Characterization of Two Neutralizing Antibodies against Rift Valley Fever Virus Gn Protein
Abstract
1. Introduction
2. Materials and Methods
2.1. Cells, Viruses, and Animals
2.2. Virus Neutralization Test (VNT)
2.3. Isolation of Gn-Specific Single Memory B Cells
2.4. Amplification of Antibody Variable Region Genes and Expression of Antibodies from Linear Expression Cassettes
2.5. Gene Construction
2.6. Enzyme-Linked Immunosorbent Assay (ELISA)
2.7. Biolayer Interferometry (BLI)
2.8. Protein Expression and Purification
2.9. Amino Acid Mapping of Epitopes by Alanine-Scanning Mutagenesis
2.10. Western Blotting
2.11. Antigen–Antibody Molecular Docking
3. Results
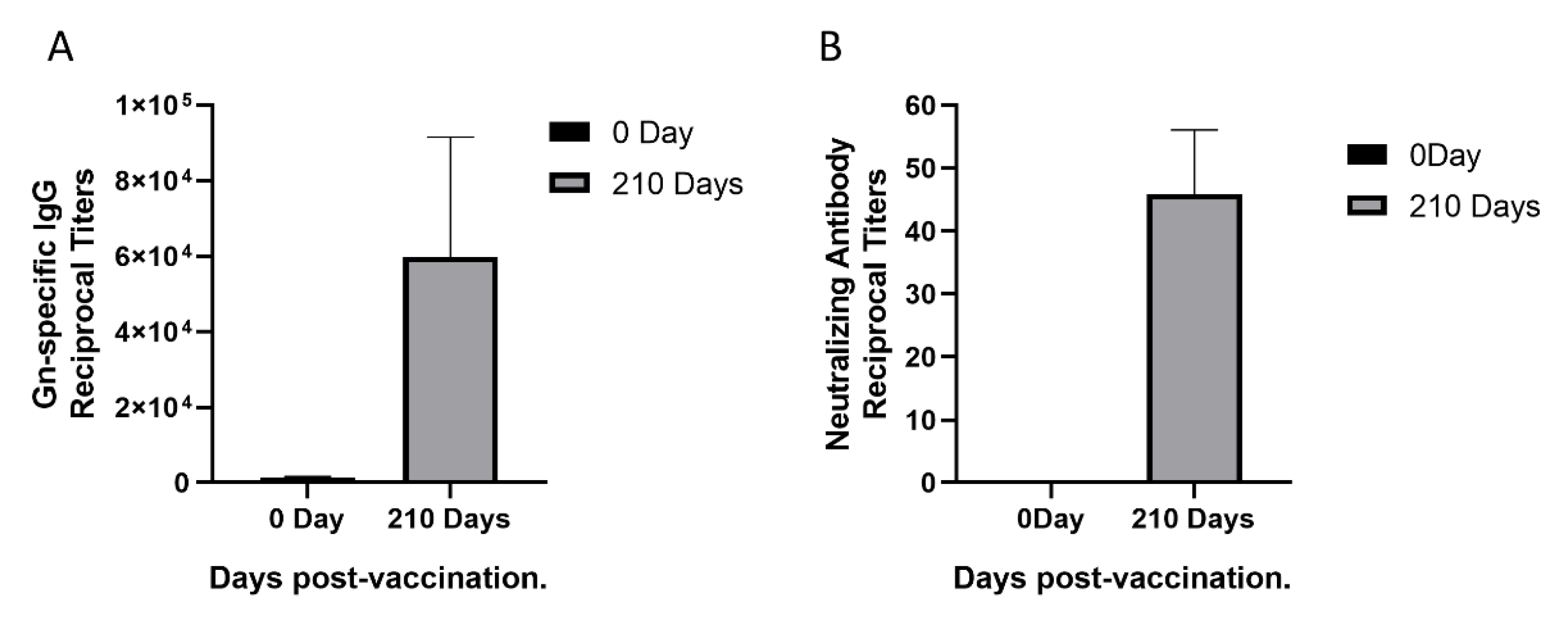
3.1. Sera Response in a Rhesus Monkey Immunized with rHAdV4-GnGcopt
3.2. Isolation and Binding Characterization of Gn-Specific Neutralizing Antibodies
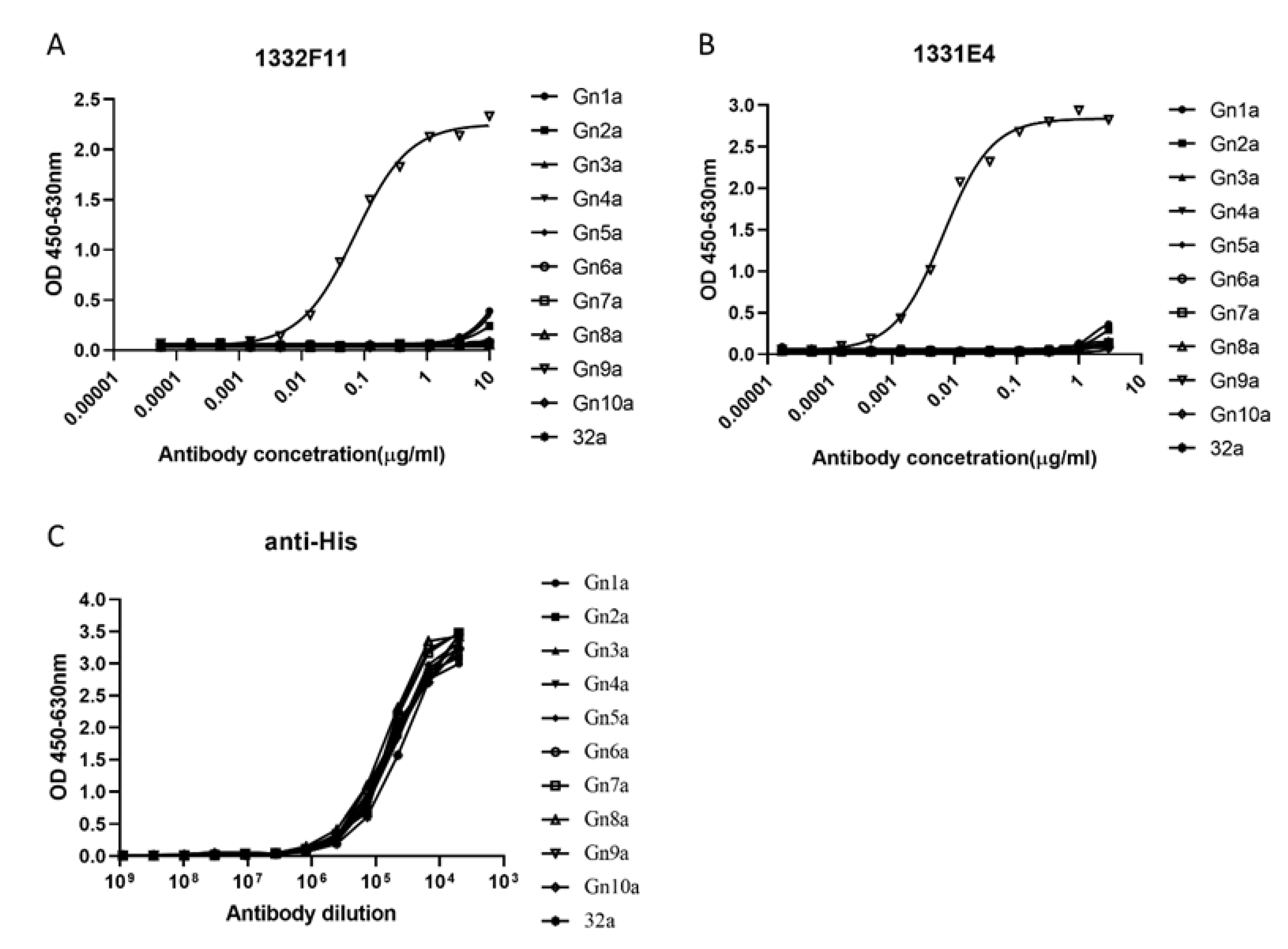
3.3. Identification of the Binding Epitopes of Two NAbs
3.4. Modeling the Interaction of NAbs and Gn
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Atkins, C.; Freiberg, A.N. Recent advances in the development of antiviral therapeutics for Rift Valley fever virus infection. Future Virol. 2017, 12, 651–665. [Google Scholar] [CrossRef]
- Murithi, R.M.; Munyua, P.; Ithondeka, P.M.; Macharia, J.M.; Hightower, A.; Luman, E.T.; Breiman, R.F.; Njenga, M.K. Rift Valley fever in Kenya: History of epizootics and identification of vulnerable districts. Epidemiol. Infect. 2011, 139, 372–380. [Google Scholar] [CrossRef]
- Laughlin, L.W.; Meegan, J.M.; Strausbaugh, L.J.; Morens, D.M.; Watten, R.H. Epidemic Rift Valley fever in Egypt: Observations of the spectrum of human illness. Trans. R. Soc. Trop. Med. Hyg. 1979, 73, 630–633. [Google Scholar] [CrossRef]
- Digoutte, J.P.; Peters, C.J. General aspects of the 1987 Rift Valley fever epidemic in Mauritania. Res. Virol. 1989, 140, 27–30. [Google Scholar] [CrossRef]
- Baba, M.; Masiga, D.K.; Sang, R.; Villinger, J. Has Rift Valley fever virus evolved with increasing severity in human populations in East Africa? Emerg. Microbes Infect. 2016, 5, e58. [Google Scholar] [CrossRef] [PubMed]
- Madani, T.A.; Al-Mazrou, Y.Y.; Al-Jeffri, M.H.; Mishkhas, A.A.; Al-Rabeah, A.M.; Turkistani, A.M.; Al-Sayed, M.O.; Abodahish, A.A.; Khan, A.S.; Ksiazek, T.G.; et al. Rift Valley fever epidemic in Saudi Arabia: Epidemiological, clinical, and laboratory characteristics. Clin. Infect. Dis. 2003, 37, 1084–1092. [Google Scholar] [CrossRef]
- Samy, A.M.; Peterson, A.T.; Hall, M. Phylogeography of Rift Valley Fever Virus in Africa and the Arabian Peninsula. PLoS Negl. Trop. Dis. 2017, 11, e0005226. [Google Scholar] [CrossRef]
- Liu, J.; Sun, Y.; Shi, W.; Tan, S.; Pan, Y.; Cui, S.; Zhang, Q.; Dou, X.; Lv, Y.; Li, X.; et al. The first imported case of Rift Valley fever in China reveals a genetic reassortment of different viral lineages. Emerg. Microbes Infect. 2017, 6, e4. [Google Scholar] [CrossRef]
- Mansfield, K.L.; Banyard, A.C.; McElhinney, L.; Johnson, N.; Horton, D.L.; Hernandez-Triana, L.M.; Fooks, A.R. Rift Valley fever virus: A review of diagnosis and vaccination, and implications for emergence in Europe. Vaccine 2015, 33, 5520–5531. [Google Scholar] [CrossRef]
- Wright, D.; Kortekaas, J.; Bowden, T.A.; Warimwe, G.M. Rift Valley fever: Biology and epidemiology. J. Gen. Virol. 2019, 100, 1187–1199. [Google Scholar] [CrossRef]
- Faburay, B.; LaBeaud, A.D.; McVey, D.S.; Wilson, W.C.; Richt, J.A. Current Status of Rift Valley Fever Vaccine Development. Vaccines (Basel) 2017, 5, 29. [Google Scholar] [CrossRef] [PubMed]
- de Boer, S.M.; Kortekaas, J.; Spel, L.; Rottier, P.J.; Moormann, R.J.; Bosch, B.J. Acid-activated structural reorganization of the Rift Valley fever virus Gc fusion protein. J. Virol. 2012, 86, 13642–13652. [Google Scholar] [CrossRef] [PubMed]
- Dessau, M.; Modis, Y. Crystal structure of glycoprotein C from Rift Valley fever virus. Proc. Natl. Acad. Sci. USA 2013, 110, 1696–1701. [Google Scholar] [CrossRef] [PubMed]
- Freiberg, A.N.; Sherman, M.B.; Morais, M.C.; Holbrook, M.R.; Watowich, S.J. Three-dimensional organization of Rift Valley fever virus revealed by cryoelectron tomography. J. Virol. 2008, 82, 10341–10348. [Google Scholar] [CrossRef]
- Huiskonen, J.T.; Overby, A.K.; Weber, F.; Grunewald, K. Electron cryo-microscopy and single-particle averaging of Rift Valley fever virus: Evidence for GN-GC glycoprotein heterodimers. J. Virol. 2009, 83, 3762–3769. [Google Scholar] [CrossRef]
- Wu, Y.; Zhu, Y.; Gao, F.; Jiao, Y.; Oladejo, B.O.; Chai, Y.; Bi, Y.; Lu, S.; Dong, M.; Zhang, C.; et al. Structures of phlebovirus glycoprotein Gn and identification of a neutralizing antibody epitope. Proc. Natl. Acad. Sci. USA 2017, 114, E7564–E7573. [Google Scholar] [CrossRef]
- Halldorsson, S.; Li, S.; Li, M.; Harlos, K.; Bowden, T.A.; Huiskonen, J.T. Shielding and activation of a viral membrane fusion protein. Nat. Commun. 2018, 9, 349. [Google Scholar] [CrossRef]
- Besselaar, T.G.; Blackburn, N.K.; Meenehan, G.M. Antigenic analysis of Rift Valley fever virus isolates: Monoclonal antibodies distinguish between wild-type and neurotropic virus strains. Res. Virol. 1991, 142, 469–474. [Google Scholar] [CrossRef]
- Besselaar, T.G.; Blackburn, N.K. The effect of neutralizing monoclonal antibodies on early events in Rift Valley fever virus infectivity. Res. Virol. 1994, 145, 13–19. [Google Scholar] [CrossRef]
- Besselaar, T.G.; Blackburn, N.K. The synergistic neutralization of Rift Valley fever virus by monoclonal antibodies to the envelope glycoproteins. Arch. Virol. 1992, 125, 239–250. [Google Scholar] [CrossRef]
- Allen, E.R.; Krumm, S.A.; Raghwani, J.; Halldorsson, S.; Elliott, A.; Graham, V.A.; Koudriakova, E.; Harlos, K.; Wright, D.; Warimwe, G.M.; et al. A Protective Monoclonal Antibody Targets a Site of Vulnerability on the Surface of Rift Valley Fever Virus. Cell Rep. 2018, 25, 3750–3758. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Ma, T.; Wu, Y.; Chen, Z.; Zeng, H.; Tong, Z.; Gao, F.; Qi, J.; Zhao, Z.; Chai, Y.; et al. Neutralization mechanism of human monoclonal antibodies against Rift Valley fever virus. Nat. Microbiol. 2019, 4, 1231–1241. [Google Scholar] [CrossRef] [PubMed]
- Ikegami, T.; Won, S.; Peters, C.J.; Makino, S. Rescue of infectious rift valley fever virus entirely from cDNA, analysis of virus lacking the NSs gene, and expression of a foreign gene. J. Virol. 2006, 80, 2933–2940. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Zhou, L.; He, B.; Zhang, X.; Ge, X.; Han, J.; Guo, X.; Yang, H. Nsp2 and GP5-M of Porcine Reproductive and Respiratory Syndrome Virus Contribute to Targets for Neutralizing Antibodies. Virol. Sin. 2019, 34, 631–640. [Google Scholar] [CrossRef]
- Sundling, C.; Phad, G.; Douagi, I.; Navis, M.; Karlsson Hedestam, G.B. Isolation of antibody V(D)J sequences from single cell sorted rhesus macaque B cells. J. Immunol. Methods 2012, 386, 85–93. [Google Scholar] [CrossRef]
- Liao, H.X.; Levesque, M.C.; Nagel, A.; Dixon, A.; Zhang, R.; Walter, E.; Parks, R.; Whitesides, J.; Marshall, D.J.; Hwang, K.K.; et al. High-throughput isolation of immunoglobulin genes from single human B cells and expression as monoclonal antibodies. J. Virol. Methods 2009, 158, 171–179. [Google Scholar] [CrossRef]
- Sherman, M.B.; Freiberg, A.N.; Holbrook, M.R.; Watowich, S.J. Single-particle cryo-electron microscopy of Rift Valley fever virus. Virology 2009, 387, 11–15. [Google Scholar] [CrossRef]
- Smith, M.R.; Schirtzinger, E.E.; Wilson, W.C.; Davis, A.S. Rift Valley Fever Virus: Propagation, Quantification, and Storage. Curr. Protoc. Microbiol. 2019, 55, e92. [Google Scholar] [CrossRef]
- Carbonetti, S.; Oliver, B.G.; Vigdorovich, V.; Dambrauskas, N.; Sack, B.; Bergl, E.; Kappe, S.H.I.; Sather, D.N. A method for the isolation and characterization of functional murine monoclonal antibodies by single B cell cloning. J. Immunol. Methods 2017, 448, 66–73. [Google Scholar] [CrossRef]
- Morrill, J.C.; Peters, C.J. Mucosal immunization of rhesus macaques with Rift Valley Fever MP-12 vaccine. J. Infect. Dis. 2011, 204, 617–625. [Google Scholar] [CrossRef]
- Ma, J.; Chen, R.; Huang, W.; Nie, J.; Liu, Q.; Wang, Y.; Yang, X. In vitro and in vivo efficacy of a Rift Valley fever virus vaccine based on pseudovirus. Hum. Vaccin. Immunother. 2019, 15, 2286–2294. [Google Scholar] [CrossRef] [PubMed]
- Caplen, H.; Peters, C.J.; Bishop, D.H. Mutagen-directed attenuation of Rift Valley fever virus as a method for vaccine development. J. Gen. Virol. 1985, 66, 2271–2277. [Google Scholar] [CrossRef] [PubMed]
- Morrill, J.C.; Mebus, C.A.; Peters, C.J. Safety and efficacy of a mutagen-attenuated Rift Valley fever virus vaccine in cattle. Am. J. Vet. Res. 1997, 58, 1104–1109. [Google Scholar] [PubMed]
- Morrill, J.C.; Jennings, G.B.; Caplen, H.; Turell, M.J.; Johnson, A.J.; Peters, C.J. Pathogenicity and immunogenicity of a mutagen-attenuated Rift Valley fever virus immunogen in pregnant ewes. Am. J. Vet. Res. 1987, 48, 1042–1047. [Google Scholar] [PubMed]
- Morrill, J.C.; Carpenter, L.; Taylor, D.; Ramsburg, H.H.; Quance, J.; Peters, C.J. Further evaluation of a mutagen-attenuated Rift Valley fever vaccine in sheep. Vaccine 1991, 9, 35–41. [Google Scholar] [CrossRef]




| Antibody | KD (M) | kon (1/Ms) | kon Error | kdis (1/s) | kdis Error | Full R2 |
|---|---|---|---|---|---|---|
| 1332F11 | 1.60 × 10−8 | 7.78 × 104 | 1.69 × 103 | 1.25 × 10−3 | 1.29 × 10−5 | 0.991524 |
| 1331E4 | 9.38 × 10−10 | 4.09 × 105 | 7.55 × 103 | 3.84 × 10−4 | 1.77 × 10−4 | 0.993053 |
| Segment | Amino Acid Sequence |
|---|---|
| Gn1a | EDPHLRNRPGKGHNYIDGMTQEDATCKPVTYAGACSSFDVLLEKG |
| Gn2a | YAGACSSFDVLLEKGKFPLFQSYAHHRTLLEAVHDTIIAKADPPS |
| Gn3a | EAVHDTIIAKADPPSCDLLSAHGNPCMKEKLVMKTHCPNDYQSAH |
| Gn4a | LVMKTHCPNDYQSAHHLNNDGKMASVKCPPKYELTEDCNFCRQMT |
| Gn5a | KYELTEDCNFCRQMTGASLKKGSYPLQDLFCQSSEDDGSKLKTKM |
| Gn6a | CQSSEDDGSKLKTKMKGVCEVGVQALKKCDGQLSTAHEVVPFAVF |
| Gn7a | GQLSTAHEVVPFAVFKNSKKVYLDKLDLKTEENLLPDSFVCFEHK |
| Gn8a | EENLLPDSFVCFEHKGQYKGTMDSGQTKRELKSFDISQCPKIGGH |
| Gn9a | LKSFDISQCPKIGGHGSKKCTGDAAFCSAYECTAQYANAYCSHAN |
| Gn10a | ECTAQYANAYCSHANGSGIVQIQVSGVWKKPLCVGYERVVVKRELS |
| Peptide | Amino Acid Sequence |
|---|---|
| Gn9a | LKSFDISQCPKIGGHGSKKCTGDAAFCSAYECTAQYANAYCSHAN |
| Gn9b | LKSFDISQCPKIGGHASKKCTGDAAFCSAYECTAQYANAYCSHAN |
| Gn9c | LKSFDISQCPKIGGHGAKKCTGDAAFCSAYECTAQYANAYCSHAN |
| Gn9d | LKSFDISQCPKIGGHGSAKCTGDAAFCSAYECTAQYANAYCSHAN |
| Gn9e | LKSFDISQCPKIGGHGSKACTGDAAFCSAYECTAQYANAYCSHAN |
| Gn9f | LKSFDISQCPKIGGHGSKKATGDAAFCSAYECTAQYANAYCSHAN |
| Gn9g | LKSFDISQCPKIGGHGSKKCAGDAAFCSAYECTAQYANAYCSHAN |
| Gn9h | LKSFDISQCPKIGGHGSKKCTADAAFCSAYECTAQYANAYCSHAN |
| Gn9i | LKSFDISQCPKIGGHGSKKCTGAAAFCSAYECTAQYANAYCSHAN |
| Gn9j | LKSFDISQCPKIGGHGSKKCTGDSAFCSAYECTAQYANAYCSHAN |
| Gn9k | LKSFDISQCPKIGGHGSKKCTGDASFCSAYECTAQYANAYCSHAN |
| Gn9l | LKSFDISQCPKIGGHGSKKCTGDAAACSAYECTAQYANAYCSHAN |
| Gn9m | LKSFDISQCPKIGGHGSKKCTGDAAFASAYECTAQYANAYCSHAN |
| Gn9n | LKSFDISQCPKIGGHGSKKCTGDAAFCAAYECTAQYANAYCSHAN |
| Gn9o | LKSFDISQCPKIGGHGSKKCTGDAAFCSSYECTAQYANAYCSHAN |
| Gn9p | LKSFDISQCPKIGGHGSKKCTGDAAFCSAAECTAQYANAYCSHAN |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hao, M.; Zhang, G.; Zhang, S.; Chen, Z.; Chi, X.; Dong, Y.; Fan, P.; Liu, Y.; Chen, Y.; Song, X.; et al. Characterization of Two Neutralizing Antibodies against Rift Valley Fever Virus Gn Protein. Viruses 2020, 12, 259. https://doi.org/10.3390/v12030259
Hao M, Zhang G, Zhang S, Chen Z, Chi X, Dong Y, Fan P, Liu Y, Chen Y, Song X, et al. Characterization of Two Neutralizing Antibodies against Rift Valley Fever Virus Gn Protein. Viruses. 2020; 12(3):259. https://doi.org/10.3390/v12030259
Chicago/Turabian StyleHao, Meng, Guanying Zhang, Shengnan Zhang, Zhengshan Chen, Xiangyang Chi, Yunzhu Dong, Pengfei Fan, Yujiao Liu, Yi Chen, Xiaohong Song, and et al. 2020. "Characterization of Two Neutralizing Antibodies against Rift Valley Fever Virus Gn Protein" Viruses 12, no. 3: 259. https://doi.org/10.3390/v12030259
APA StyleHao, M., Zhang, G., Zhang, S., Chen, Z., Chi, X., Dong, Y., Fan, P., Liu, Y., Chen, Y., Song, X., Liu, S., Yu, C., Li, J., & Xia, X. (2020). Characterization of Two Neutralizing Antibodies against Rift Valley Fever Virus Gn Protein. Viruses, 12(3), 259. https://doi.org/10.3390/v12030259




