Abstract
Mosquito-borne viruses are emerging or re-emerging globally, afflicting millions of people around the world. Aedes aegypti, the yellow fever mosquito, is the principal vector of dengue, Zika, and chikungunya viruses, and has well-established populations across tropical and subtropical urban areas of the Americas, including the southern United States. While intense arboviral epidemics have occurred in Mexico and further south in the Americas, local transmission in the United States has been minimal. Here, we study Ae. aegypti and Culex quinquefasciatus host feeding patterns and vertebrate host communities in residential environments of South Texas to identify host-utilization relative to availability. Only 31% of Ae. aegypti blood meals were derived from humans, while 50% were from dogs and 19% from other wild and domestic animals. In Cx. quinquefasciatus, 67% of blood meals were derived from chicken, 22% came from dogs, 9% from various wild avian species, and 2% from other mammals including one human, one cat, and one pig. We developed a model for the reproductive number, R0, for Zika virus (ZIKV) in South Texas relative to northern Mexico using human disease data from Tamaulipas, Mexico. We show that ZIKV R0 in South Texas communities could be greater than one if the risk of human exposure to Ae. aegypti bites in these communities is at least 60% that of Northern Mexico communities. The high utilization of non-human vertebrates and low risk of human exposure in South Texas diminishes the outbreak potential for human-amplified urban arboviruses transmitted by Ae. aegypti.
1. Introduction
Mosquito-borne viruses driven principally by Aedes aegypti, have emerged and re-emerged globally, resulting in a large burden of human disease [1]. Globalization and other anthropogenic factors have allowed this mosquito to thrive in diverse landscapes and facilitate urban transmission cycles of dengue virus (DENV), chikungunya virus (CHIKV), Zika virus (ZIKV), and others [2]. In the Americas, all four serotypes of DENV have re-emerged causing consistent epidemics from South America to Mexico and the Caribbean [3]. The Asian lineage of CHIKV first arrived in the Caribbean in 2014 and spread throughout the Americas in just a few years [4], resulting in 338,963 confirmed human cases [5]. ZIKV invaded Brazil in 2013 [6] and rapidly swept through the Americas in a similar fashion, resulting in an estimated 8.5 million cases in Brazil alone [7].
In the continental United States, Ae. aegypti is found throughout the southern states, and recent enhanced surveys of Stegomyia mosquitoes have documented the presence of this species in 26 states [8]. Despite this wide distribution of the primary vector and the Asian tiger mosquito (Ae. albopictus), a secondary vector for these viruses in many locations, the only regions experiencing autochthonous transmission of DENV, CHIKV, and ZIKV by mosquito exposure, are South Florida and South Texas [9,10]. While the Mexico cities along the U.S.—Mexico border have experienced consistent epidemics of Ae. aegypti-driven viruses, markedly fewer human cases have occurred in the communities of the Lower Rio Grande Valley (LRGV) on the Texas side of the border. For example, the state of Tamaulipas, Mexico, recorded an estimated 11,760 probable cases of DENV and 2677 cases of Dengue Hemorrhagic Fever between 2009 and 2019 [11]. In contrast, in the LRGV, local mosquito-borne DENV epidemics occurred only in 2005 and 2013 [9], and the outbreaks were associated with relatively small numbers of human cases. For example, in 2005 the LRGV documented three symptomatic cases and six asymptomatic cases of DEN with no travel history [12], compared to 7062 reported DEN cases in Tamaulipas the same year [13]. In contrast, these viruses are recorded with only isolated cases of local transmission in South Texas, including a single CHIKV case in Brownsville, TX in 2015 (Texas Department of State Health Services, 2016) and 11 cases of locally acquired ZIKV in the LRGV between 2016–2017 [14].
Aedes aegypti-driven viruses continue to have intense epidemics in the Americas, resulting in high rates of viremic humans entering the U.S. [15], but minimal local transmission has occurred [16]. This discrepancy in the magnitude of virus transmission along geo-political boundaries of the U.S.—Mexico border has attracted research attention to identify the mechanisms responsible for these patterns. This is especially perplexing given that Ae. aegypti in U.S. border communities has comparable relative abundances in residential neighborhoods to areas with a much higher burden of human disease across the border in Mexico [14,17]. Prior studies have identified several factors contributing to this discrepancy, which have identified social-ecological factors, such as window screens and air conditioning, that reduce the risk of exposure to the viruses [13,18]. However, despite evidence that housing quality is associated with virus transmission [18], there remains limited knowledge of how this influences the ability of Ae. aegypti to feed on humans and how proportional human feeding might drive virus transmission potential on both sides of the border.
This study quantifies Ae. aegypti host feeding patterns and vertebrate host availability in residential environments in South Texas, to compare the observed frequency of blood meals relative to the expected frequency in the study location. We rely on empirical data on Ae. aegypti abundance, human population density, and epidemiological data of epidemics in South Texas and in Tamaulipas, Mexico, to evaluate the risk of human-amplified urban arbovirus transmission. We present evidence contrary to the most commonly reported observation that Ae. aegypti feeds mostly on humans by showing a high utilization of dogs and other non-human hosts in South Texas, and that this contributes to a lower risk of human exposure to ZIKV, which reduces epidemic potential.
2. Materials and Methods
2.1. Study Site and Mosquito Collection
Blood-engorged mosquitoes were collected from several neighborhoods in the Lower Rio Grande Valley (LRGV) on the U.S. side of the U.S.–Mexico border (see Figure 1) from September, 2016 through December, 2018. The climate of Weslaco, Texas, which was used as a representation of the general climate for the LRGV, includes an average annual high and low temperature of Weslaco (28.7 and 17.4 °C) and Reynosa, Mexico (29.2 and 17.3 °C; climate-data.org). Average annual precipitation (in mm) is 609 for Weslaco and 532 for Reynosa. We sampled mosquitoes from eight lower-income (15,000–29,999 USD annual household income) neighborhoods (Mercedes, Mesquite (MM); Donna, Figueroa (DF); Mercedes, Chapa (MCH); Progreso Fresno/Progreso Encino (PF/PE); Indian Hills East (IHE); Indian Hills West (IHW); La Piñata (LP); Tierra Bella (TB)) and four middle-income (30,000–40,000 USD annual household income) neighborhoods (La Feria, La Bonita (LF); Mercedes, Rio Rico (MRR); McAllen, La Vista (MLV); Weslaco, Christian Court (WCT)) as described by Martin et al. [14]. We used Biogents Sentinel 2 traps (BGS2; Biogents, Germany) with BG lures at IHE, IHW, LP, TB, and DF neighborhoods, placing one trap outside homes once per week for a 24-h trapping period. We also used Autocidal Gravid Ovitraps (AGO) at PF/PE, DF, LF, MCH, MLV, MM, and MCH. One AGO was placed inside the home and an additional trap was placed outside the home, serviced weekly. More details about AGO preparation and deployment are described in Martin et al. [14]. The selection of homes was largely dictated by obtaining permission from the homeowners to place traps in and around the property. Upon collection, mosquitoes were identified morphologically based upon illustrations and dichotomous keys found in The Illustrated Key to Common Mosquitoes of Louisiana [19]. While processing mosquitoes to identify species and sex, all bloodfed mosquitoes were placed individually into nuclease-free, 1.5 mL micro-centrifuge tubes, labeled with species, sex, date and house identification number and stored at −20 or −80 °C until further processing.
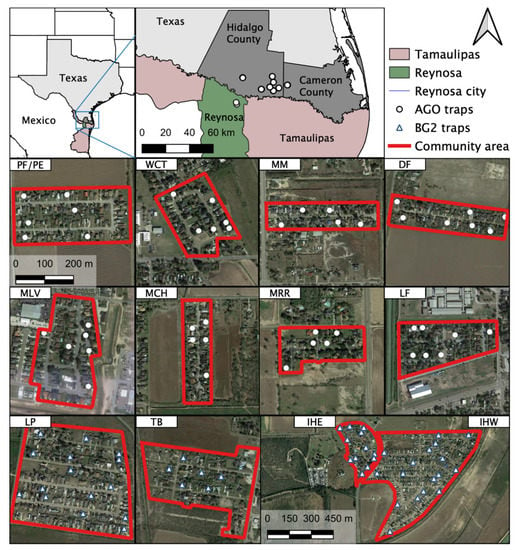
Figure 1.
Study sites and location of traps in the Lower Rio Grande Valley (LRGV), South Texas and Reynosa (municipality), Tamaulipas. The 12 study locations in the LRGV are included as individual maps: PF/PE = Progreso Fresno/Progreso Encino; WCT = Weslaco, Christian Court; MM = Mercedes, Mesquite; DF = Donna, Figueroa; MLV = McAllen, La Vista; MCH = Mercedes, Chapa; MRR = Mercedes, Rio Rico; LF = La Feria; LP = La Piñata; TB = Tierra Bella; IHE = Indian Hills East; IHW = Indian Hills West.
2.2. Blood Meal Analysis
Mosquito samples were identified under microscope, photographed, and given a Sella score (stages of blood digestion and ovary development) based upon observation of the engorged abdomen [20]. The Sella score was used to identify the engorged mosquitoes that had the highest likelihood of yielding a DNA sequence. To minimize exogenous DNA on the mosquito exoskeleton, each whole mosquito was washed in 10% bleach followed by two rinses with nuclease-free water [21,22,23,24]. On a clean, chilled microscope slide, the abdomen was carefully separated from the rest of the mosquito body, and the abdominal contents were expressed into a new, labeled, DNA-free 1.5 mL microcentrifuge tube. A homogenizing bead and 200 µL of lysis solution were added to the tube with blood and shaken for 1 min at 30 Hz in the Qiagen Tissue Lyser (Qiagen, Germantown, MD, USA). DNA was extracted using the Thermo Scientific™ Kingfisher™ Flex Purification System, along with the MagMAX Core Nucleic Acid Purification Kit (Thermo Fisher Scientific, Waltham, MA, USA) following the manufacturer’s instructions.
We adopted previously-published protocols to conduct a PCR-Sanger sequencing blood meal analysis [25,26,27]. Three primer pairs were used in a tiered approach: (I) A vertebrate cocktail targeting a 648 base pair region of the cytochrome c oxidase 1 (COI) gene, (II) blood meal (BM) primers targeting a 358 base pair region of the cytochrome b gene, and (III) ‘Herp’ primers that target a 228 base pair region of the cytochrome b gene (Table S1) [27]. This three-tiered approach has the benefits of cost efficiency, maximizing the number of identified blood meals, and increasing reliability of results [25,26,27]. First, every sample was tested using the vertebrate cocktail primers. Samples producing an amplicon of 648 bp were cleaned using ExoSAP-IT (Thermo Fisher Scientific) and submitted to Eton Bioscience (San Diego, CA, USA) for Sanger sequencing. Sequencing results were analyzed using Geneious R9 software (Biomatters, Ltd., Auckland, New Zealand). Only samples with ≥ 95% pairwise identity match to a vertebrate host sequence in NCBI, and ≥ 95% grade (a weighted score comprised of e-value, pairwise identity, and the coverage) were accepted as a confirmed result.
Based upon the outcome of the initial PCR, we continued the iterative bloodmeal analysis PCR process if there was (I) match to human basic local alignment search tool (BLAST), (II) no PCR amplicon, (III) poor sequence quality, or (IV) evidence of mixed DNA (double-nucleotide peaks in chromatograph) [27]. If we obtained any of these four outcomes, a second PCR utilizing the BM1:BM2 primers was conducted [25,27]. Finally, using the same criteria, the analysis was either concluded or subjected to a third primer pair and PCR thermal profile, the ‘Herp’ primers [25,27].
We followed the protocol of Medeiros et al. [27] for the vertebrate cocktail reaction, but modified the thermal cycling conditions as follows: after denaturation we ran nine cycles of 94 °C for 30 s, a gradient from 45 to 54 °C for 40 s, and 72 °C for 1 min. The remaining thermal cycling conditions were identical to the Medeiros protocol. For the BM and ‘Herp’ reactions, we followed the protocol of Hamer et al. [25] with the following modification: we lowered the annealing temperature for the ‘Herp’ reaction from 50 to 47 °C. The vertebrate cocktail, BM1:BM2, and ‘herp’ PCR reactions used the following reagents and quantities per reaction: 8.59 µL Nuclease-free H2O, 12.5 µL FailSafe™ PCR 2X Premix E (Lucigen, Middleton, WI, USA), 0.83 µL forward primer, 0.83 µL reverse primer, 0.25 µL FailSafe™ PCR Enzyme Mix (Lucigen), and 2 µL DNA template.
The protocol was tested using lab-raised, Aedes aegypti and Culex quinquefasciatus mosquitoes fed on defibrinated sheep blood (HemoStat Laboratories, Dixon, CA, USA). Adult female mosquitoes were offered artificial blood meals using a Hemotek membrane feeder (Hemotek Ltd., Blackburn, UK). Each specimen was observed under a dissecting light microscope to confirm species, give a Sella score, and capture a digital photograph. We also extracted DNA directly from the blood of several controls including iguana (Iguana iguana), white-tailed deer (Odocoileus virginianus), tiger (Panthera tigris), sandhill crane (Grus canadensis), and sheep (Ovis aries). These vertebrate species were selected because they are unlikely to be found in mosquito blood meals from this region and would thus minimize the risk of downstream amplicon contamination.
2.3. Molecular Verification of Mosquito Species
While most mosquitoes captured via BGS2 traps could be taxonomically classified from morphological features, those collected from the glue boards of the AGO traps are often damaged and more difficult to identify morphologically. Therefore, molecular identification of mosquito species was confirmed using a modified version of the protocol designed by Folmer et al. [28]. Briefly, a primer pair that amplifies a 710-bp fragment of the cytochrome c oxidase subunit I gene (LCO 1490 and HCO 2198; Table S2) was used with the following reagents and quantities per reaction: 8 µL Nuclease-free H2O, 12.5 µL FailSafe™ PCR 2X Premix E (Lucigen), 1 µL forward primer (LCO 1490), 1 µL reverse primer (HCO 2198), 0.5 µL FailSafe™ PCR Enzyme Mix (Lucigen), and 2 µL DNA template. The PCR thermal cycling profile included initial denaturation for three minutes at 95 °C followed by 35 cycles of 95 °C for 1 min, 45 °C for 1.5 min, and 72 °C for 2 min, followed by a final extension at 72 °C for 5 min. Amplified PCR products were purified using Exo-SAP-IT™ (ThermoFisher Scientific) and sent to Eton Bioscience (San Diego) for Sanger sequencing.
2.4. Quantitative Synthesis of Published Literature
We compiled all the published data on Ae. aegypti host feeding patterns from around the world. To systematically review the literature, we searched PubMed, Web of Science, and Google Scholar for published literature using keywords “Aedes aegypti host feeding”, and a second search of “Aedes aegypti blood meal analysis”. These queries in Web of Science yielded 50 and seven results, respectively, in PubMed yielded 13 and four results, respectively, and in Google Scholar yielded 1970 and 450 results, respectively. We also tracked references from key review papers and other primary literature. Inclusion criteria included blood meal results from wild-caught mosquitoes. Studies using laboratory-raised mosquitoes, as well as studies which indicated samples were likely from the form Ae. aegypti formosus [29,30,31], were excluded. The form formosus was excluded to allow a focus on the urban form of Ae. Aegypti, which is globally distributed.
2.5. Mosquito Relative Abundance
Female Ae. aegypti relative abundance was estimated in the LRGV and the city of Reynosa, Tamaulipas, Mexico, using AGO traps that were deployed concurrently on both sides of the border in 2017 (Figure 1). Eighty AGO traps were deployed outside residential homes in Reynosa and checked weekly between May 7 and Aug 12 (trapping data were unavailable for two weeks in this period due to adverse weather or trap failure) [32]. In the LRGV, 30 AGO traps were deployed outside residential homes during the same weeks as a subset of the data presented in a previous study [14].
2.6. Vertebrate Surveys
In order to estimate mosquito host selection, a questionnaire related to vertebrate availability was developed and conducted in the four primary communities where blood-fed mosquitoes were collected. Project personnel visited all the homes containing BG Sentinel 2 traps in these communities: 14 (out of 307 total homes present) in Indian Hills East, 10 (96) in Indian Hills West, 13 (160) in La Piñata, and seven (49) in Tierra Bella. An adult from each home was asked for the number of persons living at each residence (further categorized by age group < 5; 5–17; 18–65; > 65), the number of dogs, cats, pet birds, chickens, pigs, horses, and other animals. Of the dogs and cats, the number of them roaming outside of the property was also noted. From previous observations, we suspected that a large number of stray dogs and cats live in some of these neighborhoods, therefore a final question regarding the number of strays that the adult resident is aware of was also asked. Results from the surveys were tabulated and relative abundance calculated with 95% confidence intervals for selected vertebrates, using the Wilson/Brown method (Table S3) [33].
Populations of human, dog, cat, chicken, pig, and opossum were estimated by extrapolating the vertebrates documented from survey homes to create estimates of the number of each vertebrate per unit area in the entire community. To achieve this, the average number of vertebrates in the surveyed homes was multiplied by the total number of homes in the defined community to arrive at an estimated density of each vertebrate per unit area. This number was divided by the total estimated number of all potential hosts to obtain relative abundance. Population estimates of wild birds and wild mammals (rodents, meso-predators, etc.) were not obtained.
2.7. Human Density Estimation
We used remote sensing satellite imagery (Google Earth, California, USA) to map the communities within the LRGV using QGIS 3.4 (QGIS Development team 2019). We estimated household densities using the 2010 US census blocks shape file and extracted the information regarding number of houses, number people/house and area of the community. We also quantified household density in Nuevo Amanecer as a representative community in the city of Reynosa with prior DENV transmission activity (Rodríguez-Pérez Mario A, A. M. A., Russell Tanya L, Olguin-Rodriguez Omar, Laredo-Tiscareño Stephanie V, Garza-Hernandez Javier A, Reyes-Villanueva Filiberto. Host-seeking Aedes aegypti linked to dengue seropositive households at northeastern Mexico. Journal of Vector Borne Diseases (in press)). We used satellite imagery (Google Earth, California, USA) to quantify homes manually and census block information to identify the boundaries of the neighborhood. Nuevo Amanecer was chosen because of its known dengue endemicity.
2.8. Host Selection Indices
We estimated the Forage Ratio (FR), the frequency at which a mosquito selects a vertebrate host over other available vertebrate hosts, by dividing the observed frequency of bloodmeals divided by the expected frequency of bloodmeals of a given species [34]
where s = the percent of female mosquitoes containing blood of a particular host, and a = percent of the total available host population represented by that particular host [35]. A forage ratio of 1.0 indicates mosquitoes are feeding on hosts in equal proportion to availability, whereas values >1.0 indicate over-utilization and values <1.0 indicate under-utilization. We used the Wilson/Brown statistical method to calculate 95% confidence intervals [33].
FR = s/a,
We also estimated the human blood index (HBI), which measures the frequency at which female mosquitoes feed on human hosts and is the number of human blood meals divided by the number of engorged females [36].
2.9. Tamaulipas Human Disease Data
The General Directorate of Epidemiology, Secretariat of Health, México aggregates probable and confirmed empirical cases of DEN, CHIK, and ZIK in the state of Tamaulipas (Figure S1). Patients with history of travel outside of Tamaulipas in the month prior to onset of symptoms were not included in the modeling of . Physicians of symptomatic patients use a case definition of DEN: fever with ≥2 signs or symptoms such as retro-orbital or ocular pain, rash, headache, arthralgia, myalgia, leukopenia or hemorrhagic manifestations; CHIK: severe arthralgia, intense asymmetric, debilitating joint pain, swelling associated with tenosynovitis; and, ZIK: pruritic maculopapular rash, for differential clinical diagnosis between the three viruses and are required to report cases to the Secretary of Health of Tamaulipas. Clinical serum samples receiving laboratory confirmation were sent by sanitary jurisdictions of the state to the Molecular Biology Laboratory of the Tamaulipas State Public Health Laboratory. They were stored at −20 °C until further processing.
Nucleic acid extraction was performed using a MagNA Pure LC total nucleic acid isolation kit in a MagNA Pure LC 2.0 Instrument (Roche Applied Science, Germany). The extracted viral RNA was stored at −70 °C. We used RT-PCR to detect the presence of arboviruses using protocols previously described [37]. We used the SuperScript III Platinum® One-Step qRT-PCR System enzyme (Invitrogen, Carlsbad, CA, USA). A 7500 Fast Real-Time Thermocycler from Applied Biosystems (Foster City, CA, USA) was used, and reportable positive values were below a Ct value of 38.
For ZIKV, the primary patients that received laboratory confirmation using RT-PCR were pregnant females. For the modeling in this study, we used the empirical data of probable cases of ZIKV in the municipality of Reynosa in 2017 with no recent travel history (Figure S2).
2.10. Mathematical Modeling
The Ross MacDonald formulation of the basic reproductive number for mosquito-borne diseases [38] is defined as the average number of secondary human cases generated by an index case in an otherwise susceptible population
where m: the density of female mosquitoes to human, a: female mosquito biting rate, f: the proportion of mosquito feeding on human, b: mosquito-to-human transmission probability, c: human-to-mosquito transmission probability, EIP: extrinsic incubation period, 1/r: human average infectious period, : adult mosquito mortality rate [38]. The density of Ae. aegypti to humans is a function of mosquito density and human exposure to mosquitoes [38,39,40] m = where is mosquito density and is the human risk of exposure to Ae. aegypti. The risk of exposure to mosquitoes is a function of socioeconomic variables, such as the availability of air conditioning, which can drastically limit mosquito–human contacts and virus transmission, even when mosquitoes are abundant [18]. Studies have shown that population mobility may also play a role in individuals exposure risk to Ae. aegypti [41]. Furthermore, fine-scale variation in population susceptibility, immunity, or social structures may also be factors contributing to vector-borne disease transmission heterogeneity amongst neighboring communities [42]. Though these parameters may be hard to measure empirically, they can play a pivotal role in the risk of mosquito borne disease outbreaks.
Given the geographical proximity and similarities between the city of Reynosa and neighborhoods in the LRGV of South Texas, any difference in the risk of outbreak ), for a newly introduced Ae. Aegypti-borne disease such as Zika, between the two communities would be due to the density of mosquito to human (m) or the proportion of mosquito feeding on humans (f). Therefore, in the LRGV and Reynosa can be written as and , respectively. We have , which is rearranged as This implies that
where and are the basic reproductive numbers in the LRGV and Reynosa, respectively. and are the density of female Ae. aegypti to human in the LRGV and Reynosa, respectively; and and are the proportion of Ae. aegypti feeding on humans in the LRGV and Reynosa, respectively. We estimated the ratio . Female Ae. aegypti relative abundance was estimated using AGO data collected in 2017 during the same weeks in the city of Reynosa (5.16 female Ae. aegypti per AGO per week) and in the LRGV (4.16 female Ae. aegypti per AGO per week) [14]. So . As the proportion of feeding on human in Reynosa is not currently available, we considered a range of values informed by available data from the Americas (Table 1): LRGV, Puerto Rico, and Florida.
in Reynosa municipality was estimated using case data for the 2017 Zika epidemic and the EstimateR function from the EpiEstim R library [43,44] to estimate the time-dependent reproductive number, based on the method introduced by Cori et al. [43]. We derive using the fact that is equal to at the start of the outbreak, such as Zika, for which we do not have pre-existing immunity in the population [44]. This approach would not be applicable to endemic diseases such as dengue. The instantaneous reproduction number was computed over 4-week sliding windows using the method introduced by Cori et al. [43]. This approach uses a Bayesian inference method to propagate uncertainty of data and generation time into estimate. Following Ferguson et al. [44], we assume that the ZIKV generation time is gamma-distributed with a mean of 20.0 days and a standard deviation (s.d.) of 7.4 days. The incidence data themselves may contain many potential sources of uncertainty such as misdiagnosis, variable time-dependent case detection rate, and asymptomatic cases, which are not explicitly taken into account into our analysis.

Table 1.
Published studies of Ae. aegypti host feeding patterns.
Table 1.
Published studies of Ae. aegypti host feeding patterns.
| Feeding Patterns on Vertebrates (%) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Citation | Location | Method a | Site b | Human | Mix/Human | Dog | Cat | Other Mammal | Avian | Unknown | Total |
| [45] | Nigeria | Ab | In/Out | 7 (44%) | 1 (6%) | 8 (50%) | 16 | ||||
| [46] | Tanzania | Ab | In | 45 (100%) | 45 | ||||||
| [47] | Kenya—coast | Ab | In/Out | 165 (94%) | 1 (0.5%) | 1 (0.5%) | 9 (5%) | 176 | |||
| [48] | South Africa | Ab | Out | 3 (75%) | 1 (25%) | 4 | |||||
| [49] | India, Poona | Ab | In | 17 (81%) | 4 (19%) | 21 | |||||
| [50] | India | Ab | In | 49 (96%) | 2 (4%) | 51 | |||||
| [49] | Malaya | Ab | In | 109 (99%) | 1 (1%) | 110 | |||||
| [51] | Hawaii | Ab | Out | 339 (54%) | 117 (19%) | 21 (3%) | 71 (11%) | 3 (0.5%) | 80 (13%) | 631 | |
| [52] | Thailand | Ab | In/Out | 789 (88%) | 66 (7.4%) | 2 (2.2%) | 4 (0.5%) | 8 (1%) | 9 (1%) | 896 | |
| [53] | Puerto Rico | Ab | In | 1483 (95%) | 31 (2%) | 47 (3%) | 1561 | ||||
| [54] | Thailand—single host | Ab | In/Out | 658 (99%) | 1 | 4 (0.6%) | 1 | 664 | |||
| [54] | Thailand—mixed | Ab | In/Out | 86 (98%) | 88 | ||||||
| [55] | E. Australia | DNA | Out | 131 (75%) | 7 (4%) | 23 (13%) | 2 (1%) | 1 (0.5%) | 10 (6%) | 174 | |
| [56] | Thailand | DNA | N/A | 766 (86.1%) | 32 (3.6%) * | 18 (2%) | 39 (4.4%) | 35 (3.9%) | 890 | ||
| [57] | Puerto Rico-P | DNA | Out | 101 (76.2%) | 27 (20.8%) | 3 (2.3%) | 1 (0.8%) | 132 | |||
| [57] | Puerto Rico-R | DNA | Out | 210 (78.9%) | 1 (0.4%) | 49 (18.4%) | 3 (1.1%) | 3 (1.1%) | 266 | ||
| [58] | India | Gel precip | In/Out | 129 (87.8%) | 11 (7.5%) | 1 (0.7%) | 6 (4%) | 147 | |||
| [59] | India | Gel precip | Out | 54 (96.4%) | 2 (3.6%) | 56 | |||||
| [60] | Mexico | DNA | In/Out | 223 (98%) | 5 (2%) | 228 | |||||
| [61] | Florida—IR | DNA | Out | 111 (90.2%) | 11 (8.9%) | 1 (0.8%) | 123 | ||||
| [61] | Florida—M | DNA | Out | 8 (61.5%) | 5 (38.5%) | 13 | |||||
| [62] | Grenada | DNA | Out | 22 (70%) | 2 (6%) | 1 (3%) | 6 (18%) | 1 (3%) | 32 | ||
a Ab = precipitin test for presence of antibody, DNA = molecular identification, Gel precip = agarose gel precipitin technique. b Indoor = In, Outdoor = Out. * Samples were positive for two hosts, but the authors did not reveal which two hosts. It is assumed that one of the hosts is human.
3. Results
3.1. Blood Meal Analysis
In total, 230 bloodfed Ae. aegypti (Sella score of 2–5) [20] were collected, molecularly confirmed to species and processed for the blood meal analysis (four indoor, 226 outdoor; 181 using BGS2 traps, 49 using AGO). Of these, 186 (81%) yielded a bloodmeal analysis result which include 50% (n = 93) from dogs (Canis lupus familiaris), 31% (n = 57) from humans (Homo sapiens), 12% (n = 22) from cats (Felis catus), 3% (n = 6) from chicken (Gallus gallus) and 4% from other mammals (Table 2). Of the four Ae. aegypti collected indoors by AGO traps, three yielded a result (one human, two dogs). Bloodfed Ae. aegypti with results came from two different homes in MM, two homes in DF, one home in MCH, four homes in PF/PE, 34 homes in IHE, nine homes in IHW, 19 homes in LP, 10 homes in TB, one home in LF, two homes in MLV, and two homes in WCT. For Cx. quinquefasciatus, 124 bloodfed individuals (Sella score of 2–4) were collected, molecularly confirmed to species, and processed for bloodmeal analysis (0 indoor, 124 outdoor; 113 using BGS2 traps, 11 using AGO traps). Of these, 123 (99%) yielded a bloodmeal analysis result which included 67% (n = 82) from chicken, 22% (n = 27) were from dog, 9% (n = 11) from six wild bird species and 2% from other mammals (Table 3). Two Ae. aegypti samples had mixed bloodmeals including dog and human, while no Cx. quinquefasciatus had evidence of mixed bloodmeals. The success of the vertebrate host identification of the blooded abdomen for Ae. aegypti was significantly different across Sella scores (p = 0.0333; 92% for Sella score of two, 76% for three, 33% for four, and 25% for five). The success of the vertebrate host identification of the blooded abdomen for Cx. quinquefasciatus was not significantly different among Sella scores (p = 0.3333; 99% for Sella score of two, 100% for three, and 100% for four). The quantitative analysis of 18 published studies of Ae. aegypti host feeding patterns reveals that humans are the dominant host with an average of 83.1% (Table 1). If we only consider prior studies with outdoor mosquito collections, the average percentage of human feeding is 85%. Only two studies, one in Nigeria (Table 1) and this current study from South Texas, reveal feeding patterns where humans represent less than half of the bloodmeals.

Table 2.
Blood meal analysis results and forage ratios for Ae. aegypti.

Table 3.
Blood meal analysis results and forage ratios for Cx. quinquefasciatus.
3.2. Mosquito Relative Abundance
Mosquito sampling between May 7 to August 13, 2017 using 80 Sentinel AGO traps in Reynosa yielded an average of 5.16 (± 0.43 SEM) female Ae. aegypti per AGO per week (Figure S3). In the LRGV, 30 Sentinel AGO traps during these same weeks yielded an average of 4.16 (± 0.43 SEM) female Ae. aegypti per AGO per week [14].
3.3. Vertebrate Surveys and Population Density
We conducted a vertebrate questionnaire for 44 homes in four communities asking about all vertebrates living in the home, property, or neighborhood (Table S3). The average number of occupants per home was 4.7 (± 0.41 SEM) and the total estimated number of homes in all four communities was 612. With our vertebrate surveys, we estimated 5,146 humans per km2, 4,161 dogs per km2, 1,751 cats per km2, 2,299 chickens per km2, and 75 pigs per km2 (Table S4). Independent from the household questionnaires, our analysis of US Census data using QGIS for the combined communities in the current study where blood-fed individuals were collected and in the eight communities with AGO surveillance [14], we estimate that on average the human density was 3,597 per km2 (Table S5). In Nuevo Amanecer, Reynosa, we identified 885 homes in the neighborhood minus the soccer field open space. The area with homes is 0.27 km2 and using an average occupancy of 4.2 persons per home (based on unpublished data from co-author M. A. Rodríguez-Pérez), the estimated human density for this area is 13,767 per km2. The human density in Reynosa is between 2.7- and 3.8-fold higher than comparable low-income communities in the LRGV.
3.4. Host Selection
Forage ratios for Ae. aegypti and Cx. quinquefasciatus were calculated with host availability estimated from our vertebrate surveys in the neighborhoods where we collected engorged mosquitoes. The Ae. aegypti forage ratio (observed frequency of bloodmeals from a given host divided by the expected frequency) for dogs (1.61) was nearly twice the forage ratio for humans (0.81; Table 2). In contrast, the highest forage ratio for Cx. quinquefasciatus was on chicken (3.92; Table 3). The human blood index (total number of human blood meals divided by the total number of engorged females with a confirmed result) for Ae. aegypti and Cx. quinquefasciatus was 30.7% and 0.8% respectively.
3.5. Mathematical Modeling
Using the Ross MacDonald equation for the basic reproductive number, , and based on the 2017 cases of ZIKV in Reynosa (Figure S2) and data on Ae. aegypti collected in the LRGV and Reynosa, we estimated ZIKV in the LRGV. We started by estimating in Reynosa using case data from the 2017 Zika outbreak in Reynosa, where was 2.2 (95% Confidence Interval: 1.1—3.8) (Figure S2). A total of 330 cases of Zika were observed in Reynosa in 2017; only a subset were tested by PCR, of which 81 were confirmed positive for ZIKV RNA. Seven Zika cases were not included, given a history of travel in the prior month. Because of the geographical proximity between Reynosa and the LRGV, we assumed that all parameters, except for mosquito abundance and human biting rates, in the Ross MacDonald R0 equation were equal across the US-Mexico border. We obtained the following expression for in the LRGV
where 0.8 is the ratio between mosquito abundance in LRGV and Reynosa estimated with AGO traps, and 0.31 is the proportion of Ae. aegypti feeding on humans in LRGV. is the proportion of Ae. aegypti feeding on human in Reynosa, and is the risk of human exposure to Ae. aegypti bites in Reynosa (LRGV). Then, with the estimate and using different combinations for the unknown parameters, fRey and ERey/ELRGV, of Equation (4) we studied the conditions for the establishment, i.e., R0 > 1, of a ZIKV epidemic in the LRGV (Figure 2). Our analysis shows that if fRey = fLRGV then ZIKV outbreaks would not occur in the LRGV when the risk of human exposure to Ae. aegypti bites in the LRGV is below 60% of the risk in Reynosa (Figure 2). Below 60%, there are limited scenarios where Zika outbreaks may occur in the LRGV: for example, when the fRey is smaller than fLRGV and the human exposure risk to Ae. aegypti bites in Reynosa is fives time larger than in LRGV (Figure 2).
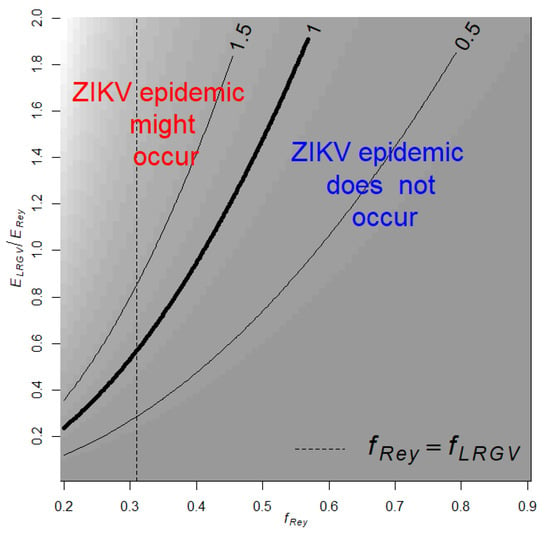
Figure 2.
Contour plot of in the LRGV as a function on the relative risk of human exposure to Ae. aegypti in the LRGV compared to Reynosa (), and the proportion of Ae. aegypti feeding on humans in Reynosa (fRey). The dashed vertical line indicates when . Background shading corresponds to contour predictions.
4. Discussion
This study documents Ae. aegypti feeding on humans only 31% of the time in the sampled communities in South Texas; instead, the majority of bloodmeals (50%) were from domestic dogs. This is an unexpectedly low rate of human feeding given that this species is ubiquitously classified as an anthropophilic species [18,63]. The quantitative synthesis of 18 published blood meal analysis studies on Ae. aegypti shows that the average percent of human blood-feeding was 83.8%. In 1967, MacDonald pointed out that “Although Ae. aegypti has been the study of a very large number of papers, there are only a few records of its host preference [49].” A half century later, this observation remains the same, given that only 21 studies have published Ae. aegypti host feeding patterns, three of which concerned the subspecies formosus, while our review of the published literature identified 86 primary publications that have reported host feeding patterns for members of the Cx. pipiens complex. The less attention to Ae. aegypti host feeding is likely due to the assumption that this species is largely anthropophilic and reluctance to conduct the expensive bloodmeal analysis for confirmation. Zooprophylaxis is the concept that the presence of incompetent hosts can ‘waste’ bites from vector species and reduce the transmission of an infectious agent [64]. Furthermore, prior studies have identified that arboviral transmission potential is impacted by host community composition and competence [65,66]. For human-amplified urban arboviruses like ZIKV, less feeding on humans and more feeding on non-competent hosts (vertebrates with a low duration and magnitude of viremia unable to re-infect Ae. aegypti), will have a zooprophylactic effect on transmission, as originally observed with cattle when describing zooprophylaxis in malaria transmission [67,68]. This has principally been considered an important phenomenon in human malaria transmission with Anopheles spp. wasting bites on cattle, something that protects humans from malaria infectious bites [69,70,71]. However, Hess and Hayes [64] determined that potential for zooprophylaxis exists in Cx. tarsalis, Cx. pipiens, Cx. quinquefasciatus and Ae. albopictus. Moreover, in East Africa the observation of non-human host feeding by Ae. aegypti led to the conclusion that these were likely to be poor vectors of yellow fever virus [29].
Non-human feeding by Ae. aegypti may have important consequences for arboviral pathogen transmission. For example, the receptivity of certain regions of the world to Ae. Aegypti-driven arboviruses might vary not simply due to the abundance of Ae. aegypti, but due to the proclivity and availability of Ae. aegypti to feed on humans relative to other hosts. In that sense, threshold indices, such as R0, that indicate when the transmission of viruses will persist, can guide management activities and even inform urban planning and home modification to further reduce the probability of Ae. aegypti feeding on humans.
Collection technique and location can influence the apparent host feeding patterns. While only 2% of the collections in this study were from inside homes, all of the specimens were collected within the residential yard. While our previous study documented Ae. aegypti inside homes of the LRGV [14], we did not target indoor collections with aspirators, given the unique socio-demographics and political climate of the LRGV, which makes indoor access challenging. Prior studies on Ae. aegypti host feeding patterns are limited, with only ten studies (56%) with indoor collections, seven (39%) with collections from residential yards, and two studies (11%) with collections in non-residential locations (Table 1). Of the published studies reporting Ae. aegypti blood meal results, most (69%) have been conducted in regions of the world where dengue is endemic (Table 1). This identifies a research gap, with a few studies such as this one reporting Ae. aegypti host feeding patterns in areas where the environment may be suitable for arboviral transmission, but risk appears to be diminished by limited access to humans, and, possibly easier access to non-human vertebrates. We also did not process blood-engorged mosquitoes collected in Reynosa in the same laboratory as the current study, which is a priority for future research.
The low rate of Ae. aegypti anthropophily in the current study could be explained by several reasons. The most parsimonious explanation is the higher availability of non-human hosts, limited opportunity for human biting, and lower human density [18,63]. In our Texas study sites, humans make up 41% of all domestic hosts in the four study sites combined. Our analysis does not account for wild birds and wild mammals which, if included, would further reduce the relative abundance of humans compared to non-human animals. Although many human-amplified urban arboviruses occur in densely populated settings, a study in Thailand found the largest dengue epidemics occurred in low to moderate population densities, where water storage and the production of mosquitoes is an additional factor driving transmission [72]. Another factor influencing the ability of Ae. aegypti to feed on humans is the integrity of the home and frequency of indoor feeding. Our recent study in nearby neighborhoods shows that the outdoor Ae. aegypti relative abundance is about eight times that of the indoor population [14]. Prior studies in the Texas–Mexico border region have shown that the presence of air conditioning units and larger lot size are associated with a lower probability of homeowners being exposed to DENV [13,18]. A final hypothesis explaining the low rate of Ae. aegypti feeding on humans is that there is a genetic basis. A genetic basis for host selection has been well documented in Culex spp. [73] and Anopheles spp. [74]. In 1967, Macdonald [49] reviewed the progress on understanding the ecology of multiple forms of Ae. Aegypti, including Ae. aegypti formosus found in east Africa which tended to be more exophilic and frequently fed on non-human hosts. More contemporary population genetics studies have confirmed that Ae. aegypti formosus is the ancestral form of the domesticated Ae. aegypti aegypti, which lives in tight association with human landscapes and is more anthropophilic [75]. The host preference of the ancestral and domesticated forms of Ae. aegypti in east Africa is considered to have a genetic basis [76,77]. Although the domesticated form of Ae. aegypti has spread around the world, Macdonald [49] postulated that, outside Africa, the plasticity of the species means the potential for non-human feeding and exophily exists, and the current study supports the ability of Ae. aegypti to adapt to an environment with lower availability of human hosts [63,78].
With Ae. aegypti feeding on non-human hosts about 70% of the time, this study highlights the potential role of Ae. aeygyti in contributing to enzootic transmission among animals or even bridge transmission of zoonotic agents to humans. Of the non-human bloodmeals, 50% were from domestic dogs. A recent study testing dogs from animal shelters in the LRGV (Edinburg, TX, USA) identified 20.9% of the dogs to be infected with dog heartworm, Dirofilaria immitis [79]. Several studies suggest Ae. aegypti as an efficient vector of D. immitis in dogs [80,81], and a study in Florida found Ae. aegypti infected with D. immitis [82]. Given that Ae. aegypti is the dominant mammalophilic mosquito species in low- and middle-income LRGV residential communities [14], these observations suggest that Ae. aegypti may play a role in D. immitis transmission, which warrants further research. Prior studies have also documented the potential spill-over of human-amplified urban arboviruses into wild or domestic animals [83,84], suggesting that Ae. aegypti could play the role of a bridge vector in this context.
The blood meal analysis results for Cx. quinquefasciatus yielded 75.6% of the bloodmeals from birds, with chickens being the dominant species (Table 3). These results are consistent with prior studies which show that Culex are principally ornithophilic [85]. Both the bloodfed Culex and Aedes were processed with the exact same protocol, and the contrasting results provide more confidence in the accuracy of the identified blood meals. The high host chicken use is consistent with Cx. quinquefasciatus host feeding patterns in tropical and subtropical regions [86]. The inclusion of Passerines as hosts by Cx. quinquefasciatus would suggest their potential role as an amplification vector for West Nile virus (WNV) in South Texas, while the observation of human feeding would suggest the potential to bridge WNV to humans. In some regions of the world, Cx. quinquefasciatus can be highly anthropophilic [87] and it was surprising to not find more human-derived bloodmeals. A good example of a study that analyzed both forage ratio (FR) and the human blood index (HBI) for Cx. quinquefasciatus mosquitoes was conducted by Garcia-Rejon et al. in Yucatan State, Mexico [35]. They found an HBI of 6.7, but the FR for humans was < 1 when compared to that of other vertebrate hosts, indicating that Cx. quinquefasciatus mosquitoes in this area under-utilized humans as hosts. In fact, species of the Passeriformes and Galliformes orders were the only hosts that had a FR >1 [35]. This ornithophilic pattern of Cx. quinquefasciatus mosquitoes was also demonstrated in College Station, Texas (95.5% blood meals on birds) [34]; and Harris County, Texas (39.1% on birds) [88].
5. Conclusions
In conclusion, we identify a potential mechanism explaining how ZIKV resulted in large epidemics in Reynosa, Tamaulipas, Mexico but did not result in widespread transmission in the LRGV of South Texas. The population of Ae. aegypti in South Texas fed on humans only 31% of the time, which is likely due to the abundance of non-human hosts in the residential neighborhood, the low human density, and social practices of minimizing risk of exposure to Ae. aegypti [18]. The high rate of non-human blood meals of Ae. aegypti occurring in the LRGV is likely reducing the risk of human-amplified urban arboviruses such as DENV, ZIKV, and CHIKV. However, the high number of blood meals from dogs and cats is concerning for zoonotic agents such as dog heartworm transmission and the potential for bridge transmission to human populations [89]. The population of Cx. quinquefasciatus in the LRGV was ornithophilic, which likely contributes to the local transmission of WNV observed in the region. This study revealed high non-human host utilization in Ae. aegypti mosquitoes, which warrants further research to determine factors driving the variation in mosquito–human contact.
Supplementary Materials
The following are available online at https://www.mdpi.com/1999-4915/12/4/453/s1, Figure S1: Probable and confirmed human cases of DENV, CHIKV and ZIKV from 2015 to 2019 in Tamaulipas, México, Figure S2: Weekly human Zika cases in Reynosa in 2017, and estimated effective reproductive number, Figure S3: Weekly Autocidal Gravid Ovitrap (AGO) counts for Ae. aegypti in the Lower Rio Grande Valley (LRGV) and Reynosa between 13 May and 12 August, 2017, Table S1: Vertebrate-specific primers used in this study, Table S2: Universal invertebrate primers used in this study, Table S3: Vertebrate densities resulting from community surveys, Table S4: Estimated vertebrate population densities based upon community surveys, Table S5: Estimated number of homes, population sizes and area in the regions of the LRGV receiving mosquito sampling in the current study and Martin et al., 2019.
Author Contributions
This study was conceived by G.L.H., J.G.E.-F., and M.A.R.-P. Data was procured by M.F.O., M.L.N.-M., J.R., A.S., J.G.E.-F., M.A.R.-P., N.A.F.-S., G.d.J.M.-G., S.D.C.A., B.d.L.R.-B., L.J.C.-D.l.c., A.G.-B., R.E.H.-G., and R.M.B.-C. Data and results were formally analyzed by M.F.O., J.G.J., and L.F.C. including additional mathematical modeling by M.L.N.-M. The methodology and protocol were refined by G.L.H., E.M., and M.L.N.-M. Study materials, laboratory equipment, instrumentation and reagents were provided by G.L.H., I.E.B.-V., M.A.R.-P., M.K.B., M.F. Mosquito samples were collected under the supervision of G.L.H., I.E.B.-V., J.G.J. and S.G.-L. Additionally, project-related activities were supervised by G.L.H., J.G.E.-F., M.A.R.-P., G.d.J.M.-G., and S.D.C.A. The first draft of this manuscript was written by M.F.O. and G.L.H. All authors approved and contributed to subsequent drafts of the manuscript and agree with the results presented. All authors have read and agreed to the published version of the manuscript.
Funding
This work was performed, in part, under the auspices of the U.S. Department of Energy by Lawrence Livermore National Laboratory under Contract DE-AC52-07NA27344 to G.L.H., M.F., M.K.B. Additional support came from NIH R21AI128953 (G.L.H. and I.E.B.-V.), K01AI128005 (G.L.H.), Texas A&M AgriLife Insect Vector Seed Grant (G.L.H. and I.E.B.-V.), CONACyT-MEXBOL (No. 295569), IPN (SIP 20181120 and 20195706) to M.A.R.-P. This publication was supported by Cooperative Agreement Number U01CK000512 (G.L.H. and I.E.B.-V.) and contract 200-2017-93141 (G.L.H. and I.E.B.-V.), funded by the Centers for Disease Control and Prevention. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the Centers for Disease Control and Prevention or the Department of Health and Human Services.
Acknowledgments
We are grateful to the residents of our study locations in the Rio Grande Valley, TX that granted us permission to trap mosquitoes on their property. We thank Ester Carbajal, Edwin Valdez, and Courtney Avila for assistance in the field and Helena Hopson, Victoria Kamilar, and Wendy Tang for assistance in the lab. We appreciate the helpful review and comments from Sarah Hamer, Roberto Barrera, Ryan Hemme, and Nga Vuong on an earlier version of this manuscript. Three anonymous reviewers provided constructive reviews which improved the manuscript. The graphical abstract was created with BioRender.com.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Huang, Y.-J.S.; Higgs, S.; Vanlandingham, D.L. Emergence and re-emergence of mosquito-borne arboviruses. Curr. Opin. Virol. 2019, 34, 104–109. [Google Scholar] [CrossRef]
- Gould, E.; Pettersson, J.; Higgs, S.; Charrel, R.; de Lamballerie, X. Emerging arboviruses: Why today? One Health 2017, 4, 1–13. [Google Scholar] [CrossRef]
- Espinal, M.A.; Andrus, J.K.; Jauregui, B.; Waterman, S.H.; Morens, D.M.; Santos, J.I.; Horstick, O.; Francis, L.A.; Olson, D. Emerging and reemerging Aedes-transmitted arbovirus infections in the region of the Americas: Implications for health policy. Am. J. Public Health 2019, 109, 387–392. [Google Scholar] [CrossRef]
- Macpherson, C.; Noël, T.; Fields, P.; Jungkind, D.; Yearwood, K.; Simmons, M.; Widjaja, S.; Mitchell, G.; Noel, D.; Bidaisee, S. Clinical and serological insights from the Asian lineage chikungunya outbreak in Grenada, 2014: An observational study. Am. J. Trop. Med. Hyg. 2016, 95, 890–893. [Google Scholar] [CrossRef]
- PAHO. Chikungunya. Available online: https://www.paho.org/hq/index.php?option=com_topics&view=article&id=343&Itemid=40931&lang=en (accessed on 12 July 2019).
- Faria, N.R.; da Silva Azevedo, R.d.S.; Kraemer, M.U.; Souza, R.; Cunha, M.S.; Hill, S.C.; Thézé, J.; Bonsall, M.B.; Bowden, T.A.; Rissanen, I. Zika virus in the Americas: Early epidemiological and genetic findings. Science 2016, 352, 345–349. [Google Scholar] [CrossRef]
- Brady, O.J.; Osgood-Zimmerman, A.; Kassebaum, N.J.; Ray, S.E.; de Araújo, V.E.; da Nóbrega, A.A.; Frutuoso, L.C.; Lecca, R.C.; Stevens, A.; de Oliveira, B.Z. The association between Zika virus infection and microcephaly in Brazil 2015–2017: An observational analysis of over 4 million births. PLoS Med. 2019, 16, e1002755. [Google Scholar] [CrossRef]
- Hahn, M.B.; Eisen, R.J.; Eisen, L.; Boegler, K.A.; Moore, C.G.; McAllister, J.; Savage, H.M.; Mutebi, J.-P. Reported distribution of Aedes (stegomyia) aegypti and Aedes (stegomyia) albopictus in the United States, 1995–2016 (diptera: Culicidae). J. Med. Entomol. 2016, 53, 1169–1175. [Google Scholar] [CrossRef]
- Thomas, D.L.; Santiago, G.A.; Abeyta, R.; Hinojosa, S.; Torres-Velasquez, B.; Adam, J.K.; Evert, N.; Caraballo, E.; Hunsperger, E.; Muñoz-Jordán, J.L. Reemergence of dengue in southern Texas, 2013. Emerg. Infect. Dis. 2016, 22, 1002. [Google Scholar] [CrossRef]
- Rey, J.R. Dengue in Florida (USA). Insects 2014, 5, 991–1000. [Google Scholar] [CrossRef]
- Secretaría de Salud, M. Dirección General de Epidemiología. 2019. Available online: https://www.gob.mx/salud/acciones-y-programas/historico-boletin-epidemiologico (accessed on 21 February 2020).
- Centers for Disease Control and Prevention. Dengue hemorrhagic fever—US-Mexico border, 2005. MMWR. Morbidity and Mortality Weekly Report. 2007. Available online: https://www.ncbi.nlm.nih.gov/pubmed/17687243 (accessed on 6 July 2019).
- Ramos, M.M.; Mohammed, H.; Zielinski-Gutierrez, E.; Hayden, M.H.; Lopez, J.L.R.; Fournier, M.; Trujillo, A.R.; Burton, R.; Brunkard, J.M.; Anaya-Lopez, L. Epidemic dengue and dengue hemorrhagic fever at the Texas–Mexico border: Results of a household-based seroepidemiologic survey, December 2005. Am. J. Trop. Med. Hyg. 2008, 78, 364–369. [Google Scholar] [CrossRef]
- Martin, E.; Medeiros, M.C.; Carbajal, E.; Valdez, E.; Juarez, J.G.; Gracia-Luna, S.; Salazar, A.; Qualls, W.A.; Hinojosa, S.; Borucki, M.K. Surveillance of Aedes aegypti indoors and outdoors using Autocidal Gravid Ovitraps in South Texas during local transmission of Zika virus, 2016 to 2018. Acta Trop. 2019, 192, 129–137. [Google Scholar] [CrossRef]
- Grubaugh, N.D.; Ladner, J.T.; Kraemer, M.U.; Dudas, G.; Tan, A.L.; Gangavarapu, K.; Wiley, M.R.; White, S.; Thézé, J.; Magnani, D.M. Genomic epidemiology reveals multiple introductions of Zika virus into the United States. Nature 2017, 546, 401. [Google Scholar] [CrossRef]
- Adams, L.E.; Martin, S.W.; Lindsey, N.P.; Lehman, J.A.; Rivera, A.; Kolsin, J.; Landry, K.; Staples, J.E.; Sharp, T.M.; Paz-Bailey, G. Epidemiology of dengue, chikungunya, and Zika virus disease in the US states and territories, 2017. Am. J. Trop. Med. Hyg. 2019, 101, 884–890. [Google Scholar] [CrossRef]
- Walker, K.R.; Williamson, D.; Carrière, Y.; Reyes-Castro, P.A.; Haenchen, S.; Hayden, M.H.; Jeffrey Gutierrez, E.; Ernst, K.C. Socioeconomic and human behavioral factors associated with Aedes aegypti (Diptera: Culicidae) immature habitat in Tucson, AZ. J. Med. Entomol. 2018, 55, 955–963. [Google Scholar] [CrossRef]
- Reiter, P.; Lathrop, S.; Bunning, M.; Biggerstaff, B.; Singer, D.; Tiwari, T.; Baber, L.; Amador, M.; Thirion, J.; Hayes, J. Texas lifestyle limits transmission of dengue virus. Emerg. Infect. Dis. 2003, 9, 86. [Google Scholar] [CrossRef]
- Fox, M. Illustrated key to common mosquitoes of Louisiana. In Mosquito Control Training Manual; Louisiana Mosquito Control Association: Baton Rouge, LA, USA, 2007; pp. 86–150. [Google Scholar]
- Sella, M. The antimalaria campaign at Fiumicino (Rome), with epidemiological and biological notes. Int. J. Public Health 1920, 1, 316–346. [Google Scholar]
- Graham, C.B.; Black Iv, W.C.; Boegler, K.A.; Montenieri, J.A.; Holmes, J.L.; Gage, K.L.; Eisen, R.J. Combining real-time polymerase chain reaction using SYBR Green I detection and sequencing to identify vertebrate bloodmeals in fleas. J. Med. Entomol. 2012, 49, 1442–1452. [Google Scholar] [CrossRef]
- Hamer, S.A.; Weghorst, A.C.; Auckland, L.D.; Roark, E.B.; Strey, O.F.; Teel, P.D.; Hamer, G.L. Comparison of DNA and carbon and nitrogen stable isotope-based techniques for identification of prior vertebrate hosts of ticks. J. Med. Entomol. 2015, 52, 1043–1049. [Google Scholar] [CrossRef]
- Greenstone, M.H.; Weber, D.C.; Coudron, T.A.; Payton, M.E.; Hu, J.S. Removing external DNA contamination from arthropod predators destined for molecular gut-content analysis. Mol. Ecol. Resour. 2012, 12, 464–469. [Google Scholar] [CrossRef]
- Hughes, G.L.; Dodson, B.L.; Johnson, R.M.; Murdock, C.C.; Tsujimoto, H.; Suzuki, Y.; Patt, A.A.; Cui, L.; Nossa, C.W.; Barry, R.M.; et al. Native microbiome impedes vertical transmission of Wolbachia in Anopheles mosquitoes. Proc. Natl. Acad. Sci. USA 2014, 111, 12498–12503. [Google Scholar] [CrossRef]
- Hamer, G.L.; Kitron, U.D.; Goldberg, T.L.; Brawn, J.D.; Loss, S.R.; Ruiz, M.O.; Hayes, D.B.; Walker, E.D. Host selection by Culex pipiens mosquitoes and West Nile virus amplification. Am. J. Trop. Med. Hyg. 2009, 80, 268–278. [Google Scholar] [CrossRef]
- Hernández-Triana, L.M.; Brugman, V.A.; Prosser, S.W.J.; Weland, C.; Nikolova, N.; Thorne, L.; De Marco, M.F.; Fooks, A.R.; Johnson, N. Molecular approaches for blood meal analysis and species identification of mosquitoes (Insecta: Diptera: Culicidae) in rural locations in southern England, United Kingdom. Zootaxa 2017, 4250, 67–76. [Google Scholar] [CrossRef]
- Medeiros, M.C.; Ricklefs, R.E.; Brawn, J.D.; Hamer, G.L. Plasmodium prevalence across avian host species is positively associated with exposure to mosquito vectors. Parasitology 2015, 142, 1612–1620. [Google Scholar] [CrossRef]
- Folmer, O.; Black, M.; Hoeh, W.; Lutz, R.; Vrijenhoek, R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotechnol. 1994, 3, 294–299. [Google Scholar]
- McClelland, G.; Weitz, B. Serological identification of the natural hosts of Aedes aegypti (L.) and some other mosquitoes (Diptera, Culicidae) caught resting in vegetation in Kenya and Uganda. Ann. Trop. Med. Parasitol. 1963, 57, 214–224. [Google Scholar] [CrossRef]
- Teesdale, C. Studies on the bionomics of Aedes aegypti (L.) in its natural habitats in a coastal region of Kenya. Bull. Entomol. Res. 1955, 46, 711–742. [Google Scholar] [CrossRef]
- Chepkorir, E.; Venter, M.; Lutomiah, J.; Mulwa, F.; Arum, S.; Tchouassi, D.; Sang, R. The occurrence, diversity and blood feeding patterns of potential vectors of dengue and yellow fever in Kacheliba, West Pokot County, Kenya. Acta Trop. 2018, 186, 50–57. [Google Scholar] [CrossRef]
- Feria-Arroyo, T.P.; Aguilar, C.; Oraby, T. A tale of two cities: Aedes Mosquito surveillance across the Texas-Mexico Border. Subtrop. Agric. Environ. 2020, 71, 12. [Google Scholar]
- Brown, L.D.; Cai, T.T.; DasGupta, A. Interval estimation for a binomial proportion. Stat. Sci. 2001, 16, 101–117. [Google Scholar]
- Komar, N.; Panella, N.A.; Golnar, A.J.; Hamer, G.L. Forage ratio analysis of the southern house mosquito in College Station, Texas. Vector Borne Zoonotic Dis. 2018, 18, 485–490. [Google Scholar] [CrossRef]
- Garcia-Rejon, J.E.; Blitvich, B.J.; Farfan-Ale, J.A.; Lorono-Pino, M.A.; Chi Chim, W.A.; Flores-Flores, L.F.; Rosado-Paredes, E.; Baak-Baak, C.; Perez-Mutul, J.; Suarez-Solis, V.; et al. Host-feeding preference of the mosquito, Culex quinquefasciatus, in Yucatan State, Mexico. J. Insect. Sci. 2010, 10, 32. [Google Scholar] [CrossRef]
- Garrett-Jones, C. The human blood index of malaria vectors in relation to epidemiological assessment. Bull. World Health Organ. 1964, 30, 241–261. [Google Scholar]
- Lanciotti, R.S.; Kosoy, O.L.; Laven, J.J.; Velez, J.O.; Lambert, A.J.; Johnson, A.J.; Stanfield, S.M.; Duffy, M.R. Genetic and serologic properties of Zika virus associated with an epidemic, Yap State, Micronesia, 2007. Emerg. Infect. Dis. 2008, 14, 1232. [Google Scholar] [CrossRef]
- Perkins, T.A.; Siraj, A.S.; Ruktanonchai, C.W.; Kraemer, M.U.; Tatem, A.J. Model-based projections of Zika virus infections in childbearing women in the Americas. Nat. Microbiol. 2016, 1, 16126. [Google Scholar] [CrossRef]
- Ndeffo-Mbah, M.L.; Pandey, A. Global risk and elimination of yellow fever epidemics. J. Inf. Dis. 2019. [Google Scholar] [CrossRef]
- Zhang, Q.; Sun, K.; Chinazzi, M.; y Piontti, A.P.; Dean, N.E.; Rojas, D.P.; Merler, S.; Mistry, D.; Poletti, P.; Rossi, L. Spread of Zika virus in the Americas. Proc. Natl. Acad. Sci. USA 2017, 114, E4334–E4343. [Google Scholar] [CrossRef]
- Reiner, R.C., Jr.; Stoddard, S.T.; Scott, T.W. Socially structured human movement shapes dengue transmission despite the diffusive effect of mosquito dispersal. Epidemics 2014, 6, 30–36. [Google Scholar] [CrossRef]
- Padmanabha, H.; Correa, F.; Rubio, C.; Baeza, A.; Osorio, S.; Mendez, J.; Jones, J.H.; Diuk-Wasser, M.A. Human social behavior and demography drive patterns of fine-scale Dengue transmission in endemic areas of Colombia. PLoS ONE 2015, 10, e0144451. [Google Scholar] [CrossRef]
- Cori, A.; Ferguson, N.M.; Fraser, C.; Cauchemez, S. A new framework and software to estimate time-varying reproduction numbers during epidemics. Am. J. Epidemiol. 2013, 178, 1505–1512. [Google Scholar] [CrossRef]
- Ferguson, N.M.; Cucunubá, Z.M.; Dorigatti, I.; Nedjati-Gilani, G.L.; Donnelly, C.A.; Basáñez, M.-G.; Nouvellet, P.; Lessler, J. Countering the Zika epidemic in Latin America. Science 2016, 353, 353–354. [Google Scholar] [CrossRef]
- Davis, G.E.; Philip, C.B. The Identification of the Blood-meal in West African Mosquitoes by means of the Precipitin Test. A preliminary Report. Am. J. Hyg. 1931, 14, 130–141. [Google Scholar] [CrossRef]
- Lumsden, W. An epidemic of virus disease in Southern Province, Tanganyika territory, in 1952–1953 II. General description and epidemiology. Trans. R. Soc. Trop. Med. Hyg. 1955, 49, 33–57. [Google Scholar] [CrossRef]
- Heisch, R.; Nelson, G.; Furlong, M. Studies in filariasis in East Africa. 1. Filariasis on the Island of Pate, Kenya. Trans. R. Soc. Trop. Med. Hyg. 1959, 53, 41–53. [Google Scholar] [CrossRef]
- Paterson, H.; Bronsden, P.; Levitt, J.; Worth, C. Some Culicine Mosquitoes (Diptera, Culicidae) at Ndumu, Republic of South. Africa. A Study of Their Host Preferences and Host Range. Med. Proc. 1964, 10, 188–192. [Google Scholar]
- Macdonald, W. Host feeding preferences. Bull. World Health Organ. 1967, 36, 597. [Google Scholar]
- Krishnamurthy, B.; Kalra, N.; Joshi, G.; Singh, N. Reconnaissance Survey of Aedes Mosquitoes in Delhi. Bull. Indian Soc. Malar. Commun. Dis. 1965, 2, 56–67. [Google Scholar]
- Tempelis, C.; Hayes, R.; Hess, A.; Reeves, W. Blood-feeding habits of four species of mosquito found in Hawaii. Am. J. Trop. Med. Hyg. 1970, 19, 335–341. [Google Scholar] [CrossRef]
- Scott, T.W.; Chow, E.; Strickman, D.; Kittayapong, P.; Wirtz, R.A.; Lorenz, L.H.; Edman, J.D. Blood-feeding patterns of Aedes aegypti (Diptera: Culicidae) collected in a rural Thai village. J. Med. Entomol. 1993, 30, 922–927. [Google Scholar] [CrossRef]
- Scott, T.W.; Amerasinghe, P.H.; Morrison, A.C.; Lorenz, L.H.; Clark, G.G.; Strickman, D.; Kittayapong, P.; Edman, J.D. Longitudinal studies of Aedes aegypti (Diptera: Culicidae) in Thailand and Puerto Rico: Blood feeding frequency. J. Med. Entomol. 2000, 37, 89–101. [Google Scholar] [CrossRef]
- Ponlawat, A.; Harrington, L.C. Blood feeding patterns of Aedes aegypti and Aedes albopictus in Thailand. J. Med. Entomol. 2005, 42, 844–849. [Google Scholar] [CrossRef]
- Jansen, C.C.; Webb, C.E.; Graham, G.C.; Craig, S.B.; Zborowski, P.; Ritchie, S.A.; Russell, R.C.; Van den Hurk, A.F. Blood sources of mosquitoes collected from urban and peri-urban environments in eastern Australia with species-specific molecular analysis of avian blood meals. Am. J. Trop. Med. Hyg. 2009, 81, 849–857. [Google Scholar] [CrossRef]
- Siriyasatien, P.; Pengsakul, T.; Kittichai, V.; Phumee, A.; Kaewsaitiam, S.; Thavara, U.; Tawatsin, A.; Asavadachanukorn, P.; Mulla, M.S. Identification of blood meal of field caught Aedes aegypti (L.) by multiplex PCR. Southeast Asian J. Trop. Med. Public Health 2010, 41, 43. [Google Scholar]
- Barrera, R.; Bingham, A.M.; Hassan, H.K.; Amador, M.; Mackay, A.J.; Unnasch, T.R. Vertebrate hosts of Aedes aegypti and Aedes mediovittatus (Diptera: Culicidae) in rural Puerto Rico. J. Med. Entomol. 2012, 49, 917–921. [Google Scholar] [CrossRef]
- Sivan, A.; Shriram, A.; Sunish, I.; Vidhya, P. Host-feeding pattern of Aedes aegypti and Aedes albopictus (Diptera: Culicidae) in heterogeneous landscapes of South Andaman, Andaman and Nicobar Islands, India. Parasitol. Res. 2015, 114, 3539–3546. [Google Scholar] [CrossRef]
- Wilson, J.J.; Sevarkodiyone, S. Host preference of blood feeding mosquitoes in rural areas of southern Tamil Nadu, India. Acad. J. Entomol. 2015, 8, 80–83. [Google Scholar]
- Baak-Baak, C.M.; Cigarroa-Toledo, N.; Cruz-Escalona, G.A.; Machain-Williams, C.; Rubi-Castellanos, R.; Torres-Chable, O.M.; Torres-Zapata, R.; Garcia-Rejon, J.E. Human blood as the only source of Aedes aegypti in churches from Merida, Yucatan, Mexico. J. Vector Borne Dis. 2018, 55, 58. [Google Scholar]
- Stenn, T.; Peck, K.J.; Rocha Pereira, G.; Burkett-Cadena, N.D. Vertebrate Hosts of Aedes aegypti, Aedes albopictus, and Culex quinquefasciatus (Diptera: Culicidae) as Potential Vectors of Zika Virus in Florida. J. Med. Entomol. 2018, 56, 10–17. [Google Scholar] [CrossRef]
- Fitzpatrick, D.M.; Hattaway, L.M.; Hsueh, A.N.; Ramos-Niño, M.E.; Cheetham, S.M. PCR-Based Bloodmeal Analysis of Aedes aegypti and Culex quinquefasciatus (Diptera: Culicidae) in St. George Parish, Grenada. J. Med. Entomol. 2019, 56, 1170–1175. [Google Scholar] [CrossRef]
- Chaves, L.F.; Harrington, L.C.; Keogh, C.L.; Nguyen, A.M.; Kitron, U.D. Blood feeding patterns of mosquitoes: Random or structured? Front. Zool. 2010, 7, 3. [Google Scholar] [CrossRef]
- Hess, A.; Hayes, R.O. Relative potentials of domestic animals for zooprophylaxis against mosquito vectors of encephalitis. Am. J. Trop. Med. Hyg. 1970, 19, 327–334. [Google Scholar] [CrossRef]
- Hamer, G.L.; Chaves, L.F.; Anderson, T.K.; Kitron, U.D.; Brawn, J.D.; Ruiz, M.O.; Loss, S.R.; Walker, E.D.; Goldberg, T.L. Fine-scale variation in vector host use and force of infection drive localized patterns of West Nile virus transmission. PLoS ONE 2011, 6, e23767. [Google Scholar] [CrossRef]
- Koolhof, I.; Carver, S. Epidemic host community contribution to mosquito-borne disease transmission: Ross River virus. Epidemiol. Infect. 2017, 145, 656–666. [Google Scholar] [CrossRef]
- Hackett, L.; Missiroli, A. The Natural Disappearance of Malaria in Certain Regions of Europe. Am. J. Hyg. 1931, 13, 57–78. [Google Scholar] [CrossRef]
- Fantini, B. Anophelism without malaria: An ecological and epidemiological puzzle. Parassitologia 1994, 36, 83–106. [Google Scholar]
- Russell, P.F.; West, L.S.; Manwell, R.D.; Macdonald, G. Practical malariology. In Practical Malariology; Oxford University Press: London, UK, 1963. [Google Scholar]
- Mahande, A.; Mosha, F.; Mahande, J.; Kweka, E. Feeding and resting behaviour of malaria vector, Anopheles arabiensis with reference to zooprophylaxis. Malar. J. 2007, 6, 100. [Google Scholar] [CrossRef]
- Iwashita, H.; Dida, G.O.; Sonye, G.O.; Sunahara, T.; Futami, K.; Njenga, S.M.; Chaves, L.F.; Minakawa, N. Push by a net, pull by a cow: Can zooprophylaxis enhance the impact of insecticide treated bed nets on malaria control? Parasit. Vectors 2014, 7, 52. [Google Scholar] [CrossRef]
- Schmidt, W.-P.; Suzuki, M.; Thiem, V.D.; White, R.G.; Tsuzuki, A.; Yoshida, L.-M.; Yanai, H.; Haque, U.; Tho, L.H.; Anh, D.D. Population density, water supply, and the risk of dengue fever in Vietnam: Cohort study and spatial analysis. PLoS Med. 2011, 8, e1001082. [Google Scholar] [CrossRef]
- Fritz, M.; Walker, E.; Miller, J.; Severson, D.; Dworkin, I. Divergent host preferences of above-and below-ground Culex pipiens mosquitoes and their hybrid offspring. Med. Vet. Entomol. 2015, 29, 115–123. [Google Scholar] [CrossRef]
- Main, B.J.; Lee, Y.; Ferguson, H.M.; Kreppel, K.S.; Kihonda, A.; Govella, N.J.; Collier, T.C.; Cornel, A.J.; Eskin, E.; Kang, E.Y. The genetic basis of host preference and resting behavior in the major African malaria vector, Anopheles arabiensis. PLoS Genet. 2016, 12, e1006303. [Google Scholar] [CrossRef]
- Kotsakiozi, P.; Evans, B.R.; Gloria-Soria, A.; Kamgang, B.; Mayanja, M.; Lutwama, J.; Le Goff, G.; Ayala, D.; Paupy, C.; Badolo, A. Population structure of a vector of human diseases: Aedes aegypti in its ancestral range, Africa. Ecol. Evolut. 2018, 8, 7835–7848. [Google Scholar] [CrossRef]
- Crawford, J.E.; Alves, J.M.; Palmer, W.J.; Day, J.P.; Sylla, M.; Ramasamy, R.; Surendran, S.N.; Black, W.C.; Pain, A.; Jiggins, F.M. Population genomics reveals that an anthropophilic population of Aedes aegypti mosquitoes in West Africa recently gave rise to American and Asian populations of this major disease vector. BMC Biol. 2017, 15, 16. [Google Scholar] [CrossRef]
- McBride, C.S.; Baier, F.; Omondi, A.B.; Spitzer, S.A.; Lutomiah, J.; Sang, R.; Ignell, R.; Vosshall, L.B. Evolution of mosquito preference for humans linked to an odorant receptor. Nature 2014, 515, 222–227. [Google Scholar] [CrossRef]
- Paris, V.; Cottingham, E.; Ross, P.A.; Axford, J.K.; Hoffmann, A.A. Effects of alternative blood sources on Wolbachia infected Aedes aegypti females within and across generations. Insects 2018, 9, 140. [Google Scholar] [CrossRef]
- Hodo, C.L.; Rodriguez, J.Y.; Curtis-Robles, R.; Zecca, I.B.; Snowden, K.F.; Cummings, K.J.; Hamer, S.A. Repeated cross-sectional study of Trypanosoma cruzi in shelter dogs in Texas, in the context of Dirofilaria immitis and tick-borne pathogen prevalence. J. Vet. Intern. Med. 2019, 33, 158–166. [Google Scholar] [CrossRef]
- Tiawsirisup, S.; Nithiuthai, S. Vector competence of Aedes aegypti (L.) and Culex quinquefasciatus (Say) for Dirofilaria immitis (Leidy). Southeast Asian J. Trop. Med. Public Health 2006, 37, 110. [Google Scholar]
- Serrão, M.L.; Labarthe, N.; Lourenço-de-Oliveira, R. Vectorial competence of Aedes aegypti (Linnaeus 1762) Rio de Janeiro strain, to Dirofilaria immitis (Leidy 1856). Mem. Inst. Oswaldo Cruz 2001, 96, 593–598. [Google Scholar] [CrossRef]
- Ledesma, N.A.; Kaufman, P.E.; Xue, R.-D.; Leyen, C.; Macapagal, M.J.; Winokur, O.C.; Harrington, L.C. Entomological and sociobehavioral components of heartworm (Dirofilaria immitis) infection in two Florida communities with a high or low prevalence of dogs with heartworm infection. J. Am. Vet. Med. Assoc. 2019, 254, 93–103. [Google Scholar] [CrossRef]
- Thoisy, B.D.; Lacoste, V.; Germain, A.; Muñoz-Jordán, J.; Colón, C.; Mauffrey, J.F.; Delaval, M.; Catzeflis, F.; Kazanji, M.; Matheus, S.; et al. Dengue infection in neotropical forest mammals. Vector Borne Zoonotic Dis. 2009, 9, 157–170. [Google Scholar] [CrossRef]
- Pauvolid-Corrêa, A.; Gonçalves Dias, H.; Marina Siqueira Maia, L.; Porfírio, G.; Oliveira Morgado, T.; Sabino-Santos, G.; Helena Santa Rita, P.; Teixeira Gomes Barreto, W.; Carvalho de Macedo, G.; Marinho Torres, J. Zika Virus Surveillance at the Human–Animal Interface in West-Central Brazil, 2017–2018. Viruses 2019, 11, 1164. [Google Scholar] [CrossRef]
- Farajollahi, A.; Fonseca, D.M.; Kramer, L.D.; Kilpatrick, A.M. “Bird biting” mosquitoes and human disease: A review of the role of Culex pipiens complex mosquitoes in epidemiology. Inf. Genet. Evolut. 2011, 11, 1577–1585. [Google Scholar] [CrossRef]
- Kent, R.J.; Reiche, A.S.G.; Morales-Betoulle, M.E.; Komar, N. Comparison of engorged Culex quinquefasciatus collection and blood-feeding pattern among four mosquito collection methods in Puerto Barrios, Guatemala, 2007. J. Am. Mosq. Control. Assoc. 2010, 26, 332–337. [Google Scholar] [CrossRef]
- Janssen, N.; Fernandez-Salas, I.; Diaz Gonzalez, E.E.; Gaytan-Burns, A.; Medina-de la Garza, C.E.; Sanchez-Casas, R.M.; Borstler, J.; Cadar, D.; Schmidt-Chanasit, J.; Jost, H. Mammalophilic feeding behaviour of Culex quinquefasciatus mosquitoes collected in the cities of Chetumal and Cancun, Yucatan Peninsula, Mexico. Trop. Med. Int. Health 2015, 20, 1488–1491. [Google Scholar] [CrossRef]
- Molaei, G.; Andreadis, T.G.; Armstrong, P.M.; Bueno, R., Jr.; Dennett, J.A.; Real, S.V.; Sargent, C.; Bala, A.; Randle, Y.; Guzman, H.; et al. Host feeding pattern of Culex quinquefasciatus (Diptera: Culicidae) and its role in transmission of West Nile virus in Harris County, Texas. Am. J. Trop. Med. Hyg. 2007, 77, 73–81. [Google Scholar] [CrossRef]
- McCall, J.W.; Genchi, C.; Kramer, L.H.; Guerrero, J.; Venco, L. Heartworm disease in animals and humans. Adv. Parasitol. 2008, 66, 193–285. [Google Scholar]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).