Serological Immunity to Smallpox in New South Wales, Australia
Abstract
1. Introduction
2. Methods
2.1. Aim 1
2.1.1. Study Population and Recruitment
2.1.2. Estimation of Neutralising Antibody Titre against Vaccinia Virus
2.1.3. Statistical Analyses
2.2. Aim 2
Data/Estimates for Projection of Waning Neutralising Antibody Titre
3. Results
3.1. GMT Levels in the Australian Population
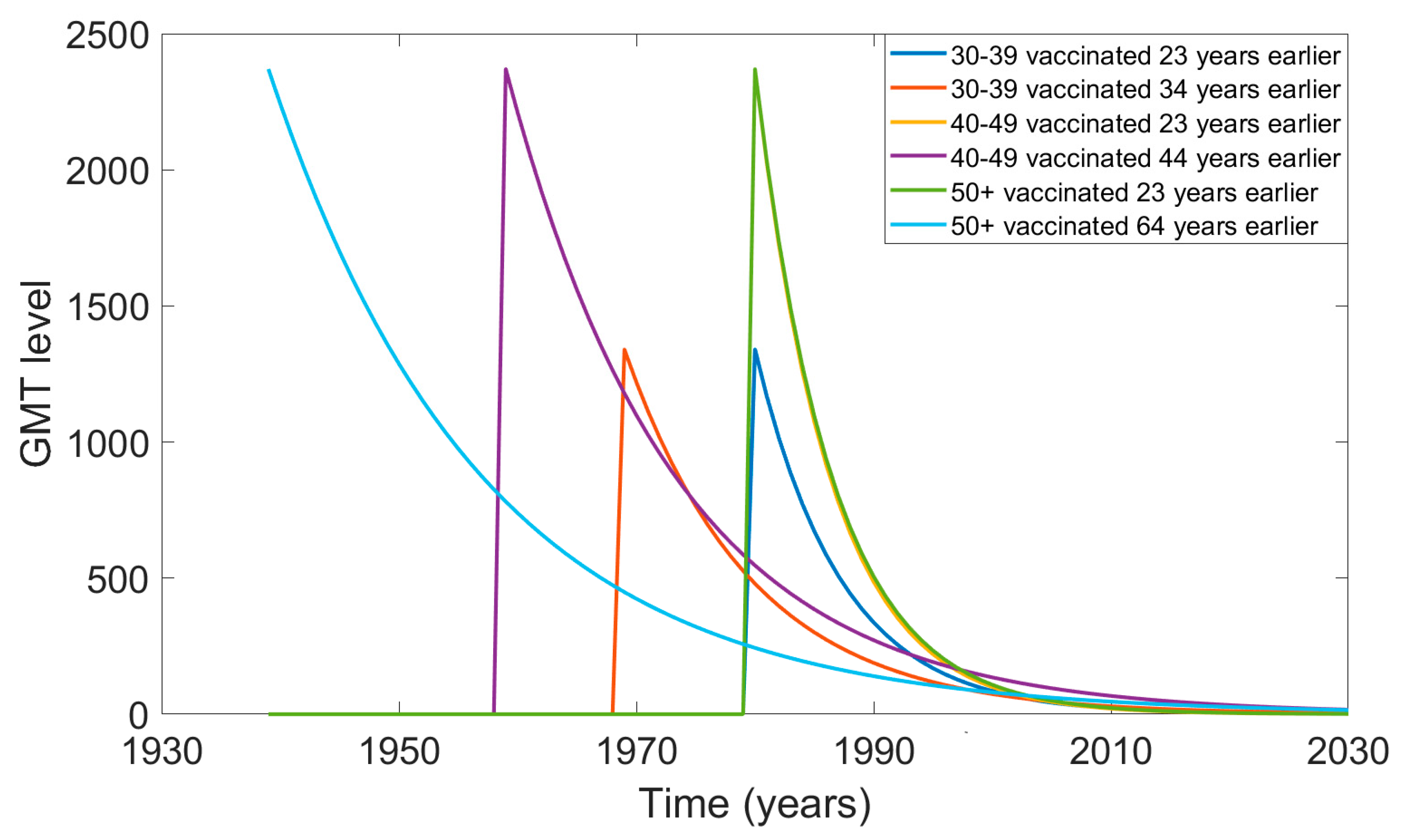
3.2. Projection of Waning Neutralising Antibody Titre over Time Since Vaccination
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Curson, P. Deadly Encounters: How Infectious Disease Helped Shape Australia; Arena Books: Edmonds, DC, USA, 2015. [Google Scholar]
- National Museum of Australia. Smallpox Epidemic. Available online: https://www.nma.gov.au/defining-moments/resources/smallpox-epidemic (accessed on 5 April 2019).
- Fenner, F.; Henderson, D.; Arita, I.; Jezek, Z.; Ladnyi, I. Smallpox and Its Eradication: The Pathogenesis, Immunology, and Pathology of Smallpox and Vaccinia; World Health Organization: Geneva, Switzerland, 1988; p. 1469. [Google Scholar]
- Boughton, C. Smallpox and Australia. Intern. Med. J. 2002, 32, 59–61. [Google Scholar] [CrossRef] [PubMed]
- NSW Health. Smallpox (Variola) Factsheet. Available online: https://www.health.nsw.gov.au/Infectious/factsheets/Pages/smallpox-variola.aspx (accessed on 14 October 2019).
- World Health Organization. Smallpox. Available online: http://www.who.int/csr/dsease/smallpox/en/ (accessed on 28 February 2019).
- Koblentz, G.D. The de novo synthesis of horsepox virus: Implications for biosecurity and recommendations for preventing the reemergence of smallpox. Health Secur. 2017, 15, 620–628. [Google Scholar] [CrossRef] [PubMed]
- Kupferschmidt, K. How Canadian researchers reconstituted an extinct poxvirus for $100,000 using mail-order DNA. Science 2017, 6. [Google Scholar] [CrossRef]
- MacIntyre, C.R.; Costantino, V.; Chen, X.; Segelov, E.; Chughtai, A.A.; Kelleher, A.; Kunasekaran, M.; Lane, J.M. Influence of Population Immunosuppression and Past Vaccination on Smallpox Reemergence. Emerg. Infect. Dis. 2018, 24, 646. [Google Scholar] [CrossRef]
- Feery, B.J. Adverse reactions after smallpox vaccination. Med. J. Aust. 1977, 2, 180–183. [Google Scholar] [CrossRef]
- Hammarlund, E.; Lewis, M.W.; Hansen, S.G.; Strelow, L.I.; Nelson, J.A.; Sexton, G.J.; Hanifin, J.M.; Slifka, M.K. Duration of antiviral immunity after smallpox vaccination. Nat. Med. 2003, 9, 1131–1137. [Google Scholar] [CrossRef]
- Rao, A.R. Smallpox; Kothari Book Depot: Mumbai, India, 1972. [Google Scholar]
- Kunasekaran, M.P.; Chen, X.; Costantino, V.; Chughtai, A.A.; MacIntyre, C.R. Evidence for Residual Immunity to Smallpox after Vaccination and Implications for Re-emergence. Mil. Med. 2019. [Google Scholar] [CrossRef]
- Amanna, I.J.; Slifka, M.K.; Crotty, S. Immunity and immunological memory following smallpox vaccination. J. Immunol. Rev. 2006, 211, 320–337. [Google Scholar] [CrossRef]
- Haralambieva, I.H.; Ovsyannikova, I.G.; Kennedy, R.B.; Larrabee, B.R.; Pankratz, V.S.; Poland, G.A. Race and sex-based differences in cytokine immune responses to smallpox vaccine in healthy individuals. Hum. Immunol. 2013, 74, 1263–1266. [Google Scholar] [CrossRef][Green Version]
- Kennedy, R.B.; Poland, G.A.; Ovsyannikova, I.G.; Oberg, A.L.; Asmann, Y.W.; Grill, D.E.; Vierkant, R.A.; Jacobson, R.M. Impaired innate, humoral, and cellular immunity despite a take in smallpox vaccine recipients. Vaccine 2016, 34, 3283–3290. [Google Scholar] [CrossRef]
- Lareau, C.; White, B.; Oberg, A.; Kennedy, R.B.; Poland, G.A.; McKinney, B. An interaction quantitative trait loci tool implicates epistatic functional variants in an apoptosis pathway in smallpox vaccine eQTL data. Genes Immun. 2016. [Google Scholar] [CrossRef] [PubMed]
- Ovsyannikova, I.G.; Haralambieva, I.H.; Kennedy, R.B.; Pankratz, V.S.; Vierkant, R.A.; Jacobson, R.M.; Poland, G.A. Impact of cytokine and cytokine receptor gene polymorphisms on cellular immunity after smallpox vaccination. Gene 2012, 510, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Sukhumvittaya, S.; Ampol, S.; Pattanapanyasat, K.; Kantakamalakul, W. Polyfunctional T Cell and Neutralizing Antibody Responses to ACAM2000TM Smallpox Vaccine Immunization in Primary-Vaccinated Individuals. Adv. Microbiol. 2016, 6, 169. [Google Scholar] [CrossRef]
- Troy, J.D.; Hill, H.R.; Ewell, M.G.; Frey, S.E. Sex difference in immune response to vaccination: A participant-level meta-analysis of randomized trials of IMVAMUNE® smallpox vaccine. Vaccine 2015, 33, 5425–5431. [Google Scholar] [CrossRef] [PubMed]
- Australian Government Department of Health. CDPLAN Emergency Response Plan for Communicable Disease Incidents of National Significance. Available online: https://www.health.gov.au/internet/main/publishing.nsf/Content/7A38C92C483C8B77CA25805E001A402D/$File/CDPLAN.pdf (accessed on 12 September 2019).
- StataCorp. Stata Statistical Software: Release 14; StataCorp LP: College Station, TX, USA, 2015. [Google Scholar]
- Mack, T.M.; Noble, J., Jr.; Thomas, D.B. A prospective study of serum antibody and protection against smallpox. Am. J. Trop. Med. Hyg. 1972, 21, 214–218. [Google Scholar] [CrossRef] [PubMed]
- Taub, D.D.; Ershler, W.B.; Janowski, M.; Artz, A.; Key, M.L.; McKelvey, J.; Muller, D.; Moss, B.; Ferrucci, L.; Duffey, P.L. Immunity from smallpox vaccine persists for decades: A longitudinal study. Am. J. Med. 2008, 121, 1058–1064. [Google Scholar] [CrossRef]
- Orr, N.; Forman, M.; Marcus, H.; Lustig, S.; Paran, N.; Grotto, I.; Klement, E.; Yehezkelli, Y.; Robin, G.; Reuveny, S. Clinical and immune responses after revaccination of israeli adults with the Lister strain of vaccinia virus. J. Infect. Dis. 2004, 190, 1295–1302. [Google Scholar] [CrossRef]
- Saito, T.; Fujii, T.; Kanatani, Y.; Saijo, M.; Morikawa, S.; Yokote, H.; Takeuchi, T.; Kuwabara, N. Clinical and immunological response to attenuated tissue-cultured smallpox vaccine LC16m8. J. Am. Med. Assoc. 2009, 301, 1025–1033. [Google Scholar] [CrossRef]
- Stienlauf, S.; Shoresh, M.; Solomon, A.; Lublin-Tennenbaum, T.; Atsmon, Y.; Meirovich, Y.; Katz, E. Kinetics of formation of neutralizing antibodies against vaccinia virus following re-vaccination. Vaccine 1999, 17, 201–204. [Google Scholar] [CrossRef]
- Haselow, D. Vaccination-related side effects, humoral immunity, and adverse events during the civilian smallpox vaccination campaign, Arkansas, 2003. Public Health Nurs. 2016, 33, 129–138. [Google Scholar] [CrossRef]
- World Health Organization. Summary Report on First, Second and Third Generation Smallpox Vaccines. Available online: https://www.who.int/immunization/sage/meetings/2013/november/2_Smallpox_vaccine_review_updated_11_10_13.pdf?ua=1 (accessed on 2 February 2019).
- Antia, A.; Ahmed, H.; Handel, A.; Carlson, N.E.; Amanna, I.J.; Antia, R.; Slifka, M. Heterogeneity and longevity of antibody memory to viruses and vaccines. PLoS Biol. 2018, 16, e2006601. [Google Scholar] [CrossRef] [PubMed]
- MacIntyre, C.R.; Costantino, V.; Kunasekaran, M.P. Health system capacity in Sydney, Australia in the event of a biological attack with smallpox. PLoS ONE 2019, 14, e0217704. [Google Scholar] [CrossRef] [PubMed]
- MacIntyre, C.R.; Heslop, D.; Nand, D.; Schramm, C.; Butel, M.; Rawlinson, W.; Baker, M.; Kiedrzynski, T.; Nelson, C.; Rosewell, A.; et al. Exercise Mataika: White Paper on response to a smallpox bioterrorism release in the Pacific. Glob. Biosecur. 2019, 1, 91. [Google Scholar] [CrossRef]
- Mooi, F.; Van Der Maas, N.; De Melker, H. Pertussis resurgence: Waning immunity and pathogen adaptation–two sides of the same coin. Epidemiol. Infect. 2014, 142, 685–694. [Google Scholar] [CrossRef] [PubMed]
- Mossong, J.; Nokes, D.J.; Edmunds, W.J.; Cox, M.J.; Ratnam, S.; Muller, C.P. Modeling the impact of subclinical measles transmission in vaccinated populations with waning immunity. Am. J. Epidemiol. 1999, 150, 1238–1249. [Google Scholar] [CrossRef]
- Heffernan, J.; Keeling, M.J. Implications of vaccination and waning immunity. Proc. R. Soc. Lond. B Biol. Sci. 2009. [Google Scholar] [CrossRef]
- Hughes, C.M.; Newman, F.K.; Davidson, W.B.; Olson, V.A.; Smith, S.K.; Holman, R.C.; Yan, L.; Frey, S.E.; Belshe, R.B.; Karem, K.L. Analysis of variola and vaccinia virus neutralization assays for smallpox vaccines. Clin. Vaccine Immunol. 2012, 19, 1116–1118. [Google Scholar] [CrossRef]
- El-Ad, B.; Roth, Y.; Winder, A.; Tochner, Z.; Lublin-Tennenbaum, T.; Katz, E.; Schwartz, T. The persistence of neutralizing antibodies after revaccination against smallpox. J. Infect. Dis. 1990, 161, 446–448. [Google Scholar] [CrossRef]
- Gallwitz, S.; Schutzbank, T.; Heberling, R.L.; Kalter, S.; Galpin, J.E. Smallpox: Residual antibody after vaccination. J. Clin. Microbiol. 2003, 41, 4068–4070. [Google Scholar] [CrossRef]
- Hatakeyama, S.; Moriya, K.; Saijo, M.; Morisawa, Y.; Kurane, I.; Koike, K.; Kimura, S.; Morikawa, S. Persisting humoral antiviral immunity within the Japanese population after the discontinuation in 1976 of routine smallpox vaccinations. Clin. Diagn. Lab. Immunol. 2005, 12, 520–524. [Google Scholar] [CrossRef]
- Lane, J.M. The current and future landscape of smallpox vaccines. Glob. Biosecurity 2019, 1. [Google Scholar] [CrossRef]
- Yinka-Ogunleye, A.; Aruna, O.; Dalhat, M.; Ogoina, D.; McCollum, A.; Disu, Y.; Mamadu, I.; Akinpelu, A.; Ahmad, A.; Burga, J.; et al. Outbreak of human monkeypox in Nigeria in 2017-18: A clinical and epidemiological report. Lancet Infect. Dis. 2019, 19, 872–879. [Google Scholar] [CrossRef]
- Fine, P.; Jezek, Z.; Grab, B.; Dixon, H. The transmission potential of monkeypox virus in human populations. Int. J. Epidemiol. 1988, 17, 643–650. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, J.; Mitra, A.; Mukherjee, M. The minimum protective level of antibodies in smallpox. Bull. World Health Organ. 1975, 52, 307. [Google Scholar] [PubMed]
- Plotkin, S.A. Correlates of protection induced by vaccination. Clin. Vaccine Immunol. 2010, 17, 1055–1065. [Google Scholar] [CrossRef] [PubMed]
- Thakur, A.; Pedersen, L.E.; Jungersen, G. Immune markers and correlates of protection for vaccine induced immune responses. Vaccine 2012, 30, 4907–4920. [Google Scholar] [CrossRef]
- Offit, P.A.; Quarles, J.; Gerber, M.A.; Hackett, C.J.; Marcuse, E.K.; Kollman, T.R.; Gellin, B.G.; Landry, S. Addressing parents’ concerns: Do multiple vaccines overwhelm or weaken the infant’s immune system. Pediatrics 2002, 109, 124–129. [Google Scholar] [CrossRef]
- Eichner, M. Analysis of historical data suggests long-lasting protective effects of smallpox vaccination. Am. J. Epidemiol. 2003, 158, 717–723. [Google Scholar] [CrossRef]
- Nishiura, H.; Eichner, M. Estimation of the duration of vaccine-induced residual protection against severe and fatal smallpox based on secondary vaccination failure. Infection 2006, 34, 241–246. [Google Scholar] [CrossRef]
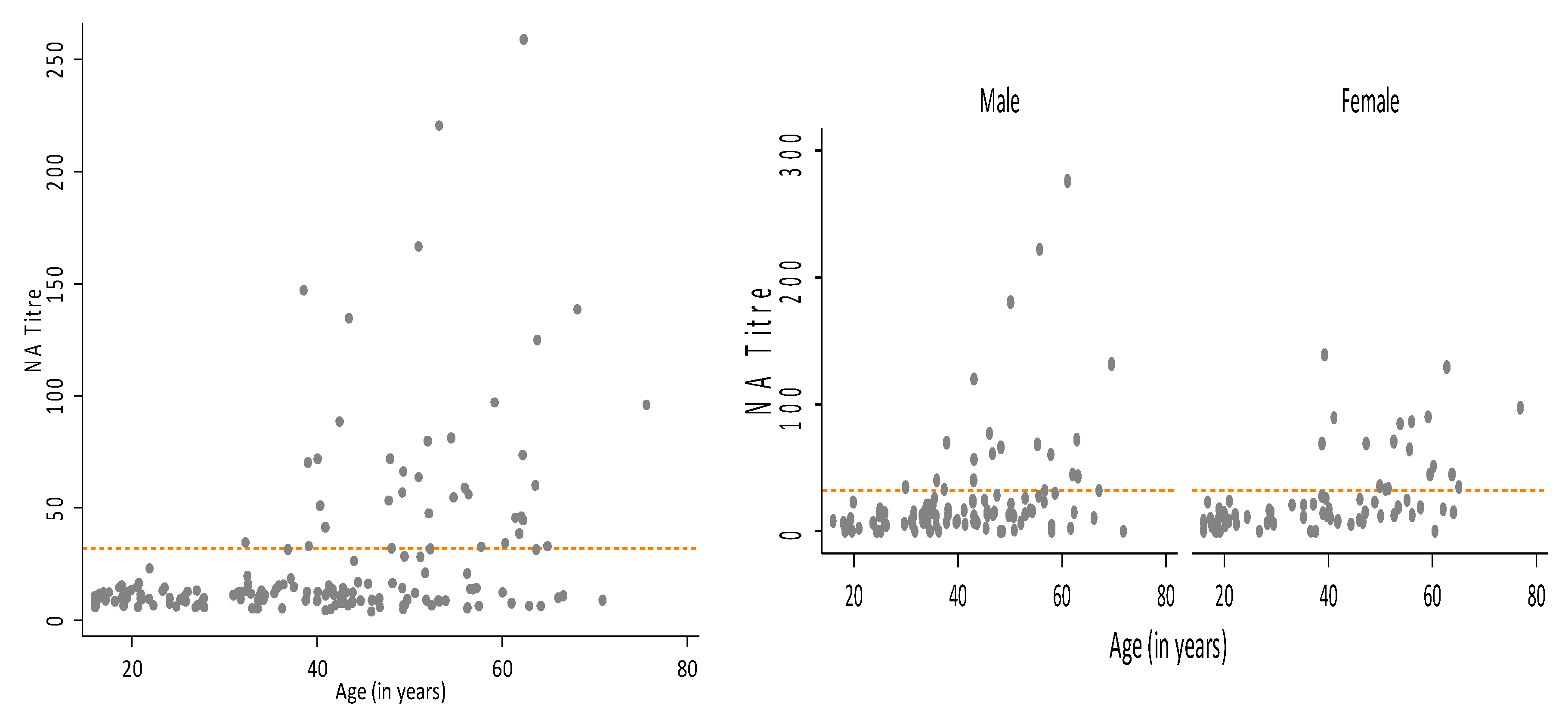
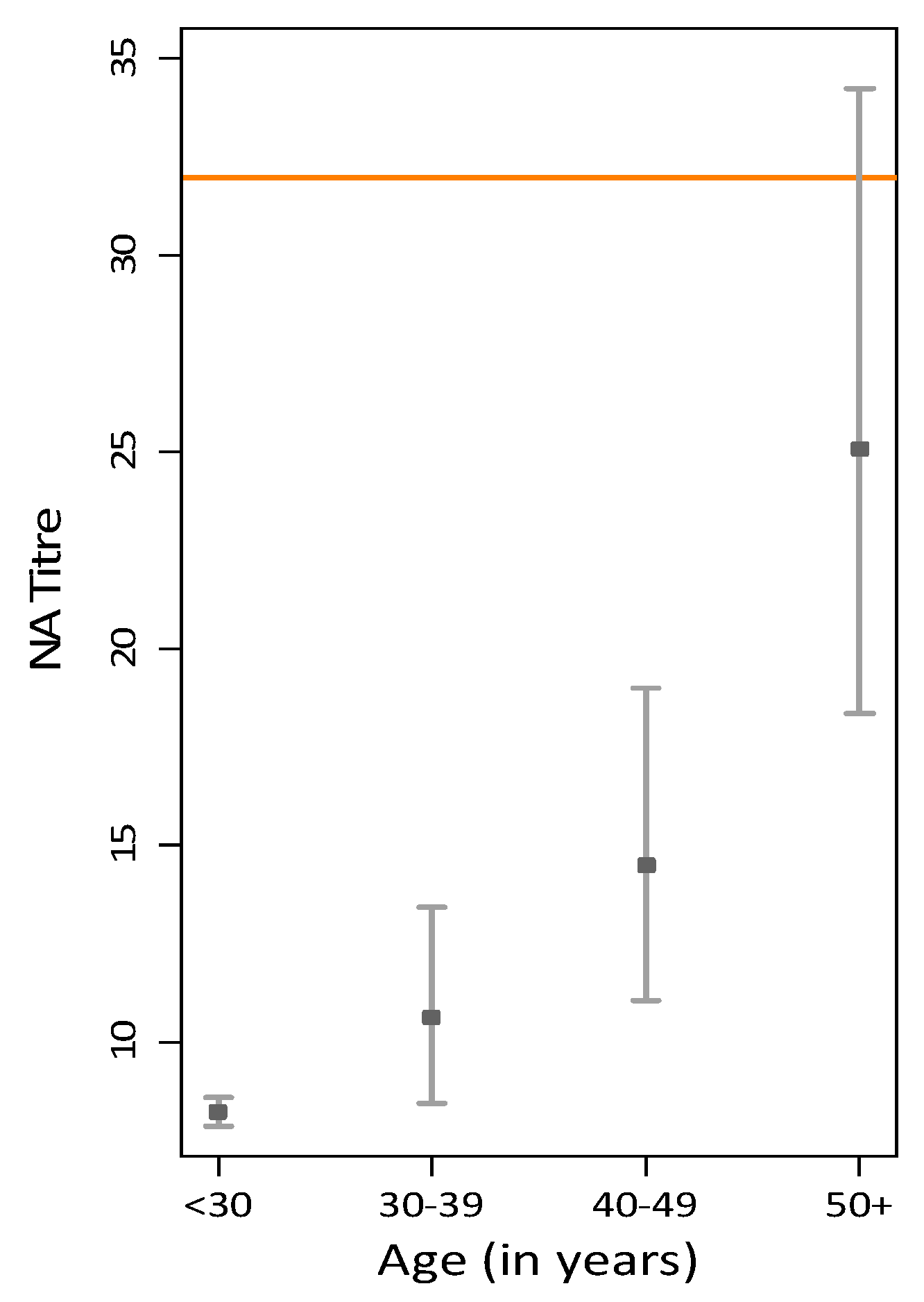
| Age (Years) | GMT Total Sample | n | % Seropositive | GMT for Seropositive | 95% CI |
|---|---|---|---|---|---|
| <30 | 8.21 | 0 | 0.00% | - | - |
| 30–39 | 10.63 | 3 | 9.09% | 56.3 | (6.84, 463.62) |
| 40–49 | 14.49 | 11 | 24.44% | 61.74 | (48.09, 79.26) |
| 50+ | 25.07 | 24 | 48.00% | 68.33 | (52.87, 88.31) |
| Age (Years) | GMT Following Vaccination (First Year after Vaccination) [28] | GMT at the Shortest Time Since Vaccination t = 23 Years | GMT at the Longest Time Since Vaccination (at 1 Year Old) t = 34, 44, and 64 Years Depending on the Age Group |
|---|---|---|---|
| 30–39 | GMT (1) = 1340 | GMT (23) = 56 | GMT (34) = 56 |
| 40–49 | GMT (1) = 2370 | GMT (23) = 62 | GMT (44) = 62 |
| 50+ | GMT (1) = 2370 | GMT (23) = 68 | GMT (64) = 68 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costantino, V.; Trent, M.J.; Sullivan, J.S.; Kunasekaran, M.P.; Gray, R.; MacIntyre, R. Serological Immunity to Smallpox in New South Wales, Australia. Viruses 2020, 12, 554. https://doi.org/10.3390/v12050554
Costantino V, Trent MJ, Sullivan JS, Kunasekaran MP, Gray R, MacIntyre R. Serological Immunity to Smallpox in New South Wales, Australia. Viruses. 2020; 12(5):554. https://doi.org/10.3390/v12050554
Chicago/Turabian StyleCostantino, Valentina, Mallory J. Trent, John S. Sullivan, Mohana P. Kunasekaran, Richard Gray, and Raina MacIntyre. 2020. "Serological Immunity to Smallpox in New South Wales, Australia" Viruses 12, no. 5: 554. https://doi.org/10.3390/v12050554
APA StyleCostantino, V., Trent, M. J., Sullivan, J. S., Kunasekaran, M. P., Gray, R., & MacIntyre, R. (2020). Serological Immunity to Smallpox in New South Wales, Australia. Viruses, 12(5), 554. https://doi.org/10.3390/v12050554





