Natural Products-Derived Chemicals: Breaking Barriers to Novel Anti-HSV Drug Development
Abstract
1. Introduction
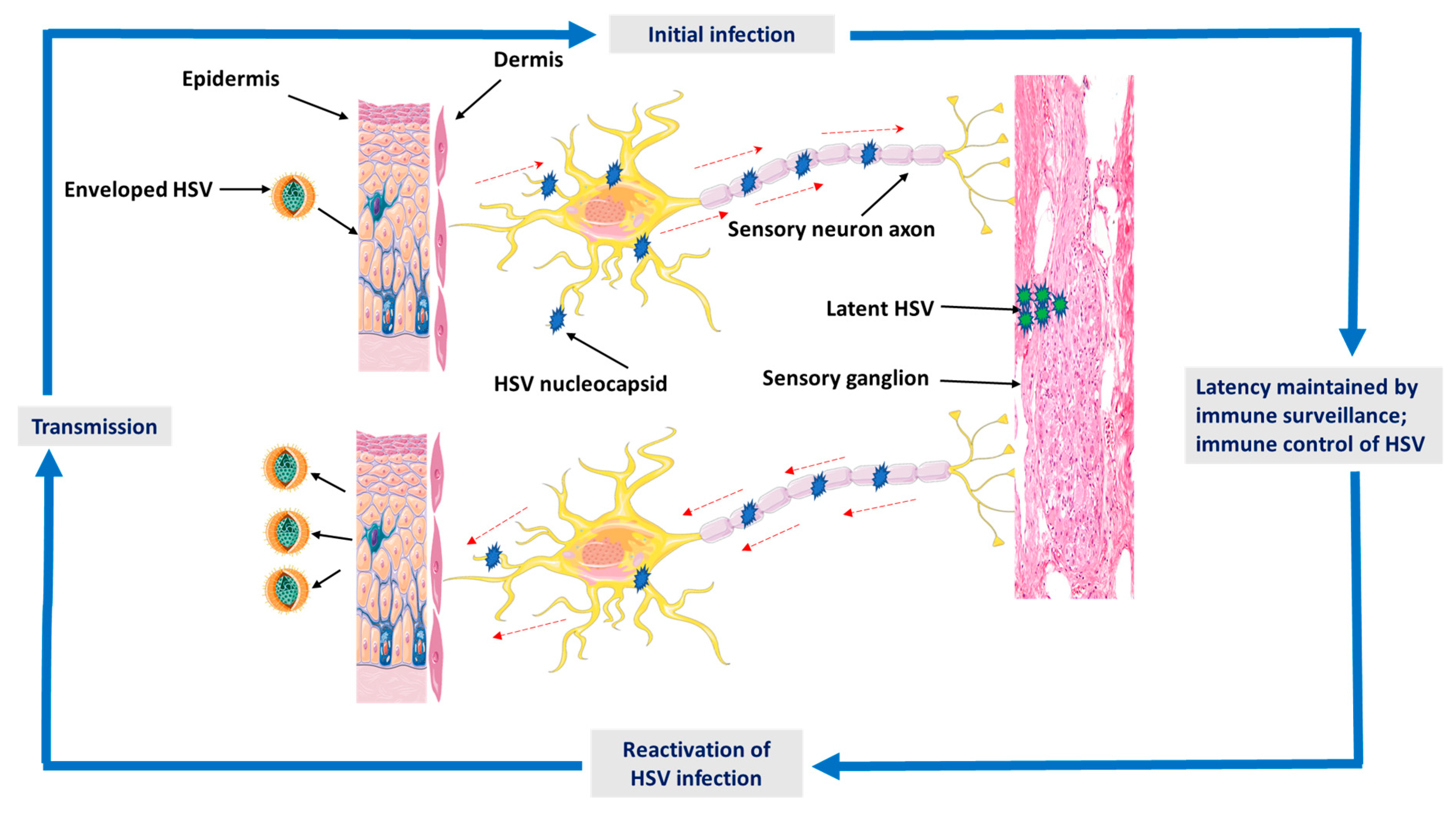
2. Epidemiology and Pathogenesis of HSV Infection
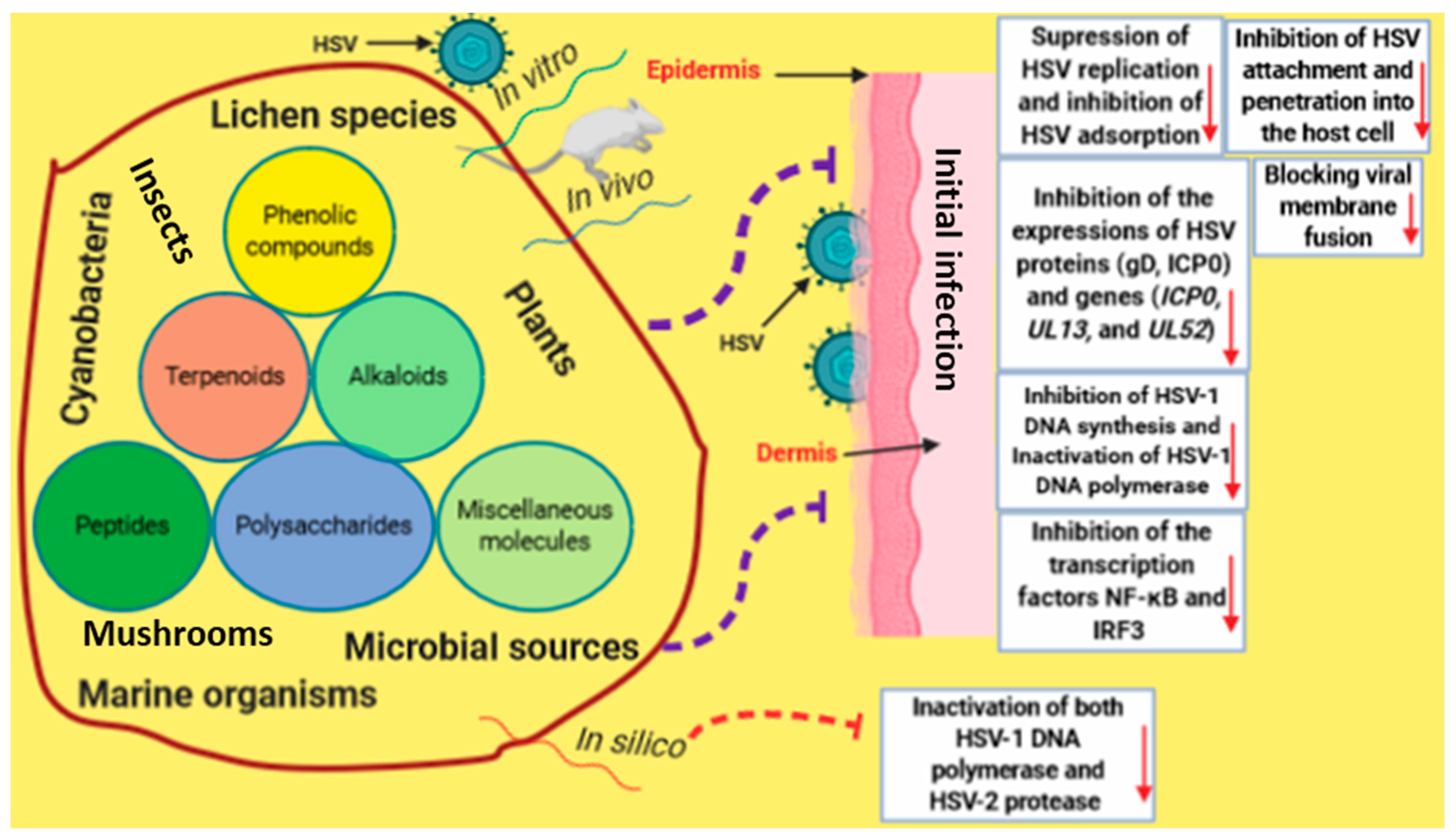
3. Natural Products-Derived Molecules with Anti-HSV-1 and Anti-HSV-2 Properties
4. Natural Products Targeting Enzymes Implicated in HSV Replication
5. General Discussion
6. Take-Home Messages
7. Concluding Remarks and Future Insights
Author Contributions
Funding
Conflicts of Interest
References
- Parker, F.; Nye, R.N. Studies on Filterable Viruses: II. Cultivation of Herpes Virus. Am J Pathol. 1925, 1, 337–340. [Google Scholar]
- Nahmias, A.J.; Dowdle, W.R. Antigenic and biologic differences in herpesvirus hominis. Prog. Med. Virol. 1968, 10, 110–159. [Google Scholar]
- Sanders, J.E.; Garcia, S.E. Pediatric herpes simplex virus infections: An evidence-based approach to treatment. Pediatr. Emerg. Med. Pract. 2014, 11, 1–19. [Google Scholar]
- Miller, A.S.; Bennett, J.S. Challenges in the care of young infants with suspected neonatal herpes simplex virus. Hosp. Pediatr. 2015, 5, 106–108. [Google Scholar] [CrossRef]
- Widener, R.W.; Whitley, R.J. Herpes simplex virus. Handb. Clin. Neurol. 2014, 123, 251–263. [Google Scholar]
- Akinyi, B.; Odhiambo, C.; Otieno, F.; Inzaule, S.; Oswago, S.; Kerubo, E.; Ndivo, R.; Zeh, C. Prevalence, incidence and correlates of HSV-2 infection in an HIV incidence adolescent and adult cohort study in western Kenya. PLoS ONE. 2017, 12, e017890. [Google Scholar] [CrossRef]
- Memish, Z.A.; Almasri, M.; Chentoufi, A.A.; Al-Tawfiq, J.A.; Al-Shangiti, A.M.; Al-Kabbani, K.M.; Otaibi, B.; Assirri, A.; Yezli, S. Seroprevalence of Herpes Simplex Virus Type 1 and Type 2 and Coinfection with HIV and Syphilis: The First National Seroprevalence Survey in Saudi Arabia. Sex. Trans. Dis. 2015, 42, 526–532. [Google Scholar] [CrossRef]
- Birkmann, A.; Zimmermann, H. HSV antivirals - current and future treatment options. Curr. Opin. Virol. 2016, 18, 9–13. [Google Scholar] [CrossRef]
- Kenny, K.; Leung, W.; Stephanson, K.; Ross, S. Clinical practice in prevention of neonatal HSV infection: A survey of obstetrical care providers in Alberta. J. Obstet. Gynaecol. Can. 2013, 35, 131–137. [Google Scholar] [CrossRef]
- Johnston, C.; Koelle, D.M.; Wald, A. Current status and prospects for development of an HSV vaccine. Vaccine. 2014, 32, 1553–1560. [Google Scholar] [CrossRef]
- Zhu, X.P.; Muhammad, Z.S.; Wang, J.G.; Lin, W.; Guo, S.K.; Zhang, W. HSV-2 vaccine: current status and insight into factors for developing an efficient vaccine. Viruses. 2014, 6, 371–390. [Google Scholar] [CrossRef]
- Hassan, S.T.S.; Šudomová, M.; Masarčíková, R. Herpes simplex virus infection: an overview of the problem, pharmacologic therapy and dietary measures. Ceska Slov. Farm. 2017, 66, 95–102. [Google Scholar]
- Knipe, D.M.; Cliffe, A. Chromatin control of herpes simplex virus lytic and latent infection. Nat. Rev. Microbiol. 2008, 6, 211–221. [Google Scholar] [CrossRef]
- Roizman, B.; Whitley, R.J. An inquiry into the molecular basis of HSV latency and reactivation. Annu. Rev. Microbiol. 2013, 67, 355–374. [Google Scholar] [CrossRef]
- Cliffe, A.R.; Garber, D.A.; Knipe, D.M. Transcription of the herpes simplex virus latency-associated transcript promotes the formation of facultative heterochromatin on lytic promoters. J. Virol. 2009, 83, 8182–8190. [Google Scholar] [CrossRef]
- Cliffe, A.R.; Arbuckle, J.H.; Vogel, J.L.; Geden, M.J.; Rothbart, S.B.; Cusack, C.L.; Strahl, B.D.; Kristie, T.M.; Deshmukh, M. Neuronal Stress Pathway Mediating a Histone Methyl/Phospho Switch is Required for Herpes Simplex Virus Reactivation. Cell Host. Microbe. 2015, 18, 649–658. [Google Scholar] [CrossRef]
- Johnston, C.; Corey, L. Current Concepts for Genital Herpes Simplex Virus Infection: Diagnostics and Pathogenesis of Genital Tract Shedding. Clin. Microbiol. Rev. 2016, 29, 149–161. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, Y.; Li, Q. Characteristics of herpes simplex virus infection and pathogenesis suggest a strategy for vaccine development. Rev. Med. Virol. 2019, 29, 2054. [Google Scholar] [CrossRef]
- Nicoll, M.P.; Proença, J.T.; Efstathiou, S. The molecular basis of herpes simplex virus latency. FEMS Microbiol. Rev. 2012, 36, 684–705. [Google Scholar] [CrossRef]
- Mancini, M.; Vidal, S.M. Insights into the pathogenesis of herpes simplex encephalitis from mouse models. Mamm. Genome. 2018, 29, 425–445. [Google Scholar] [CrossRef]
- Egan, K.P.; Wu, S.; Wigdahl, B.; Jennings, S.R. Immunological control of herpes simplex virus infections. J. Neurovirol. 2013, 19, 328–345. [Google Scholar] [CrossRef] [PubMed]
- Vlietinck, A.J.; De Bruyne, T.; Vanden Berghe, D.A. Plant substances as antiviral agents. Curr. Org. Chem. 1997, 1, 307–344. [Google Scholar]
- Cheng, C.-L.; Xu, H.-X. Antiviral agents from traditional Chinese medicine against herpes simplex virus. J. Trad. Med. 2005, 22, 133–137. [Google Scholar]
- Chattopadhyay, D. Ethnomedicinal antivirals: scope and opportunity. In Modern Phytomedicine: Turning Medicinal Plants into Drugs; Ahmad, I., Aqil, F., Owais, M., Eds.; Wiley-VCH Verlag GmbH & Co: Weinheim, Germany, 2006; pp. 313–339. [Google Scholar]
- Hassan, S.T.; Masarčíková, R.; Berchová, K. Bioactive natural products with anti-herpes simplex virus properties. J. Pharm. Pharmacol. 2015, 67, 1325–1336. [Google Scholar] [CrossRef]
- Savi, L.A.; Barardi, C.R.; Simões, C.M. Evaluation of antiherpetic activity and genotoxic effects of tea catechin derivatives. J. Agric. Food Chem. 2006, 54, 2552–2557. [Google Scholar] [CrossRef]
- Lyu, S.Y.; Rhim, J.Y.; Park, W.B. Antiherpetic activities of flavonoids against herpes simplex virus type 1 (HSV-1) and type 2 (HSV-2) in vitro. Arch. Pharm. Res. 2005, 28, 1293–1301. [Google Scholar] [CrossRef]
- Lee, S.; Lee, H.H.; Shin, Y.S.; Kang, H.; Cho, H. The anti-HSV-1 effect of quercetin is dependent on the suppression of TLR-3 in Raw 264.7 cells. Arch. Pharm. Res. 2017, 40, 623–630. [Google Scholar] [CrossRef]
- Medini, F.; Megdiche, W.; Mshvildadze, V.; Pichette, A.; Legault, J.; St-Gelais, A.; Ksouri, R. Antiviral-guided fractionation and isolation of phenolic compounds from Limonium densiflorum hydroalcoholic extract. C. R. Chim. 2016, 19, 726–732. [Google Scholar] [CrossRef]
- Pradhan, P.; Nguyen, M.L. Herpes simplex virus virucidal activity of MST-312 and epigallocatechin gallate. Virus Res. 2018, 2, 93–98. [Google Scholar] [CrossRef]
- Li, J.J.; Chen, G.D.; Fan, H.X.; Hu, D.; Zhou, Z.Q.; Lan, K.H.; Zhang, H.P.; Maeda, H.; Yao, X.S.; Gao, H. Houttuynoid M, an Anti-HSV Active Houttuynoid from Houttuynia cordata Featuring a Bis-houttuynin Chain Tethered to a Flavonoid Core. J. Nat. Prod. 2017, 80, 3010–3013. [Google Scholar] [CrossRef]
- Li, T.; Liu, L.; Wu, H.; Chen, S.; Zhu, Q.; Gao, H.; Yu, X.; Wang, Y.; Su, W.; Yao, X.; et al. Anti-herpes simplex virus type 1 activity of Houttuynoid A, a flavonoid from Houttuynia cordata Thunb. Antiviral. Res. 2017, 144, 273–280. [Google Scholar] [CrossRef]
- Argenta, D.F.; Silva, I.T.; Bassani, V.L.; Koester, L.S.; Teixeira, H.F.; Simões, C.M. Antiherpes evaluation of soybean isoflavonoids. Arch. Virol. 2015, 160, 2335–2342. [Google Scholar] [CrossRef]
- Čulenová, M.; Sychrová, A.; Hassan, S.T.S.; Berchová-Bímová, K.; Svobodová, P.; Helclová, A.; Michnová, H.; Hošek, J.; Vasilev, H.; Suchý, P.; et al. Multiple In vitro biological effects of phenolic compounds from Morus alba root bark. J. Ethnopharmacol. 2019, 248, 112296. [Google Scholar] [CrossRef]
- Fritz, D.; Venturi, C.R.; Cargnin, S.; Schripsema, J.; Roehe, P.M.; Montanha, J.A.; von Poser, G.L. Herpes virus inhibitory substances from Hypericum connatum Lam., a plant used in southern Brazil to treat oral lesions. J. Ethnopharmacol. 2007, 113, 517–520. [Google Scholar] [CrossRef]
- Ojha, D.; Das, R.; Sobia, P.; Dwivedi, V.; Ghosh, S.; Samanta, A.; Chattopadhyay, D. Pedilanthus tithymaloides Inhibits HSV Infection by Modulating NF-κB Signaling. PLoS ONE. 2015, 10, e0139338. [Google Scholar] [CrossRef]
- De Oliveira, A.; Prince, D.; Lo, C.Y.; Lee, L.H.; Chu, T.C. Antiviral activity of theaflavin digallate against herpes simplex virus type 1. Antiviral. Res. 2015, 118, 56–67. [Google Scholar] [CrossRef]
- Likhitwitayawuid, K.; Chaiwiriya, S.; Sritularak, B.; Lipipun, V. Antiherpetic flavones from the heartwood of Artocarpus gomezianus. Chem. Biodivers. 2006, 3, 1138–1143. [Google Scholar] [CrossRef]
- El-Toumy, S.A.; Saliba, J.Y.; El-Kashak, W.A.; Marty, C.; Bedoux, G.; Bourgougnon, N. Antiviral effect of polyphenol rich plant extracts on herpes simplex virus type 1. Food Sci. Human Wellness. 2018, 7, 91–101. [Google Scholar] [CrossRef]
- Li, Y.; Leung, K.T.; Yao, F.; Ooi, L.S.M.; Ooi, V.E.C. Antiviral flavans from the leaves of Pithecellobium clypearia. J. Nat. Prod. 2006, 69, 833–835. [Google Scholar] [CrossRef]
- Boff, L.; Silva, I.T.; Argenta, D.F.; Farias, L.M.; Alvarenga, L.F.; Pádua, R.M.; Braga, F.C.; Leite, J.P.; Kratz, J.M.; Simões, C.M. Strychnos pseudoquina A. St. Hil.: a Brazilian medicinal plant with promising in vitro antiherpes activity. J. Appl. Microbiol. 2016, 121, 1519–1529. [Google Scholar] [CrossRef]
- Uozaki, M.; Yamasaki, H.; Katsuyama, Y.; Higuchi, M.; Higuti, T.; Koyama, A.H. Antiviral effect of octyl gallate against DNA and RNA viruses. Antiviral Res. 2007, 73, 85–91. [Google Scholar] [CrossRef]
- Kesharwani, A.; Polachira, S.K.; Nair, R.; Mishra, N.N.; Gupta, S.K. Anti-HSV-2 activity of Terminalia chebula Retz extract and its constituents, chebulagic and chebulinic acids. BMC Complement Altern. Med. 2017, 17, 110. [Google Scholar] [CrossRef]
- Lavoie, S.; Côte, I.; Pichette, A.; Gauthier, C.; Quellet, M.; Nagau-Lavoie, F.; Mshvildadze, V.; Legault, J. Chemical composition and anti-herpes simplex virus type 1 (HSV-1) activity of extracts from Cornus canadensis. BMC Complement. Altern. Med. 2017, 17, 123. [Google Scholar] [CrossRef]
- Hassan, S.T.S.; Švajdlenka, E.; Berchová-Bímová, K. Hibiscus sabdariffa L. and Its Bioactive Constituents Exhibit Antiviral Activity against HSV-2 and Anti-enzymatic Properties against Urease by an ESI-MS Based Assay. Molecules. 2017, 22, 722. [Google Scholar] [CrossRef]
- Hassan, S.T.S.; Šudomová, M.; Berchová-Bímová, K.; Šmejkal, K.; Echeverría, J. Psoromic Acid, a Lichen-Derived Molecule, Inhibits the Replication of HSV-1 and HSV-2, and Inactivates HSV-1 DNA Polymerase: Shedding Light on Antiherpetic Properties. Molecules. 2019, 24, 2912. [Google Scholar] [CrossRef]
- Thongchuai, B.; Tragoolpua, Y.; Sangthong, P.; Trisuwan, K. Antiviral carboxylic acids and naphthoquinones from the stems of Rhinacanthus nasutus. Tetrahedron Lett. 2015, 56, 5161–5163. [Google Scholar] [CrossRef]
- He, Y.C.; Lu, Z.H.; Shi, P.; Hao, J.C.; Zhao, Z.J.; Xie, H.T.; Mao, P.; Chen, S.J. Anti-herpes simplex virus activities of bioactive extracts from Antrodia camphorata mycelia. Antivir. Ther. 2016, 21, 377–383. [Google Scholar] [CrossRef]
- Huang, Z.; Nong, X.; Ren, Z.; Wang, J.; Zhang, X.; Qi, S. Anti-HSV-1, antioxidant and antifouling phenolic compounds from the deep-sea-derived fungus Aspergillus versicolor SCSIO 41502. Bioorg. Med. Chem. Lett. 2017, 27, 787–791. [Google Scholar] [CrossRef]
- Ma, F.; Shen, W.; Zhang, X.; Li, M.; Wang, Y.; Zou, Y.; Li, Y.; Wang, H. Anti-HSV Activity of Kuwanon X from Mulberry Leaves with Genes Expression Inhibitory and HSV-1 Induced NF-κB Deactivated Properties. Biol. Pharm. Bull. 2016, 39, 1667–1674. [Google Scholar] [CrossRef]
- Cavalcanti, J.F.; de Araujo, M.F.; Gonçalves, P.B.; Romeiro, N.C.; Villela Romanos, M.T.; Curcino Vieira, I.J.; Braz-Filho, R.; de Carvalho, M.G.; Sanches, M.N.G. Proposed anti-HSV compounds isolated from Simira species. Nat. Prod. Res. 2018, 32, 2720–2723. [Google Scholar] [CrossRef]
- Flores, D.J.; Lee, L.H.; Adams, S.D. Inhibition of Curcumin-Treated Herpes Simplex Virus 1 and 2 in Vero Cells. Adv. Microbiol. 2016, 06, 276–287. [Google Scholar] [CrossRef]
- Rajtar, B.; Skalicka-Woźniak, K.; Świątek, Ł.; Stec, A.; Boguszewska, A.; Polz-Dacewicz, M. Antiviral effect of compounds derived from Angelica archangelica L. on Herpes simplex virus-1 and Coxsackievirus B3 infections. Food Chem. Toxicol. 2017, 109, 1026–1031. [Google Scholar]
- Benzekri, R.; Bouslama, L.; Papetti, A.; Hammami, M.; Smaoui, A.; Limam, F. Anti HSV-2 activity of Peganum harmala (L.) and isolation of the active compound. Microb. Pathog. 2018, 114, 291–298. [Google Scholar] [CrossRef]
- Hutterer, C.; Milbradt, J.; Hamilton, S.; Zaja, M.; Leban, J.; Henry, C.; Vitt, D.; Steingruber, M.; Sonntag, E.; Zeitträger, I.; et al. Inhibitors of dual-specificity tyrosine phosphorylation-regulated kinases (DYRK) exert a strong anti-herpesviral activity. Antiviral. Res. 2017, 143, 113–121. [Google Scholar] [CrossRef]
- Zalilawati, M.R.; Andriani, Y.; Shaari, K.; Bourgougnon, N.; Ali, A.M.; Muhammad, T.S.T.; Mohamad, H. Induction of apoptosis and anti HSV-1 activity of 3-(Phenethylamino) demethyl(oxy)aaptamine from a Malaysian Aaptos aaptos. J. Chem. Pharm. Res. 2015, 7, 330–341. [Google Scholar]
- Hassan, S.T.S.; Berchová-Bímová, K.; Šudomová, M.; Malaník, M.; Šmejkal, K.; Rengasamy, K.R.R. In Vitro Study of Multi-Therapeutic Properties of Thymus bovei Benth. Essential Oil and Its Main Component for Promoting Their Use in Clinical Practice. J. Clin. Med. 2018, 7, 283. [Google Scholar] [CrossRef]
- Brezáni, V.; Leláková, V.; Hassan, S.T.S.; Berchová-Bímová, K.; Nový, P.; Klouček, P.; Maršík, P.; Dall’Acqua, S.; Hošek, J.; Šmejkal, K. Anti-Infectivity against Herpes Simplex Virus and Selected Microbes and Anti-Inflammatory Activities of Compounds Isolated from Eucalyptus globulus Labill. Viruses. 2018, 10, 360. [Google Scholar] [CrossRef]
- Liao, H.B.; Huang, G.H.; Yu, M.H.; Lei, C.; Hou, A.J. Five Pairs of Meroterpenoid Enantiomers from Rhododendron capitatum. J. Org. Chem. 2017, 82, 1632–1637. [Google Scholar] [CrossRef]
- Cagno, V.; Sgorbini, B.; Sanna, C.; Cagliero, C.; Ballero, M.; Civra, A.; Donalisio, M.; Bicchi, C.; Lembo, D.; Rubiolo, P. In vitro anti-herpes simplex virus-2 activity of Salvia desoleana Atzei & V. Picci essential oil. PLoS ONE 2017, 12, e0172322. [Google Scholar]
- Ghannadi, A.; Fattahian, K.; Shokoohinia, Y.; Behbahani, M.; Shahnoush, A. Anti-Viral Evaluation of Sesquiterpene Coumarins from Ferula assa-foetida against HSV-1. Iran. J. Pharm. Res. 2014, 13, 523–530. [Google Scholar]
- Krawczyk, E.; Łuczak, M.; Kobus, M.; Bańka, D.; Daniewski, W. Antiviral Activity of N-Benzoylphenylisoserinates of Lactarius Sesquiterpenoid Alcohols in vitro. Planta Med. 2003, 69, 552–554. [Google Scholar] [PubMed]
- Rezeng, C.; Yuan, D.; Long, J.; Suonan, D.; Yang, F.; Li, W.; Tong, L.; Jiumei, P. Alantolactone exhibited anti-herpes simplex virus 1 (HSV-1) action in vitro. Biosci. Trends. 2015, 9, 420–422. [Google Scholar] [CrossRef] [PubMed]
- Tsai, Y.C.; Cheng, Y.B.; Lo, I.W.; Cheng, H.H.; Lin, C.J.; Hwang, T.L.; Kuo, Y.C.; Liou, S.S.; Huang, Y.Z.; Kuo, Y.H.; et al. Seven new sesquiterpenoids from the fruits of Schisandra sphenanthera. Chem. Biodivers. 2014, 11, 1053–1068. [Google Scholar] [CrossRef] [PubMed]
- Rédei, D.; Kúsz, N.; Rafai, T.; Bogdanov, A.; Burián, K.; Csorba, A.; Mándi, A.; Kurtán, T.; Vasas, A.; Hohmann, J. 14-Noreudesmanes and a phenylpropane heterodimer from sea buckthorn berry inhibit Herpes simplex type 2 virus replication. Tetrahedron. 2019, 75, 1364–1370. [Google Scholar] [CrossRef]
- Zhang, L.B.; Liao, H.B.; Zhu, H.Y.; Yu, M.H.; Lei, C.; Hou, A.J. Antiviral clerodane diterpenoids from Dodonaea viscosa. Tetrahedron. 2016, 72, 8036–8041. [Google Scholar] [CrossRef]
- Soares, A.R.; Abrantes, J.L.; Lopes Souza, T.M.; Leite Fontes, C.F.; Pereira, R.C.; de Palmer Paixão Frugulhetti, I.C.; Teixeira, V.L. In vitro antiviral effect of meroditerpenes isolated from the Brazilian seaweed Stypopodium zonale (Dictyotales). Planta Med. 2007, 73, 1221–1224. [Google Scholar] [CrossRef]
- Krawczyk, E.; Luczak, M.; Kniotek, M.; Nowaczyk, M. Cytotoxic, antiviral (in-vitro and in-vivo), immunomodulatory activity and influence on mitotic divisions of three taxol derivatives: 10-Deacetyl-baccatin III, methyl (N-benzoyl-(2′R,3′S)-3′-phenylisoserinate) and N-benzoyl-(2′R,3′S)-3′-phenylisoserine. J. Pharm. Pharmacol. 2005, 57, 791–797. [Google Scholar] [CrossRef]
- Wiart, C.; Kumar, K.; Yusof, M.Y.; Hamimah, H.; Fauzi, Z.M.; Sulaiman, M. Antiviral properties of ent-labdene diterpenes of Andrographis paniculata nees, inhibitors of herpes simplex virus type 1. Phytother. Res. 2005, 19, 1069–1070. [Google Scholar] [CrossRef]
- Barbosa, J.P.; Pereira, R.C.; Abrantes, J.L.; Cirne dos Santos, C.C.; Rebello, M.A.; Frugulhetti, I.C.; Texeira, V.L. In vitro antiviral diterpenes from the Brazilian brown alga Dictyota pfaffii. Planta Med. 2004, 70, 856–860. [Google Scholar] [CrossRef]
- Isaka, M.; Chinthanom, P.; Srichomthong, K.; Thummarukcharoen, T. Lanostane triterpenoids from fruiting bodies of the bracket fungus Fomitopsis feei. Tetrahedron Lett. 2017, 58, 1758–1761. [Google Scholar] [CrossRef]
- Lv, X.J.; Li, Y.; Ma, S.G.; Qu, J.; Liu, Y.B.; Li, Y.H.; Zhang, D.; Li, L.; Yu, S.S. Antiviral Triterpenes from the Twigs and Leaves of Lyonia ovalifolia. J. Nat. Prod. 2016, 79, 2824–2837. [Google Scholar] [CrossRef] [PubMed]
- Hassan, S.T.S.; Berchová-Bímová, K.; Petráš, J.; Hassan, K.T.S. Cucurbitacin B interacts synergistically with antibiotics against Staphylococcus aureus clinical isolates and exhibits antiviral activity against HSV-1. S. Afr. J. Bot. 2017, 108, 90–94. [Google Scholar] [CrossRef]
- Da Rosa Guimarães, T.; Quiroz, C.G.; Borges, C.R.; de Oliveira, S.Q.; de Almeida, M.T.; Bianco, É.M.; Moritz, M.I.; Carraro, J.L.; Palermo, J.A.; Cabrera, G.; et al. Anti HSV-1 activity of halistanol sulfate and halistanol sulfate C isolated from Brazilian marine sponge Petromica citrina (Demospongiae). Mar. Drugs. 2013, 11, 4176–4192. [Google Scholar] [CrossRef] [PubMed]
- Laconi, S.; Madeddu, M.A.; Pompei, R. Autophagy activation and antiviral activity by a licorice triterpene. Phytother. Res. 2014, 28, 1890–1892. [Google Scholar] [CrossRef]
- Ikeda, T.; Yokomizo, K.; Okawa, M.; Tsuchihashi, R.; Kinjo, J.; Nohara, T.; Uyeda, M. Anti-herpes virus type 1 activity of oleanane-type triterpenoids. Biol. Pharm. Bull. 2005, 28, 1779–1781. [Google Scholar] [CrossRef]
- Li, Y.; Jiang, R.; Ooi, L.S.; But, P.P.; Ooi, V.E. Antiviral triterpenoids from the medicinal plant Schefflera heptaphylla. Phytother. Res. 2007, 21, 466–470. [Google Scholar] [CrossRef]
- Mukherjee, H.; Ojha, D.; Bag, P.; Chandel, H.S.; Bhattacharyya, S.; Chatterjee, T.K.; Mukherjee, P.K.; Chakraborti, S.; Chattopadhyay, D. Anti-herpes virus activities of Achyranthes aspera: an indian ethnomedicine, and its triterpene acid. Microbiol. Res. 2013, 168, 238–244. [Google Scholar] [CrossRef]
- Zhou, M.; Xu, M.; Ma, X.X.; Zheng, K.; Yang, K.; Yang, C.R.; Wang, Y.F.; Zhang, Y.J. Antiviral triterpenoid saponins from the roots of Ilex asprella. Planta Med. 2012, 78, 1702–1705. [Google Scholar] [CrossRef]
- Liu, F.; Wang, Y.-N.; Li, Y.; Ma, S.-G.; Qu, J.; Liu, Y.-B.; Niu, C.-S.; Tang, Z.H.; Li, Y.-H.; Li, L.; et al. Triterpenoids from the twigs and leaves of Rhododendron latoucheae by HPLC‒MS‒SPE‒NMR. Tetrahedron. 2019, 75, 296–307. [Google Scholar] [CrossRef]
- Sun, Y.L.; Wang, J.; Wang, Y.F.; Zhang, X.Y.; Nong, X.H.; Chen, M.Y.; Xu, X.; Qi, S.H. Cytotoxic and Antiviral Tetramic Acid Derivatives from the Deep-Sea-Derived Fungus Trichobotrys effuse DFFSCS021. Tetrahedron. 2015, 71, 9328–9332. [Google Scholar] [CrossRef]
- Álvarez, Á.L.; Habtemariam, S.; Abdel Moneim, A.E.; Melón, S.; Dalton, K.P.; Parra, F. A spiroketal-enol ether derivative from Tanacetum vulgare selectively inhibits HSV-1 and HSV-2 glycoprotein accumulation in Vero cells. Antiviral Res. 2015, 119, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Pongmuangmul, S.; Phumiamorn, S.; Sanguansermsri, P.; Wongkattiya, N.; Fraser, I.H.; Sanguansermsri, D. Anti-herpes simplex virus activities of monogalactosyl diglyceride and digalactosyl diglyceride from Clinacanthus nutans, a traditional Thai herbal medicine. Asian Pac. J. Trop. Biomed. 2016, 6, 192–197. [Google Scholar] [CrossRef]
- Ma, F.W.; Kong, S.Y.; Tan, H.S.; Wu, R.; Xia, B.; Zhou, Y.; Xu, H.X. Structural characterization and antiviral effect of a novel polysaccharide PSP-2B from Prunellae spica. Carbohydr. Polym. 2016, 152, 699–709. [Google Scholar] [CrossRef]
- Jin, F.; Zhuo, C.; He, Z.; Wang, H.; Liu, W.; Zhang, R.; Wang, Y. Anti-herpes simplex virus activity of polysaccharides from Eucheuma gelatinae. World J. Microbiol. Biotechnol. 2015, 31, 453–460. [Google Scholar] [CrossRef]
- Sahera, F.M.; Mohsen, M.S.A.; El-Sayed, O.H. Chemical structure and antiviral activity of sulfated polysaccharides from Surgassium latifolium. In Proceedings of the Medical Research Day, Faculty of Medicine, Jazan University, Al Maarefah Rd, Jazan, Saudi Arabia, June 2011. [Google Scholar]
- Zhu, W.; Chiu, L.C.; Ooi, V.E.; Chan, P.K.; Ang, P.O., Jr. Antiviral property and mechanisms of a sulphated polysaccharide from the brown alga Sargassum patens against Herpes simplex virus type 1. Phytomedicine. 2006, 13, 695–701. [Google Scholar] [CrossRef]
- Lee, J.-B.; Takeshita, A.; Hayashi, K.; Hayashi, T. Structures and antiviral activities of polysaccharides from Sargassum trichophyllum. Carbohydr. Polym. 2001, 86, 995–999. [Google Scholar] [CrossRef]
- Bedoux, G.; Caamal-Fuentes, E.; Boulho, R.; Marty, C.; Bourgougnon, N.; Freile-Pelegrín, Y.; Robledo, D. Antiviral and Cytotoxic Activities of Polysaccharides Extracted from Four Tropical Seaweed Species. Nat. Prod. Commun. 2017, 12, 807–811. [Google Scholar] [CrossRef]
- Hardouin, K.; Bedoux, G.; Burlot, A.-S.; Donnay-Moreno, C.; Bergé, J.-P.; Nyvall-Collén, P.; Bourgougnon, N. Enzyme-assisted extraction (EAE) for the production of antiviral and antioxidant extracts from the green seaweed Ulva armoricana (Ulvales, Ulvophyceae). Algal Res. 2016, 16, 233–239. [Google Scholar] [CrossRef]
- Vanderlei, E.; Eloy, Y.; de Araújo, I.; Quinderé, A.; Fontes, B.; Mendes, G.; Cavalcanti, J.; Romanos, M.; Benevides, N. Structural features, molecular weight and anti-HSV activity of sulfated polysaccharides from three red seaweeds. J. Chem. Pharm. Res. 2016, 8, 164–170. [Google Scholar]
- Bouhlal, R.; Haslin, C.; Chermann, J.-C.; Colliec-Jouault, S.; Sinquin, C.; Simon, G.; Cerantola, S.; Riadi, H.; Bourgougnon, N. Antiviral Activities of Sulfated Polysaccharides Isolated from Sphaerococcus coronopifolius (Rhodophytha, Gigartinales) and Boergeseniella thuyoides (Rhodophyta, Ceramiales). Marine Drugs. 2011, 9, 1187–1209. [Google Scholar] [CrossRef]
- Saha, S.; Navidb, M.H.; Bandyopahyay, S.S.; Schitzlerb, P.; Ray, B. Sulfated polysaccharides from Laminaria angustata: Structural features and in vitro antiviral activities. Carbohydr. Polym. 2012, 87, 123–130. [Google Scholar] [CrossRef]
- Lopes, N.; Ray, S.; Espada, S.F.; Bomfim, W.A.; Ray, B.; Faccin-Galhardi, L.C.; Linhares, R.E.C.; Nozawa, C. Green seaweed Enteromorpha compressa (Chlorophyta, Ulvaceae) derived sulphated polysaccharides inhibit herpes simplex virus. Int. J. Biol. Macromol. 2017, 102, 605–612. [Google Scholar] [CrossRef] [PubMed]
- Karmakar, P.; Pujol, C.A.; Damonte, E.B.; Ghosh, T.; Ray, B. Polysaccharides from Padina tetrastromatica: Structural features, chemical modification and antiviral activity. Carbohydr. Polym. 2010, 80, 513–520. [Google Scholar] [CrossRef]
- Adhikari, U.; Mateu, C.G.; Chattopadhyay, K.; Pujol, C.A.; Damonte, E.B.; Ray, B. Structure and antiviral activity of sulfated fucans from Stoechospermum marginatum. Phytochemistry. 2006, 67, 2474–2482. [Google Scholar] [CrossRef]
- Mandal, P.; Mateu, C.G.; Chattopadhyay, K.; Pujol, C.A.; Damonte, E.B.; Ray, B. Structural features and antiviral activity of sulphated fucans from the brown seaweed Cystoseira indica. Antivir. Chem. Chemother. 2007, 18, 153–162. [Google Scholar] [CrossRef]
- Lee, J.B.; Hayashi, K.; Hashimoto, M.; Nakano, T.; Hayashi, T. Novel antiviral fucoidan from sporophyll of Undaria pinnatifida (Mekabu). Chem. Pharm. Bull. 2004, 52, 1091–1094. [Google Scholar] [CrossRef]
- Chattopadhyay, K.; Mateu, C.G.; Mandal, P.; Pujol, C.A.; Damonte, E.B.; Ray, B. Galactan sulfate of Grateloupia indica: Isolation, structural features and antiviral activity. Phytochemistry. 2007, 68, 1428–1435. [Google Scholar] [CrossRef]
- Matsuhiro, B.; Conte, A.F.; Damonte, E.B.; Kolender, A.A.; Matulewicz, M.C.; Mejías, E.G.; Pujol, C.A.; Zúñiga, E.A. Structural analysis and antiviral activity of a sulfated galactan from the red seaweed Schizymenia binderi (Gigartinales, Rhodophyta). Carbohydr. Res. 2005, 340, 2392–2402. [Google Scholar] [CrossRef]
- Carlucci, M.J.; Pujol, C.A.; Ciancia, M.; Noseda, M.D.; Matulewicz, M.C.; Damonte, E.B.; Cerezo, A.S. Antiherpetic and anticoagulant properties of carrageenans from the red seaweed Gigartina skottsbergii and their cyclized derivatives: correlation between structure and biological activity. Int. J. Biol. Macromol. 1997, 20, 97–105. [Google Scholar] [CrossRef]
- Li, Z.; Liu, J.; Zhao, Y. Possible mechanism underlying the antiherpetic activity of a proteoglycan isolated from the mycelia of Ganoderma lucidum in vitro. J. Biochem. Mol. Biol. 2005, 38, 34–40. [Google Scholar] [CrossRef]
- Dong, C.X.; Hayashi, K.; Lee, J.B.; Hayashi, T. Characterization of structures and antiviral effects of polysaccharides from Portulaca oleracea L. Chem. Pharm. Bull. 2010, 58, 507–510. [Google Scholar] [CrossRef] [PubMed]
- Lopes, N.; Faccin-Galhardi, L.C.; Espada, S.F.; Pacheco, A.C.; Ricardo, N.M.; Linhares, R.E.; Nozawa, C. Sulfated polysaccharide of Caesalpinia ferrea inhibits herpes simplex virus and poliovirus. Int. J. Biol. Macromol. 2013, 60, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.B.; Tanikawa, T.; Hayashi, K.; Asagi, M.; Kasahara, Y.; Hayashi, T. Characterization and biological effects of two polysaccharides isolated from Acanthopanax sciadophylloides. Carbohydr. Polym. 2015, 116, 159–166. [Google Scholar] [CrossRef]
- Kanekiyo, K.; Lee, J.B.; Hayashi, K.; Takenaka, H.; Hayakawa, Y.; Endo, S.; Hayashi, T. Isolation of an antiviral polysaccharide, nostoflan, from a terrestrial cyanobacterium, Nostoc flagelliforme. J. Nat. Prod. 2005, 68, 1037–1041. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, P.; Adhikari, U.; Ghosal, P.K.; Pujol, C.A.; Carlucci, M.J.; Damonte, E.B.; Ray, B. In vitro anti-herpetic activity of sulfated polysaccharide fractions from Caulerpa racemosa. Phytochemistry. 2004, 65, 3151–3157. [Google Scholar] [CrossRef]
- Cavicchioli, V.Q.; Carvalho, O.V.; Paiva, J.C.; Todorov, S.D.; Silva Júnior, A.; Nero, L.A. Inhibition of herpes simplex virus 1 (HSV-1) and poliovirus (PV-1) by bacteriocins from Lactococcus lactis subsp. lactis and Enterococcus durans strains isolated from goat milk. Int. J. Antimicrob. Agents. 2018, 51, 33–37. [Google Scholar]
- Quintana, V.M.; Torres, N.I.; Wachsman, M.B.; Sinko, P.J.; Castilla, V.; Chikindas, M. Antiherpes simplex virus type 2 activity of the antimicrobial peptide subtilosin. J. Appl. Microbiol. 2014, 117, 1253–1259. [Google Scholar] [CrossRef]
- Liang, X.; Nong, X.H.; Huang, Z.H.; Qi, S.H. Antifungal and Antiviral Cyclic Peptides from the Deep-Sea-Derived Fungus Simplicillium obclavatum EIODSF 020. J. Agric. Food Chem. 2017, 65, 5114–5121. [Google Scholar] [CrossRef]
- Ma, X.; Nong, X.-H.; Ren, Z.; Wang, J.; Liang, X.; Wang, L.; Qi, S.-H. Antiviral peptides from marine gorgonian-derived fungus Aspergillus sp. SCSIO 41501. Tetrahedron Lett. 2017, 58, 1151–1155. [Google Scholar] [CrossRef]
- Gong, M.; Piraino, F.; Yan, N.; Zhang, J.; Xia, M.; Ma, J.; Cheng, J.; Liu, X. Purification, partial characterization and molecular cloning of the novel antiviral protein RC28. Peptides. 2009, 30, 654–659. [Google Scholar] [CrossRef]
- Vilas Boas, L.C.P.; de Lima, L.M.P.; Migliolo, L.; Mendes, G.d.S.; de Jesus, M.G.; Franco, O.L.; Silva, P.A. Linear antimicrobial peptides with activity against herpes simplex virus 1 and Aichi virus. Biopolym. 2017, 108, e22871. [Google Scholar] [CrossRef] [PubMed]
- El-Fakharany, E.M.; Uversky, V.N.; Redwan, E.M. Comparative Analysis of the Antiviral Activity of Camel, Bovine, and Human Lactoperoxidases Against Herpes Simplex Virus Type 1. Appl. Biochem. Biotechnol. 2017, 182, 294–310. [Google Scholar] [CrossRef] [PubMed]
- Levendosky, K.; Mizenina, O.; Martinelli, E.; Jean-Pierre, N.; Kizima, L.; Rodriguez, A.; Kleinbeck, K.; Bonnaire, T.; Robbiani, M.; Zydowsky, T.M.; et al. Griffithsin and carrageenan combination to target herpes simplex virus 2 and human papillomavirus. Antimicrob. Agents Chemother. 2015, 59, 7290–7298. [Google Scholar] [CrossRef] [PubMed]
- Albiol Matanic, V.C.; Castilla, V. Antiviral activity of antimicrobial cationic peptides against Junin virus and herpes simplex virus. Int. J. Antimicrob. Agents. 2004, 23, 382–389. [Google Scholar] [CrossRef] [PubMed]
- Waxman, L.; Darke, P.L. The herpesvirus proteases as targets for antiviral chemotherapy. Antivir. Chem. Chemother. 2000, 11, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Reardon, J.E. Herpes simplex virus type 1 DNA polymerase. Mechanism-based affinity chromatography. J. Biol. Chem. 1990, 265, 7112–7115. [Google Scholar] [PubMed]
- Valencia, F.; Veselenak, R.L.; Bourne, N. In vivo evaluation of antiviral efficacy against genital herpes using mouse and guinea pig models. Methods Mol. Biol. 2013, 1030, 315–326. [Google Scholar]
- Osada, N.; Kohara, A.; Yamaji, T.; Hirayama, N.; Kasai, F.; Sekizuka, T.; Kuroda, M.; Hanada, K. The genome landscape of the african green monkey kidney-derived Vero cell line. DNA Res. 2014, 21, 673–683. [Google Scholar] [CrossRef]
- D’Aiuto, L.; Williamson, K.; Dimitrion, P.; McNulty, J.; Brown, C.E.; Dokuburra, C.B.; Nielsen, A.J.; Lin, W.J.; Piazza, P.; Schurdak, M.E.; et al. Comparison of three cell-based drug screening platforms for HSV-1 infection. Antiviral Res. 2017, 142, 136–140. [Google Scholar] [CrossRef]
- Cotarelo, M.; Catalán, P.; Sánchez-Carrillo, C.; Menasalvas, A.; Cercenado, E.; Tenorio, A.; Bouza, E. Cytopathic effect inhibition assay for determining the in-vitro susceptibility of herpes simplex virus to antiviral agents. J. Antimicrob. Chemother. 1999, 44, 705–708. [Google Scholar] [CrossRef]
- Thi, T.N.; Deback, C.; Malet, I.; Bonnafous, P.; Ait-Arkoub, Z.; Agut, H. Rapid determination of antiviral drug susceptibility of herpes simplex virus types 1 and 2 by real-time PCR. Antiviral Res. 2006, 69, 152–157. [Google Scholar] [CrossRef] [PubMed]
- McClain, D.S.; Fuller, A.O. Cell-specific kinetics and efficiency of herpes simplex virus type 1 entry are determined by two distinct phases of attachment. Virology. 1994, 198, 690–702. [Google Scholar] [CrossRef] [PubMed]
- Silva, I.T.; Costa, G.M.; Stoco, P.H.; Schenkel, E.P.; Reginatto, F.H.; Simões, C.M.O. In vitro antiherpes effects of a c-glycosylflavonoid enriched fraction of Cecropia glaziovii Sneth. Lett. Appl. Microbiol. 2010, 51, 143–148. [Google Scholar] [CrossRef]
- Klysik, K.; Pietraszek, A.; Karewicz, A.; Nowakowska, M. Acyclovir in the Treatment of Herpes Viruses—A Review. Curr. Med. Chem. 2018. [Google Scholar] [CrossRef]
- Ouyang, J.; Sun, F.; Feng, W.; Xie, Y.; Ren, L.; Chen, Y. Antimicrobial Activity of Galangin and Its Effects on Murein Hydrolases of Vancomycin-Intermediate Staphylococcus aureus (VISA) Strain Mu50. Chemother. 2018, 63, 20. [Google Scholar] [CrossRef]
- Céliz, G.; Daz, M.; Audisio, M.C. Antibacterial activity of naringin derivatives against pathogenic strains. J. Appl. Microb. 2011, 111, 731. [Google Scholar] [CrossRef]
- Pujol, C.A.; Carlucci, M.J.; Matulewicz, M.C.; Damonte, E.B. Natural sulfated polysaccharides for the prevention and control of viral infections. Top. Heterocycl. Chem. 2007, 11, 259–281. [Google Scholar]
- Choi, J.H.; Jang, A.Y.; Lin, S.; Lim, S.; Kim, D.; Park, K.; Han, S.M.; Yeo, J.H.; Seo, H.S. Melittin, a honeybee venom‑derived antimicrobial peptide, may target methicillin‑resistant Staphylococcus aureus. Mol. Med. Rep. 2015, 12, 6483. [Google Scholar] [CrossRef]
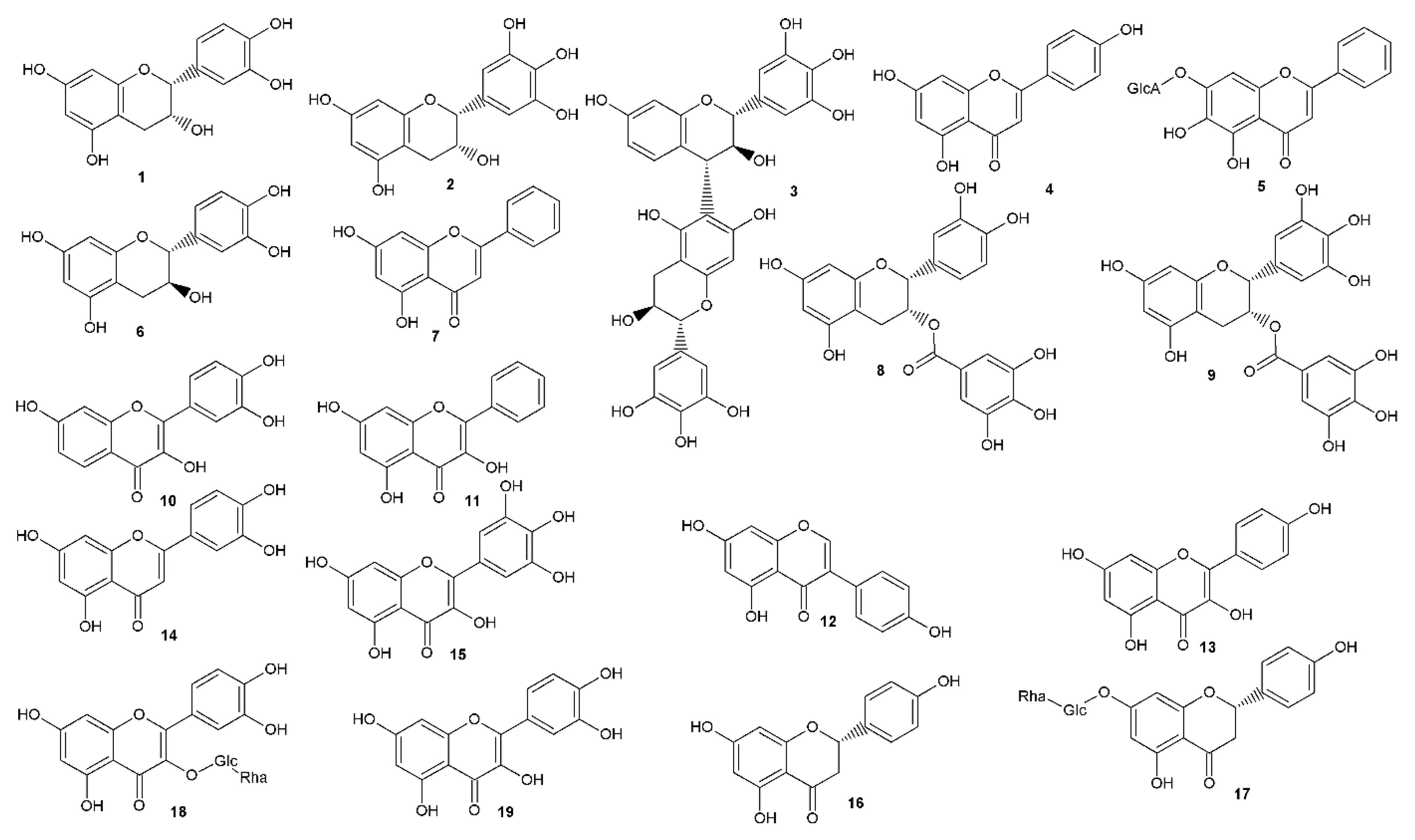
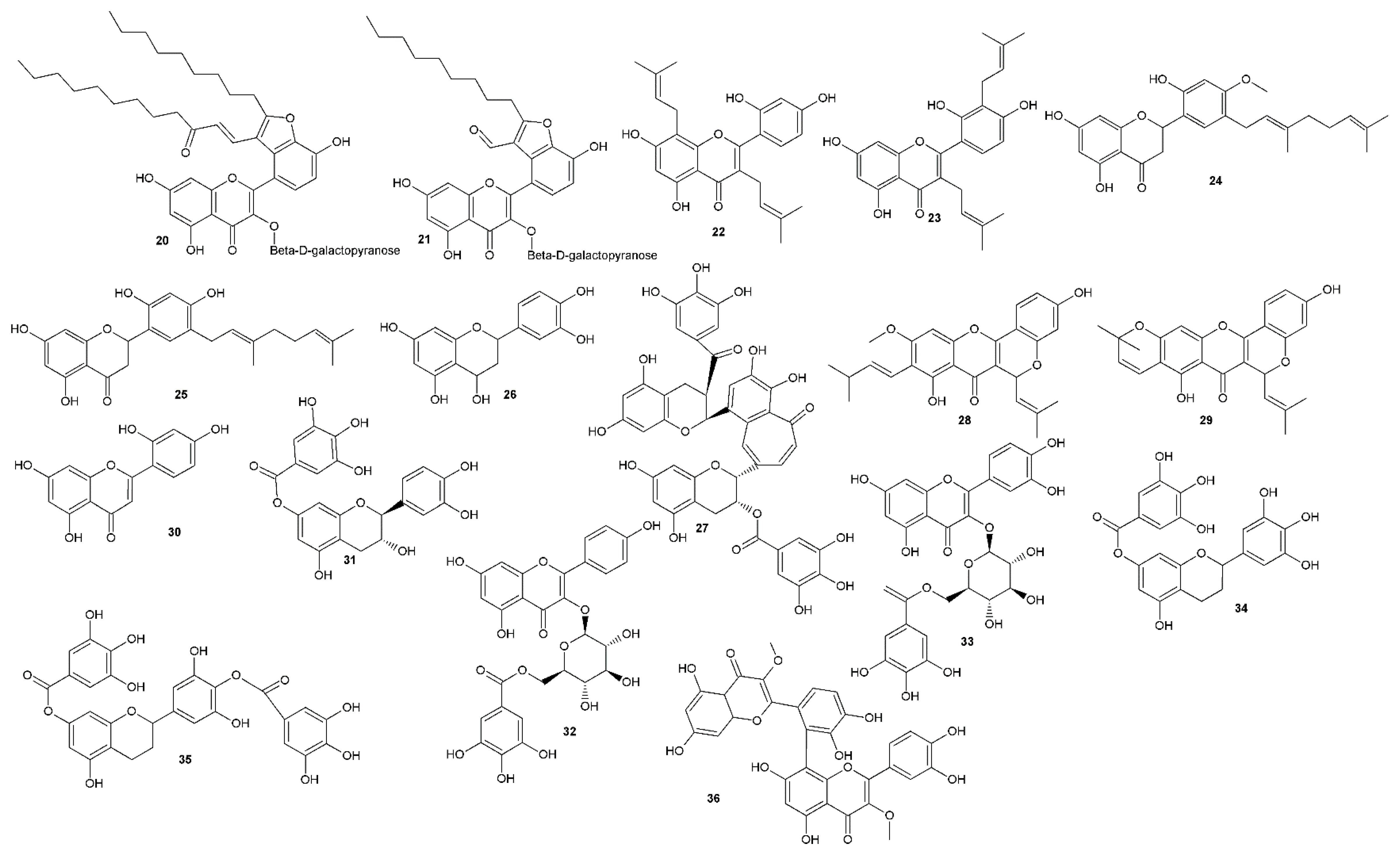
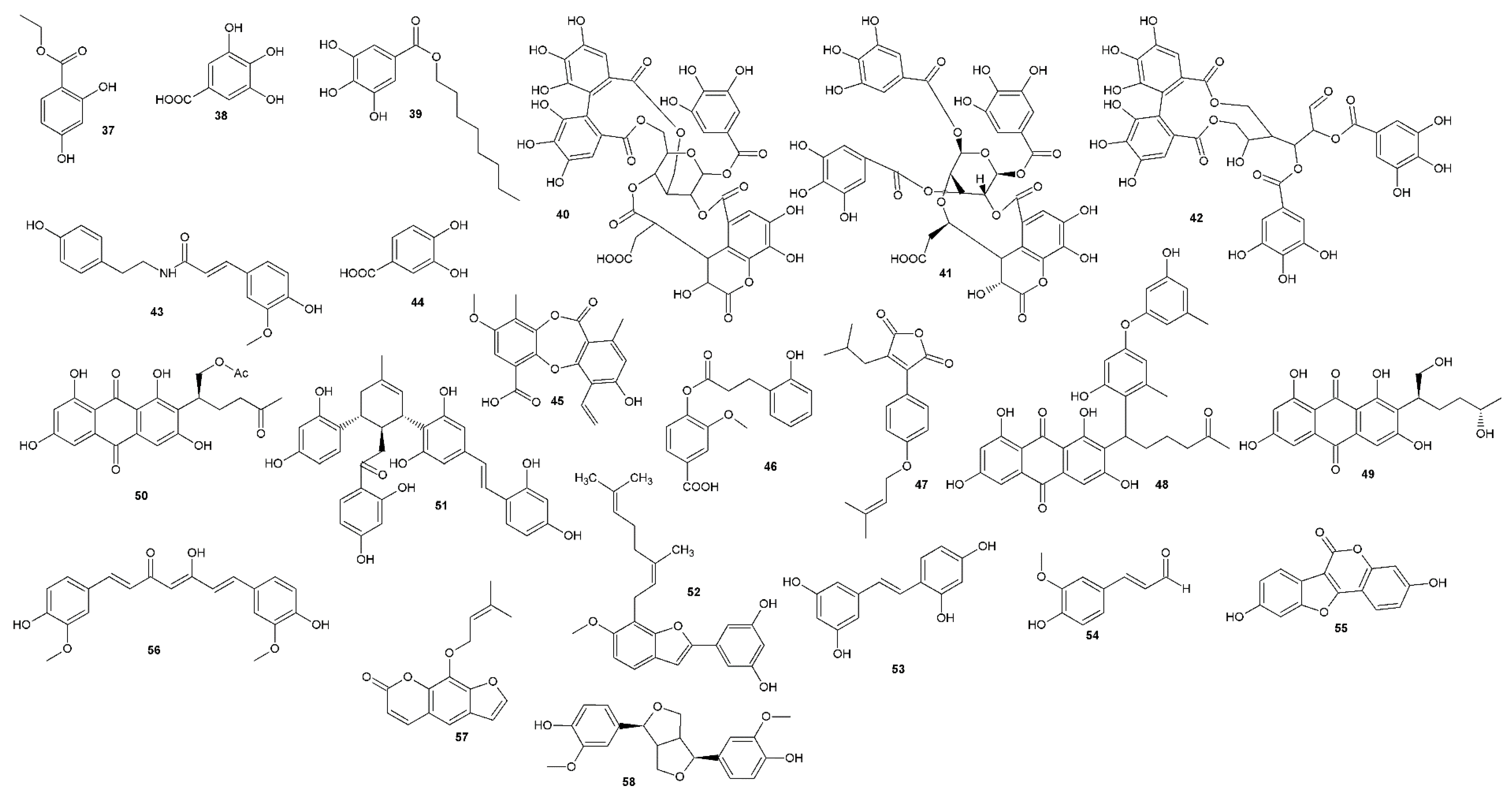
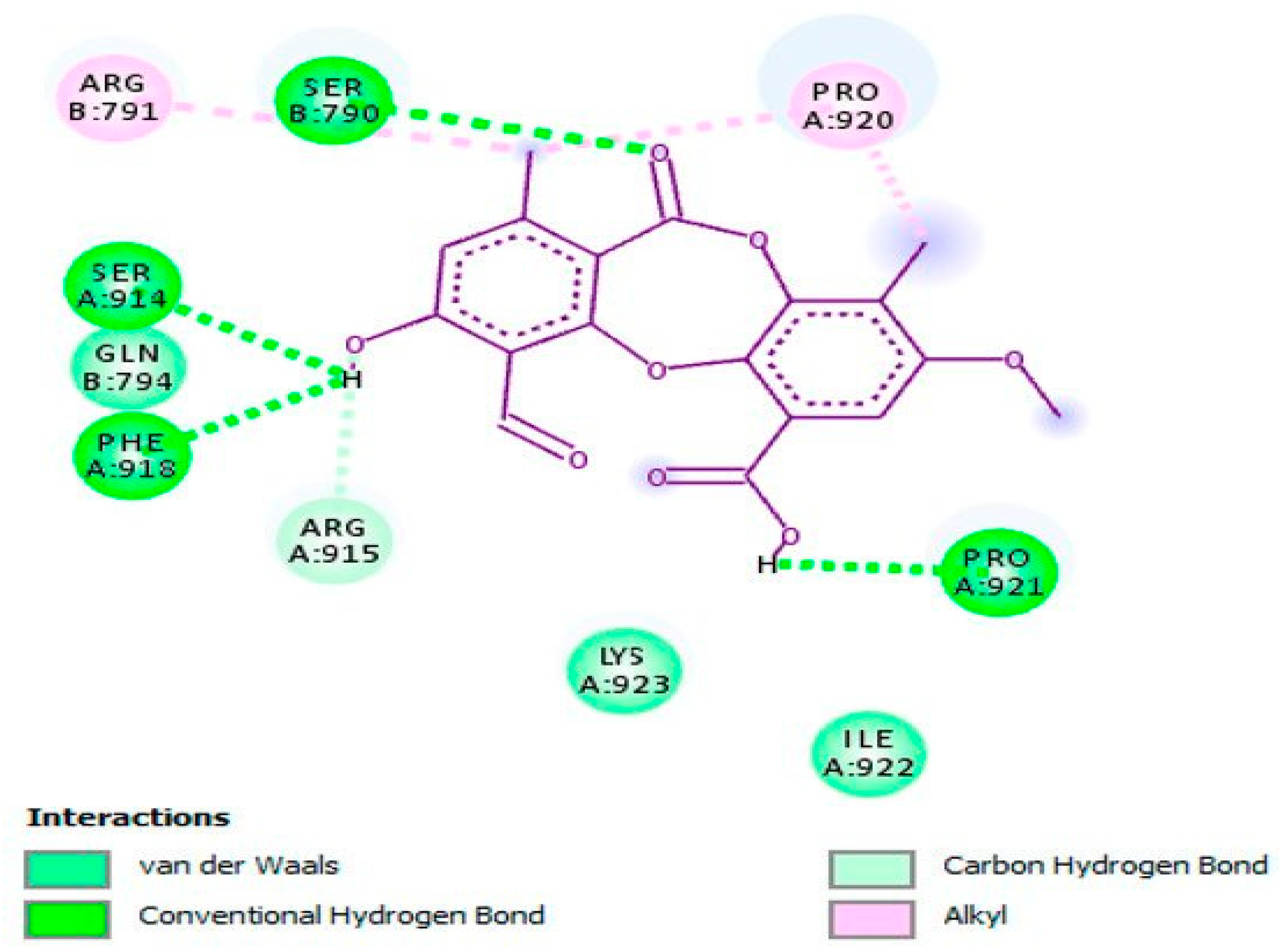
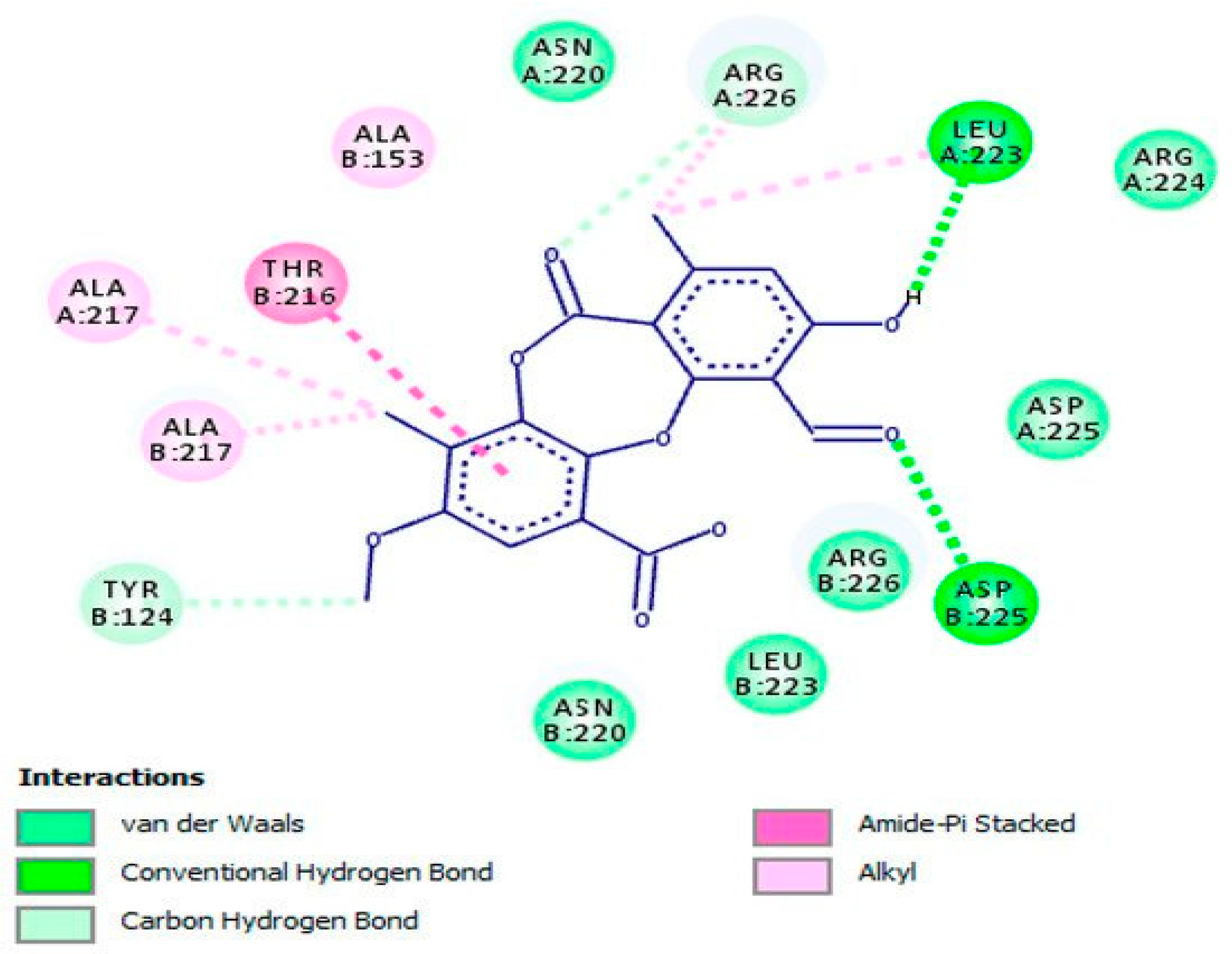
| Compound | Antiherpetic and Cytotoxicity Assays, Strains, Cells, and Reference Agents | Results | Additional Information | Source | |
|---|---|---|---|---|---|
| Flavonoids | Epicatechin (1) | MTT cell viability ACV SI = 4.5 | HSV-1, SI = 6.0 (29-R) | Dietary phenolics | [26] |
| Epigallocatechin (2) | HSV-1, SI = 5.2 (KOS), 12.8 (29-R) | ||||
| Robinetinidol(4α-6)gallocatechin (3) | HSV-1 SI = 5.2 (KOS), 5.0 (29-R) | ||||
| Apigenin (4) | CPE, PRA, YRA ACV for HSV-1 EC50 = 50 μM, SI = 10; HSV-2, EC50 = 50 μM, SI = 10 | HSV-1, EC50 = 5 μM, SI = 50; HSV-2, EC50 N/A, SI N/A | Dietary phenolics, green tea, propolis, some flavonoid rich medicinal plants. Flavanols and flavonols appear to be more active than flavones. Furthermore, treatment of Vero cells with ECG (8) and galangin (11) before virus adsorption led to a slight enhancement of inhibition, indicating that an intracellular effect may be involved. | [27] | |
| Baicalin (5) | HSV-1 EC50 = 5 μM, SI= 200; HSV-2 EC50 N/A, SI N/A | ||||
| Catechin hydrate (6) | HSV-1, EC50 = 4 μM, SI = 250; HSV-2, EC50 N/A, SI N/A | ||||
| Chrysin (7) | HSV-1 EC50 = 2.5 μM, SI= 4; HSV-2 EC50 N/A, SI N/A | ||||
| Epicatechin (1) | HSV-1, EC50 = 2.5 μM, SI = 40; HSV-2, EC50 = 35 μM, SI = 2.9 | ||||
| Epicatechin gallate (8) | HSV-1, EC50 = 4 μM, SI = 125; HSV-2, EC50 = 63 μM, SI = 7.9 | ||||
| Epigallocatechin (2) | HSV-1, EC50 = 2.5 μM, SI = 100; HSV-2, EC50 N/A, SI N/A | ||||
| Epigallocatechin gallate (9) | HSV-1, EC50 = 2.5 μM, SI = 40; HSV-2, EC50 N/A, SI N/A | ||||
| Fisetin (10) | HSV-1 EC50 2.5 μM, SI = 40; HSV-2 EC50 N/A, SI N/A | ||||
| Galangin (11) | HSV-1 EC50 2.5 μM, SI = 400; HSV-2 EC50 N/A, SI N/A | ||||
| Genistein (12) | HSV-1 EC50 5 μM, SI = 50; HSV-2 EC50 50 μM, SI = 5 | ||||
| Kaempferol (13) | HSV-1 EC50 15 μM, SI = 3.3; HSV-2 EC50 N/A, SI N/A | ||||
| Luteolin (14) | HSV-1 EC50 5 μM, SI = 20; HSV-2 EC50 N/A, SI N/A | ||||
| Myricetin (15) | HSV-1 EC50 5 μM, SI = 20; HSV-2 EC50 N/A, SI N/A | ||||
| Naringenin (16) | HSV-1 EC50 4 μM, SI = 187.5; HSV-2 EC50 22.5 μM, SI 33.3 | ||||
| Naringin (17) | HSV-1 EC50 2.5 μM, SI = 400; HSV-2 EC50 N/A, SI N/A | ||||
| Rutin (18) | HSV-1 EC50 5 μM, SI = 2000; HSV-2 EC50 N/A, SI N/A | ||||
| Quercetin (19) | HSV-1 EC50 5 μM, SI = 20; HSV-2 EC50 35 μM, SI = 2.9 | ||||
| Quercetin (19) | Raw 264.7 and Vero cells, HSV-1 PRA, Western blot analysis, quantitative RT-PCR | Reduction in plaque formation of 90% at 30 µg/mL | Inhibition of the expressions of HSV proteins (gD, ICP0) and genes (ICP0, UL13, UL52). Specific suppression of the expression of TLR-3, inhibition of transcriptional factors NF-κB and IRF3. | [28] | |
| Epigallocatechin gallate (9) | IP (%) | IP: 100% | Dietary phenolic, green tea component | [29] | |
| % PFU | At 1 μM cca 40%, at 5 µM cca 5% | [30] | |||
| Houttuynoid M (20) | PFA ACV IC50 0.15 μM; SI>1333 | IC50 17.72 μM; SI> 11.29 IC50 12.42 μM; SI> 16.10 | Houttuynia cordata | [31] | |
| Houttuynoid A (21) | |||||
| 1. β-galactosidase assay - the activity of enzyme measured in cell lysates 2. PRA 3. Progeny HSV-1 yield assay - effect on HSV-1 multiplication | 1. HSV-1 (F) IC50 23.50 ± 1.82 μM, CC50 166.36 ± 9.27 μM 2. HSV-1 (F) IC50 of 21.08 μM 3. HSV-1 (F) multiplication reduced by 100% at 75 μM | Possible mechanism—blocking viral membrane fusion | [32] | ||
| Genistein (12) | Vero cells, HSV-1 (KOS), HSV-1 (29 R), HSV-2 (333) PRA ACV: IC50 2.44 μM, SI >1818 (KOS), NA (29 R), IC50 3.30 μM, SI >303 (333) | IC50 (μM); SI: HSV-1 (KOS)/HSV-1 (29 R)/HSV-2 (333) 14.02, 3.88/7.76, 7.01/14.12, 6.95 | Isoflavonoid, soya beans, alfalfa | [33] | |
| Kuwanon C (22) | Vero cells, HSV-1 PRA ACV IC50 1.45 μg/mL; SI 144.8 | HSV-1 IC50 0.91 ± 0.43 μg/mL; SI 230.8 | In silico analysis along with antibacterial and anti-inflammatory effects | [34] | |
| Kuwanon T (23) | HSV-1 IC50 0.64 ± 0.52 μg/mL; SI 328.1 | ||||
| Kuwanon U (24) | HSV-1 IC50 1.93 ± 1.13 μg/mL; SI 108.8 | ||||
| Kuwanon E (25) | Vero cells, HSV-2 TRA ACV IC50 1.65 μg/mL; SI 127.3 | HSV-2 IC50 1.61 ± 0.31 μg/mL; SI 130.4 | |||
| Luteoforol (26) | Vero cells, HSV-1 (KOS, VR733) CPE as 50% tissue culture infective dose (TCID50/50 μL) ACV reduced the titer by 3.16 log10 against strain KOS and by 3 log10 against strain VR733 | Reduced the titer by 2.9 log10 against strain KOS and by 3.18 log10 against strain VR733 | Hypericum connatum | [35] | |
| Luteolin (14) | Vero cells, HSV-2 PRA ACV EC50 2.6 μg/mL, SI= 42.53 | HSV-2 EC50 22.4 μg/mL, SI = 12.43 | Dietary flavonoid | [36] | |
| Theaflavin-3,3′-digallate (27) | Vero cells, HSV-1 PRA Flow cytometry antiviral assay Fluorescence confocal microscopy | EC50 20 μM; SI = 5.625 | Green tea | [37] | |
| Cycloartocarpin (28) | Vero cells, HSV-1 (KOS), HSV-2 (186) PRA ACV HSV-1 IC50 1.5 μM; HSV-2 IC50 2.9 μM | HSV-1 IC50 28.2 μM; HSV-2 IC50 23.5 μM | Prenylated phenolics Morus spp., Artocarpus spp. | [38] | |
| Isocyclomorusin (29) | HSV-1 IC50 30.4 μM; HSV-2 IC50 27.2 μM | ||||
| Norartocarpetin (30) | HSV-1 IC50 63 μM; HSV-2 IC50 52.2 μM | ||||
| Catechin-7-gallate (31) | Vero cells, HSV-1 CPE ACV CC50 >200 ± 0.4 μg/mL | CC50 43.2 ± 2.3 μg/mL | Dietary phenols Low activity, questionable results. | [39] | |
| Kaempferol-3-O-6′´-O-galloyl-β-D-glucopyranoside (32) | CC50 124.1 ± 1.2 μg/mL | ||||
| Kaempferol (13) | CC50 76.1 ± 0.2 μg/mL | ||||
| Quercetin-3-O-6´´-O-galloyl-β-D-glucopyranoside (33) | CC50 175.6 ± 0.9 μg/mL | ||||
| Quercetin (19) | CC50 78.1 ± 0.8 μg/mL | ||||
| 7-O-galloyltricetiflavan (34) | Vero cells, HSV-1 CPE ACV IC50 0.25 μg/mL | IC50 30 μg/mL | Pithecellobium clypearia Other viruses tested | [40] | |
| 7,4′-di-O-galloyltricetiflavan (35) | IC50 20 μg/mL | ||||
| Strychnobiflavone (36) | Vero cells, HSV-1 (KOS), HSV-2 (333) PRA Post infection treatment ACV HSV-1 IC50 1.38 μg/mL, SI > 1.449; HSV-2 IC50 3.23 μg/mL, SI > 619 | HSV-1 (KOS) IC50 11.82 μg/mL, SI = 22.61; HSV-2 (strain 333) IC50 6.31 μg/mL, SI = 42.33 | Strychnos pseudoquina | [41] | |
| Derivatives of phenolic acids | Ethyl 2,4-dihydroxybenzoate (37) | Vero cells, HSV-1 PRA ACV IC50 1.45 μg/mL; SI 144.8 | HSV-1 IC50 1.32 ± 0.44 μg/mL; SI 159.1 | In silico analysis; antibacterial and anti-inflammatory effects | [34] |
| Gallic acid (38) | Vero cells, HSV-1 CPE ACV CC50 > 200 ± 0.4 μg/mL | CC50 49.8 ± 0.4 μg/mL | Dietary phenols Low activity, questionable results. | [39] | |
| IP (%) | IP: 100 % | Dietary phenolics | [29] | ||
| Alkyl derivatives of gallic acid Octyl gallate (39) | HEp-2 and Vero cells, HSV-1 CPE | Octyl gallate directly inactivates HSV-1 (virucidal activity). 39 suppresses both the intracellular multiplication and the release of the virus. 39 selectively accelerates the death of the virus-infected cells. The addition of the compound (39), even at 6 h post-infection, completely abolished the formation of progeny virus in the infected cells. | Other viruses tested including HSV-1: Inhibition was enhanced by the compounds with a higher number of carbons in the alkyl moieties, maximum at 12 (lauryl gallate), however, cytotoxicity was increased. | [42] | |
| Chebulagic acid (40) | IPF ACV IC50 29.04 ± 1.04 μg/mL | HSV-2 IC50 1.41 ± 0.51 μg/mL | Dose-dependently potent in vitro direct anti-viral activity. Effective prevention of the attachment as well as penetration of the HSV-2 to Vero cells. | [43] | |
| Chebulinic acid (41) | HSV-2 IC50 0.06 ± 0.002 μg/mL | ||||
| Tellimagrandin I (42) | IPF At 0.75 μg/mL ACV completely protected Vero cells against infection | EC50 of 2.6 μM for the direct mode, 5.0 μM for the absorption mode. | Ellagitannin—Cornus spp., Eucalyptus spp., Melaleuca styphelioides | [44] | |
| N-trans-ferulolyl tyramine (43) | IP (%) | IP: 100% | Dietary phenolics | [29] | |
| Protocatechuic acid (44) | Vero cells, HSV-2 ACV EC50 1.43 μg/mL, SI= 140 | EC50 0.92 µg/mL, SI = 217 | Dietary phenolic, metabolite of gut degradation of phenolics | [45] | |
| Psoromic acid (45) | Vero cells, HSV-1, HSV-2 ACV for HSV-1 IC50 2.6 μM; SI 119.2; for HSV-2 EC50 2.8 μM; SI 110.7 | HSV-1 IC50 1.9 μM; SI 163.2 HSV-2 EC50 2.7 μM; SI 114.8 | Study of synergy with ACV and inhibition of HSV-1 DNA polymerase (in vitro and in silico assays). | [46] | |
| Rhinacanthinic acid C (46) | Vero cells, HSV-2 PRA ACV ED50 14.67 μg/mL | ED50 58.98 μg/mL | Rhinacanthus nasutus | [47] | |
| Anthrones | Antrodin A (47) | Vero cells, HSV-1, HSV-2 PRA ACV HSV-1 IC50 2.1 μg/mL, SI = 61.9, HSV-2 IC50 2.9 μg/mL, SI = 44.8 | HSV-1 IC50 5.8 μg/mL, SI= 18.97, HSV-2 IC50 5.5 μg/mL, SI= 20.0 | Antrodia camphorate Additive effect of 47 with ACV | [48] |
| Aspergilol H (48) | HSV-1 PRA ACV EC50 3.0 μM | HSV-1 EC50 4.68 μM | Deep-sea fungus Aspergillus versicolor | [49] | |
| Aspergilol I (49) | HSV-1 EC50 6.25 μM | ||||
| Coccoquinone A (50) | HSV-1 EC50 3.12 μM | ||||
| Stilbenoids and 2-arylbenzofurans | Kuwanon X (51) | Vero cells, HSV-1 (15577 and clinical strains), HSV-2 (333) PRA ACV IC50 0.1 µg/mL for all strains | HSV-1 IC50 2.2 and 1.5 μg/mL; HSV-2 IC50 2.5 µg/mL | Prenylated phenol, Morus spp. 51 did not inactivate cell-free HSV-1 particles but inhibited cellular adsorption and penetration of HSV-1 viral particles. Following viral penetration, 51 reduced the expression of HSV-1 IE and L genes and decreased the synthesis of HSV-1 DNA. Furthermore, 51 inhibited the HSV-1-induced nuclear factor (NF)-κB activation through blocking the nuclear translocation and DNA binding of NF-κB. | [50] |
| Mulberrofuran B (52) | Vero cells, HSV-2 TRA ACV IC50 1.65 μg/mL; SI 127.3 | HSV-2 IC50 0.93 ± 0.23 μg/mL; SI 225.8 | In silico analysis; antibacterial and anti-inflammatory effects | [34] | |
| Oxyresveratrol (53) | Vero cells, HSV-1 (KOS), HSV-2 (186) PRA ACV HSV-1 IC50 1.5 μM; HSV-2 IC50 2.9 μM | HSV-1 IC50 42.8 μM; HSV-2 IC50 42.5 μM | Stilbenoid Morus spp., Artocarpus spp. | [38] | |
| Other phenolics | Coniferyl aldehyde (54) | Vero cells, HSV-1, HSV-2 ACV HSV-1 EC50 0.8 μg/mL | HSV-1 EC50 6.39 μg/mL, SI = 78.3 HSV-2 EC50 41.2 μg/mL, SI = 12.1 | Phenolic, Quercus suber, Simira glaziovii, S. eleiezeriana | [51] |
| Coumestrol (55) | Vero cells, HSV-1 (KOS), HSV-1 (29 R), HSV-2 (333) PRA ACV: IC50 2.44 μM, SI >1818 (KOS), NA (29 R), IC50 3.30 μM, SI >303 (333) | IC50 (μM), SI: HSV-1 (KOS)/HSV-1 (29 R)/HSV-2(333) 11.62, 9.6/3.34, 31.52/35.53, 28.14 | Coumestan, soya beans, alfalfa | [33] | |
| Curcumin (56) | CPA, PRA, viral adsorption assay, viral penetration assay | At 30 µM, 85% inhibition of HSV-1 and 68% of HSV-2 CPE, PRA 92% for HSV-1 and 88% for HSV-2 | Curcuma longa Inhibits HSV adsorption and replication | [52] | |
| Vero cells, HSV-1 CPE ACV CC50 > 200 ± 0.4 μg/mL | CC50 49.8 ± 0.4 μg/mL | Dietary phenols Low activity, questionable results | [39] | ||
| Imperatorin (57) | Vero cells, HSV-1 CPE ACV – full inhibition of replication of HSV-1 at 250 μg/mL | 57 decreases titer of HSV-1 by 55.6% at 31.25 μg/mL | Furanocoumarin of Apiaceae family | [53] | |
| Pinoresinol (58) | IP (%) | IP: 26% | Dietary phenolics | [29] |
| Compound | Antiherpetic and Cytotoxicity Assays, Strains, Cells, and Reference Agents | Results | Additional Information | Source |
|---|---|---|---|---|
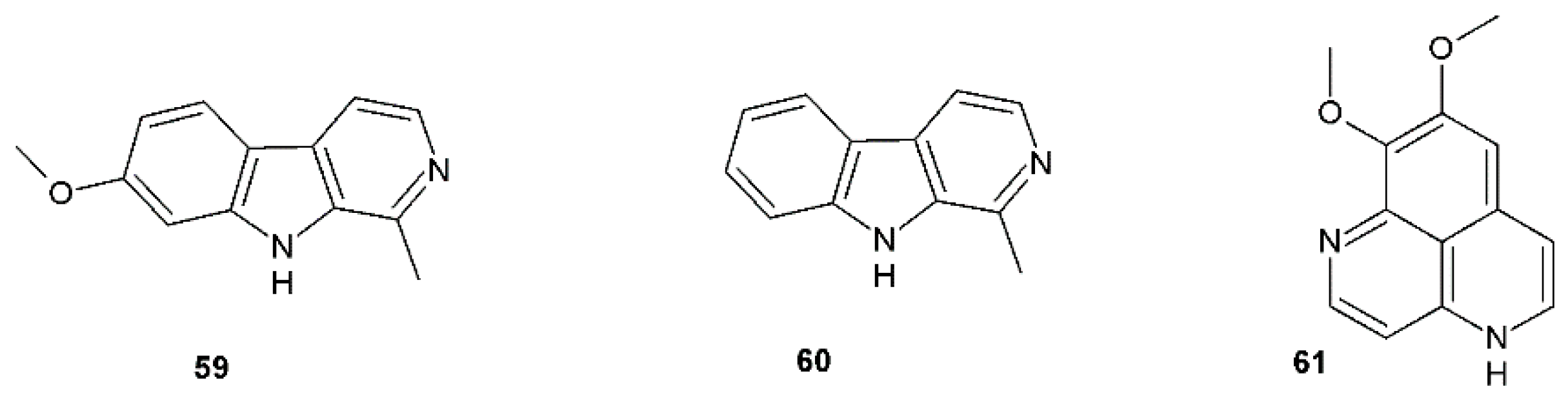
| Harmine (59) | Vero cells, HSV-2 PRA ACV CC50 and IC50 > 3.000 mg/mL and 0.1 μg/mL, respectively, SI > 30.000 | CC50 and IC50 12.5 μg/mL and 0.3 μg/mL, respectively, SI = 41.6 | Peganum harmala, Banisteriopsis caapi, Passiflora incarnata | [54] |
| Human foreskin fibroblasts (HFF), HSV-1 (166vVP22-GFP) GFP-based reporter assay Cidofovir at 3 μM reduced to 20% | At 3.3 μM, 59 reduced HSV-1 replication to approx. 50%, at 10 μM to approx. 5% | 59 inhibited viral protein expressed as a dual-specificity tyrosine phosphorylation-regulated kinase inhibitor. | [55] | |
| Harmane (60) | Vero cells, HSV-1, HSV-2 PRA ACV HSV-1 EC50 0.8 μg/mL | HSV-1 EC50 4.9 μg/mL, SI = 11.8 HSV-2 EC50 71.8 μg/mL, SI = 24.7 | P. harmala, B. caapi, P. incarnata | [51] |
| Aaptamine (61) (8,9-dimethoxy-1H-benzo[d,e][1,6]-naphthyridin) | Vero cells, HSV-1 CPE | EC50 7.0 µg/mL | Marine sponge Aaptos spp. | [56] |
| Compound | Antiherpetic and Cytotoxicity Assays, Strains, Cells, and Reference Agents | Results | Additional Information | Source | |
|---|---|---|---|---|---|
| Monoterpenes | Geraniol (62) | Vero cells, HSV-2 ACV (EC50 1.94 µg/mL; SI = 108.25 | HSV-2 EC50 1.92 µg/mL SI = 109.38 | Thymus bovei Benth. essential oil, typical monoterpene of Lamiaceae | [57] |
| Cypellocarpin C (63) | Vero cells, HSV-1 (KOS), HSV-2 (clinical isolates) PRA, TRI ACV HSV-1 IC50 1.92 ± 0.23 μg/mL, SI >109.4, HSV-2 IC50 1.75 ± 0.33, SI > 120.0 | HSV-1 IC50 0.96 ± 0.12, SI > 218.8 | 63 is a cross-metabolite of monoterpenic glycoside and a methylchromone), Eucalyptus globulus | [58] | |
| (+)-rhodonoid C (64) | Vero cells, HSV-1 CPE ACV IC50 4.2 μM, SI > 100 | IC50 80.6 ± 4.7 μM, SI = 2.7 | 64 is a cross-metabolite of monoterpene and polyketide Rhododendron spp. | [59] | |
| Sesquiterpenes | β-caryophyllene (65) | Vero cells, HSV-1, HSV-2 (clinical isolates), (HSV-2 ACV-resistant) PRA Time-of-addition assay Virus inactivation assay ACV HS2-2 0.14 μg/mL; SI = 1178; HSV-2 (acyclovir-resistant) EC50 71.84 μg/mL, SI = 2.29 | HSV-2 EC50 5.38 μg/mL, SI = 9.10 HSV-2 (acyclovir resistant) EC50 5.02 μg/mL, SI = 9.76 | Bicyclic sesquiterpene, common occurrence, for example, cloves | [60] |
| Kellerin (66) | Vero cells, HSV-1 (KOS) PRA ACV at 2.5 µg/mL, 82% of plaque reduction | 66 at 2.5 µg/mL, 65% | 66 is a cross-metabolite of sesquiterpene and coumarin Gum resin of Ferula assa foetida No cytotoxic effect up to 10 µg/mL | [61] | |
| Lactarorufin A 8-[N-benzoyl-(2′R,3′S)-3′-phenylisoserinate] (67) | Vero cells, HSV-1 (MacIntyre strain) CPE ACV IC50 1 µg/mL, SI ˃ 250 | HSV-1 IC50 17.3 µg/mL, SI = 16 | Taxol-N-benzoylphenyl-isoserinates of sesquiterpenoid alcohols and sesquiterpenoids Lactarius mushroom | [62] | |
| Isolactarorufin 8-[N-benzoyl-(2′R,3′S)-3′-phenylisoserinate] (68) | HSV-1 IC50 21.9 µg/mL, SI = 17.4 | ||||
| Furandiol 8-[N-benzoyl-(2′R,3′S)-3′-phenylisoserinate] (69) | HSV-1 IC50 15 µg/mL, SI = 19.3 | ||||
| Isovellerol 13-[N-benzoyl-(2′R,3′S)-3′-phenylisoserinate] (70) | HSV-1 IC50 7.8 µg/mL, SI = 13.9 | ||||
| 5-deoxylactarolid B 8-[N-benzoyl-(2′R,3′S)-3′-phenylisoserinate] (71) | HSV-1 IC50 3.4 µg/mL, SI = 31.7 | ||||
| Isolactarorufin 8-epi-[N-benzoyl-(2′R,3′S)-3′-phenylisoserinate] (72) | HSV-1 IC50 4.2 µg/mL, SI = 18.4 | ||||
| Alantolactone (73) | Vero cells, HSV-1 CPE Ribavirin as a positive control | At 10-6–10-8 g/mL showed an antiviral effect | Sesquiterpene Inula helenium | [63] | |
| (-)-15-methoxy-3,6-peroxocupar-1-ene (74) | Vero cells, HSV-1 (KOS strain, VR-1493) PRA ACV at 2.5 μM 96.96% | Anti HSV-1at 10 μg/mL 43.93 % | Sesquiterpene Schisandra sphenanthera | [64] | |
| (R)-6,9-dihydroxy-1-oxo-14-noreudesm-5,7,9-triene (75) | Vero cells, HSV-2 CPE inhibition method Quantitative PCR | 2 log10 reduction in HSV-2 yield at conc. 12.5 µM, IC50 6.25 μM | 14-Noreudesmane sesquiterpene Elaeagnus rhamnoides | [65] | |
| Diterpenes | Simirane A (76) | Vero cells, HSV-1, HSV-2 PRA ACV HSV-1 EC50 0.8 μg/mL | HSV-1 EC50 4.61 μg/mL, SI = 7.01 HSV-2 EC50 3.73 μg/mL, SI = 8.7 | Erythroxylane diterpene, Simira eliezeriana | [51] |
| Dodovisnoid D (77) | Vero cells, HSV-1 CPE ACV IC50 4.2 μM, SI > 100 | IC50 5.5 μM, SI = 2.8 | Clerodane diterpenes Dodonaea viscosa | [66] | |
| Dodovisnoid F (78) | IC50 23.0 μM, SI = 4.7 | ||||
| Atomaric acid (79) | Vero cells, HSV-1 (ACR-29) CPE ACV EC50 1.2 μM, SI > 716.6 | EC50 1.28 µM, SI > 353.1 | Meroditerpenes from Brazilian seaweed Stypopodium zonale (Dictyotales) Atomaric acid (79) and epitanodiol (80) may be selectively targeted to HSV-1 replication Low effect on HIV-1 reverse transcriptase | [67] | |
| Epitaondiol (80) | EC50 1.34 µM, SI > 361.9 | ||||
| 10-deacetyl-baccatin III (81) | Vero cells, HSV-1 (MacIntyre strain) CPE ACV IC50 1 µg/mL, SI ˃ 250 | HSV-1 IC50 52.7 µg/mL, SI ˃ 9.5 | The activity may be associated with their influence on mitotic division. 113 and 114 are included for comparison of effect (non-terpenoid compounds) | [68] | |
| Andrographolide (82) | Vero cells, HSV-1 PRA ACV IC50 < 1 µg/mL | IC50 8.28 µg/mL | Ent-labdane diterpenes Andrographis paniculata No cytotoxic effect at virucidal concentration. | [69] | |
| Neoandrographolide (83) | IC50 7.97 µg/mL | ||||
| 14-deoxy-11,12-didehydroandrographolide (84) | IC50 11.1 µg/mL | ||||
| 10,18-diacetoxy-8-hydroxy-2, 6-dolabelladiene (85) | Vero cells, HSV-1 CPE ACV at 15 µM, 79% of CPE | At 50 µM, 89% of CPE | Dollabene diterpenes brown alga Dictyota pfaffi Effect on HIV-1 reverse transcriptase. | [70] | |
| 10-acetoxy-8,18-di-hydroxy-2,6-dolabelladiene (86) | At 50 µM, 87% of CPE | ||||
| Steroids | Fomitopsin D (87) | Vero cells, HSV-1 Green fluorescent protein (GFP) expression ACV IC50 2.18 μg/mL | HSV-1 IC50 17 μg/mL | Steroid Fungus Fomitopsis | [71] |
| Lyonifoloside A (88) | Vero cells, HSV-1 (F strain VR 733) CPE ACV EC50 0.41 μM, SI > 244 | EC50 11.1 μM, SI = 2.1 | 9,10-seco-cycloartanes 88–90, lanosterol derivatives 91–93 Lyonia ovalifolia | [72] | |
| Lyonifolic acid A (89) | EC50 3.7 μM, SI = 4.3 | ||||
| Lyofoligenic acid (90) | EC50 11.1 μM, SI = 5.2 | ||||
| Lyonifolic acid C (91) | EC50 2.1 μM, SI = 7.6 | ||||
| Lyonifoloside M (92) | EC50 6.4 μM, SI = 3.0 | ||||
| Lyonifoloside P (93) | EC50 14.3 μM, SI > 7.0 | ||||
| Cucurbitacin B (94) | Vero cells, HSV-1 (KOS) PRA Acyclovir IC50 1.74 μM, SI > 132.2 | IC50 0.94 μM, SI = 127.7 | Cucurbitane steroid Cucurbitaceae | [73] | |
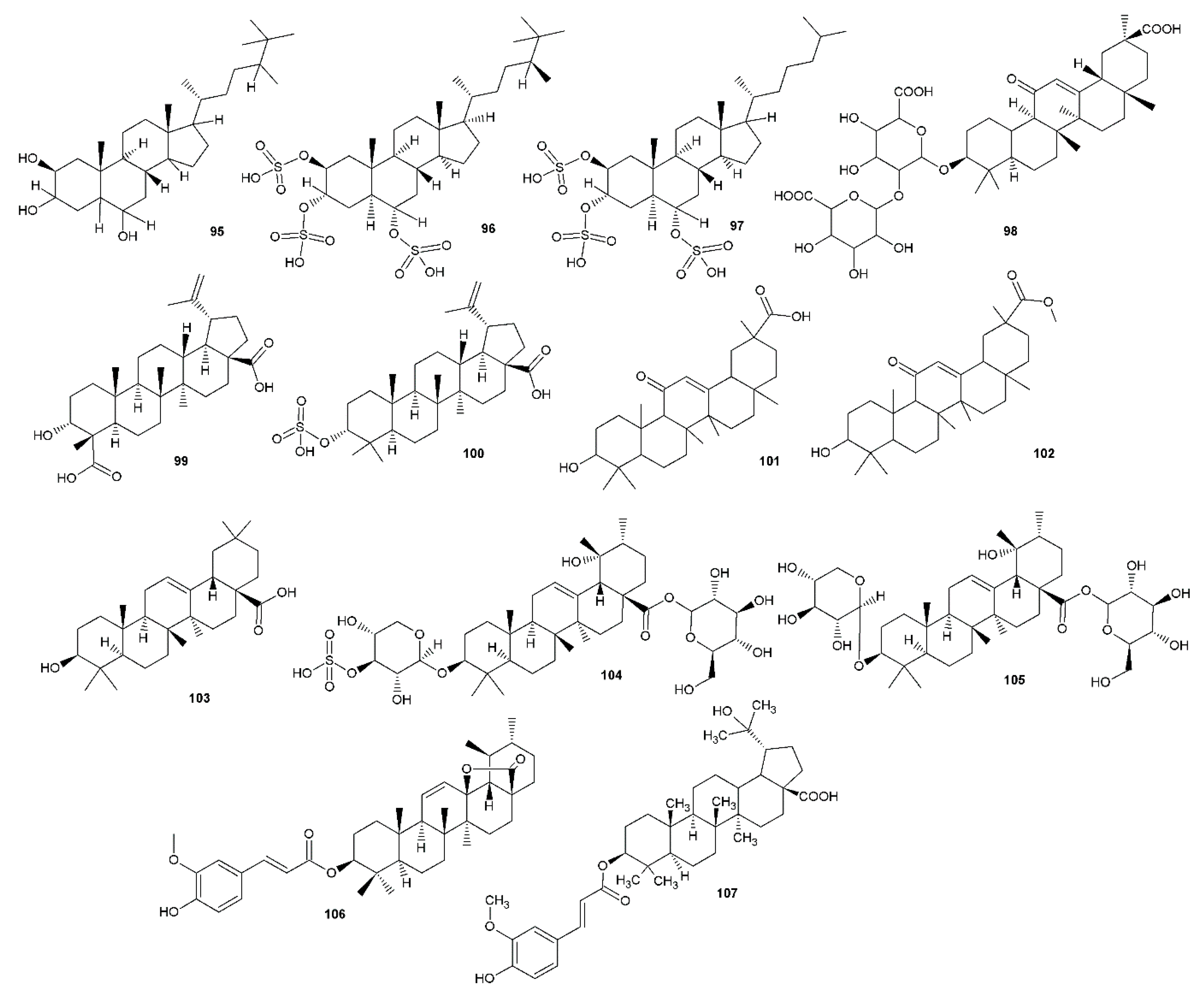
| TSH (halistanol (95) rich fraction) | Vero cells, HSV-1 (KOS) PRA Effects on HSV-1 attachment and penetration ACV IC50 3.45 ± 0.42 μg/mL, SI > 580 | IC50 2.87 ± 0.78 μg/mL, SI = 15.53 | Brazilian marine sponge Petromica citrina (Demospongiae) The observed anti-HSV-1 activity was found to be mediated by the inhibition of virus attachment and by the penetration into Vero cells, the virucidal effect on virus particles, and by the impairment in levels of ICP27 and gD proteins of HSV-1. Synergic effect with acyclovir | [74] | |
| Halistanol sulfate (96) | IC50 5.63 ± 1.37 μg/mL, SI = 2.46 | ||||
| Halistanol sulfate C (97) | IC50 6.09 ± 1.51 μg/mL, SI = 1.95 | ||||
| Pentacyclic triterpenes | Glycyrrhizic acid (98) | HeLa cells, HSV-1 CPE | At 1 and 2 mM, the inhibition ranged from about 78% to 85% | Oleanane triterpene Glycyrrhizha spp. 24h pre-treatment strongly enhanced the antiviral activity of 98 (at 2 mM), with a viral inhibition that rise as high as 95%–98%. 98 is a strong inducer of the autophagy activator Beclin 1 (connected to resistance to HSV-1 infection). | [75] |
| Glycyrrhetic acid (101) and its methylester (102) | Vero cells, HSV-1 strain (KOS) PRA ACV IC50 1.1 ± 0.09 µM, SI> 400 | IC50 21.7 ± 0.06 and 8.1 ± 0.2 µM, respectively. SI = 3.9 and > 26, respectively. | Oleanane triterpene Glycyrrhiza spp. The hydroxylation at C-21 seems to be responsible for the reduction of anti-HSV-1 activity, the C-29 hydroxy group would eliminate the anti-HSV-1 activity. C-20 methoxy or carboxy groups should be responsible for the enhancement of activity. | [76] | |
| 3α-hydroxylup-20(29)-ene-23,28-dioic acid (99) | Vero cells, HSV-1 (15577) CPE ACV IC50 0.25 µg/mL, SI > 2000 | IC50 31.3 µg/mL, SI = 3.8 | Lupane triterpenes Schefflera heptaphylla Other viruses tested | [77] | |
| 3-epi-betulinic acid 3-O-sulphate (100) | IC50 20 µg/mL, SI = 5 | ||||
| Oleanolic acid (103) | Vero cells, HSV-1 (strain F), HSV-2 (strain G) CPE Viral inactivation or virucidal assay Viral penetration assay Time response assay Amplification of viral DNA by PCR ACV HSV-1 EC50 2.1 ± 0.1 μg/mL, SI = 61.9; HSV-2 EC50 2.9 ± 0.1 μg/mL, SI = 44.8 | HSV-1 EC50 6.8 ± 1.24 μg/mL, SI = 14.4 HSV-2 EC50 7.8 ± 1.4 μg/mL, SI = 12.6 | Common oleanane triterpene Possible inhibition of the early stage of HSV multiplication | [78] | |
| Asprellanoside A (104) | Vero cells, HSV-1 PRA ACV total inhibitory concentration (TIC) 0.0043 mM | TIC 0.14 mM | Sulphur containing triterpenoid saponins Ilex asprella | [79] | |
| Oblonganoside H (105) | TIC 0.18 mM | ||||
| Tereticornate (106) | Vero cells, HSV-1 (KOS), HSV-2 (clinical isolates) PRA, TRI ACV HSV-1 IC50 1.92 ± 0.23 μg/mL, SI >109.4, HSV-2 IC50 1.75 ± 0.33, SI > 120.0 | HSV-1 IC50 0.96 ± 0.12, SI > 218.8 | 106 - triterpene Eucalyptus globulus | [58] | |
| 3β-O-trans-ferulyl-20-hydroxy-lup-28-oic acid (107) | Vero cells, HSV-1 (F strain VR 733) CPE inhibition method ACV IC50 0.41 ± 0.3 µM, SI > 243.9 | IC50 0.71 ± 0.06, SI 5.2 | Triterpene Rhododendron latoucheae | [80] | |
| Compound | Antiherpetic and Cytotoxicity Assays, Strains, Cells, and Reference Agents | Results | Additional Information | Source |
|---|---|---|---|---|
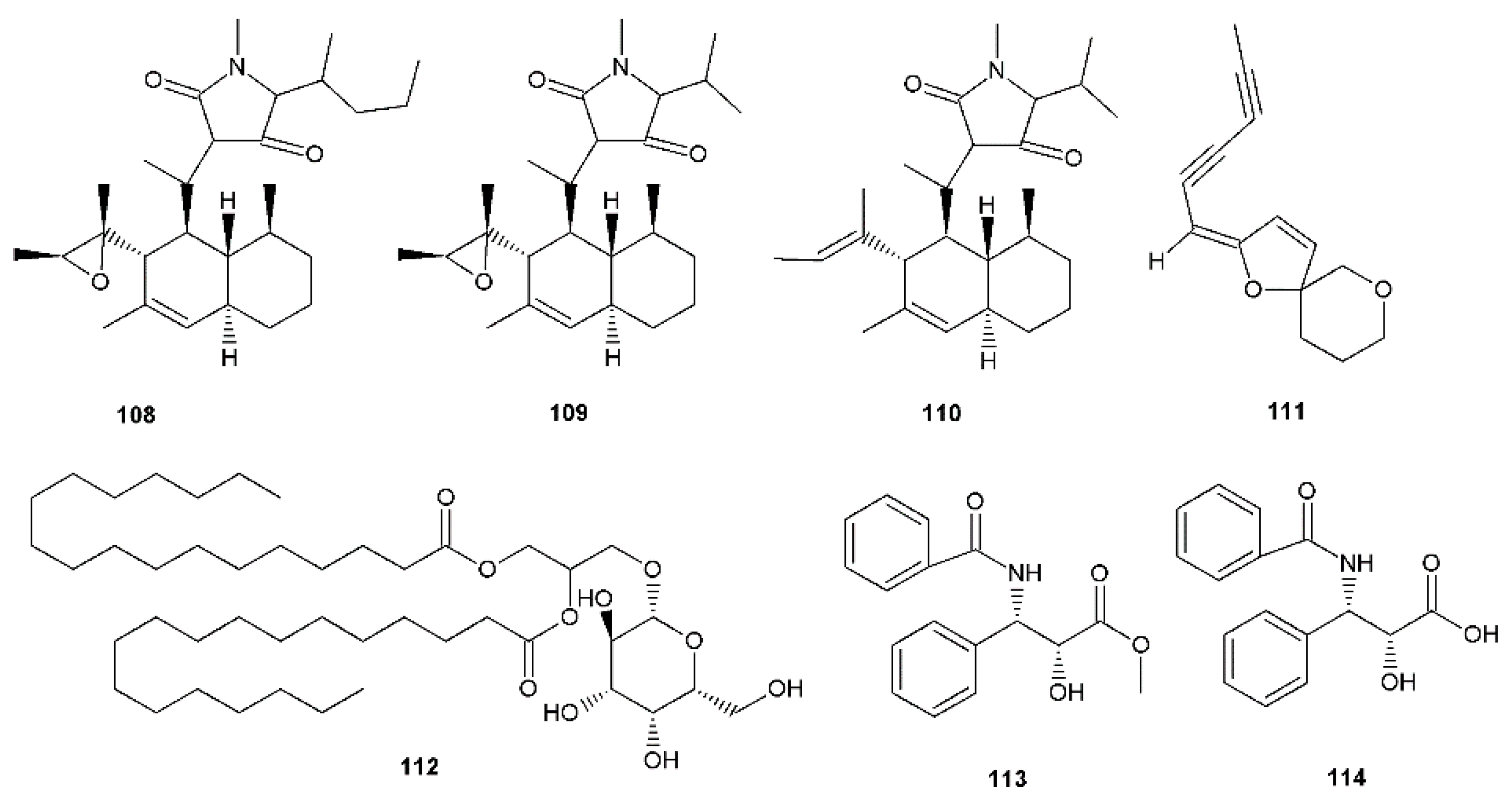
| Trichobotrysin A (108) | Vero cells, HSV-1 PRA ACV IC50 3.50 μM | IC50 3.08 μM | Deep-sea-derived fungus Trichobotrys effuse Tetramic acid derivatives | [81] |
| Trichobotrysin B (109) | IC50 9.37 μM | |||
| Trichobotrysin D (110) | IC50 3.12 μM | |||
| (E)-2-(2,4-hexa-diynyliden)-1,6-dioxaspiro[4.5] dec-3-ene (111) | Vero cells, HSV-1 (clinical isolate with >99% homology to isolate SK087 US4–6 genes), HSV-2 (clinical isolate >99% homology to isolate 99-62039 US4 gene) CPE, YRA Time-of-addition, adsorption inhibition, virucidal, penetration inhibition assays Macromolecular synthesis inhibition analysis ACV HSV-1 EC50 0.9 μg/mL; SI > 1000 / HSV-2 EC50 0.7 μg/mL; SI > 1000 | EC50, SI: HSV-1/HSV-2 0.146 μg/mL; > 205 / 0.127 μg/mL; > 236 | Tanacetum vulgare Spiroketal-enol ether derivative. Mechanism of antiviral activity elucidated on petroleum ether extract and 111 (inhibition of viral gene expression). | [82] |
| Monogalactosyl diglyceride (112) and digalactosyl diglyceride (DGDG) | Vero cells, HSV-1, HSV-2 PRA ACV HSV-1 IC50 0.64 μg/mL and HSV-2 IC50 0.80 μg/mL | HSV-1 IC50 36.00 μg/mL for 112 and 40.00 μg/mL for DGDG, respectively. HSV-2 IC50 41.00 μg/mL for 112 and 43.20 μg/mL for DGDG, respectively. | Clinacanthus nutans | [83] |
| Methyl (N-benzoyl-(2′R,3′S)-3′-phenylisoserinate) (113) | Vero cells, HSV-1 (MacIntyre strain) CPE ACV IC50 1 µg/mL, SI ˃ 250 | HSV-1 IC50 10.7 µg/mL, SI ˃ 46.7 | Taxol derivatives. The activity may be associated with their influence on mitotic division. | [68] |
| N-benzoyl-(2′R,3′S)-3′-phenylisoserine (114) | HSV-1 IC50 21.7 µg/mL, SI ˃ 23 |
| Compound | Antiherpetic and Cytotoxicity Assays, Strains, Cells, and Reference Agents | Results | Additional Information | Source |
|---|---|---|---|---|
| PSP-B2 polysaccharide from Prunellae Spica (Prunella vulgaris L.) | Vero cells, HSV-1, HSV-2 PRA ACV HSV-1 IC50 0.78 µM, HSV-2 1.32 μM | HSV-1 IC50 69 μg/mL HSV-2 IC50 49 μg/mL | No cytotoxicity even at 1600 μg/mL | [84] |
| Eucheuma gelatinae (seaweed) polysaccharide | Vero cells, HSV-1 PRA ACV EC50 HSV-1(strain F), HSV-2 (strain 333), HSV-1 (strain 106), HSV-1 (strain 153), and HSV-1 (strain blue) 0.78, 0.71, 9.60, 21.11, and 23.50 μg/mL, respectively | HSV-1/F, HSV-2/333, HSV-1/106, HSV-1/153, and HSV-1/blue EC50 0.65, 2.12, 1.11, 1.24, and 1.48 μg/mL, respectively | Effect via activity on early HSV-1 infection. Inhibition of viral DNA synthesis. | [85] |
| Sulfated polysaccharide SP-III from Sargassum latifolium | Vero cells, HSV-1 PRA | SP-III 33% and 81% inhibition at 20 μg/mL and 40 μg/mL, respectively. | Glucuronic acid, mannose, glucose, xylose and fucose. | [86] |
| Sulfated polysaccharide SP-2a from Sargassum patens (Kütz.) Agardh | Vero cells, HSV-1 (15577 strain, clinical strain, DM2.1 strain-ACV resistant) PRA Determination of extracellular virucidal activity Time of addition experiment Virus adsorption assay | Inhibition of replication of both the acyclovir-sensitive and -resistant strains of HSV-1, in a dose-dependent manner, EC50 1.5–5.3 μg/mL | Fucose, xylose, mannose, glucose, galactose, galactosamine Extracellular virucidal activity only against the ACV-sensitive strains. This compound might inhibit the attachment of the virus to its host cell. | [87] |
| ST-F polysaccharide from marine brown algae Sargassum trichophyllum | Vero cells, HSV-2 (UW264 strain) PRA Time of addition experiment | Added to the medium during infection and throughout the incubation (Experiment A) or immediately after viral infection (Experiment B), IC50 18 and 410 μg/mL, respectively. SI > 280 and >12 for A and B, respectively. | (Fucose and galactose) The main antiviral target of ST-F might be virus adsorption and/or penetration step(s) on the host cell surface. Low cytotoxicity | [88] |
| SPs - sulfated polysaccharides from brown sea algae Sargassum fluitans and red sea algae Solieria filiformis | Vero cells, HSV-1 Neutral red dye method ACV EC50 15.4 ± 5.6 μg/mL | S. fluitans EC50 42.8 ± 4.3 μg/mL and S. filiformis EC50 136.0 ± 12 μg/mL Without cytotoxicity (1–200 μg/mL) | The activity observed suggests that the degree of sulfation, molecular weight, and carbohydrate nature of these polysaccharides may affect the activity | [89] |
| Polysaccharide fractions C1p and C4p from chlorophyta Ulva armoricana | Vero cells, HSV-1 (wild-type strain 17, sensitive to ACV) CPE ACV EC50 0.3 µg/mL | EC50 373.0 ± 20.7 and 320.9 ± 6 µg/mL | Activities correlated to amounts of rhamnose, uronic acids and degree of sulfation. | [90] |
| Sp-Am polysaccharide from Acanthophora muscoides | Vero cells, HSV-1, HSV-2 CPE | HSV-1 IC50 1.63 μg/mL, SI = 3.5 HSV-2 IC50 3.5 μg/mL, SI = 99.9 | Sulfated polysaccharides from marine seaweeds The possible mechanism of the effect - the inhibition of virus adsorption. | [91] |
| SP-Gb polysaccharide from Gracila riabirdiae | HSV-1 IC50 0.75 μg/mL, SI = 1.25 HSV-2 IC50 82.2 μg/mL, SI = 94.40 | |||
| SP-Sf polysaccharide from Solieria filiformis | HSV-1 IC50 0.6 μg/mL, SI = 1.6 HSV-2 IC50 74.9 μg/mL, SI = 97.5 | |||
| PSC polysaccharide from marine seaweed Sphaerococcus coronopifolius | Vero cells, HSV-1 (wild type strain 17, sensitive to ACV) CPE Time of addition assay Virus adsorption assay ACV SI ˃ 500 | EC50 4.1 μg/mL, SI = 61 | Galactose, 3,6-anhydrogalactose, uronic acids, sulfated The adsorption step of HSV-1 to the host cell possible mechanism of action. | [92] |
| PBT polysaccharide from marine seaweed Boergeseniella thuyoides | EC50 17.2 μg/mL, SI = 14.5 | |||
| Sulfated xylogalactofucans and alginic acids from brown algae Laminaria angustata | RC-37 cells, HSV-1 (KOS) PRA Time of addition assay Virus adsorption assay Virucidal assay | IC50 0.21–25 μg/mL, SI ˃ 40 ˃ 3225 | Possible inhibiting HSV attachment to cells by direct interaction with viral particles. | [93] |
| SU1F1 polysaccharide from green algae Enteromorpha compressa | HEp-2 cells, HSV-1 (clinical isolate) PRA Time-of-addition assay Inhibition of adsorption assay Inhibition of penetration assay Virucidal assay Acyclovir IC50 2100 μg/mL, SI = 1.21 | IC50 28.25 μg/mL, SI = 35.3 | Chemically altered- sulfated ulvan Broad mechanism of action. | [94] |
| Sulfated fucoidans (S1-S3) from marine brown alga Padina tetrastomatica | Vero cells, HSV-1 (strains F and B2006), HSV-2 (strain MS) PRA Virucidal assay Effect of treatment period on the antiviral activity | HSV-1 and HSV-2 with IC50 in range of 0.30–1.05 μg/mL B2006 S3 IC50 0.6 μg/mL | Active during the virus adsorption period Degree of sulfation affects the activity | [95] |
| Polysaccharides from brown seaweed Stoechospermum marginatum | Vero cells, HSV-1 (strains F, TK- B2006 and filed strains, syncytial variants arising after selection with a natural carrageenan, syn 13-8 and 14-1), HSV-2 (MS) PRA | HSV-1 (F) EC50 1.15-50 μg/mL, SI = ˃20 ˃ 869 HSV-2 (MS) EC50 0.78 μg/mL, SI= 0.57 ˃50 F3 B2006, Field, 13-8 and 14-1 strains EC50 0.95, 1.52, 4.52 μg/mL, SI ˃1053, ˃658, ˃221, ˃176 | Sulfated fucans Active during the virus adsorption period. No direct virucidal activity. No correlation between the antiviral and anticoagulant activity. | [96] |
| CiWE CiF3 polysaccharides from brown seaweed Cystoseira indica | Vero cells, HSV-1 (strain F), HSV-2 (strain MS) PRA | IC50 values in the range of 0.5–2.8 μg/mL | Sulfated fucans Degree of sulfation affects the activity. No correlation between the antiviral and anticoagulant activity. | [97] |
| Sulfated polysaccharide (fucoidan) from brown algae Undaria pinnatifida (Mekabu) | Vero cells, HSV-1 (strain HF), HSV-2 (strain UW-268) PRA A) 1 h after the viral infection, B) immediately after infection | HSV-1 IC50 and SI A) 2.5 μg/mL, ˃800; B) 14 μg/mL, ˃140 HSV-2 IC50 and SI A) 2.6 μg/mL, ˃770; B) 5.1 μg/mL, ˃390 | Fucose, galactose Other viruses tested. | [98] |
| Polysaccharides (GiWE and F3) from red seaweed Grateloupia indica | Vero cells, HSV-1 (strain F, TK- B2006 and filed strains, syncytial variants arising after selection with natural carrageenan, syn 13-8 and 14-1), HSV-2 (MS) PRA | HSV-1 (F) IC50 0.27 μg/mL and HSV-2 (MS), IC50 0.31 μg/mL F3 B2006, Field, 13-8 and 14-1 strains EC50 0.89, 0.87, 1.06, 0.81 μg/mL, SI ˃ 1123, ˃1149, ˃943, ˃1234 No direct virucidal activity at 40 μg/mL | Sulfated galactans Degree of sulfation affects the activity. Possible ability to interfere with the replication cycle. | [99] |
| Polysaccharide from Schizymenia binderi | Vero cells, HSV-1 (strains F, TK− (B2006), (Field)), HSV-2 (G) PRA | EC50 0.21-0.76 μg/mL SI > 1000 for all assays No cytotoxicity at 1000 μg/mL | Sulfated galactan Interference with the initial adsorption of viruses to cells, no virucidal activity at 100 μg/mL. | [100] |
| Polysaccharide from red seaweed Gigartina skottsbergii | Vero cells, HSV-1 (strains F, TK− (B2006), (Field), clinical isolates 1213 LCR/94, 374 LCR/94 and 1180 BE/94), HSV-2 (G, clinical isolate 244 BE/94) PRA | 1C3 HSV-1 (F) and HSV-2 (G) EC50 0.7 and 0.5 µg/mL, respectively, SI ˃ 1408 and ˃2128 1T1 HSV-1 (F) and HSV-2 (G) EC50 0.6 and 0.4 µg/mL, respectively, SI ˃ 1538 and ˃2439 Clinical isolates EC50 0.19–2.18 µg/mL | Carrageenans Lack of anticoagulant activity No virucidal activity, effect on virus adsorption | [101] |
| Proteoglycan GLPG from Ganoderma lucidum (Agaromycetes)—lingzhi mushroom | Vero cells, HSV-1, HSV-2 CPE Virus yield inhibition assay | HSV-1 and HSV-2 EC50 48 and 56 µg/mL, respectively, SI ˃ 42 and >36 No cytotoxicity at 2000 µg/mL | Proteoglycan GLPG (carbohydrate: protein ratio of 10.4: 1) The antiviral activity may be due to its inhibiting HSV attachment to cells, in addition, its inhibition of viral penetration would augment its antiviral activity. | [102] |
| Polysaccharide RP from Portulaca oleracea | Vero cells, HSV-2 (UW268 strain) PRA Time-of-addition assay Virus adsorption and penetration assay | A: RP added during infection and throughout the incubation thereafter B: RP added immediately after viral infection. A: EC50 210 µg/mL, SI = 33 B: EC50 320 µg/mL, SI = 22 | Pectic polysaccharide (RP) Activity against influenza virus tested on MDCK cells. | [103] |
| Polysaccharide SPLCf from Caesalpinia ferrera | HEp-2 cells, HSV-1 (clinical isolate) PRA Time-of-addition assay Inhibition of adsorption assay Inhibition of penetration Virucidal activity HSV-1 cell to cell spread assay ACV IC50 2100 μg/mL, SI >1.21 | IC50 405 μg/mL, SI > 7.4. | Sulfated polysaccharide SPLCf showed the effect on several stages of the HSV replication—virus adsorption, the effect on virus particles and the expression of viral protein. | [104] |
| Polysaccharides (ANP, AAP) from Acanthopanax sciadophylloides | Vero cells, HSV-2 (UW264 strain) PRA In vivo anti-HSV-2 effects on female BALB/c mice ACV In vivo: Polysaccharides (1 mg/20 μL) or ACV (0.2 mg/20 μL) administered intravaginally twice per day from 3 days before infection to 7 days post-infection. | ANP and AAP IC50 52 and 620 μg/mL when added to the medium during infection and throughout the incubation thereafter HSV-2 IC50 67 and 580 μg/mL when added to the medium immediately after infection. | Acanthopanax sciadophylloides Virus titers in the vaginal region were decreased by the administration of AAP. | [105] |
| Acidic polysaccharide (nostoflan) from terrestrial cyanobacterium Nostoc flagelliforme | Vero cells, HSV-1 (HF strain) and HSV-2 (UW268 strain) PRA | A: added during infection and throughout the incubation thereafter B: added immediately after viral infection A: HSV-1 IC50 0.37 μg/mL, SI = 13000 A: HSV-2 IC50 2.9 μg/mL, SI = 2700 B: HSV-1 IC50 ˃100 μg/mL, SI = <49 B: HSV-2 IC50 7.7 μg/mL, SI = 1000 | Other antiviral activity tested. No anti-thrombin activity. | [106] |
| Polysaccharide sulfate fraction from Caulerpa racemosa | Vero cells, HSV-1 strain F, TK- B2006 and field strains), HSV-2 G strain) PRA | HSV-1 (strain F, TK- B2006 and field strains) EC50 4.2, 2.4, 2.2 µg/mL, SI ˃ 238, ˃417, ˃454 HSV-2 (strain G) EC50 3.0 µg/mL, SI ˃ 333 No cytotoxic effects up to 1000 µg/mL | Galactose, glucose, arabinose, and xylose as the major components. | [107] |
| Compound | Antiherpetic and Cytotoxicity Assays, Strains, Cells, and Reference Agents | Results | Additional Information | Source |
|---|---|---|---|---|
| Bacteriocins (semi-purified) | Vero cells, HSV-1 (strain EK) CPE Viral adsorption assay | Before adsorption IC50 235.6 μg/mL, SI 4.6 (for GEn14) After adsorption IC50 24.0 μg/mL, SI 17.8 (for GEn17) | Bacteria from goat milk Enterococcus durans (GEn09, GEn12, GEn14, and GEn17). | [108] |
| Subtilosin | Vero cells, HSV-2 (strain G) PRA Virucidal assay Time-of-addition assay Indirect immunofluorescence assay | At 200 μg/mL a reduction over 99.9% in virus titer EC50 18.2 μg/mL, SI 17.4 | Bacillus amyloliquefaciens Cyclic peptide Antiviral and virucidal effect. This compound affects late stages of the viral replicative cycle such as viral glycoprotein intracellular transport. | [109] |
| Simplicilliumtide J | Vero cells, HSV-1 PRA ACV IC50 3.0 µM | IC50 14.0 µM | Deep-Sea-Derived Fungus Simplicillium obclavatum Cyclic peptides | [110] |
| Verlamelin A | IC50 16.7 µM | |||
| Verlamelin B | IC50 15.6 µM | |||
| Aspergillipeptide D | Vero cells, HSV-1 (strain 15577, ACV resistant clinical isolates HSV-1-106 and HSV-1-153) CPE ACV IC50 3 µM | HSV-1 (strain 15577) IC50 9.5 µM | Marine gorgonian-derived fungus Aspergillus sp. No cytotoxicity at concentrations tested. Aspergillipeptide D showed activity against acyclovir-resistant HSV-1-106 and HSV-1-153. | [111] |
| Aspergillipeptide E | HSV-1 (strain 15577) IC50 19.8 µM | |||
| RC28 (28.25 kDa) protein | BGMK cells, HSV-1 (KOS) CPE | IC50 0.078 mg/mL, SI > 32 | Edible mushroom Rozites caperata (Cortinarious caperata) | [112] |
| Pa-MAP | Vero cells, HSV-1 Virus titer reduction method Virucidal assay ACV at 20 μg/mL PI 99% | EC50 83 μg/mL, SI ˃ 5 | Polar fish Pleuronectes americanus | [113] |
| Bovine lactoperoxidase | Vero cells, HSV-1 PRA | At 0.5 mg/mL 100% antiviral effect | Milk hemoprotein | [114] |
| Griffithsin | Vero cells, HeLa cells, HSV-2 (strain G) PrestoBlue cell viability reagent - reading of fluorescence Flow cytometry Inhibition of adsorption assay In vivo HSV-2 murine model | EC50 5.8 μg/mL (230 nM) Griffithsin/carrageenan combination EC50 3.4 ng/mL | Red alga Griffithsia A lectin with high affinity for mannose-rich N-linked glycans. Griffithsin may block viral entry by binding to HSV-2 glycoprotein D. The griffithsin/carrageenan combination product but not GRFT or CG alone, reduced HSV-2 vaginal infection in mice when given an hour before challenge. | [115] |
| Melittin | Vero cells, HSV-1 Virus yield inhibition assay | EC50 1.35 μM; SI 6.3 | Cationic 26 amino acids peptide isolated from insects—the main component of bee venom | [116] |
| Chemical Class | Compound | Mechanisms of Action or Types of Inhibition | Structure–Activity Relationship (SAR) |
|---|---|---|---|
| Flavan-3-ol (flavonoid) | Epicatechin gallate (ECG) (8) | Inhibition of viral adsorption. | — |
| Flavonol (flavonoid) | Galangin (11) | Inhibition of viral adsorption. | — |
| Flavonol (flavonoid) | Quercetin (19) | Inhibition of the expressions of HSV proteins (gD, ICP0) and genes (ICP0, UL13, UL52). Additionally, this molecule suppressed the expression of TLR-3 and inhibited the transcriptional factors NF-κB and IRF3. | — |
| Flavonoid | Houttuynoid A (21) | Blocking viral membrane fusion. | — |
| Phenolics | kuwanon C (22), kuwanon T (23), kuwanon U (24), kuwanon E (25), and ethyl 2,4-dihydroxybenzoate (37) | Inhibition of HSV-1 and HSV-2 replication (in vitro) and inactivation of HSV-1 DNA polymerase and HSV-2 protease (proposed as competitive inhibitors via in silico assay). | Hydroxyl, carbonyl, and methyl groups along with phenyl ring (proposed as functional groups via in silico assays). |
| Alkyl derivatives of gallic acid | Octyl gallate (39) | Inhibition of multiplication of HSV-1 and suppression of formation of virus progeny at early stages (within 6 h post-infection) in the infected cells. | Alkyl moieties. |
| Tannins | Chebulagic acid (40) and chebulinic acid (41) | Avoiding the attachment and penetration of HSV-2 into Vero cells. | — |
| β-orcinol depsidone, a type of phenolic compound | Psoromic acid (45) | Inhibition of HSV-1 and HSV-2 replication and inactivation of HSV-1 DNA polymerase (competitive inhibitor via in vitro and in silico experiments). Also, via in silico assay, inactivates HSV-2 protease (competitive inhibitor). | Hydroxyl, carbonyl, and methyl groups along with phenyl ring (proposed as functional groups via in silico assays). |
| Stilbene derivative | Kuwanon X (51) | Anti-HSV activity through multiple modes of action (impeded cellular adsorption and penetration of HSV-1 viral particles). After viral penetration, this agent decreased the expression of HSV-1 IE and L genes and diminished the synthesis of HSV-1 DNA. Moreover, this molecule prevented the HSV-1-induced nuclear factor (NF)-κB activation via obstructing the nuclear translocation and DNA binding of NF-κB. | — |
| Flavonoid | Curcumin (56) | Inhibition of adsorption and replication of HSV. | Hydroxyl groups (assessed as functional groups). |
| Alkaloid | Harmine (59) | Inhibition of viral protein expression. | — |
| Monoterpenoid | Geraniol (62) | Inhibition of HSV-2 replication (in vitro assay) and inactivation of HSV-2 protease (in silico assay). | Hydroxyl and methyl groups (proposed as functional groups via in silico assay). |
| Steroids | Halistanol sulfate (96) and halistanol sulfate C (97) | Suppression of HSV-1 attachment and penetration into the host cells. These substances also impair the levels of ICP27 and gD proteins of HSV-1. | Sulfate groups (assessed as functional groups). |
| Triterpene glycoside | Glycyrrhizic acid (98) | The compound was detected to be an effective inducer of the autophagy activator Beclin 1, which creates a resistance to HSV-1 replication. | Carboxyl and hydroxyl groups along with sugar moiety (assessed as functional groups). |
| Triterpenoid | Methylester of glycyrrhetic acid (102) | Inhibition of HSV-1 replication. | Methoxy and carboxy groups at C-20 were noted to be responsible for the enhanced inhibitory activity against HSV-1 replication. |
| Pentacyclic triterpenoid | Oleanolic acid (103) | Inhibition of HSV-1 and HSV-2 multiplication at the early stage. | — |
| Spiroketal-enol ether derivative | (E)-2-(2,4-hexa-diynyliden)-1,6-dioxaspiro[4.5] dec-3-ene (111) | Suppression of viral gene expression and reduction of viral protein accumulation within infected cells. | — |
| Taxol derivatives | Methyl (N-benzoyl-(2′R,3′S)-3′-phenylisoserinate) (113) and N-benzoyl-(2′R,3′S)-3′-phenylisoserine (114) | Inhibition of HSV-1 replication (the inhibitory activity might be related to the impact on the mitotic division). | — |
| Polysaccharides | Polysaccharides and sulfated polysaccharides | Multiple mechanisms of action (inhibition of HSV replication, inhibition of virus adsorption, suppression of gene expression, suppression of HSV attachment and penetration into the host cell). | Sugar moieties and sulfate groups. |
| Cyclic peptide | Subtilosin | This antiherpetic agent alters the late stages of the viral replicative cycle such as viral glycoprotein intracellular transport. | — |
| Peptide | Griffithsin | Blocking viral entry by attaching with HSV-2 glycoprotein D. | — |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Treml, J.; Gazdová, M.; Šmejkal, K.; Šudomová, M.; Kubatka, P.; Hassan, S.T.S. Natural Products-Derived Chemicals: Breaking Barriers to Novel Anti-HSV Drug Development. Viruses 2020, 12, 154. https://doi.org/10.3390/v12020154
Treml J, Gazdová M, Šmejkal K, Šudomová M, Kubatka P, Hassan STS. Natural Products-Derived Chemicals: Breaking Barriers to Novel Anti-HSV Drug Development. Viruses. 2020; 12(2):154. https://doi.org/10.3390/v12020154
Chicago/Turabian StyleTreml, Jakub, Markéta Gazdová, Karel Šmejkal, Miroslava Šudomová, Peter Kubatka, and Sherif T. S. Hassan. 2020. "Natural Products-Derived Chemicals: Breaking Barriers to Novel Anti-HSV Drug Development" Viruses 12, no. 2: 154. https://doi.org/10.3390/v12020154
APA StyleTreml, J., Gazdová, M., Šmejkal, K., Šudomová, M., Kubatka, P., & Hassan, S. T. S. (2020). Natural Products-Derived Chemicals: Breaking Barriers to Novel Anti-HSV Drug Development. Viruses, 12(2), 154. https://doi.org/10.3390/v12020154








