-resistant Acinetobacter baumannii. Nat. Rev. Microbiol. 2007, 5, 939–951. [Google Scholar] [CrossRef]
- Magiorakos, A.P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef]
- Wand, M.E.; Bock, L.J.; Bonney, L.C.; Sutton, J.M. Retention of virulence following adaptation to colistin in Acinetobacter baumannii reflects the mechanism of resistance. J. Antimicrob. Chemother. 2015, 70, 2209–2216. [Google Scholar] [CrossRef]
- Cai, Y.; Chai, D.; Wang, R.; Liang, B.B.; Bai, N. Colistin resistance of Acinetobacter baumannii: Clinical reports, mechanisms and antimicrobial strategies. J. Antimicrob. Chemother. 2012, 67, 1607–1615. [Google Scholar] [CrossRef]
- Nordmann, P.; Poirel, L. Emerging carbapenemases in Gram-negative aerobes. Clin. Microbiol. Infect. 2002, 8, 321–331. [Google Scholar] [CrossRef]
- Burrowes, B.; Harper, D.R.; Anderson, J.; McConville, M.; Enright, M.C. Bacteriophage therapy: Potential uses in the control of antibiotic-resistant pathogens. Expert Rev. Anti-Infect. Ther. 2011, 9, 775–785. [Google Scholar] [CrossRef]
- Parisien, A.; Allain, B.; Zhang, J.; Mandeville, R.; Lan, C.Q. Novel alternatives to antibiotics: Bacteriophages, bacterial cell wall hydrolases, and antimicrobial peptides. J. Appl. Microbiol. 2008, 104, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Hua, Y.; Luo, T.; Yang, Y.; Dong, D.; Wang, R.; Wang, Y.; Xu, M.; Guo, X.; Hu, F.; He, P. Phage Therapy as a Promising New Treatment for Lung Infection Caused by Carbapenem-Resistant Acinetobacter baumannii in Mice. Front. Microbiol. 2017, 8, 2659. [Google Scholar] [CrossRef] [PubMed]
- Regeimbal, J.M.; Jacobs, A.C.; Corey, B.W.; Henry, M.S.; Thompson, M.G.; Pavlicek, R.L.; Quinones, J.; Hannah, R.M.; Ghebremedhin, M.; Crane, N.J.; et al. Personalized therapeutic cocktail of wild environmental phages rescues mice from Acinetobacter baumannii wound infections. Antimicrob. Agents Chemother. 2016, 60, 5806–5816. [Google Scholar] [CrossRef] [PubMed]
- LaVergne, S.; Hamilton, T.; Biswas, B.; Kumaraswamy, M.; Schooley, R.T.; Wooten, D. Phage therapy for a multidrug-resistant Acinetobacter baumannii craniectomy site infection. Open Forum Infect. Dis. 2018, 5, ofy064. [Google Scholar] [CrossRef] [PubMed]
- Schooley, R.T.; Biswas, B.; Gill, J.J.; Hernandez-Morales, A.; Lancaster, J.; Lessor, L.; Barr, J.J.; Reed, S.L.; Rohwer, F.; Benler, S.; et al. Development and use of personalized bacteriophage-based therapeutic cocktails to treat a patient with a disseminated resistant Acinetobacter baumannii Infection. Antimicrob. Agents Chemother. 2017, 61, e00954-17. [Google Scholar] [CrossRef] [PubMed]
- Abedon, S.T.; Kuhl, S.J.; Blasdel, B.G.; Kutter, E.M. Phage treatment of human infections. Bacteriophage 2011, 1, 66–85. [Google Scholar] [CrossRef]
- Garcia, P.; Monjardin, C.; Martin, R.; Madera, C.; Soberon, N.; Garcia, E.; Meana, A.; Suarez, J.E. Isolation of new Stenotrophomonas bacteriophages and genomic characterization of temperate phage S1. Appl. Environ. Microbiol. 2008, 74, 7552–7560. [Google Scholar] [CrossRef]
- Adams, M.H. Bacteriophages; Interscience Publishers: New York, NY, USA, 1959. [Google Scholar]
- Ackermann, H.W. Phage classification and characterization. Methods Mol. Biol. 2009, 501, 127–140. [Google Scholar]
- Adriaenssens, E.M.; Sullivan, M.B.; Knezevic, P.; van Zyl, L.J.; Sarkar, B.L.; Dutilh, B.E.; Alfenas-Zerbini, P.; Lobocka, M.; Tong, Y.; Brister, J.R.; et al. Taxonomy of prokaryotic viruses: 2018–2019 update from the ICTV Bacterial and Archaeal Viruses Subcommittee. Arch. Virol. 2020, 165, 1253–1260. [Google Scholar] [CrossRef]
- Skurnik, M.; Strauch, E. Phage therapy: Facts and fiction. Int. J. Med. Microbiol. 2006, 296, 5–14. [Google Scholar] [CrossRef]
- Shao, Q.; Trinh, J.T.; Zeng, L. High-resolution studies of lysis-lysogeny decision-making in bacteriophage lambda. J. Biol. Chem. 2019, 294, 3343–3349. [Google Scholar] [CrossRef] [PubMed]
- Feiner, R.; Argov, T.; Rabinovich, L.; Sigal, N.; Borovok, I.; Herskovits, A.A. A new perspective on lysogeny: Prophages as active regulatory switches of bacteria. Nat. Rev. Microbiol. 2015, 13, 641–650. [Google Scholar] [CrossRef] [PubMed]
- Bobay, L.M.; Rocha, E.P.; Touchon, M. The adaptation of temperate bacteriophages to their host genomes. Mol. Biol. Evol. 2013, 30, 737–751. [Google Scholar] [CrossRef] [PubMed]
- Cuenca Mdel, S.; Molina-Santiago, C.; Gomez-Garcia, M.R.; Ramos, J.L. A Pseudomonas putida double mutant deficient in butanol assimilation: A promising step for engineering a biological biofuel production platform. FEMS Microbiol. Lett. 2016, 363, fnw018. [Google Scholar] [CrossRef]
- Boyd, E.F. Bacteriophage-encoded bacterial virulence factors and phage-pathogenicity island interactions. Adv. Virus Res. 2012, 82, 91–118. [Google Scholar]
- Chen, Y.; Yang, L.; Yang, D.; Song, J.; Wang, C.; Sun, E.; Gu, C.; Chen, H.; Tong, Y.; Tao, P.; et al. Specific Integration of Temperate Phage Decreases the Pathogenicity of Host Bacteria. Front. Cell. Infect. Microbiol. 2020, 10, 14. [Google Scholar] [CrossRef]
- Snitkin, E.S.; Zelazny, A.M.; Montero, C.I.; Stock, F.; Mijares, L.; Program, N.C.S.; Murray, P.R.; Segre, J.A. Genome-wide recombination drives diversification of epidemic strains of Acinetobacter baumannii. Proc. Natl. Acad. Sci. USA 2011, 108, 13758–13763. [Google Scholar] [CrossRef]
- Edlin, G.; Lin, L.; Bitner, R. Reproductive Fitness of P1, P2, and Mu-Lysogens of Escherichia coli. J. Virol. 1977, 21, 560–564. [Google Scholar] [CrossRef]
- Shaikh, N.; Tarr, P.I. Escherichia coli O157: H7 shiga toxin-encoding bacteriophages: Integrations, excisions, truncations, and evolutionary implications. J. Bacteriol. 2003, 185, 6495. [Google Scholar] [CrossRef]
- Wang, X.X.; Kim, Y.; Ma, Q.; Hong, S.H.; Pokusaeva, K.; Sturino, J.M.; Wood, T.K. Cryptic prophages help bacteria cope with adverse environments. Nat. Commun. 2010, 1, 147. [Google Scholar] [CrossRef]
- Mahony, J.; McGrath, S.; Fitzgerald, G.F.; van Sinderen, D. Identification and Characterization of Lactococcal-Prophage-Carried Superinfection Exclusion Genes. Appl. Environ. Microbiol. 2008, 74, 6206–6215. [Google Scholar] [CrossRef] [PubMed]
- Costa, A.R.; Monteiro, R.; Azeredo, J. Genomic analysis of Acinetobacter baumannii prophages reveals remarkable diversity and suggests profound impact on bacterial virulence and fitness. Sci. Rep. 2018, 8, 15346. [Google Scholar] [CrossRef] [PubMed]
- Summers, W.C. Bacteriophage therapy. Annu. Rev. Microbiol. 2001, 55, 437–451. [Google Scholar] [CrossRef] [PubMed]
- Jonczyk, E.; Klak, M.; Miedzybrodzki, R.; Gorski, A. The influence of external factors on bacteriophages-review. Folia Microbiol. 2011, 56, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Bertani, G. Lysogeny at mid-twentieth century: P1, P2, and other experimental, systems. J. Bacteriol. 2004, 186, 595–600. [Google Scholar] [CrossRef] [PubMed]
- Baker, P.M.; Farmer, J.J. New Bacteriophage-Typing System for Yersinia enterocolitica, Yersinia kristensenii, Yersinia frederiksenii, and Yersinia intermedia: Correlation with serotyping, biotyping, and antibiotic susceptibility. J. Clin. Microbiol. 1982, 15, 491–502. [Google Scholar] [CrossRef]
- Lin, N.T.; Chiou, P.Y.; Chang, K.C.; Chen, L.K.; Lai, M.J. Isolation and characterization of phi AB2: A novel bacteriophage of Acinetobacter baumannii. Res. Microbiol. 2010, 161, 308–314. [Google Scholar] [CrossRef]
- Jamalludeen, N.; Johnson, R.P.; Friendship, R.; Kropinski, A.M.; Lingohr, E.J.; Gyles, C.L. Isolation and characterization of nine bacteriophages that lyse O149 enterotoxigenic Escherichia coli. Vet. Microbiol. 2007, 124, 47–57. [Google Scholar] [CrossRef]
- Karumidze, N.; Kusradze, I.; Rigvava, S.; Goderdzishvili, M.; Rajakumar, K.; Alavidze, Z. Isolation and Characterisation of Lytic Bacteriophages of Klebsiella pneumoniae and Klebsiella oxytoca. Curr. Microbiol. 2013, 66, 251–258. [Google Scholar] [CrossRef]
- Oliveira, A.; Sillankorva, S.; Quinta, R.; Henriques, A.; Sereno, R.; Azeredo, J. Isolation and characterization of bacteriophages for avian pathogenic E. coli strains. J. Appl. Microbiol. 2009, 106, 1919–1927. [Google Scholar] [CrossRef]
- Pickard, D.J. Preparation of bacteriophage lysates and pure DNA. Methods Mol. Biol. 2009, 502, 3–9. [Google Scholar] [PubMed]
- Sambrook, J.; Russell, D.W. Molecular Cloning: A Laboratory Manual, 3rd ed.; Cold Spring Harbor Laboratory: Cold Spring Harbor, NY, USA, 2001. [Google Scholar]
- Sambrook, J.; Russell, D.W. Purification of Bacteriophage λ Particles by Centrifugation through a Glycerol Step Gradient. CSH Protoc. 2006, 2006. [Google Scholar] [CrossRef] [PubMed]
- Kwiatek, M.; Mizak, L.; Parasion, S.; Gryko, R.; Olender, A.; Niemcewicz, M. Characterization of five newly isolated bacteriophages active against Pseudomonas aeruginosa clinical strains. Folia Microbiol. 2015, 60, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Prestinaci, F.; Pezzotti, P.; Pantosti, A. Antimicrobial resistance: A global multifaceted phenomenon. Pathog. Glob. Health 2015, 109, 309–318. [Google Scholar] [CrossRef]
- Carver, T.; Berriman, M.; Tivey, A.; Patel, C.; Bohme, U.; Barrell, B.G.; Parkhill, J.; Rajandream, M.A. Artemis and ACT: Viewing, annotating and comparing sequences stored in a relational database. Bioinformatics 2008, 24, 2672–2676. [Google Scholar] [CrossRef]
- Carver, T.; Harris, S.R.; Berriman, M.; Parkhill, J.; McQuillan, J.A. Artemis: An integrated platform for visualization and analysis of high-throughput sequence-based experimental data. Bioinformatics 2012, 28, 464–469. [Google Scholar] [CrossRef]
- Kearse, M.; Moir, R.; Wilson, A.; Stones-Havas, S.; Cheung, M.; Sturrock, S.; Buxton, S.; Cooper, A.; Markowitz, S.; Duran, C.; et al. Geneious Basic: An integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 2012, 28, 1647–1649. [Google Scholar] [CrossRef]
- Aziz, R.K.; Bartels, D.; Best, A.A.; DeJongh, M.; Disz, T.; Edwards, R.A.; Formsma, K.; Gerdes, S.; Glass, E.M.; Kubal, M.; et al. The RAST server: Rapid annotations using subsystems technology. BMC Genom. 2008, 9, 75. [Google Scholar] [CrossRef]
- Garneau, J.R.; Depardieu, F.; Fortier, L.C.; Bikard, D.; Monot, M. PhageTerm: A tool for fast and accurate determination of phage termini and packaging mechanism using next-generation sequencing data. Sci. Rep. 2017, 7, 8292. [Google Scholar] [CrossRef]
- Alva, V.; Nam, S.Z.; Soding, J.; Lupas, A.N. The MPI bioinformatics Toolkit as an integrative platform for advanced protein sequence and structure analysis. Nucleic Acids Res. 2016, 44, W410–W415. [Google Scholar] [CrossRef]
- Kallio, M.A.; Tuimala, J.T.; Hupponen, T.; Klemela, P.; Gentile, M.; Scheinin, I.; Koski, M.; Kaki, J.; Korpelainen, E.I. Chipster: User-friendly analysis software for microarray and other high-throughput data. BMC Genom. 2011, 12, 507. [Google Scholar] [CrossRef] [PubMed]
- Katoh, K.; Standley, D.M. MAFFT Multiple Sequence Alignment Software Version 7: Improvements in Performance and Usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Meier-Kolthoff, J.P.; Goker, M. VICTOR: Genome-based phylogeny and classification of prokaryotic viruses. Bioinformatics 2017, 33, 3396–3404. [Google Scholar] [CrossRef]
- Wyres, K.L.; Cahill, S.M.; Holt, K.E.; Hall, R.M.; Kenyon, J.J. Identification of Acinetobacter baumannii loci for capsular polysaccharide (KL) and lipooligosaccharide outer core (OCL) synthesis in genome assemblies using curated reference databases compatible with Kaptive. Microb. Genom. 2020, 6, e000339. [Google Scholar] [CrossRef]
- Goffartroskam, J. Quantitative study of sensitive and lysogenic bacteria surviving infection of a Staphylococcus by a temperate bacteriophage. Antonie Leeuwenhoek 1965, 31, 249–261. [Google Scholar] [CrossRef]
- Bae, T.; Baba, T.; Hiramatsu, K.; Schneewind, O. Prophages of Staphylococcus aureus Newman and their contribution to virulence. Mol. Microbiol. 2006, 62, 1035–1047. [Google Scholar] [CrossRef]
- Linz, B.; Mukhta, N.; Shabbir, M.Z.; Rivera, I.; Ivanov, Y.V.; Tahir, Z.; Yaqub, T.; Harvill, E.T. Virulent Epidemic Pneumonia in Sheep Caused by the Human Pathogen Acinetobacter baumannii. Front. Microbiol. 2018, 9, 9. [Google Scholar] [CrossRef]
- Hare, J.M.; Ferrell, J.C.; Witkowski, T.A.; Grice, A.N. Prophage induction and differential RecA and UmuDAb transcriptome regulation in the DNA damage responses of Acinetobacter baumannii and Acinetobacter baylyi. PLoS ONE 2014, 9, e93861. [Google Scholar] [CrossRef]
- Stark, W.M. Making serine integrases work for us. Curr. Opin. Microbiol. 2017, 38, 130–136. [Google Scholar] [CrossRef]
- Yoshida, M.; Yoshida-Takashima, Y.; Nunoura, T.; Takai, K. Genomic characterization of a temperate phage of the psychrotolerant deep-sea bacterium Aurantimonas sp. Extremophiles 2015, 19, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Narendra, U.; Iype, L.E.; Cox, M.M.; Rice, P.A. Crystal structure of a Flp recombinase—Holliday junction complex: Assembly of an active oligomer by helix swapping. Mol. Cell 2000, 6, 885–897. [Google Scholar] [CrossRef]
- Sherratt, D.J.; Soballe, B.; Barre, F.X.; Filipe, S.; Lau, I.; Massey, T.; Yates, J. Recombination and chromosome segregation. Philos. Trans. R. Soc. B Biol. Sci. 2004, 359, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Van Duyne, G.D. A structural view of cre-loxp site-specific recombination. Annu. Rev. Biophys. Biomol. Struct. 2001, 30, 87–104. [Google Scholar] [CrossRef]
- Barer, M.R.; Harwood, C.R. Bacterial viability and culturability. Adv. Microb. Physiol. 1999, 41, 93–137. [Google Scholar]
- Cleverley, R.M.; Rutter, Z.J.; Rismondo, J.; Corona, F.; Tsui, H.T.; Alatawi, F.A.; Daniel, R.A.; Halbedel, S.; Massidda, O.; Winkler, M.E.; et al. The cell cycle regulator GpsB functions as cytosolic adaptor for multiple cell wall enzymes. Nat. Commun. 2019, 10, 261. [Google Scholar] [CrossRef]
- Lionnet, T.; Spiering, M.M.; Benkovic, S.J.; Bensimon, D.; Croquette, V. Real-time observation of bacteriophage T4 gp41 helicase reveals an unwinding mechanism. Proc. Natl. Acad. Sci. USA 2007, 104, 19790–19795. [Google Scholar] [CrossRef]
- Mitsunobu, H.; Zhu, B.; Lee, S.J.; Tabor, S.; Richardson, C.C. Flap endonuclease of bacteriophage T7: Possible roles in RNA primer removal, recombination and host DNA breakdown. Bacteriophage 2014, 4, e28507. [Google Scholar] [CrossRef]
- Shi, J.; Gao, X.; Tian, T.; Yu, Z.; Gao, B.; Wen, A.; You, L.; Chang, S.; Zhang, X.; Zhang, Y.; et al. Structural basis of Q-dependent transcription antitermination. Nat. Commun. 2019, 10, 2925. [Google Scholar] [CrossRef]
- Duffy, C.; Feiss, M. The large subunit of bacteriophage λ’s terminase plays a role in DNA translocation and packaging termination. J. Mol. Biol. 2002, 316, 547–561. [Google Scholar] [CrossRef]
- Rajagopala, S.V.; Casjens, S.; Uetz, P. The protein interaction map of bacteriophage lambda. BMC Microbiol. 2011, 11, 213. [Google Scholar] [CrossRef] [PubMed]
- Katsura, I.; Hendrix, R.W. Length determination in bacteriophage lambda tails. Cell 1984, 39, 691–698. [Google Scholar] [CrossRef]
- Kropinski, A.M.; Waddell, T.; Meng, J.; Franklin, K.; Ackermann, H.W.; Ahmed, R.; Mazzocco, A.; Yates, J., 3rd; Lingohr, E.J.; Johnson, R.P. The host-range, genomics and proteomics of Escherichia coli O157:H7 bacteriophage rV5. Virol. J. 2013, 10, 76. [Google Scholar] [CrossRef] [PubMed]
- Dedrick, R.M.; Jacobs-Sera, D.; Bustamante, C.A.; Garlena, R.A.; Mavrich, T.N.; Pope, W.H.; Reyes, J.C.; Russell, D.A.; Adair, T.; Alvey, R.; et al. Prophage-mediated defence against viral attack and viral counter-defence. Nat. Microbiol. 2017, 2, 16251. [Google Scholar] [CrossRef] [PubMed]
- MacLean, L.L.; Perry, M.B.; Chen, W.; Vinogradov, E. The structure of the polysaccharide O-chain of the LPS from Acinetobacter baumannii strain ATCC 17961. Carbohydr. Res. 2009, 344, 474–478. [Google Scholar] [CrossRef]
- Senchenkova, S.N.; Shashkov, A.S.; Popova, A.V.; Shneider, M.M.; Arbatsky, N.P.; Miroshnikov, K.A.; Volozhantsev, N.V.; Knirel, Y.A. Structure elucidation of the capsular polysaccharide of Acinetobacter baumannii AB5075 having the KL25 capsule biosynthesis locus. Carbohydr. Res. 2015, 408, 8–11. [Google Scholar] [CrossRef]
- Lopez-Leal, G.; Santamaria, R.I.; Cevallos, M.A.; Gonzalez, V.; Castillo-Ramirez, S. Prophages encode antibiotic resistance genes in Acinetobacter baumannii. Microb. Drug Resist. 2020. [Google Scholar] [CrossRef]
- Turner, D.; Wand, M.E.; Sutton, J.M.; Centron, D.; Kropinski, A.M.; Reynolds, D.M. Genome sequence of vB_AbaS_TRS1, a viable prophage isolated from acinetobacter baumannii strain A118. Genome Announc. 2016, 4, 4. [Google Scholar] [CrossRef]
- Dedrick, R.M.; Guerrero-Bustamante, C.A.; Garlena, R.A.; Russell, D.A.; Ford, K.; Harris, K.; Gilmour, K.C.; Soothill, J.; Jacobs-Sera, D.; Schooley, R.T.; et al. Engineered bacteriophages for treatment of a patient with a disseminated drug-resistant Mycobacterium abscessus. Nat. Med. 2019, 25, 730–733. [Google Scholar] [CrossRef]
- Abedon, S.T. Phage therapy dosing: The problem(s) with multiplicity of infection (MOI). Bacteriophage 2016, 6, e1220348. [Google Scholar] [CrossRef]
| Bacteriophage | fEg-Aba01 | fLi-Aba02 | fLi-Aba03 |
|---|---|---|---|
| Capsid size, nm | 58.4 ± 4 | 63 ± 2 | 58.6 ± 5 |
| Tail length, nm | 156 ± 9 | 136 ± 1.8 | 142 ± 0.8 |
| Tail width, nm | 10.8 ± 0.3 | 9 ± 0.2 | 8.5 ± 0.9 |
| Genome size, bp | 33,779 | 35,053 | 34,931 |
| GC content (%) | 40.1 | 40.2 | 40.1 |
| n genes | 52 | 53 | 54 |
| n tRNAs | 1 | 1 | 1 |
| Gene | Location | Predicted Function | Amino Acids |
|---|---|---|---|
| g25fEg-Aba01 | 20438-21397 | Integrase | 319 |
| g27fLi-Aba02 | 22436-21477 | 319 | |
| g27fLi-Aba03 | 22436-21477 | 319 | |
| g26cfEg-Aba01 | 21614-21363 | Excisionase | 83 |
| g28cfLi-Aba02 | 22653-22402 | 83 | |
| g28cfLi-Aba03 | 22653-22402 | 83 | |
| g27cfEg-Aba01 | 22353-21667 | Arginine repressor | 228 |
| g29cfLi-Aba02 | 23392-22706 | 228 | |
| g29cfLi-Aba03 | 23392-22706 | 228 | |
| g28cfEg-Aba01 | 23182-22595 | ADP-ribosyltransferase | 195 |
| g30cfLi-Aba02 | 24429-23392 | 345 | |
| g30cfLi-Aba03 | 24267-23392 | 291 | |
| g29cfEg-Aba01 | 23494-23258 | Antitoxin | 78 |
| g31cfLi-Aba02 | 24741-24505 | 78 | |
| g31cfLi-Aba03 | 24579-24343 | 78 | |
| g30cfEg-Aba01 | 24113-23478 | Xre family transcriptional regulator | 211 |
| g32cfLi-Aba02 | 25360-24725 | 211 | |
| g32cfLi-Aba03 | 25198-24563 | 211 | |
| g31cfEg-Aba01 | 24406-24116 | Hypothetical protein | 96 |
| g33cfLi-Aba02 | 25653-25363 | 96 | |
| g33cfLi-Aba03 | 25491-25201 | 96 | |
| g32cfEg-Aba01 | 25397-24657 | Repressor protein CI | 246 |
| g34cfLi-Aba02 | 26596-25904 | 230 | |
| g34cfLi-Aba03 | 26434-25742 | 230 | |
| g33fEg-Aba01 | 25663-25421 | Cro repressor | 80 |
| g35fLi-Aba02 | 26910-26725 | 61 | |
| g35fLi-Aba03 | 26748-26521 | 75 |
| Region of Difference | Location in fEg-Aba01 | Description |
|---|---|---|
| RoD-01 | 18065..18066 | A 207 bp in-frame deletion in g20 of fEg-Aba01 that causes a 69 amino acid truncation of Gp20. |
| RoD-02 | 13550..13551 | A 832 bp deletion in fEg-Aba01 that generates a fusion of genes corresponding to g18 and g20 of phages fLi-Aba02 and fLi-Aba03. The deletion includes the gene g19 of the latter. |
| RoD-03 | 6915..6925 | Five nucleotide substitutions in a stretch of 11 bp within g10 of AbPK1 prophage causing one (Arg-to-Lys) neutral substitution. |
| RoD-04 | 32618 | A silent one nucleotide substitution in g50 of fEg-Aba01 |
| RoD-05 | 32453..32454 | A 67 bp deletion in g50 of fEg-Aba01 causing a frame shift mutation and truncating the 3’-end of g48 |
| RoD-06 | 24177 | A one bp substitution in gene g32c causing a Ser to Phe substitution in the AbPK1 protein |
| RoD-07 | 22613..22772 | A stretch of three variants of 27 bp repetitions (6–13 repetitions) in the 3’end of gene g28c. Also out of frame deletions in the fEg-Aba01 gene causing frame shift and 3’-end truncation. Identical full length region is present in fLi-Aba02, and the #5707 and #5920 prophages |
| RoD-08 | 21103 | A silent one bp substitution in the integrase gene g27 of AbPK1 prophage |
| Strain | MLST | LOS-Type | K-Type | Prophage |
|---|---|---|---|---|
| #5707 | 1114, 1841 | OCL1 | KL25 | Yes |
| #5907 | 1806, 208 | OCL1 | KL2 | Partial |
| #5920 | 1114, 1841 | OCL1 | KL25 | Yes |
| #6597 | 1816, 195 | OCL1 | KL3 | No |
| AbPK1 | 452 | OCL1 | KL3 | Yes |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Badawy, S.; Pajunen, M.I.; Haiko, J.; Baka, Z.A.M.; Abou-Dobara, M.I.; El-Sayed, A.K.A.; Skurnik, M. Identification and Functional Analysis of Temperate Siphoviridae Bacteriophages of Acinetobacter baumannii. Viruses 2020, 12, 604. https://doi.org/10.3390/v12060604
Badawy S, Pajunen MI, Haiko J, Baka ZAM, Abou-Dobara MI, El-Sayed AKA, Skurnik M. Identification and Functional Analysis of Temperate Siphoviridae Bacteriophages of Acinetobacter baumannii. Viruses. 2020; 12(6):604. https://doi.org/10.3390/v12060604
Chicago/Turabian StyleBadawy, Shimaa, Maria I. Pajunen, Johanna Haiko, Zakaria A. M. Baka, Mohamed I. Abou-Dobara, Ahmed K. A. El-Sayed, and Mikael Skurnik. 2020. "Identification and Functional Analysis of Temperate Siphoviridae Bacteriophages of Acinetobacter baumannii" Viruses 12, no. 6: 604. https://doi.org/10.3390/v12060604
APA StyleBadawy, S., Pajunen, M. I., Haiko, J., Baka, Z. A. M., Abou-Dobara, M. I., El-Sayed, A. K. A., & Skurnik, M. (2020). Identification and Functional Analysis of Temperate Siphoviridae Bacteriophages of Acinetobacter baumannii. Viruses, 12(6), 604. https://doi.org/10.3390/v12060604






