Sea Lice (Lepeophtheirus salmonis) Infestation Reduces the Ability of Peripheral Blood Monocytic Cells (PBMCs) to Respond to and Control Replication of Salmonid Alphavirus in Atlantic Salmon (Salmo salar L.)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethical Statement
2.2. Cell Lines
2.3. Virus Propagation
2.4. Experimental Design
2.4.1. In Vivo Experiment
2.4.2. Ex Vivo Experiments
2.5. Graphics and Statistical Analysis
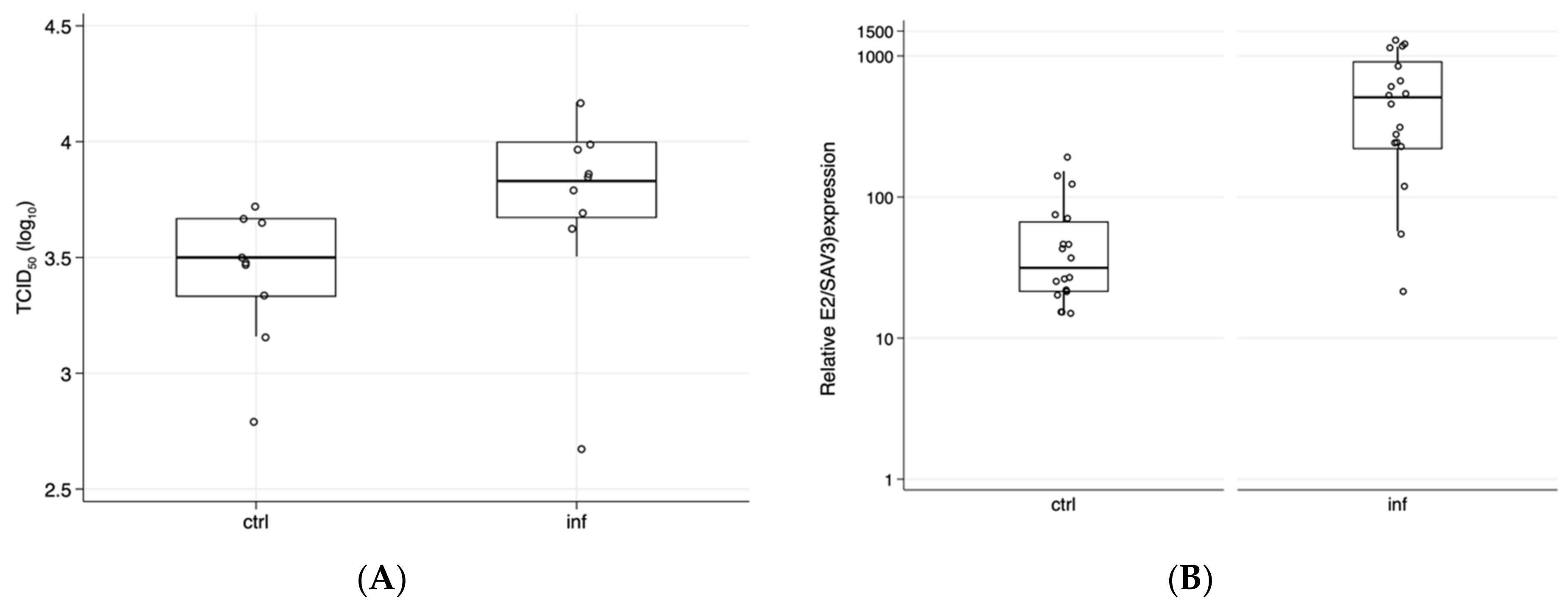
3. Results
Author Contributions
Funding
Conflicts of Interest
References
- McDonagh:, V. Salmon Lice Costing Norway NOK 5 Billion A Year. 2019. Available online: https://www.fishfarmermagazine.com/news/salmon-lice-costing-norway-nok-5-billion-a-year/ (accessed on 16 September 2020).
- Abolofia, J.; Wilen, J.E.; Asche, F. The cost of lice: Quantifying the impacts of parasitic sea lice on farmed salmon. Mar. Resour. Econ. 2017, 32, 329–349. [Google Scholar] [CrossRef]
- Barker, S.E.; Bricknell, I.R.; Covello, J.; Purcell, S.; Fast, M.D.; Wolters, W.; Bouchard, D.A. Sea lice, Lepeophtheirus salmonis (Kroyer 1837), infected Atlantic salmon (Salmo salar L.) are more susceptible to infectious salmon anemia virus. PLoS ONE 2019, 14, e0209178. [Google Scholar] [CrossRef] [Green Version]
- Hamre, L.A.; Eichner, C.; Caipang, C.M.; Dalvin, S.T.; Bron, J.E.; Nilsen, F.; Boxshall, G.; Skern-Mauritzen, R. The Salmon louse Lepeophtheirus salmonis (copepoda: Caligidae) life cycle has only two chalimus stages. PLoS ONE 2013, 8, e73539. [Google Scholar] [CrossRef] [Green Version]
- Fast, M.D. Fish immune responses to parasitic copepod (namely sea lice) infection. Dev. Comp. Immunol. 2014, 43, 300–312. [Google Scholar] [CrossRef] [PubMed]
- Holm, H.; Santi, N.; Kjoglum, S.; Perisic, N.; Skugor, S.; Evensen, O. Difference in skin immune responses to infection with salmon louse (Lepeophtheirus salmonis) in Atlantic salmon (Salmo salar L.) of families selected for resistance and susceptibility. Fish Shellfish Immunol. 2015, 42, 384–394. [Google Scholar] [CrossRef] [PubMed]
- Holm, H.J.; Skugor, S.; Bjelland, A.K.; Radunovic, S.; Wadsworth, S.; Koppang, E.O.; Evensen, O. Contrasting expression of immune genes in scaled and scaleless skin of Atlantic salmon infected with young stages of Lepeophtheirus salmonis. Dev. Comp. Immunol. 2017, 67, 153–165. [Google Scholar] [CrossRef] [PubMed]
- Tadiso, T.M.; Krasnov, A.; Skugor, S.; Afanasyev, S.; Hordvik, I.; Nilsen, F. Gene expression analyses of immune responses in Atlantic salmon during early stages of infection by salmon louse (Lepeophtheirus salmonis) revealed bi-phasic responses coinciding with the copepod-chalimus transition. BMC Genom. 2011, 12, 141. [Google Scholar] [CrossRef] [Green Version]
- Robledo, D.; Gutiérrez, A.P.; Barría, A.; Lhorente, J.P.; Houston, R.D.; Yáñez, J.M. Discovery and functional annotation of quantitative trait loci affecting resistance to sea lice in Atlantic salmon. Front. Genet. 2019, 10, 56. [Google Scholar] [CrossRef] [Green Version]
- Umasuthan, N.; Xue, X.; Caballero-Solares, A.; Kumar, S.; Westcott, J.D.; Chen, Z.; Fast, M.D.; Skugor, S.; Nowak, B.F.; Taylor, R.G.; et al. Transcriptomic profiling in fins of Atlantic salmon parasitized with sea lice: Evidence for an early imbalance between chalimus-induced immunomodulation and the host’s defense response. Int. J. Mol. Sci. 2020, 21, 2417. [Google Scholar] [CrossRef] [Green Version]
- Skugor, S.; Glover, K.A.; Nilsen, F.; Krasnov, A. Local and systemic gene expression responses of Atlantic salmon (Salmo salar L.) to infection with the salmon louse (Lepeophtheirus salmonis). BMC Genom. 2008, 9, 498. [Google Scholar] [CrossRef] [Green Version]
- Carvalho, L.A.; Whyte, S.K.; Braden, L.M.; Purcell, S.L.; Manning, A.J.; Muckle, A.; Fast, M.D. Impact of co-infection with Lepeophtheirus salmonis and Moritella viscosa on inflammatory and immune responses of Atlantic salmon (Salmo salar). J. Fish Dis. 2020, 43, 459–473. [Google Scholar] [CrossRef] [PubMed]
- Figueroa, C.; Bustos, P.; Torrealba, D.; Dixon, B.; Soto, C.; Conejeros, P.; Gallardo, J.A. Coinfection takes its toll: Sea lice override the protective effects of vaccination against a bacterial pathogen in Atlantic salmon. Sci. Rep. UK 2017, 7. [Google Scholar] [CrossRef] [PubMed]
- Taksdal, T.; Olsen, A.B.; Bjerkas, I.; Hjortaas, M.J.; Dannevig, B.H.; Graham, D.A.; McLoughlin, M.F. Pancreas disease in farmed Atlantic salmon, Salmo salar L., and rainbow trout, Oncorhynchus mykiss (Walbaum), in Norway. J. Fish Dis. 2007, 30, 545–558. [Google Scholar] [CrossRef] [PubMed]
- Strauss, J.H.; Strauss, E.G. The alphaviruses: Gene expression, replication, and evolution. Microbiol. Rev. 1994, 58, 491–562. [Google Scholar] [CrossRef]
- Weston, J.H.; Welsh, M.D.; McLoughlin, M.F.; Todd, D. Salmon pancreas disease virus, an alphavirus infecting farmed Atlantic salmon, Salmo salar L. Virology 1999, 256, 188–195. [Google Scholar] [CrossRef] [Green Version]
- Fringuelli, E.; Rowley, H.M.; Wilson, J.C.; Hunter, R.; Rodger, H.; Graham, D.A. Phylogenetic analyses and molecular epidemiology of European salmonid alphaviruses (SAV) based on partial E2 and nsP3 gene nucleotide sequences. J. Fish Dis. 2008, 31, 811–823. [Google Scholar] [CrossRef]
- Hodneland, K.; Bratland, A.; Christie, K.E.; Endresen, C.; Nylund, A. New subtype of salmonid alphavirus (SAV), Togaviridae, from Atlantic salmon Salmo salar and rainbow trout Oncorhynchus mykiss in Norway. Dis. Aquat. Organ. 2005, 66, 113–120. [Google Scholar] [CrossRef]
- Jensen, B.B.; Gu, J.; Sindre, H. The Health Situation in Norwegian Aquaculture 2018; Norwegian Veterinary Institute: Oslo, Norway, 2019; pp. 37–41. [Google Scholar]
- Petterson, E.; Sandberg, M.; Santi, N. Salmonid alphavirus associated with Lepeophtheirus salmonis (Copepoda: Caligidae) from Atlantic salmon, Salmo salar L. J. Fish Dis. 2009, 32, 477–479. [Google Scholar] [CrossRef]
- Xu, C.; Guo, T.C.; Mutoloki, S.; Haugland, O.; Marjara, I.S.; Evensen, O. Alpha interferon and not gamma interferon inhibits salmonid alphavirus subtype 3 replication in vitro. J. Virol. 2010, 84, 8903–8912. [Google Scholar] [CrossRef] [Green Version]
- Guo, T.C.; Johansson, D.X.; Haugland, O.; Liljestrom, P.; Evensen, O. A 6K-deletion variant of salmonid alphavirus is non-viable but can be rescued through RNA recombination. PLoS ONE 2014, 9, e100184. [Google Scholar] [CrossRef] [Green Version]
- Reed, L.J.; Muench, H. A simple method of estimating fifty per cent endpoints. Am. J. Epidemiol. 1938, 27, 493–497. [Google Scholar] [CrossRef]
- Froystad, M.K.; Rode, M.; Berg, T.; Gjoen, T. A role for scavenger receptors in phagocytosis of protein-coated particles in rainbow trout head kidney macrophages. Dev. Comp. Immunol. 1998, 22, 533–549. [Google Scholar] [CrossRef]
- Xu, C.; Evensen, O.; Munang’andu, H.M. Transcriptome analysis shows that IFN-I treatment and concurrent SAV3 infection enriches MHC-I antigen processing and presentation pathways in Atlantic salmon-derived macrophage/dendritic cells. Viruses 2019, 11, 464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(T)(-Delta Delta C) method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Fast, M.D.; Burka, J.F.; Johnson, S.C.; Ross, N.W. Enzymes released from Lepeophtheirus salmonis in response to mucus from different salmonids. J. Parasitol. 2003, 89, 7–13. [Google Scholar] [CrossRef]
- Braden, L.M.; Barker, D.E.; Koop, B.F.; Jones, S.R. Comparative defense-associated responses in salmon skin elicited by the ectoparasite Lepeophtheirus salmonis. Comp. Biochem. Physiol. Part D Genom. Proteom. 2012, 7, 100–109. [Google Scholar] [CrossRef]
- Fast, M.D.; Ross, N.W.; Mustafa, A.; Sims, D.E.; Johnson, S.C.; Conboy, G.A.; Speare, D.J.; Johnson, G.; Burka, J.F. Susceptibility of rainbow trout Oncorhynchus mykiss, Atlantic salmon Salmo salar and coho salmon Oncorhynchus kisutch to experimental infection with sea lice Lepeophtheirus salmonis. Dis. Aquat. Organ 2002, 52, 57–68. [Google Scholar] [CrossRef] [Green Version]
- Lamichhane, P.P.; Samarasinghe, A.E. The role of innate leukocytes during influenza virus infection. J. Immunol. Res. 2019, 2019. [Google Scholar] [CrossRef] [Green Version]
- Nikitina, E.; Larionova, I.; Choinzonov, E.; Kzhyshkowska, J. Monocytes and macrophages as viral targets and reservoirs. Int. J. Mol. Sci. 2018, 19, 2821. [Google Scholar] [CrossRef] [Green Version]
- Lewis, D.L.; Barker, D.E.; McKinley, R.S. Modulation of cellular innate immunity by Lepeophtheirus salmonis secretory products. Fish Shellfish Immunol. 2014, 38, 175–183. [Google Scholar] [CrossRef]
- Herbein, G.; Varin, A. The macrophage in HIV-1 infection: From activation to deactivation? Retrovirology 2010, 7, 33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fjelldal, P.G.; Hansen, T.J.; Karlsen, O. Effects of laboratory salmon louse infection on osmoregulation, growth and survival in Atlantic salmon. Conserv. Physiol. 2020, 8, coaa023. [Google Scholar] [CrossRef] [PubMed]
- Vargas-Chacoff, L.; Munoz, J.L.P.; Saravia, J.; Oyarzun, R.; Pontigo, J.P.; Gonzalez, M.P.; Mardones, O.; Hawes, C.; Pino, J.; Wadsworth, S.; et al. Neuroendocrine stress response in Atlantic salmon (Salmo salar) and Coho salmon (Oncorynchus kisutch) during sea lice infestation. Aquaculture 2019, 507, 329–340. [Google Scholar] [CrossRef]
- Muralidharan, S.; Mandrekar, P. Cellular stress response and innate immune signaling: Integrating pathways in host defense and inflammation. J. Leukoc. Biol. 2013, 94, 1167–1184. [Google Scholar] [CrossRef] [Green Version]
- Tveiten, H.; Bjørn, P.A.; Johnsen, H.K.; Finstad, B.; McKinley, R.S. Effects of the sea louse Lepeophtheirus salmonis on temporal changes in cortisol, sex steroids, growth and reproductive investment in Arctic charr Salvelinus alpinus. J. Fish Biol. 2010, 76, 2318–2341. [Google Scholar] [CrossRef] [PubMed]
- Fast, M.D.; Ross, N.W.; Muise, D.M.; Johnson, S.C. Differential gene expression in atlantic salmon infected with Lepeophtheirus salmonis. J. Aquat. Anim. Health 2006, 18, 116–127. [Google Scholar] [CrossRef]
- Gadan, K.; Marjara, I.S.; Sundh, H.; Sundell, K.; Evensen, O. Slow release cortisol implants result in impaired innate immune responses and higher infection prevalence following experimental challenge with infectious pancreatic necrosis virus in Atlantic salmon (Salmo salar) parr. Fish Shellfish Immunol. 2012, 32, 637–644. [Google Scholar] [CrossRef]
- Fast, M.D.; Hosoya, S.; Johnson, S.C.; Afonso, L.O. Cortisol response and immune-related effects of Atlantic salmon (Salmo salar Linnaeus) subjected to short- and long-term stress. Fish Shellfish Immunol. 2008, 24, 194–204. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gamil, A.A.A.; Gadan, K.; Gislefoss, E.; Evensen, Ø. Sea Lice (Lepeophtheirus salmonis) Infestation Reduces the Ability of Peripheral Blood Monocytic Cells (PBMCs) to Respond to and Control Replication of Salmonid Alphavirus in Atlantic Salmon (Salmo salar L.). Viruses 2020, 12, 1450. https://doi.org/10.3390/v12121450
Gamil AAA, Gadan K, Gislefoss E, Evensen Ø. Sea Lice (Lepeophtheirus salmonis) Infestation Reduces the Ability of Peripheral Blood Monocytic Cells (PBMCs) to Respond to and Control Replication of Salmonid Alphavirus in Atlantic Salmon (Salmo salar L.). Viruses. 2020; 12(12):1450. https://doi.org/10.3390/v12121450
Chicago/Turabian StyleGamil, Amr A. A., Koestan Gadan, Elisabeth Gislefoss, and Øystein Evensen. 2020. "Sea Lice (Lepeophtheirus salmonis) Infestation Reduces the Ability of Peripheral Blood Monocytic Cells (PBMCs) to Respond to and Control Replication of Salmonid Alphavirus in Atlantic Salmon (Salmo salar L.)" Viruses 12, no. 12: 1450. https://doi.org/10.3390/v12121450
APA StyleGamil, A. A. A., Gadan, K., Gislefoss, E., & Evensen, Ø. (2020). Sea Lice (Lepeophtheirus salmonis) Infestation Reduces the Ability of Peripheral Blood Monocytic Cells (PBMCs) to Respond to and Control Replication of Salmonid Alphavirus in Atlantic Salmon (Salmo salar L.). Viruses, 12(12), 1450. https://doi.org/10.3390/v12121450





