In Vitro Evaluation of a Phage Cocktail Controlling Infections with Escherichia coli
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Growth Conditions
2.2. Phage Isolation, Purification, and Propagation
2.3. Morphology of Phages and Analysis of Phage Bacteria Interactions
2.4. Host Range and Efficiency of Plating (EOP) Analysis
2.5. One-Step Growth Assays
2.6. Stability of Phages
2.7. Identification of a Suitable E. coli Strain Serving as a Basis for In Vivo Experiments
2.8. Lytic Efficacy of Phages and Phage Preparations
2.9. In Vitro Biofilm Model
2.10. Isolation of Phage-Resistant Variants
2.11. Analysis of the Carbon Consumption of Phage-Resistant Variants Using the Biolog GN2 MicroPlateTM Assay
2.12. Growth and Swimming Motility of Phage-Resistant Variants
2.13. DNA Isolation from Phages and Bacteria
2.14. Library Preparation and Whole Genome Sequencing
2.15. Bioinformatic Analyses
3. Results
3.1. Identification and Characterization of Bacteriophages Active against E. coli Field Isolates of Human and Animal Origin
3.1.1. Six Phages were Isolated That Represent Different Morphological Subgroups of Myoviruses and Show Overlapping Host Ranges and Different Growth Characteristics
3.1.2. All Bacteriophages Showed Lytic Activity under Conditions Relevant for In Vivo Investigation
3.2. Effectivity of the Phages against Model Strain E28
3.2.1. E. coli Strain E28 was Shown to Allow Selective Quantification and Distinction from Other E. coli Strains, thus Being a Suitable Model Strain for In Vivo Experiments
3.2.2. Phage Combinations Show Dose Dependent Lysis of E. coli Model Strain E28
3.2.3. The Six-Phage Preparation Effectively Reduces Biofilm Formation by E28 and DH5α but Not by ECOR10
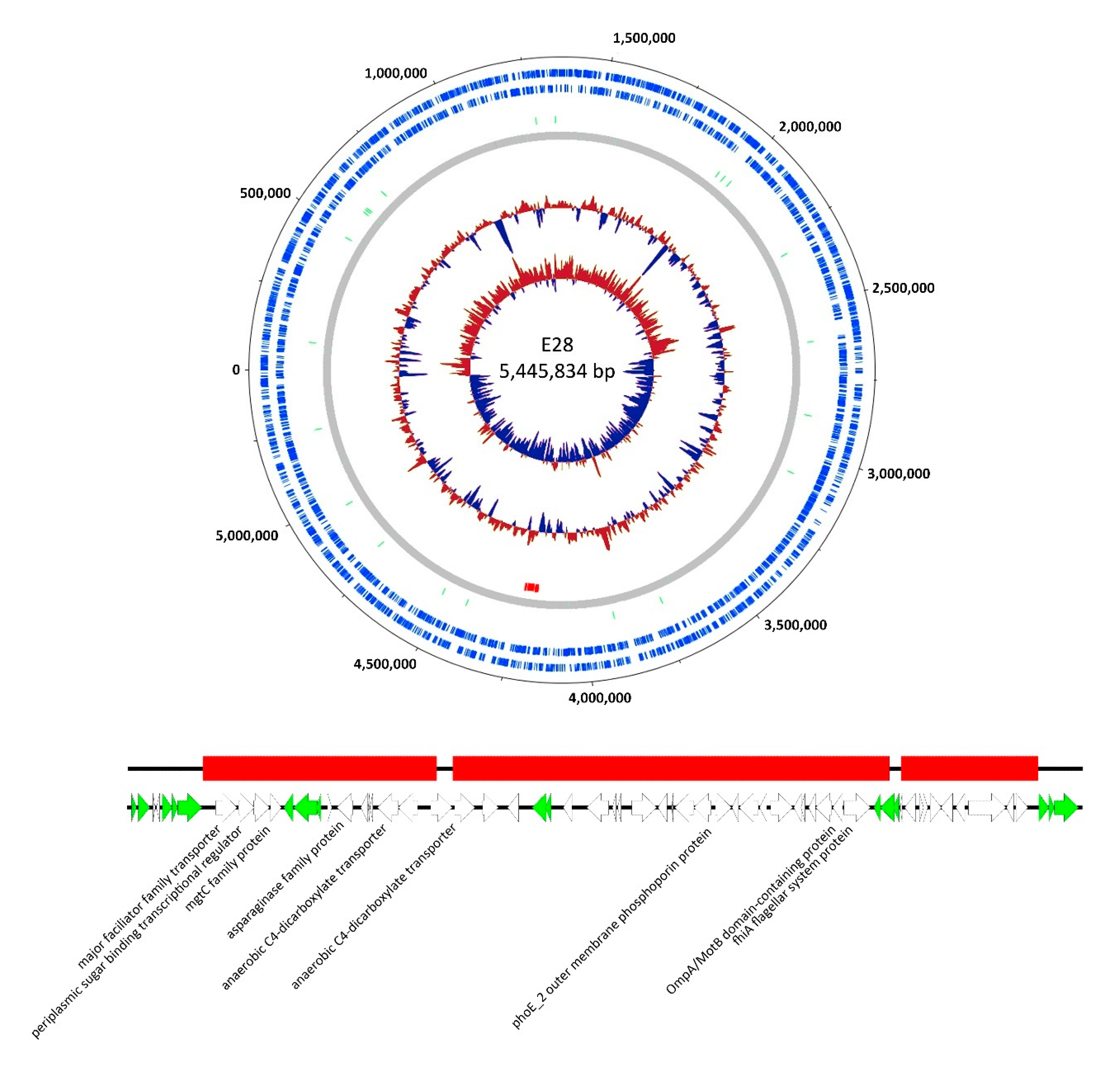
3.3. Genomic Analysis of G28
3.4. Phenotypic and Genotypic Characterization of Phage-Resistant Variants of E. coli E28
3.4.1. Some Phage-Resistant Strain E28 Variants Show Decreased Growth, Reduced Metabolic Ability, and No Motility
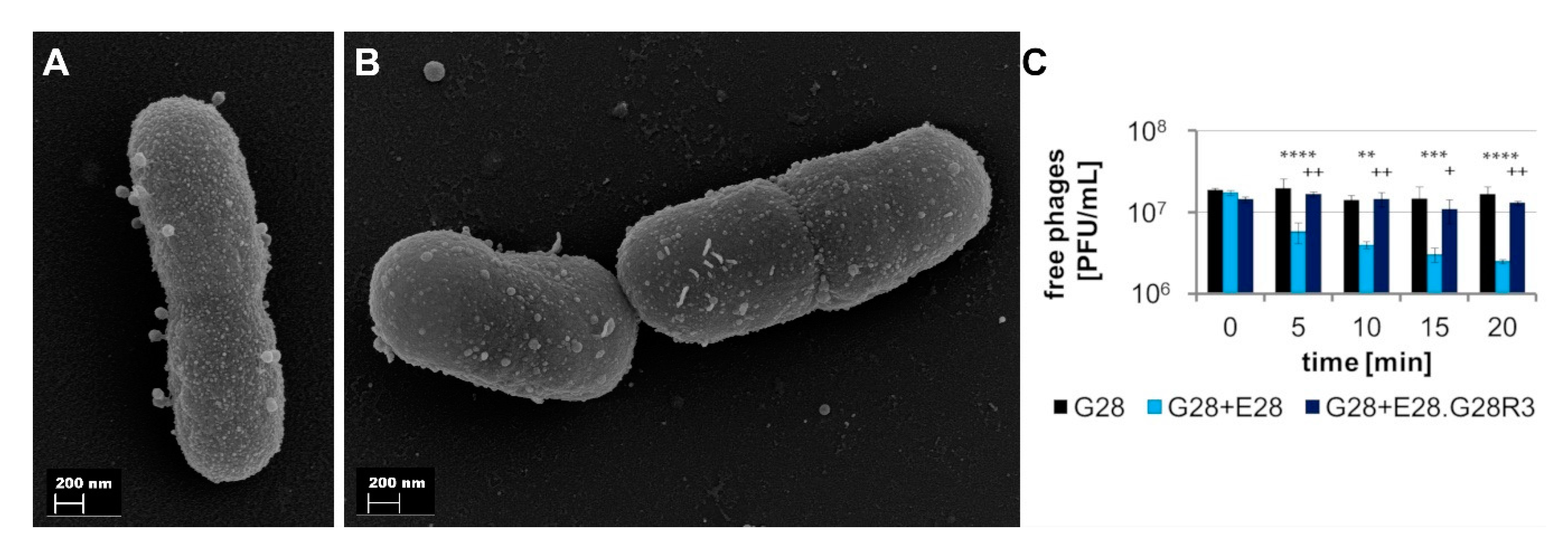
3.4.2. E28.G28R3 Shows Altered Adsorption Behavior and Changes in the Genome
4. Discussion
4.1. Selection of an E. coli Model Strain and Cocktail Composition for a Subsequent Animal Experiment Testing Phage Application against Intestinal Colonization of APEC
4.2. Cocktail Composition Based on Diversity of Phages
4.3. Phages Show Sufficient In Vitro Stability for Testing In Vivo
4.4. In Vitro Determination of an Efficient Cocktail against E28
4.5. Phages Were Composed to Prevent the Development of Resistance
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nolan, L.K.; Barnes, H.J.; Vaillancourt, J.P.; Abdul-Aziz, T.; Logue, C.M. Colibacillosis. In Diseases of Poultry, 13th ed.; Swayne, D.E., Glisson, J.R., McDougald, L.R., Nolan, L.K., Suarez, D.L., Nair, V.L., Eds.; Wiley-Blackwell: Oxford, UK, 2013; pp. 751–805. [Google Scholar]
- Ewers, C.; Antao, E.M.; Diehl, I.; Philipp, H.C.; Wieler, L.H. Intestine and environment of the chicken as reservoirs for extraintestinal pathogenic Escherichia coli strains with zoonotic potential. Appl. Environ. Microbiol. 2009, 75, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Schouler, C.; Schaeffer, B.; Bree, A.; Mora, A.; Dahbi, G.; Biet, F.; Oswald, E.; Mainil, J.; Blanco, J.; Moulin-Schouleur, M. Diagnostic strategy for identifying avian pathogenic Escherichia coli based on four patterns of virulence genes. J. Clin. Microbiol. 2012, 50, 1673–1678. [Google Scholar] [CrossRef] [PubMed]
- Dziva, F.; Stevens, M.P. Colibacillosis in poultry: Unravelling the molecular basis of virulence of avian pathogenic Escherichia coli in their natural hosts. Avian Pathol. 2008, 37, 355–366. [Google Scholar] [CrossRef] [PubMed]
- Levy, S.B. Antibiotic resistance: Consequences of inaction. Clin. Infect. Dis. 2001, 33 (Suppl. 3), S124–S129. [Google Scholar] [CrossRef]
- Overdevest, I.; Willemsen, I.; Rijnsburger, M.; Eustace, A.; Xu, L.; Hawkey, P.; Heck, M.; Savelkoul, P.; Vandenbroucke-Grauls, C.; van der Zwaluw, K.; et al. Extended-spectrum beta-lactamase genes of Escherichia coli in chicken meat and humans, The Netherlands. Emerg. Infect. Dis. 2011, 17, 1216–1222. [Google Scholar] [CrossRef]
- Ewers, C.; Li, G.; Wilking, H.; Kiessling, S.; Alt, K.; Antao, E.M.; Laturnus, C.; Diehl, I.; Glodde, S.; Homeier, T.; et al. Avian pathogenic, uropathogenic, and newborn meningitis-causing Escherichia coli: How closely related are they? Int. J. Med. Microbiol. 2007, 297, 163–176. [Google Scholar] [CrossRef] [PubMed]
- Abedon, S.T.; Garcia, P.; Mullany, P.; Aminov, R. Editorial: Phage therapy: Past, present and future. Front. Microbiol. 2017, 8, 981. [Google Scholar] [CrossRef]
- Pirnay, J.-P.; Verbeken, G.; Rose, T.; Jennes, S.; Zizi, M.; Huys, I.; Lavigne, R.; Merabishvili, M.; Vaneechoutte, M.; Buckling, A.; et al. Introducing yesterday’s phage therapy in today’s medicine. Future Virol. 2012, 7, 379–390. [Google Scholar] [CrossRef]
- Chan, B.K.; Abedon, S.T.; Loc-Carrillo, C. Phage cocktails and the future of phage therapy. Future Microbiol. 2013, 8, 769–783. [Google Scholar] [CrossRef]
- Johnson, R.P.; Gyles, C.L.; Huff, W.E.; Ojha, S.; Huff, G.R.; Rath, N.C.; Donoghue, A.M. Bacteriophages for prophylaxis and therapy in cattle, poultry and pigs. Anim. Health Res. Rev. 2008, 9, 201–215. [Google Scholar]
- Colavecchio, A.; Goodridge, L.D. Phage therapy approaches to reducing pathogen persistence and transmission in animal production environments: Opportunities and challenges. Microbiol. Spectr. 2017, 5, 1–14. [Google Scholar]
- Abedon, S.T.; Kuhl, S.J.; Blasdel, B.G.; Kutter, E.M. Phage treatment of human infections. Bacteriophage 2011, 1, 66–85. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, C.; Costa, A.R.; Silva, F.; Oliveira, A. Bacteriophages and their derivatives for the treatment and control of food-producing animal infections. Crit. Rev. Microbiol. 2017, 43, 583–601. [Google Scholar] [CrossRef] [PubMed]
- Grant, A.; Parveen, S.; Schwarz, J.; Hashem, F.; Vimini, B. Reduction of Salmonella in ground chicken using a bacteriophage. Poult. Sci. 2017, 96, 2845–2852. [Google Scholar] [CrossRef]
- Lau, G.L.; Sieo, C.C.; Tan, W.S.; Hair-Bejo, M.; Jalila, A.; Ho, Y.W. Efficacy of a bacteriophage isolated from chickens as a therapeutic agent for colibacillosis in broiler chickens. Poult. Sci. 2010, 89, 2589–2596. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.; Sereno, R.; Azeredo, J. In vivo efficiency evaluation of a phage cocktail in controlling severe colibacillosis in confined conditions and experimental poultry houses. Vet. Microbiol. 2010, 146, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Tsonos, J.; Oosterik, L.H.; Tuntufye, H.N.; Klumpp, J.; Butaye, P.; De Greve, H.; Hernalsteens, J.P.; Lavigne, R.; Goddeeris, B.M. A cocktail of in vitro efficient phages is not a guarantee for in vivo therapeutic results against avian colibacillosis. Vet. Microbiol. 2014, 171, 470–479. [Google Scholar] [CrossRef]
- Oliveira, A.; Sillankorva, S.; Quinta, R.; Henriques, A.; Sereno, R.; Azeredo, J. Isolation and characterization of bacteriophages for avian pathogenic E. coli strains. J. Appl. Microbiol. 2009, 106, 1919–1927. [Google Scholar]
- Kittler, S.; Wittmann, J.; Mengden, R.A.L.P.; Klein, G.; Rohde, C.; Lehnherr, H. The use of bacteriophages as One-Health approach to reduce multidrug-resistant bacteria. Sustain. Chem. Pharm. 2017, 5, 80–83. [Google Scholar] [CrossRef]
- Kittler, S.; Mengden, R.; Korf, I.H.E.; Bierbrodt, A.; Wittmann, J.; Plotz, M.; Jung, A.; Lehnherr, T.; Rohde, C.; Lehnherr, H.; et al. Impact of bacteriophage-supplemented drinking water on the E. coli population in the chicken gut. Pathogens 2020, 9, 293. [Google Scholar] [CrossRef]
- Toma, C.; Higa, N.; Iyoda, S.; Rivas, M.; Iwanaga, M. The long polar fimbriae genes identified in Shiga toxin-producing Escherichia coli are present in other diarrheagenic E. coli and in the standard E. coli collection of reference (ECOR) strains. Res. Microbiol. 2006, 157, 153–161. [Google Scholar] [CrossRef]
- Amor, K.; Heinrichs, D.E.; Frirdich, E.; Ziebell, K.; Johnson, R.P.; Whitfield, C. Distribution of core oligosaccharide types in lipopolysaccharides from Escherichia coli. Infect. Immun. 2000, 68, 1116–1124. [Google Scholar] [CrossRef] [PubMed]
- Braun, S.D.; Ahmed, M.F.; El-Adawy, H.; Hotzel, H.; Engelmann, I.; Weiss, D.; Monecke, S.; Ehricht, R. Surveillance of extended-spectrum beta-lactamase-producing Escherichia coli in dairy cattle farms in the Nile Delta, Egypt. Front. Microbiol. 2016, 7, 1020. [Google Scholar] [CrossRef]
- Kropinski, A.M.; Mazzocco, A.; Waddell, T.E.; Lingohr, E.; Johnson, R.P. Enumeration of bacteriophages by double agar overlay plaque assay. Methods Mol. Biol. 2009, 501, 69–76. [Google Scholar] [PubMed]
- Dreiseikelmann, B.; Bunk, B.; Sproer, C.; Rohde, M.; Nimtz, M.; Wittmann, J. Characterization and genome comparisons of three Achromobacter phages of the family Siphoviridae. Arch. Virol. 2017, 162, 2191–2201. [Google Scholar] [CrossRef] [PubMed]
- Wittmann, J.; Dreiseikelmann, B.; Rohde, M.; Meier-Kolthoff, J.P.; Bunk, B.; Rohde, C. First genome sequences of Achromobacter phages reveal new members of the N4 family. Virol. J. 2014, 11, 14. [Google Scholar] [CrossRef]
- Washizaki, A.; Yonesaki, T.; Otsuka, Y. Characterization of the interactions between Escherichia coli receptors, LPS and OmpC, and bacteriophage T4 long tail fibers. Microbiologyopen 2016, 5, 1003–1015. [Google Scholar] [CrossRef]
- Kaliniene, L.; Klausa, V.; Truncaite, L. Low-temperature T4-like coliphages vB_EcoM-VR5, vB_EcoM-VR7 and vB_EcoM-VR20. Arch. Virol. 2010, 155, 871–880. [Google Scholar] [CrossRef]
- Jurczak-Kurek, A.; Gasior, T.; Nejman-Falenczyk, B.; Bloch, S.; Dydecka, A.; Topka, G.; Necel, A.; Jakubowska-Deredas, M.; Narajczyk, M.; Richert, M.; et al. Biodiversity of bacteriophages: Morphological and biological properties of a large group of phages isolated from urban sewage. Sci. Rep. 2016, 6, 34338. [Google Scholar] [CrossRef]
- Kaliniene, L.; Truncaite, L.; Simoliunas, E.; Zajanckauskaite, A.; Vilkaityte, M.; Kaupinis, A.; Skapas, M.; Meskys, R. Molecular analysis of the low-temperature Escherichia coli phage vB_EcoS_NBD2. Arch. Virol. 2018, 163, 105–114. [Google Scholar] [CrossRef]
- Lee, H.; Ku, H.-J.; Lee, D.-H.; Kim, Y.-T.; Shin, H.; Ryu, S.; Lee, J.-H. Characterization and genomic study of the novel bacteriophage HY01 infecting both Escherichia coli O157:H7 and Shigella flexneri: Potential as a biocontrol agent in food. PLoS ONE 2016, 11, e0168985. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Breidt, F. Escherichia coli O157:H7 bacteriophage Φ241 isolated from an industrial cucumber fermentation at high acidity and salinity. Front. Microbiol. 2015, 6, 67. [Google Scholar] [CrossRef] [PubMed]
- Wittmann, J.; Dreiseikelmann, B.; Rohde, C.; Rohde, M.; Sikorski, J. Isolation and characterization of numerous novel phages targeting diverse strains of the ubiquitous and opportunistic pathogen Achromobacter xylosoxidans. PLoS ONE 2014, 9, e86935. [Google Scholar] [CrossRef] [PubMed]
- Vaas, L.A.; Sikorski, J.; Michael, V.; Goker, M.; Klenk, H.P. Visualization and curve-parameter estimation strategies for efficient exploration of phenotype microarray kinetics. PLoS ONE 2012, 7, e34846. [Google Scholar] [CrossRef] [PubMed]
- Chibeu, A.; Lingohr, E.J.; Masson, L.; Manges, A.; Harel, J.; Ackermann, H.W.; Kropinski, A.M.; Boerlin, P. Bacteriophages with the ability to degrade uropathogenic Escherichia coli biofilms. Viruses 2012, 4, 471–487. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Xu, Y.; Xu, J.; Yu, X.; Huang, X.; Liu, G.; Liu, X. Identification of novel bacteriophage vB_EcoP-EG1 with lytic activity against planktonic and biofilm forms of uropathogenic Escherichia coli. Appl. Microbiol. Biotechnol. 2019, 103, 315–326. [Google Scholar] [PubMed]
- Denes, T.; den Bakker, H.C.; Tokman, J.I.; Guldimann, C.; Wiedmann, M. Selection and characterization of phage-resistant mutant strains of Listeria monocytogenes reveal host genes linked to phage adsorption. Appl. Environ. Microbiol. 2015, 81, 4295–4305. [Google Scholar]
- Schmidt, I.; Riedel, T.; Schober, I.; Bunk, B.; Sproer, C.; Bierbrodt, A.; Lehnherr, H.; Wittmann, J. Genome sequence of Escherichia coli E28, a multidrug-resistant strain isolated from a chicken carcass, and its spontaneously inducible prophage. Genome Announc. 2017, 5. [Google Scholar] [CrossRef]
- Seemann, T. Prokka: Rapid prokaryotic genome annotation. Bioinformatics 2014, 30, 2068–2069. [Google Scholar] [CrossRef]
- Rutherford, K.; Parkhill, J.; Crook, J.; Horsnell, T.; Rice, P.; Rajandream, M.A.; Barrell, B. Artemis: Sequence visualization and annotation. Bioinformatics 2000, 16, 944–945. [Google Scholar] [CrossRef]
- Lesnik, E.A.; Sampath, R.; Levene, H.B.; Henderson, T.J.; McNeil, J.A.; Ecker, D.J. Prediction of rho-independent transcriptional terminators in Escherichia coli. Nucleic Acids Res. 2001, 29, 3583–3594. [Google Scholar] [CrossRef] [PubMed]
- Lowe, T.M.; Eddy, S.R. tRNAscan-SE: A program for improved detection of transfer RNA genes in genomic sequence. Nucleic Acids Res. 1997, 25, 955–964. [Google Scholar] [CrossRef] [PubMed]
- Laslett, D.; Canback, B. ARAGORN, a program to detect tRNA genes and tmRNA genes in nucleotide sequences. Nucleic Acids Res. 2004, 32, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Center for Genomic Epidemiology. Available online: http://www.genomicepidemiology.org/ (accessed on 2 December 2020).
- Bortolaia, V.; Kaas, R.S.; Ruppe, E.; Roberts, M.C.; Schwarz, S.; Cattoir, V.; Philippon, A.; Allesoe, R.L.; Rebelo, A.R.; Florensa, A.F.; et al. ResFinder 4.0 for predictions of phenotypes from genotypes. J. Antimicrob. Chemother. 2020, 75, 3491–3500. [Google Scholar] [CrossRef]
- Joensen, K.G.; Scheutz, F.; Lund, O.; Hasman, H.; Kaas, R.S.; Nielsen, E.M.; Aarestrup, F.M. Real-time whole-genome sequencing for routine typing, surveillance, and outbreak detection of verotoxigenic Escherichia coli. J. Clin. Microbiol. 2014, 52, 1501–1510. [Google Scholar]
- Koboldt, D.C.; Chen, K.; Wylie, T.; Larson, D.E.; McLellan, M.D.; Mardis, E.R.; Weinstock, G.M.; Wilson, R.K.; Ding, L. VarScan: Variant detection in massively parallel sequencing of individual and pooled samples. Bioinformatics 2009, 25, 2283–2285. [Google Scholar] [CrossRef]
- Thorvaldsdottir, H.; Robinson, J.T.; Mesirov, J.P. Integrative Genomics Viewer (IGV): High-performance genomics data visualization and exploration. Brief. Bioinform. 2013, 14, 178–192. [Google Scholar] [CrossRef]
- Ackermann, H.-W. Frequency of morphological phage descriptions in the year 2000. Arch. Virol. 2001, 146, 843–857. [Google Scholar]
- Ciolacu, L.; Stessl, B.; Bolocan, A.S.; Oniciuc, E.A.; Wagner, M.; Rychli, K.; Nicolau, A.I. Tracking foodborne pathogenic bacteria in raw and ready-to-eat food illegally sold at the eastern EU border. Foodborne Pathog. Dis. 2016, 13, 148–155. [Google Scholar] [CrossRef]
- Zogaj, X.; Nimtz, M.; Rohde, M.; Bokranz, W.; Romling, U. The multicellular morphotypes of Salmonella typhimurium and Escherichia coli produce cellulose as the second component of the extracellular matrix. Mol. Microbiol. 2001, 39, 1452–1463. [Google Scholar] [CrossRef]
- Son, H.M.; Duc, H.M.; Masuda, Y.; Honjoh, K.I.; Miyamoto, T. Application of bacteriophages in simultaneously controlling Escherichia coli O157:H7 and extended-spectrum beta-lactamase producing Escherichia coli. Appl. Microbiol. Biotechnol. 2018, 102, 10259–10271. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, M.J.; Petty, N.K.; Beatson, S.A. Easyfig: A genome comparison visualiser. Bioinformatics 2011, 27, 1009–1010. [Google Scholar] [CrossRef] [PubMed]
- Pirnay, J.P.; Blasdel, B.G.; Bretaudeau, L.; Buckling, A.; Chanishvili, N.; Clark, J.R.; Corte-Real, S.; Debarbieux, L.; Dublanchet, A.; De Vos, D.; et al. Quality and safety requirements for sustainable phage therapy products. Pharm. Res. 2015, 32, 2173–2179. [Google Scholar] [CrossRef] [PubMed]
- Koskella, B.; Meaden, S. Understanding bacteriophage specificity in natural microbial communities. Viruses 2013, 5, 806–823. [Google Scholar] [CrossRef]
- Kalatzis, P.G.; Bastias, R.; Kokkari, C.; Katharios, P. Isolation and characterization of two lytic bacteriophages, phiSt2 and phiGrn1; Phage therapy application for biological control of Vibrio alginolyticus in aquaculture live feeds. PLoS ONE 2016, 11, e0151101. [Google Scholar] [CrossRef]
- Wernicki, A.; Nowaczek, A.; Urban-Chmiel, R. Bacteriophage therapy to combat bacterial infections in poultry. Virol. J. 2017, 14, 179. [Google Scholar] [CrossRef]
- Gill, J.J.; Hyman, P. Phage choice, isolation, and preparation for phage therapy. Curr. Pharm. Biotechnol. 2010, 11, 2–14. [Google Scholar] [CrossRef]
- Pouillot, F.; Chomton, M.; Blois, H.; Courroux, C.; Noelig, J.; Bidet, P.; Bingen, E.; Bonacorsi, S. Efficacy of bacteriophage therapy in experimental sepsis and meningitis caused by a clone O25b:H4-ST131 Escherichia coli strain producing CTX-M-15. Antimicrob. Agents Chemother. 2012, 56, 3568–3575. [Google Scholar] [CrossRef]
- Kakabadze, E.; Makalatia, K.; Grdzelishvili, N.; Bakuradze, N.; Goderdzishvili, M.; Kusradze, I.; Phoba, M.F.; Lunguya, O.; Lood, C.; Lavigne, R.; et al. Selection of potential therapeutic bacteriophages that lyse a CTX-M-15 extended spectrum beta-lactamase producing Salmonella enterica Serovar Typhi strain from the Democratic Republic of the Congo. Viruses 2018, 10, 172. [Google Scholar] [CrossRef]
- Skaradzinska, A.; Sliwka, P.; Kuzminska-Bajor, M.; Skaradzinski, G.; Rzasa, A.; Friese, A.; Roschanski, N.; Murugaiyan, J.; Roesler, U.H. The efficacy of isolated bacteriophages from pig farms against ESBL/AmpC-producing Escherichia coli from pig and turkey farms. Front. Microbiol. 2017, 8, 530. [Google Scholar] [CrossRef]
- Amarillas, L.; Rubi-Rangel, L.; Chaidez, C.; Gonzalez-Robles, A.; Lightbourn-Rojas, L.; Leon-Felix, J. Isolation and characterization of phiLLS, a novel phage with potential biocontrol agent against multidrug-resistant Escherichia coli. Front. Microbiol. 2017, 8, 1355. [Google Scholar] [CrossRef] [PubMed]
- Foschino, R.; Perrone, F.; Galli, A. Characterization of two virulent Lactobacillus fermentum bacteriophages isolated from sour dough. J. Appl. Bacteriol. 1995, 79, 677–683. [Google Scholar] [CrossRef]
- Chang, H.-C.; Chen, C.-R.; Lin, J.-W.; Shen, G.-H.; Chang, K.-M.; Tseng, Y.-H.; Weng, S.-F. Isolation and characterization of novel giant Stenotrophomonas maltophilia phage φSMA5. Appl. Environ. Microbiol. 2005, 71, 1387–1393. [Google Scholar] [CrossRef] [PubMed]
- Raya, R.R.; Varey, P.; Oot, R.A.; Dyen, M.R.; Callaway, T.R.; Edrington, T.S.; Kutter, E.M.; Brabban, A.D. Isolation and characterization of a new T-even bacteriophage, CEV1, and determination of its potential to reduce Escherichia coli O157:H7 levels in sheep. Appl. Environ. Microbiol. 2006, 72, 6405–6410. [Google Scholar] [CrossRef]
- Bao, H.; Zhang, H.; Wang, R. Isolation and characterization of bacteriophages of Salmonella enterica serovar Pullorum. Poult. Sci. 2011, 90, 2370–2377. [Google Scholar]
- Park, M.; Lee, J.H.; Shin, H.; Kim, M.; Choi, J.; Kang, D.H.; Heu, S.; Ryu, S. Characterization and comparative genomic analysis of a novel bacteriophage, SFP10, simultaneously inhibiting both Salmonella enterica and Escherichia coli O157:H7. Appl. Environ. Microbiol. 2012, 78, 58–69. [Google Scholar] [CrossRef]
- Gallet, R.; Kannoly, S.; Wang, I.N. Effects of bacteriophage traits on plaque formation. BMC Microbiol. 2011, 11, 181. [Google Scholar] [CrossRef]
- Muller-Merbach, M.; Kohler, K.; Hinrichs, J. Environmental factors for phage-induced fermentation problems: Replication and adsorption of the Lactococcus lactis phage P008 as influenced by temperature and pH. Food Microbiol. 2007, 24, 695–702. [Google Scholar] [CrossRef]
- Chhibber, S.; Kaur, T.; Kaur, S. Essential role of calcium in the infection process of broad-spectrum methicillin-resistant Staphylococcus aureus bacteriophage. J. Basic Microbiol. 2014, 54, 775–780. [Google Scholar]
- Niu, Y.D.; Stanford, K.; Kropinski, A.M.; Ackermann, H.W.; Johnson, R.P.; She, Y.M.; Ahmed, R.; Villegas, A.; McAllister, T.A. Genomic, proteomic and physiological characterization of a T5-like bacteriophage for control of Shiga toxin-producing Escherichia coli O157:H7. PLoS ONE 2012, 7, e34585. [Google Scholar] [CrossRef]
- Jung, L.S.; Ding, T.; Ahn, J. Evaluation of lytic bacteriophages for control of multidrug-resistant Salmonella Typhimurium. Ann. Clin. Microbiol. Antimicrob. 2017, 16, 66. [Google Scholar] [CrossRef] [PubMed]
- Richter, W.; Werner, E.; Bähr, H. Grundwerte der Tiergesundheit und Tierhaltung; Enke: Stuttgart, Germany, 1992. [Google Scholar]
- Cieplak, T.; Soffer, N.; Sulakvelidze, A.; Nielsen, D.S. A bacteriophage cocktail targeting Escherichia coli reduces E. coli in simulated gut conditions, while preserving a non-targeted representative commensal normal microbiota. Gut Microbes 2018, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, M.; Karimi Torshizi, M.A.; Rahimi, S.; Dennehy, J.J. Prophylactic bacteriophage administration more effective than post-infection administration in reducing Salmonella enterica serovar enteritidis shedding in quail. Front. Microbiol. 2016, 7, 1253. [Google Scholar] [CrossRef]
- Oliveira, A.; Sereno, R.; Nicolau, A.; Azeredo, J. The influence of the mode of administration in the dissemination of three coliphages in chickens. Poult. Sci. 2009, 88, 728–733. [Google Scholar] [CrossRef]
- Svab, D.; Falgenhauer, L.; Rohde, M.; Szabo, J.; Chakraborty, T.; Toth, I. Identification and characterization of T5-Like bacteriophages representing two novel subgroups from food products. Front. Microbiol. 2018, 9, 202. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Ryu, S. Characterization of a T5-like coliphage, SPC35, and differential development of resistance to SPC35 in Salmonella enterica serovar typhimurium and Escherichia coli. Appl. Environ. Microbiol. 2011, 77, 2042–2050. [Google Scholar] [CrossRef] [PubMed]
- Kocharunchitt, C.; Ross, T.; McNeil, D.L. Use of bacteriophages as biocontrol agents to control Salmonella associated with seed sprouts. Int. J. Food Microbiol. 2009, 128, 453–459. [Google Scholar] [CrossRef]
- O’Flynn, G.; Coffey, A.; Fitzgerald, G.F.; Ross, R.P. The newly isolated lytic bacteriophages st104a and st104b are highly virulent against Salmonella enterica. J. Appl. Microbiol. 2006, 101, 251–259. [Google Scholar]
- Henry, M.; Lavigne, R.; Debarbieux, L. Predicting in vivo efficacy of therapeutic bacteriophages used to treat pulmonary infections. Antimicrob. Agents Chemother. 2013, 57, 5961–5968. [Google Scholar] [CrossRef]
- Sun, H.; Tang, J.W.; Yao, X.H.; Wu, Y.F.; Wang, X.; Feng, J. Effects of dietary inclusion of fermented cottonseed meal on growth, cecal microbial population, small intestinal morphology, and digestive enzyme activity of broilers. Trop. Anim. Health Prod. 2013, 45, 987–993. [Google Scholar] [CrossRef]
- Pires, D.P.; Dotsch, A.; Anderson, E.M.; Hao, Y.; Khursigara, C.M.; Lam, J.S.; Sillankorva, S.; Azeredo, J. A genotypic analysis of five P. aeruginosa strains after biofilm Infection by phages targeting different cell surface receptors. Front. Microbiol. 2017, 8, 1229. [Google Scholar] [CrossRef]
- Nzakizwanayo, J.; Hanin, A.; Alves, D.R.; McCutcheon, B.; Dedi, C.; Salvage, J.; Knox, K.; Stewart, B.; Metcalfe, A.; Clark, J.; et al. Bacteriophage can prevent encrustation and blockage of urinary catheters by Proteus mirabilis. Antimicrob. Agents Chemother. 2015, 60, 1530–1536. [Google Scholar] [CrossRef] [PubMed]
- Alves, D.R.; Booth, S.P.; Scavone, P.; Schellenberger, P.; Salvage, J.; Dedi, C.; Thet, N.T.; Jenkins, A.T.A.; Waters, R.; Ng, K.W.; et al. Development of a high-throughput ex-vivo burn wound model using porcine skin, and its application to evaluate new approaches to control wound infection. Front. Cell. Infect. Microbiol. 2018, 8, 196. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, K.V.G.; Ribeiro, C.; Dias, R.S.; Cardoso, S.A.; de Paula, S.O.; Zanuncio, J.C.; de Oliveira, L.L. Bacteriophage isolated from sewage eliminates and prevents the establishment of Escherichia coli biofilm. Adv. Pharm. Bull. 2018, 8, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Maes, S.; Vackier, T.; Nguyen Huu, S.; Heyndrickx, M.; Steenackers, H.; Sampers, I.; Raes, K.; Verplaetse, A.; De Reu, K. Occurrence and characterisation of biofilms in drinking water systems of broiler houses. BMC Microbiol. 2019, 19, 77. [Google Scholar] [CrossRef]
- Fong, S.A.; Drilling, A.; Morales, S.; Cornet, M.E.; Woodworth, B.A.; Fokkens, W.J.; Psaltis, A.J.; Vreugde, S.; Wormald, P.J. Activity of bacteriophages in removing biofilms of Pseudomonas aeruginosa isolates from chronic rhinosinusitis patients. Front. Cell. Infect. Microbiol. 2017, 7, 418. [Google Scholar] [CrossRef]
- Drilling, A.; Morales, S.; Jardeleza, C.; Vreugde, S.; Speck, P.; Wormald, P.J. Bacteriophage reduces biofilm of Staphylococcus aureus ex vivo isolates from chronic rhinosinusitis patients. Am. J. Rhinol. Allergy 2014, 28, 3–11. [Google Scholar] [CrossRef]
- Alves, D.R.; Perez-Esteban, P.; Kot, W.; Bean, J.E.; Arnot, T.; Hansen, L.H.; Enright, M.C.; Jenkins, A.T. A novel bacteriophage cocktail reduces and disperses Pseudomonas aeruginosa biofilms under static and flow conditions. Microb. Biotechnol. 2016, 9, 61–74. [Google Scholar]
- Azeredo, J.; Sutherland, I.W. The use of phages for the removal of infectious biofilms. Curr. Pharm. Biotechnol. 2008, 9, 261–266. [Google Scholar] [CrossRef]
- Chibani-Chennoufi, S.; Sidoti, J.; Bruttin, A.; Kutter, E.; Sarker, S.; Brüssow, H. In vitro and In vivo bacteriolytic activities of Escherichia coli phages: Implications for phage therapy. Antimicrob. Agents Chemother. 2004, 48, 2558–2569. [Google Scholar]
- Hooton, S.P.; Atterbury, R.J.; Connerton, I.F. Application of a bacteriophage cocktail to reduce Salmonella Typhimurium U288 contamination on pig skin. Int. J. Food Microbiol. 2011, 151, 157–163. [Google Scholar] [CrossRef]
- Tanji, Y.; Shimada, T.; Yoichi, M.; Miyanaga, K.; Hori, K.; Unno, H. Toward rational control of Escherichia coli O157:H7 by a phage cocktail. Appl. Microbiol. Biotechnol. 2004, 64, 270–274. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Liu, X.; Li, Y.; Han, W.; Lei, L.; Yang, Y.; Zhao, H.; Gao, Y.; Song, J.; Lu, R.; et al. A method for generation phage cocktail with great therapeutic potential. PLoS ONE 2012, 7, e31698. [Google Scholar] [CrossRef] [PubMed]
- Nobrega, F.L.; Vlot, M.; de Jonge, P.A.; Dreesens, L.L.; Beaumont, H.J.E.; Lavigne, R.; Dutilh, B.E.; Brouns, S.J.J. Targeting mechanisms of tailed bacteriophages. Nat. Rev. Genet. 2018, 16, 760–773. [Google Scholar]
- Yu, F.; Mizushima, S. Roles of lipopolysaccharide and outer membrane protein OmpC of Escherichia coli K-12 in the receptor function for bacteriophage T4. J. Bacteriol. 1982, 151, 718–722. [Google Scholar] [CrossRef] [PubMed]
- Montag, D.; Hashemolhosseini, S.; Henning, U. Receptor-recognizing proteins of T-even type bacteriophages. The receptor-recognizing area of proteins 37 of phages T4 TuIa and TuIb. J. Mol. Biol. 1990, 216, 327–334. [Google Scholar] [PubMed]
- Trojet, S.N.; Caumont-Sarcos, A.; Perrody, E.; Comeau, A.M.; Krisch, H.M. The gp38 adhesins of the T4 superfamily: A complex modular determinant of the phage’s host specificity. Genome Biol. Evol. 2011, 3, 674–686. [Google Scholar] [CrossRef] [PubMed]
- Gauger, E.J.; Leatham, M.P.; Mercado-Lubo, R.; Laux, D.C.; Conway, T.; Cohen, P.S. Role of motility and the flhDC operon in Escherichia coli MG1655 colonization of the mouse intestine. Infect. Immun. 2007, 75, 3315–3324. [Google Scholar] [CrossRef]
- Wang, X.; Wood, T.K. IS5 inserts upstream of the master motility operon flhDC in a quasi-Lamarckian way. ISME J. 2011, 5, 1517–1525. [Google Scholar] [CrossRef]
- Wei, B.L.; Brun-Zinkernagel, A.M.; Simecka, J.W.; Pruss, B.M.; Babitzke, P.; Romeo, T. Positive regulation of motility and flhDC expression by the RNA-binding protein CsrA of Escherichia coli. Mol. Microbiol. 2001, 40, 245–256. [Google Scholar] [CrossRef]
- Hampton, H.G.; McNeil, M.B.; Paterson, T.J.; Ney, B.; Williamson, N.R.; Easingwood, R.A.; Bostina, M.; Salmond, G.P.; Fineran, P.C. CRISPR-Cas gene-editing reveals RsmA and RsmC act through FlhDC to repress the SdhE flavinylation factor and control motility and prodigiosin production in Serratia. Microbiology 2016, 162, 1047–1058. [Google Scholar] [CrossRef]
- Fahrner, K.A.; Berg, H.C. Mutations that stimulate flhDC expression in Escherichia coli K-12. J. Bacteriol. 2015, 197, 3087–3096. [Google Scholar] [CrossRef] [PubMed]
- Chang, D.E.; Smalley, D.J.; Tucker, D.L.; Leatham, M.P.; Norris, W.E.; Stevenson, S.J.; Anderson, A.B.; Grissom, J.E.; Laux, D.C.; Cohen, P.S.; et al. Carbon nutrition of Escherichia coli in the mouse intestine. Proc. Natl. Acad. Sci. USA 2004, 101, 7427–7432. [Google Scholar] [CrossRef] [PubMed]
- Leatham, M.P.; Stevenson, S.J.; Gauger, E.J.; Krogfelt, K.A.; Lins, J.J.; Haddock, T.L.; Autieri, S.M.; Conway, T.; Cohen, P.S. Mouse intestine selects nonmotile flhDC mutants of Escherichia coli MG1655 with increased colonizing ability and better utilization of carbon sources. Infect. Immun. 2005, 73, 8039–8049. [Google Scholar] [CrossRef] [PubMed]
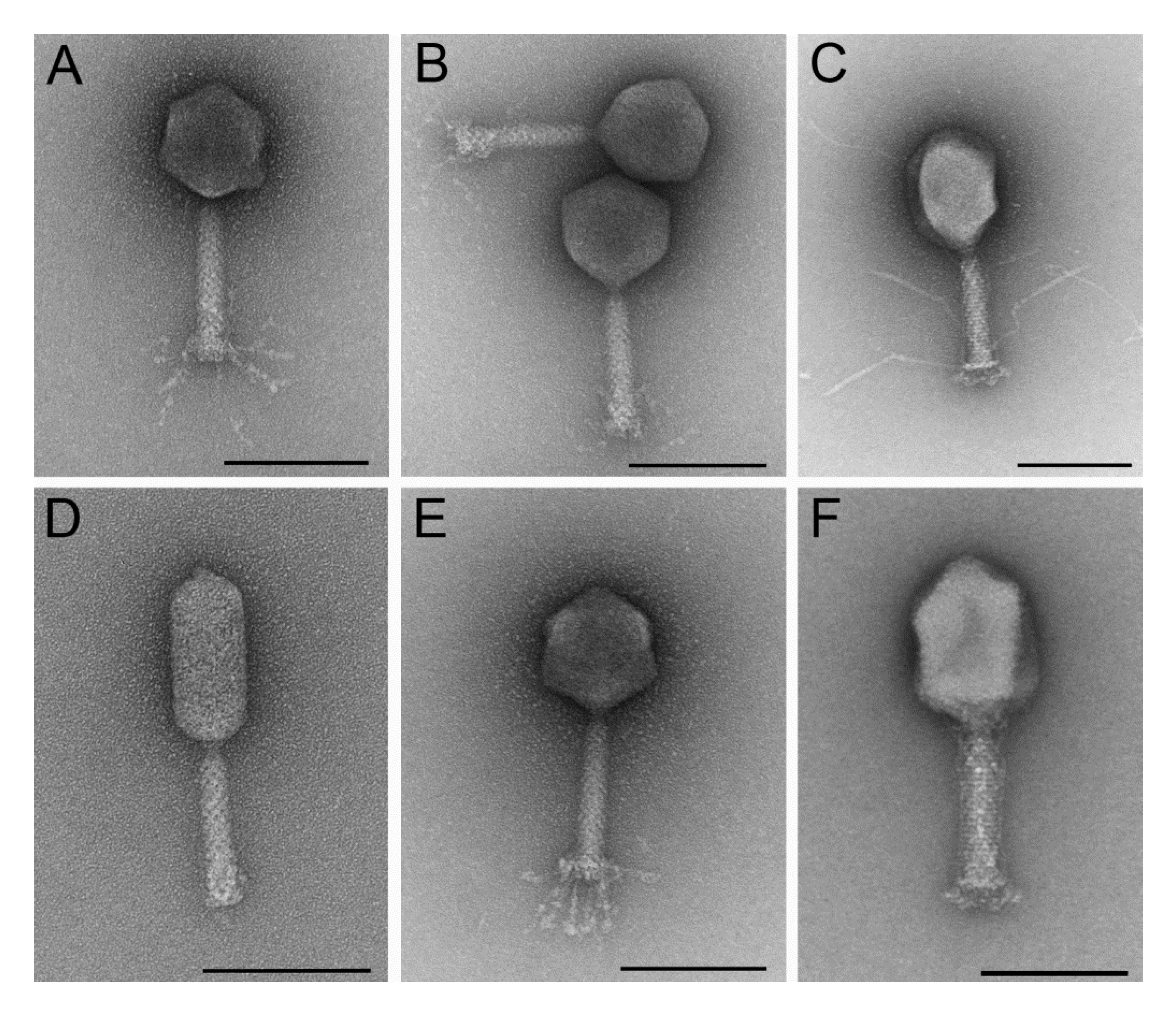
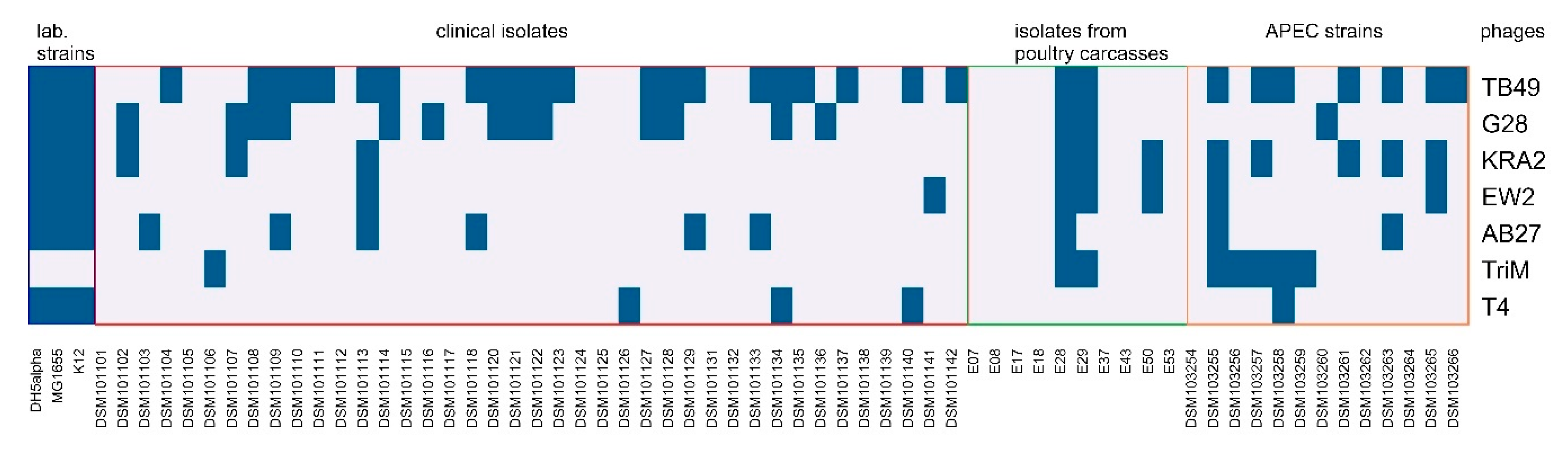
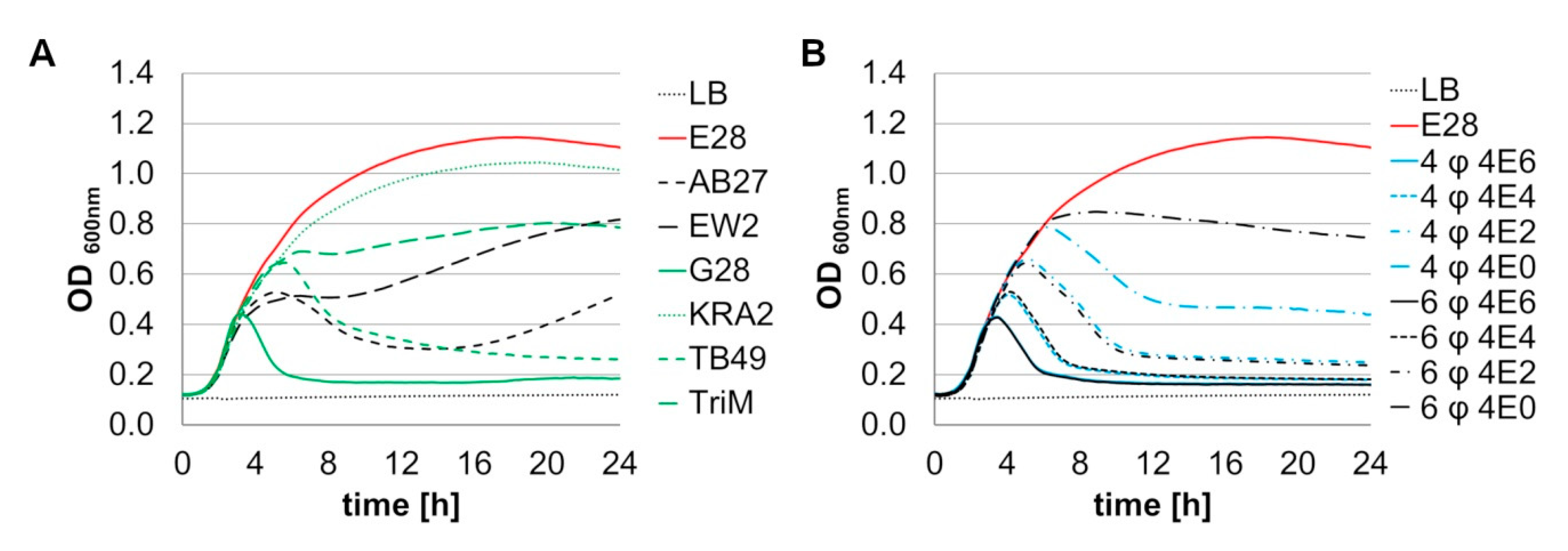
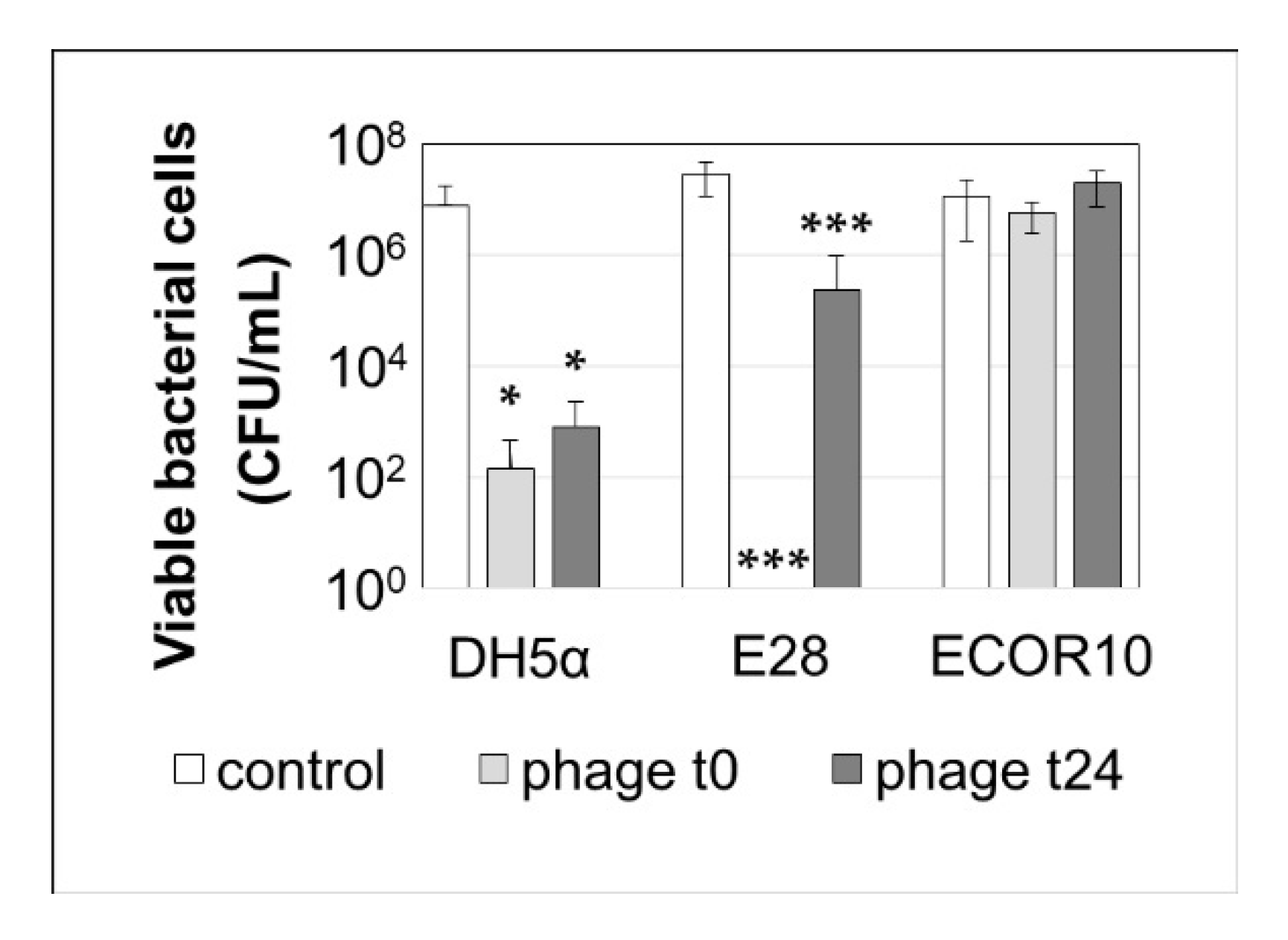
| Strain | E28 (WT) | E28.AB27R2 | E28.EW2R3 | E28.G28R3 | E28.KRA2R3 | E28.TB49R2c | E28.TriMR3 | |
|---|---|---|---|---|---|---|---|---|
| sensitivity against phages | ||||||||
| G28 | + | + | + | − | + | (−) | + | |
| EW2 | + | + | − | (−) | − | (−) | + | |
| KRA2 | + | + | + | + | − | + | + | |
| AB27 | + | (−) | + | + | + | + | + | |
| TriM | + | + | + | − | + | (−) | − | |
| TB49 | + | + | + | (−) | + | − | + | |
| metabolic activity | ||||||||
| negative | 36 | 38 | 36 | 48 | 36 | 48 | 36 | |
| positive | 51 | 47 | 50 | 6 | 51 | 3 | 51 | |
| reduced | 2 | 0 | 25 | 0 | 28 | 0 | ||
| increased | 0 | 1 | 8 | 0 | 8 | 0 | ||
| growth behavior | ||||||||
| swimming motility diameter [mm] | 26.3 ± 4.5 | 24.0 ± 7.7 | 19.0 ± 9.0 | 1.0 ± 0.0 | 20.0 ± 5.4 | 1.0 ± 0.0 | 33.3 ± 4.9 | |
| growth (OD600nm, 24 h at 37 °C) | 1.33 ± 0.04 | 1.03 ± 0.01 | 1.33 ± 0.05 | 0.53 ± 0.02 | 1.34 ± 0.05 | 0.59 ± 0.03 | 1.34 ± 0.06 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Korf, I.H.E.; Kittler, S.; Bierbrodt, A.; Mengden, R.; Rohde, C.; Rohde, M.; Kroj, A.; Lehnherr, T.; Fruth, A.; Flieger, A.; et al. In Vitro Evaluation of a Phage Cocktail Controlling Infections with Escherichia coli. Viruses 2020, 12, 1470. https://doi.org/10.3390/v12121470
Korf IHE, Kittler S, Bierbrodt A, Mengden R, Rohde C, Rohde M, Kroj A, Lehnherr T, Fruth A, Flieger A, et al. In Vitro Evaluation of a Phage Cocktail Controlling Infections with Escherichia coli. Viruses. 2020; 12(12):1470. https://doi.org/10.3390/v12121470
Chicago/Turabian StyleKorf, Imke H. E., Sophie Kittler, Anna Bierbrodt, Ruth Mengden, Christine Rohde, Manfred Rohde, Andrea Kroj, Tatiana Lehnherr, Angelika Fruth, Antje Flieger, and et al. 2020. "In Vitro Evaluation of a Phage Cocktail Controlling Infections with Escherichia coli" Viruses 12, no. 12: 1470. https://doi.org/10.3390/v12121470
APA StyleKorf, I. H. E., Kittler, S., Bierbrodt, A., Mengden, R., Rohde, C., Rohde, M., Kroj, A., Lehnherr, T., Fruth, A., Flieger, A., Lehnherr, H., & Wittmann, J. (2020). In Vitro Evaluation of a Phage Cocktail Controlling Infections with Escherichia coli. Viruses, 12(12), 1470. https://doi.org/10.3390/v12121470






