Recombinant Myxoma Virus Expressing Walleye Dermal Sarcoma Virus orfC Is Attenuated in Rabbits
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Viruses
2.3. Viral Growth Curves
2.4. Detection of Exogenous Protein Production by MYXVorfC
2.5. Apoptosis in Cell Culture
2.6. Rabbits
2.7. Sample Collection
2.8. Detection of Virus in Tissues
2.9. Histopathology
2.10. Statistical Analysis
3. Results
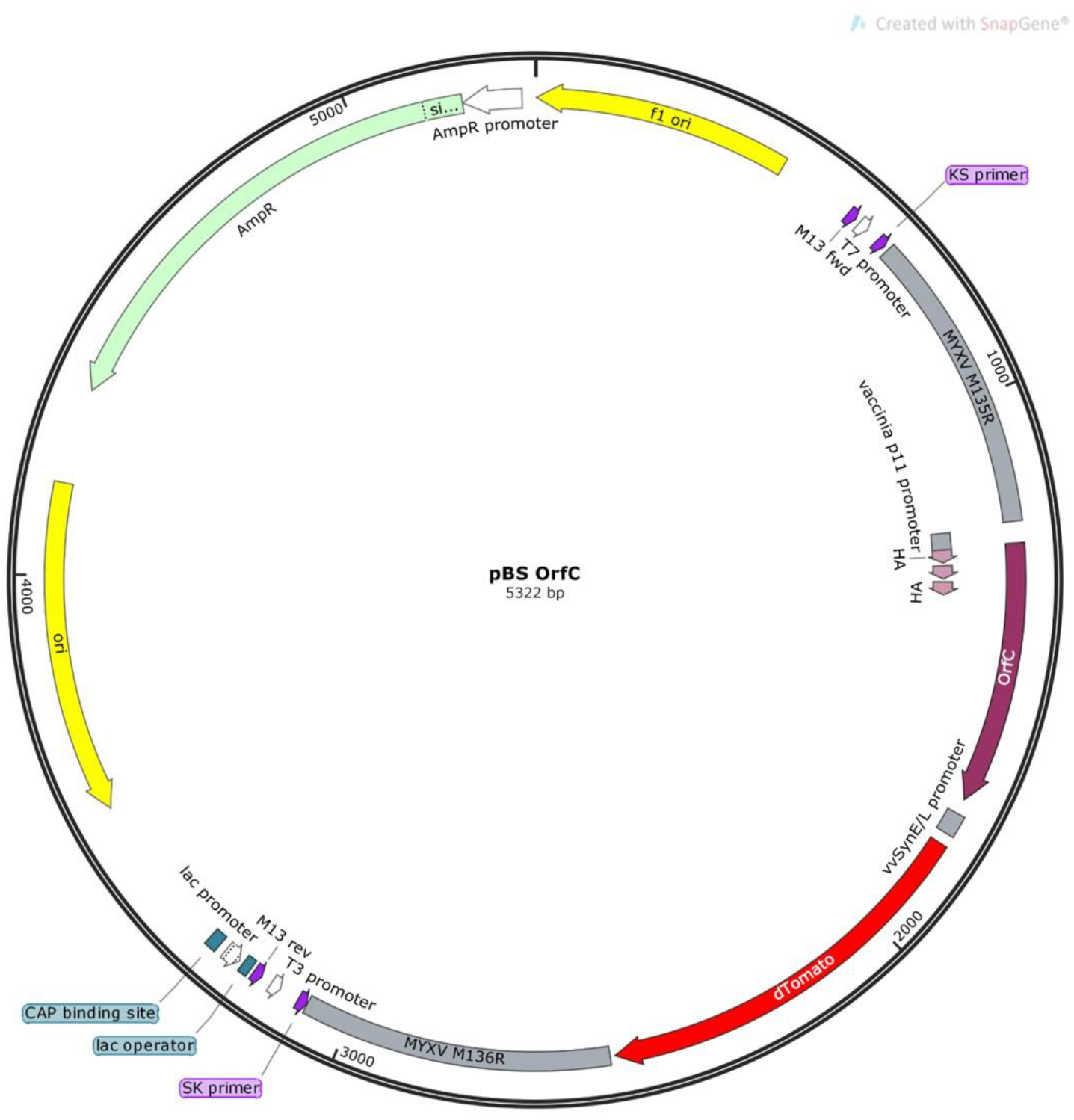
3.1. Validation of MYXVorfC Construction
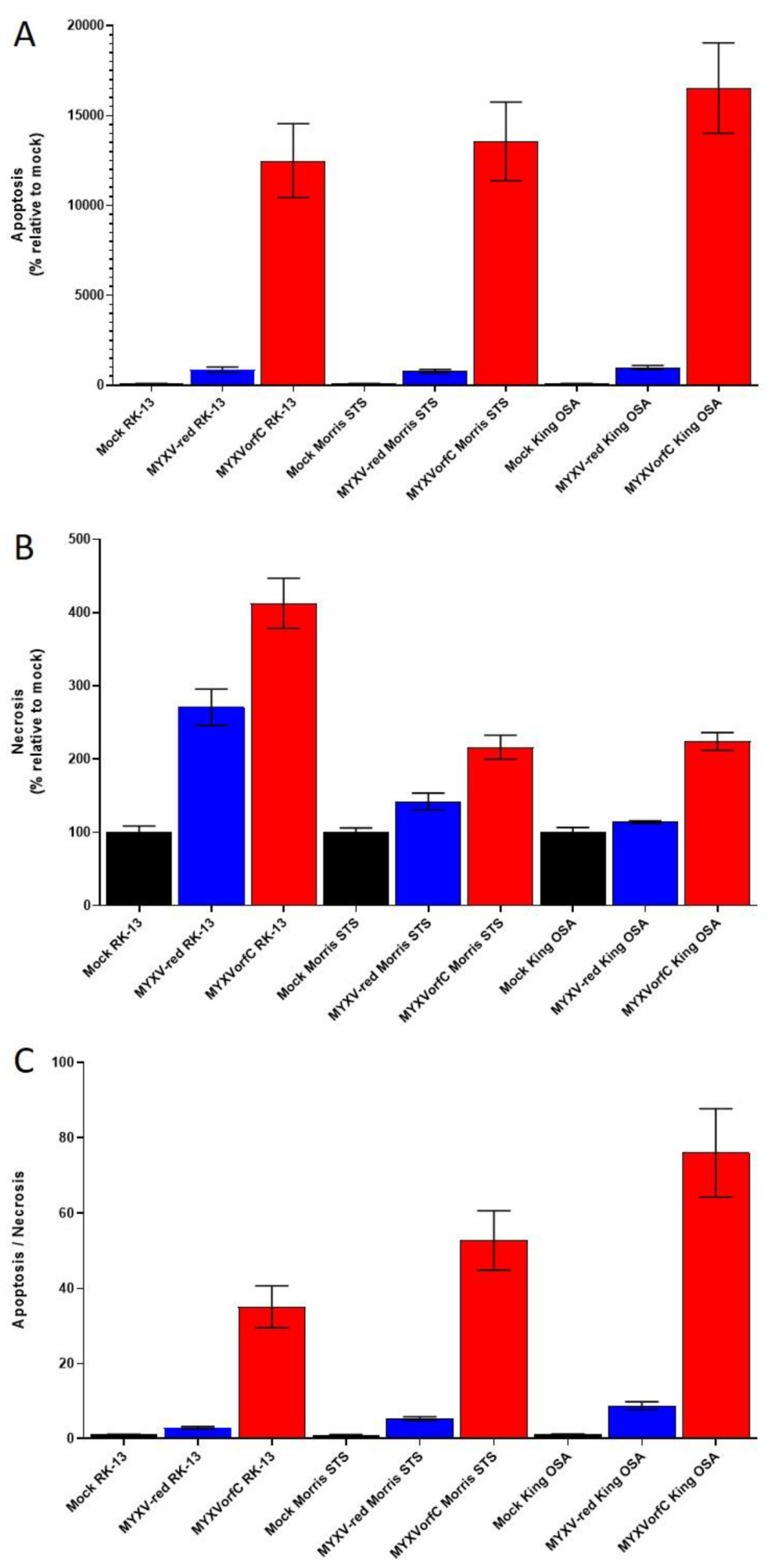
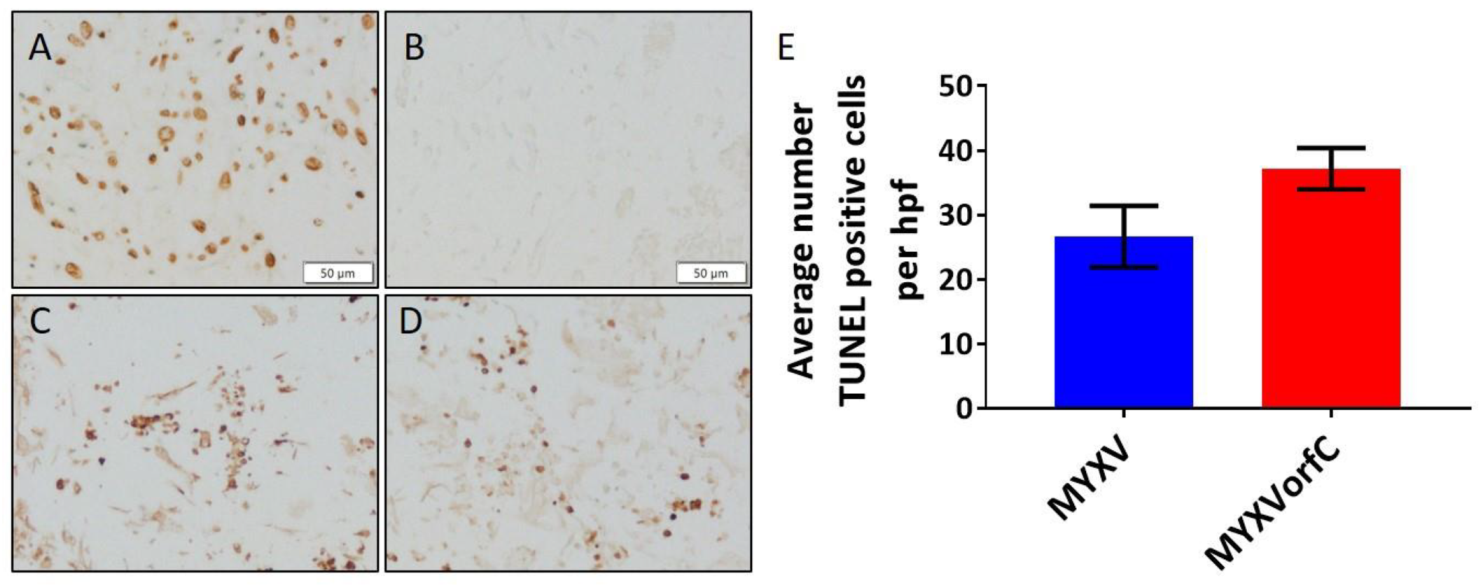
3.2. Apoptosis Is Induced By MYXVorfC
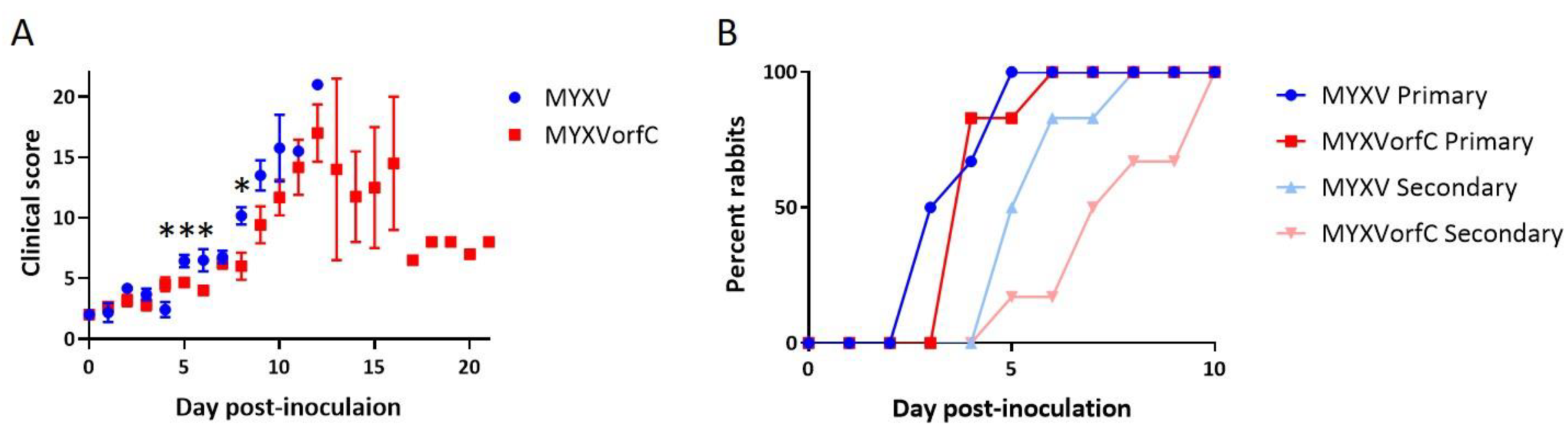
3.3. MYXVorfC Is Attenulated in Rabbits
3.4. MYXVorfC Tissue Tropism Is Not Altered
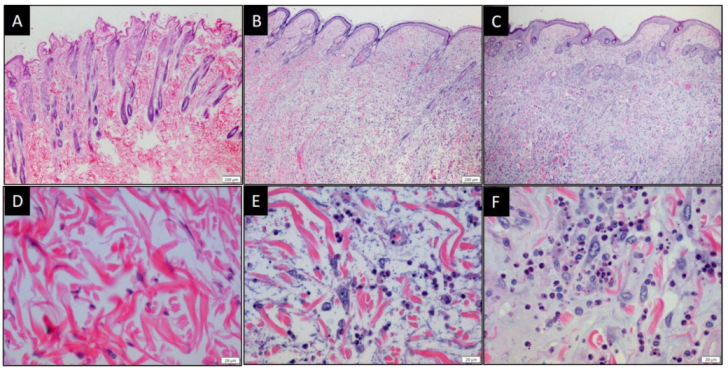
3.5. MYXV and MYXVorfC Induce Similar Histologic Lesions
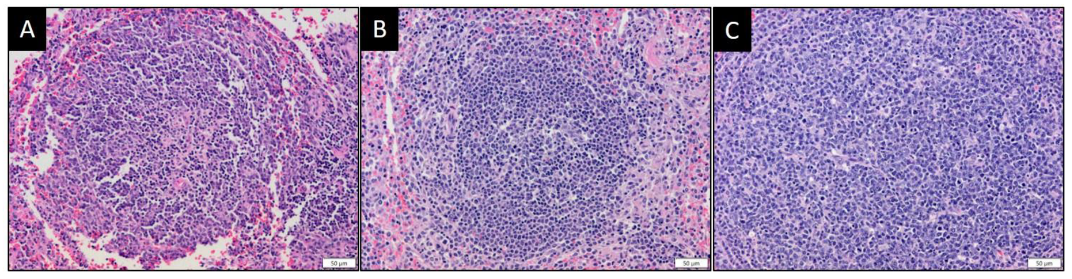
3.6. MYXVorfC Stimulated Follicular Hyperplasia in Lymphoid Tissues
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Pol, J.; Kroemer, G.; Galluzzi, L. First oncolytic virus approved for melanoma immunotherapy. Oncoimmunology 2016, 5, e1115641. [Google Scholar] [CrossRef] [PubMed]
- Andrewes, C.H.; Harisijades, S. Propagation of myxoma virus in one-day old mice. Br. J. Exp. Pathol. 1955, 36, 18–21. [Google Scholar] [PubMed]
- Fenner, F.; Woodroffe, G.M. The pathogenesis of infectious myxomatosis: The mechanism of infection and the immunological response in the European rabbit (Oryctolagus cuniculus). Br. J. Exp. Pathol. 1953, 34, 400–411. [Google Scholar] [PubMed]
- Fenner, F. Adventures with poxviruses of vertebrates. FEMS Microbiol. Rev. 2000, 24, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Gorski, J.; Mizak, B.; Chrobocinska, M. Control of rabbit myxomatosis in Poland. Rev. Sci. Tech. 1994, 13, 869–879. [Google Scholar] [CrossRef] [PubMed]
- Jackson, E.W.; Dorn, C.R.; Saito, J.K.; McKercher, D.G. Absence of serological evidence of myxoma virus infection in humans exposed during an outbreak of myxomatosis. Nature 1966, 211, 313–314. [Google Scholar] [CrossRef]
- McCabe, V.J.; Tarpey, I.; Spibey, N. Vaccination of cats with an attenuated recombinant myxoma virus expressing feline calicivirus capsid protein. Vaccine 2002, 20, 2454–2462. [Google Scholar] [CrossRef]
- McCabe, V.J.; Spibey, N. Potential for broad-spectrum protection against feline calicivirus using an attenuated myxoma virus expressing a chimeric FCV capsid protein. Vaccine 2005, 23, 5380–5388. [Google Scholar] [CrossRef]
- Pignolet, B.; Boullier, S.; Gelfi, J.; Bozzetti, M.; Russo, P.; Foulon, E.; Meyer, G.; Delverdier, M.; Foucras, G.; Bertagnoli, S. Safety and immunogenicity of myxoma virus as a new viral vector for small ruminants. J. Gen. Virol. 2008, 89, 1371–1379. [Google Scholar] [CrossRef]
- MacNeill, A.L.; Moldenhauer, T.; Doty, R.; Mann, T. Myxoma virus induces apoptosis in cultured feline carcinoma cells. Res. Vet. Sci. 2012, 93, 1036–1038. [Google Scholar] [CrossRef]
- Urbasic, A.S.; Hynes, S.; Somrak, A.; Contakos, S.; Rahman, M.M.; Liu, J.; MacNeill, A.L. Oncolysis of canine tumor cells by myxoma virus lacking the serp2 gene. Am. J. Vet. Res. 2012, 73, 1252–1261. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Ma, Y.; Barrett, J.W.; Gao, X.; Loh, J.; Barton, E.; Virgin, H.W.; McFadden, G. Disruption of Erk-dependent type I interferon induction breaks the myxoma virus species barrier. Nat. Immunol. 2004, 5, 1266–1274. [Google Scholar] [CrossRef] [PubMed]
- Woo, Y.; Kelly, K.J.; Stanford, M.M.; Galanis, C.; Chun, Y.S.; Fong, Y.; McFadden, G. Myxoma virus is oncolytic for human pancreatic adenocarcinoma cells. Ann. Surg. Oncol. 2008, 15, 2329–2335. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Barrett, J.W.; Stanford, M.; Werden, S.J.; Johnston, J.B.; Gao, X.; Sun, M.; Cheng, J.Q.; McFadden, G. Infection of human cancer cells with myxoma virus requires Akt activation via interaction with a viral ankyrin-repeat host range factor. Proc. Natl. Acad. Sci. USA 2006, 103, 4640–4645. [Google Scholar] [CrossRef] [PubMed]
- Bartee, E.; McFadden, G. Human cancer cells have specifically lost the ability to induce the synergistic state caused by tumor necrosis factor plus interferon-beta. Cytokine 2009, 47, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Lun, X.; Yang, W.; Alain, T.; Shi, Z.Q.; Muzik, H.; Barrett, J.W.; McFadden, G.; Bell, J.; Hamilton, M.G.; Senger, D.L.; et al. Myxoma virus is a novel oncolytic virus with significant antitumor activity against experimental human gliomas. Cancer Res. 2005, 65, 9982–9990. [Google Scholar] [CrossRef] [PubMed]
- Lun, X.; Alain, T.; Zemp, F.J.; Zhou, H.; Rahman, M.M.; Hamilton, M.G.; McFadden, G.; Bell, J.; Senger, D.L.; Forsyth, P.A. Myxoma virus virotherapy for glioma in immunocompetent animal models: Optimizing administration routes and synergy with rapamycin. Cancer Res. 2010, 70, 598–608. [Google Scholar] [CrossRef]
- Stanford, M.M.; Shaban, M.; Barrett, J.W.; Werden, S.J.; Gilbert, P.A.; Bondy-Denomy, J.; Mackenzie, L.; Graham, K.C.; Chambers, A.F.; McFadden, G. Myxoma virus oncolysis of primary and metastatic B16F10 mouse tumors in vivo. Mol. Ther. 2008, 16, 52–59. [Google Scholar] [CrossRef]
- Thomas, D.L.; Doty, R.; Tosic, V.; Liu, J.; Kranz, D.M.; McFadden, G.; Macneill, A.L.; Roy, E.J. Myxoma virus combined with rapamycin treatment enhances adoptive T cell therapy for murine melanoma brain tumors. Cancer Immunol. Immunother. 2011, 60, 1461–1472. [Google Scholar] [CrossRef]
- Doty, R.A.; McFadden, G.; Roy, E.J.; MacNeill, A.L. Histological evaluation of intratumoral myxoma virus treatment in an immunocompetent mouse model of melanoma. Oncolytic Virotherapy 2013, 2, 1–17. [Google Scholar]
- Nudson, W.A.; Rovnak, J.; Buechner, M.; Quackenbush, S.L. Walleye dermal sarcoma virus Orf C is targeted to the mitochondria. J. Gen. Virol. 2003, 84, 375–381. [Google Scholar] [CrossRef]
- Rovnak, J.; Quackenbush, S.L. Walleye dermal sarcoma virus: Molecular biology and oncogenesis. Viruses 2010, 2, 1984–1999. [Google Scholar] [CrossRef] [PubMed]
- Gentschev, I.; Adelfinger, M.; Josupeit, R.; Rudolph, S.; Ehrig, K.; Donat, U.; Weibel, S.; Chen, N.G.; Yu, Y.A.; Zhang, Q.; et al. Preclinical evaluation of oncolytic vaccinia virus for therapy of canine soft tissue sarcoma. PLoS ONE 2012, 7, e37239. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wennier, S.; Reinhard, M.; Roy, E.; MacNeill, A.; McFadden, G. Myxoma virus expressing interleukin-15 fails to cause lethal myxomatosis in European rabbits. J. Virol. 2009, 83, 5933–5938. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rice, A.D.; Gray, S.A.; Li, Y.; Damon, I.; Moyer, R.W. An efficient method for generating poxvirus recombinants in the absence of selection. Viruses 2011, 3, 217–232. [Google Scholar] [CrossRef] [PubMed]
- Condit, R.C.; Motyczka, A. Isolation and preliminary characterization of temperature-sensitive mutants of vaccinia virus. Virology 1981, 113, 224–241. [Google Scholar] [CrossRef]
- Rice, A.D.; Adams, M.M.; Lindsey, S.F.; Swetnam, D.M.; Manning, B.R.; Smith, A.J.; Burrage, A.M.; Wallace, G.; Macneill, A.L.; Moyer, R.W. Protective Properties of Vaccinia Virus-Based Vaccines: Skin Scarification Promotes a Nonspecific Immune Response That Protects against Orthopoxvirus Disease. J. Virol. 2014, 88, 7753–7763. [Google Scholar] [CrossRef]
- MacNeill, A.L.; Turner, P.C.; Moyer, R.W. Mutation of the Myxoma virus SERP2 P1-site to prevent proteinase inhibition causes apoptosis in cultured RK-13 cells and attenuates disease in rabbits, but mutation to alter specificity causes apoptosis without reducing virulence. Virology 2006, 356, 12–22. [Google Scholar] [CrossRef]
- MacNeill, A.L.; Weishaar, K.M.; Séguin, B.; Powers, B.E. Safety of an oncolytic myxoma virus in dogs with soft tissue sarcoma. Viruses 2018, 10, 398. [Google Scholar] [CrossRef]
- Kinn, V.G.; Hilgenberg, V.A.; MacNeill, A.L. Myxoma virus therapy for human embryonal rhabdomyosarcoma in a nude mouse model. Oncolytic Virotherapy 2016, 5, 59–71. [Google Scholar]
| Viral DNA Targets of Primers | |||
|---|---|---|---|
| Virus | Tissue | Multigene | M135-M136 |
| MYXV | Primary lesion | 6/6 (100%) | 6/6 (100%) |
| Secondary lesion | 6/6 (100%) | 6/6 (100%) | |
| Heart | 6/6 (100%) | 6/6 (100%) | |
| Kidney | 6/6 (100%) | 6/6 (100%) | |
| Liver | 6/6 (100%) | 6/6 (100%) | |
| Lung | 6/6 (100%) | 6/6 (100%) | |
| Spleen | 6/6 (100%) | 6/6 (100%) | |
| MYXVorfC | Primary lesion | 6/6 (100%) | 6/6 (100%) |
| Secondary lesion | 6/6 (100%) | 6/6 (100%) | |
| Heart | 3/6 (50%) | 3/6 (50%) | |
| Kidney | 5/6 (83%) | 5/6 (83%) | |
| Liver | 6/6 (100%) | 5/6 (83%) | |
| Lung | 6/6 (100%) | 5/6 (83%) | |
| Spleen | 4/6 (67%) | 3/6 (50%) | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ashton, L.V.; Quackenbush, S.L.; Castle, J.; Wilson, G.; McCoy, J.; Jordan, M.; MacNeill, A.L. Recombinant Myxoma Virus Expressing Walleye Dermal Sarcoma Virus orfC Is Attenuated in Rabbits. Viruses 2020, 12, 517. https://doi.org/10.3390/v12050517
Ashton LV, Quackenbush SL, Castle J, Wilson G, McCoy J, Jordan M, MacNeill AL. Recombinant Myxoma Virus Expressing Walleye Dermal Sarcoma Virus orfC Is Attenuated in Rabbits. Viruses. 2020; 12(5):517. https://doi.org/10.3390/v12050517
Chicago/Turabian StyleAshton, Laura V., Sandra L. Quackenbush, Jake Castle, Garin Wilson, Jasmine McCoy, Mariah Jordan, and Amy L. MacNeill. 2020. "Recombinant Myxoma Virus Expressing Walleye Dermal Sarcoma Virus orfC Is Attenuated in Rabbits" Viruses 12, no. 5: 517. https://doi.org/10.3390/v12050517
APA StyleAshton, L. V., Quackenbush, S. L., Castle, J., Wilson, G., McCoy, J., Jordan, M., & MacNeill, A. L. (2020). Recombinant Myxoma Virus Expressing Walleye Dermal Sarcoma Virus orfC Is Attenuated in Rabbits. Viruses, 12(5), 517. https://doi.org/10.3390/v12050517





