Longitudinal Analysis of Peripheral and Colonic CD161+ CD4+ T Cell Dysfunction in Acute HIV-1 Infection and Effects of Early Treatment Initiation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Study Approval
2.3. Biopsy Processing and Calculation of Absolute Number of Colonic T Cell Subset
2.4. Flow Cytometry
2.5. Functional Assays
2.6. Statistical Analysis
3. Results
3.1. CD161+ CD4+ T Cells Express RORγt and Produce Th1 and Th17 Cytokines in Response to IL-12 and IL-18 Stimulation
3.2. CD161+ CD4+ T Cells Have a Diverse TCR Repertoire
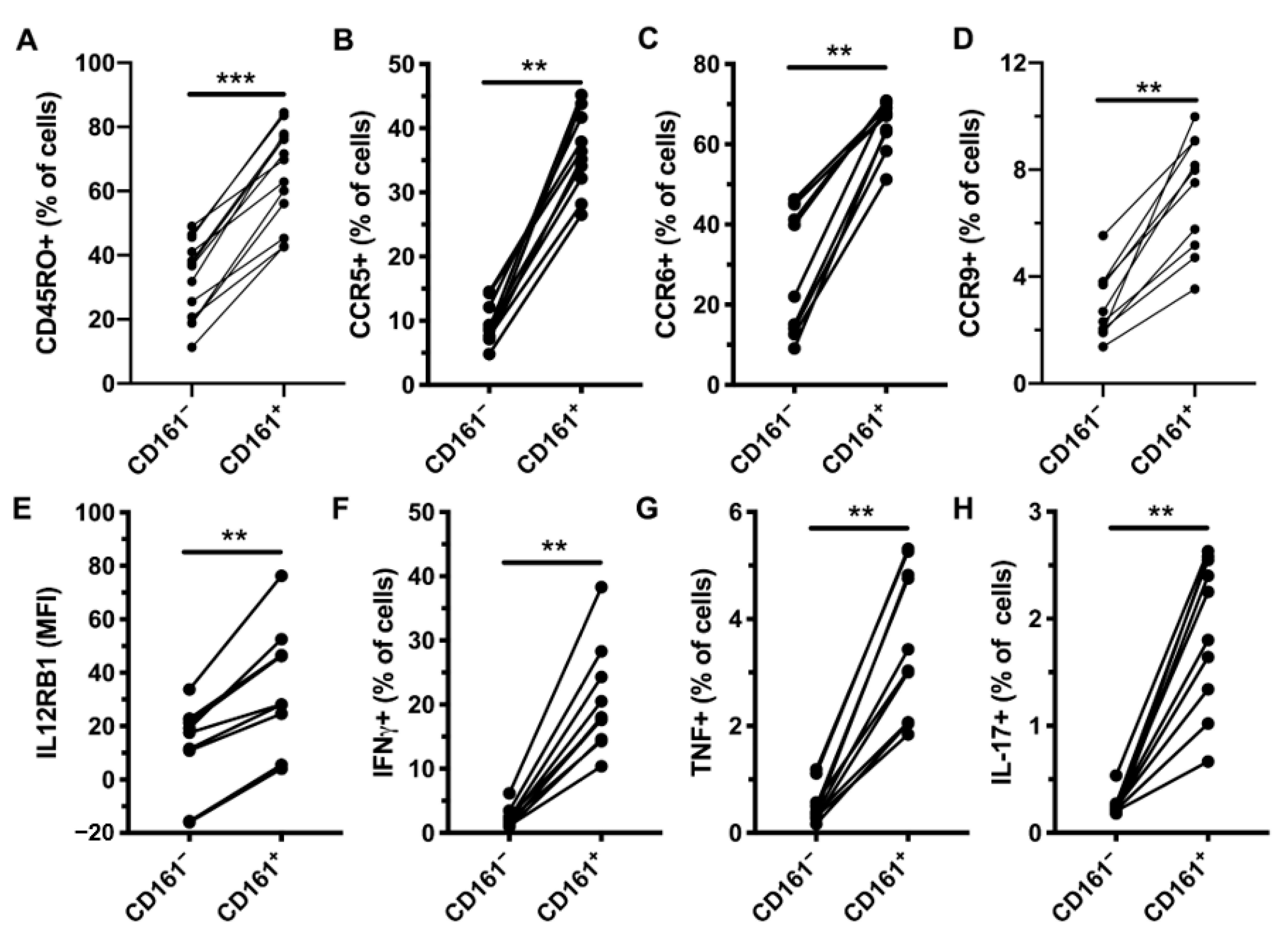
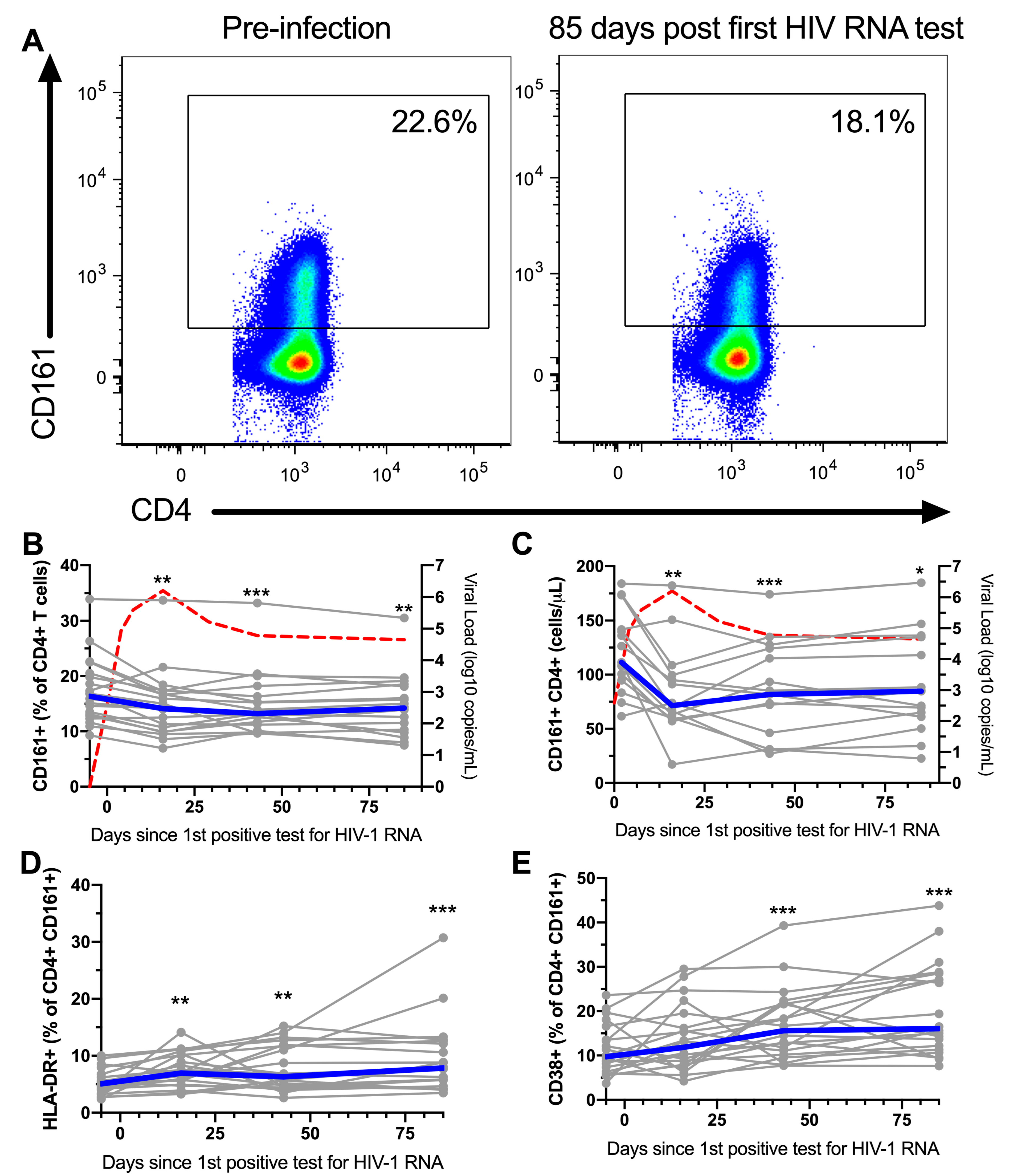
3.3. CD161+ CD4+ T Cells Decline in Peripheral Blood During AHI, Have an Activated Phenotype and Decreased Functional Capacity
3.4. Pre-Infection Levels of CCR5+ CD161+ CD4+ T Cells are Inversely Associated with CD4 Nadir
3.5. ART Initiation During AHI Restores the Phenotype and Functional Response, but not Frequency of Peripheral Blood CD161+ CD4+ T Cells
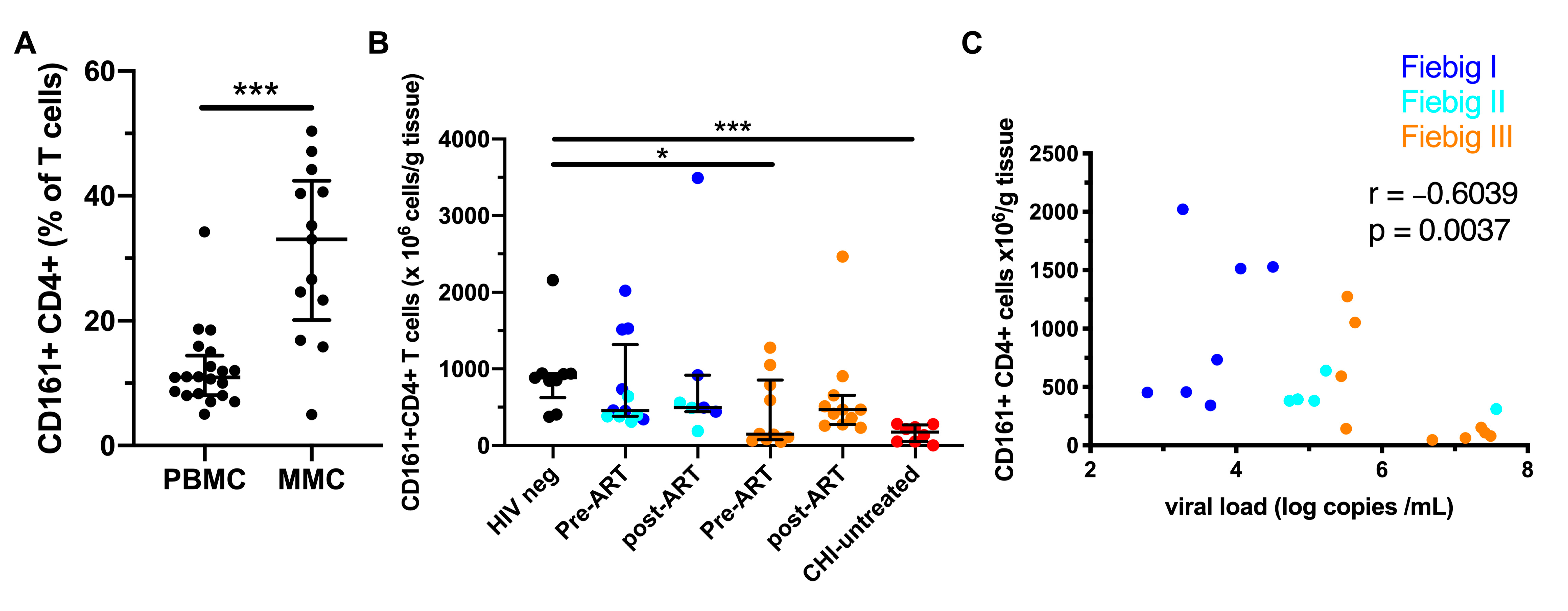
3.6. Early ART Initiation Restores CD161+ CD4+ T Cells in the Colonic Mucosa
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Disclaimer
Conflicts of Interest
Abbreviations
| TCR | T cell receptor |
| IL | Interleukin |
| CCR | C-C chemokine receptor |
| CXCR | C-X-C chemokine receptor |
| NAAT | Nucleic acid amplification test |
| IRBs | Institutional Review Boards |
| PBMCs | Peripheral blood mononuclear cells |
| MMCs | Mucosal mononuclear cells |
| ROR | RAR-related orphan receptor |
| PLZF | Promyelocytic leukemia zinc finger |
| Eomes | Eomesodermin |
| T-bet | T-box expressed in T cells |
| TIGIT | T cell immunoreceptor with Ig and ITAM domains |
| PD-1 | Programmed cell death protein 1 |
| IFNγ | Interferon gamma |
| TNF | Tumor necrosis factor |
| MAIT | Mucosal-associated invariant T |
| iNKT | Invariant natural killer T |
| CHI | Chronic HIV-1 infection |
| AHI | Acute HIV-1 infection |
| ART | Anti-retroviral treatment |
| VL | Viral load |
| PCA | Principal Component Analysis |
References
- Lanier, L.L.; Chang, C.; Phillips, J.H. Human NKR-P1A. A disulfide-linked homodimer of the C-type lectin superfamily expressed by a subset of NK and T lymphocytes. J. Immunol. 1994, 153, 2417–2428. [Google Scholar]
- Aldemir, H.; Prod’Homme, V.; Dumaurier, M.J.; Retiere, C.; Poupon, G.; Cazareth, J.; Bihl, F.; Braud, V.M. Cutting Edge: Lectin-Like Transcript 1 Is a Ligand for the CD161 Receptor. J. Immunol. 2005, 175, 7791–7795. [Google Scholar] [CrossRef] [Green Version]
- Maggi, L.; Santarlasci, V.; Capone, M.; Peired, A.; Frosali, F.; Crome, S.Q.; Querci, V.; Fambrini, M.; Eliotta, F.; Levings, M.K.; et al. CD161 is a marker of all human IL-17-producing T-cell subsets and is induced by RORC. Eur. J. Immunol. 2010, 40, 2174–2181. [Google Scholar] [CrossRef] [PubMed]
- Cosmi, L.; De Palma, R.; Santarlasci, V.; Maggi, L.; Capone, M.; Frosali, F.; Rodolico, G.; Querci, V.; Abbate, G.; Angeli, R.; et al. Human interleukin 17-producing cells originate from a CD161+CD4+ T cell precursor. J. Exp. Med. 2008, 205, 1903–1916. [Google Scholar] [CrossRef] [PubMed]
- Kleinschek, M.A.; Boniface, K.; Sadekova, S.; Grein, J.; Murphy, E.E.; Turner, S.P.; Raskin, L.; Desai, B.; Faubion, W.A.; Malefyt, R.D.W.; et al. Circulating and gut-resident human Th17 cells express CD161 and promote intestinal inflammation. J. Exp. Med. 2009, 206, 525–534. [Google Scholar] [CrossRef] [PubMed]
- Dusseaux, M.; Martin, E.; Serriari, N.; Péguillet, I.; Premel, V.; Louis, D.; Milder, M.; Le Bourhis, L.; Soudais, C.; Treiner, E.; et al. Human MAIT cells are xenobiotic-resistant, tissue-targeted, CD161hi IL-17–secreting T cells. Blood 2011, 117, 1250–1259. [Google Scholar] [CrossRef]
- Exley, M.; Porcelli, S.; Furman, M.; Garcia, J.; Balk, S. CD161 (NKR-P1A) costimulation of CD1d-dependent activation of human T cells expressing invariant V alpha 24 J alpha Q T cell receptor alpha chains. J. Exp. Med. 1998, 188, 867–876. [Google Scholar] [CrossRef] [Green Version]
- Fergusson, J.R.; Smith, K.E.; Fleming, V.M.; Rajoriya, N.; Newell, E.W.; Simmons, R.; Marchi, E.; Björkander, S.; Kang, Y.H.; Swadling, L.; et al. CD161 Defines a Transcriptional and Functional Phenotype across Distinct Human T Cell Lineages. Cell Rep. 2014, 9, 1075–1088. [Google Scholar] [CrossRef] [Green Version]
- Gosselin, A.; Monteiro, P.; Chomont, N.; Diaz-Griffero, F.; Said, E.A.; Fonseca, S.; Wacleche, V.S.; El-Far, M.; Boulassel, M.-R.; Routy, J.-P.; et al. Peripheral Blood CCR4+CCR6+ and CXCR3+CCR6+CD4+T Cells Are Highly Permissive to HIV-1 Infection. J. Immunol. 2010, 184, 1604–1616. [Google Scholar] [CrossRef] [Green Version]
- Prendergast, A.; Prado, J.G.; Kang, Y.H.; Chen, F.; Riddell, L.A.; Luzzi, G.; Goulder, P.; Klenerman, P. HIV-1 infection is characterized by profound depletion of CD161+ Th17 cells and gradual decline in regulatory T cells. AIDS 2010, 24, 491–502. [Google Scholar] [CrossRef]
- Li, X.; Liu, Z.; Li, Q.; Hu, R.; Zhao, L.; Yang, Y.; Zhao, J.; Huang, Z.; Gao, H.; Li, L.; et al. CD161+ CD4+ T Cells Harbor Clonally Expanded Replication-Competent HIV-1 in Antiretroviral Therapy-Suppressed Individuals. mBio 2019, 10, e02121-19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boily-Larouche, G.; Omollo, K.; Cheruiyot, J.; Njoki, J.; Kimani, M.; Kimani, J.; Oyugi, J.; Lajoie, J.; Fowke, K.R. CD161 identifies polyfunctional Th1/Th17 cells in the genital mucosa that are depleted in HIV-infected female sex workers from Nairobi, Kenya. Sci. Rep. 2017, 7, 11123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McGary, C.S.; Alvarez, X.; Harrington, S.; Cervasi, B.; Ryan, E.S.; Iriele, R.I.; Paganini, S.; Harper, J.L.; Easley, K.A.; Silvestri, G.; et al. The loss of CCR6+ and CD161+ CD4+ T-cell homeostasis contributes to disease progression in SIV-infected rhesus macaques. Mucosal Immunol. 2017, 10, 1082–1096. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robb, M.L.; Eller, L.A.; Kibuuka, H.; Rono, K.; Maganga, L.; Nitayaphan, S.; Kroon, E.; Sawe, F.K.; Sinei, S.; Sriplienchan, S.; et al. Prospective Study of Acute HIV-1 Infection in Adults in East Africa and Thailand. N. Engl. J. Med. 2016, 374, 2120–2130. [Google Scholar] [CrossRef] [PubMed]
- Andrade, B.B.; Phanuphak, N.; De Souza, M.; Paris, R.; Arroyo, M.; Trichavaroj, R.; Sirivichayakul, S.; Shikuma, C.; Phanuphak, P.; Kim, J.H. Incidence and Characterization of Acute HIV-1 Infection in a High-Risk Thai Population. JAIDS J. Acquir. Immune Defic. Syndr. 2008, 49, 151–155. [Google Scholar] [CrossRef]
- Schuetz, A.; Deleage, C.; Sereti, I.; Rerknimitr, R.; Phanuphak, N.; Phuang-Ngern, Y.; Estes, J.D.; Sandler, N.G.; Sukhumvittaya, S.; Marovich, M.; et al. Initiation of ART during Early Acute HIV Infection Preserves Mucosal Th17 Function and Reverses HIV-Related Immune Activation. PLoS Pathog. 2014, 10, e1004543. [Google Scholar] [CrossRef] [Green Version]
- Lal, K.G.; Leeansyah, E.; Sandberg, J.K.; Eller, M. OMIP-046: Characterization of invariant T cell subset activation in humans. Cytom. Part A 2018, 93, 499–503. [Google Scholar] [CrossRef]
- Dias, J.; Leeansyah, E.; Sandberg, J.K. Multiple layers of heterogeneity and subset diversity in human MAIT cell responses to distinct microorganisms and to innate cytokines. Proc. Natl. Acad. Sci. USA 2017, 114, E5434–E5443. [Google Scholar] [CrossRef] [Green Version]
- Lal, K.G.; Kim, D.; Costanzo, M.C.; Creegan, M.; Leeansyah, E.; Dias, J.; Paquin-Proulx, D.; Eller, L.A.; Schuetz, A.; Phuang-Ngern, Y.; et al. Dynamic MAIT cell response with progressively enhanced innateness during acute HIV-1 infection. Nat. Commun. 2020, 11, 272. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.J.; Barnitz, R.A.; Kreslavsky, T.; Brown, F.D.; Moffett, H.; Lemieux, M.E.; Kaygusuz, Y.; Meissner, T.; Holderried, T.A.W.; Chan, S.; et al. Stable inhibitory activity of regulatory T cells requires the transcription factor Helios. Science 2015, 350, 334–349. [Google Scholar] [CrossRef] [Green Version]
- Ananworanich, J.; Schuetz, A.; Vandergeeten, C.; Sereti, I.; De Souza, M.; Rerknimitr, R.; Dewar, R.; Marovich, M.; Van Griensven, F.; Sekaly, R.; et al. Impact of Multi-Targeted Antiretroviral Treatment on Gut T Cell Depletion and HIV Reservoir Seeding during Acute HIV Infection. PLoS ONE 2012, 7, e33948. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guadalupe, M.; Reay, E.; Sankaran, S.; Prindiville, T.; Flamm, J.; McNeil, A.; Dandekar, S. Severe CD4+ T-Cell Depletion in Gut Lymphoid Tissue during Primary Human Immunodeficiency Virus Type 1 Infection and Substantial Delay in Restoration following Highly Active Antiretroviral Therapy. J. Virol. 2003, 77, 11708–11717. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mehandru, S.; Poles, M.A.; Tenner-Racz, K.; Horowitz, A.; Hurley, A.; Hogan, C.; Boden, D.; Racz, P.; Markowitz, M. Primary HIV-1 Infection Is Associated with Preferential Depletion of CD4+ T Lymphocytes from Effector Sites in the Gastrointestinal Tract. J. Exp. Med. 2004, 200, 761–770. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sobkowiak, M.J.; Davanian, H.; Heymann, R.; Gibbs, A.; Emgård, J.; Dias, J.; Aleman, S.; Krüger-Weiner, C.; Moll, M.; Tjernlund, A.; et al. Tissue-resident MAIT cell populations in human oral mucosa exhibit an activated profile and produce IL-17. Eur. J. Immunol. 2019, 49, 133–143. [Google Scholar] [CrossRef] [Green Version]
- Lu, B.; Liu, M.; Wang, J.; Fan, H.; Yang, D.; Zhang, L.; Gu, X.; Nie, J.; Chen, Z.; Corbett, A.J.; et al. IL-17 production by tissue-resident MAIT cells is locally induced in children with pneumonia. Mucosal Immunol. 2020, 13, 824–835. [Google Scholar] [CrossRef] [Green Version]
- Gibbs, A.; Leeansyah, E.; Introini, A.; Paquin-Proulx, D.; Hasselrot, K.; Andersson, E.; Broliden, K.; Sandberg, J.K.; Tjernlund, A. MAIT cells reside in the female genital mucosa and are biased towards IL-17 and IL-22 production in response to bacterial stimulation. Mucosal Immunol. 2017, 10, 35–45. [Google Scholar] [CrossRef] [Green Version]
- Moreira-Teixeira, L.; Resende, M.; Coffre, M.; Devergne, O.; Herbeuval, J.-P.; Hermine, O.; Schneider, E.; Rogge, L.; Ruemmele, F.M.; Dy, M.; et al. Proinflammatory Environment Dictates the IL-17–Producing Capacity of Human Invariant NKT Cells. J. Immunol. 2011, 186, 5758–5765. [Google Scholar] [CrossRef] [Green Version]
- Cano-Gamez, E.; Soskic, B.; Roumeliotis, T.I.; So, E.; Smyth, D.J.; Baldrighi, M.; Willé, D.; Nakic, N.; Esparza-Gordillo, J.; Larminie, C.G.C.; et al. Single-cell transcriptomics identifies an effectorness gradient shaping the response of CD4+ T cells to cytokines. Nat. Commun. 2020, 11, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Gutierrez-Arcelus, M.; Teslovich, N.; Mola, A.R.; Polidoro, R.B.; Nathan, A.; Kim, H.; Hannes, S.; Slowikowski, K.; Watts, G.F.M.; Korsunsky, I.; et al. Lymphocyte innateness defined by transcriptional states reflects a balance between proliferation and effector functions. Nat. Commun. 2019, 10, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Sprent, J.; Surh, C.D. Normal T cell homeostasis: The conversion of naive cells into memory-phenotype cells. Nat. Immunol. 2011, 12, 478–484. [Google Scholar] [CrossRef]
- Planas, D.; Zhang, Y.; Monteiro, P.; Goulet, J.P.; Gosselin, A.; Grandvaux, N.; Hope, T.J.; Fassati, A.; Routy, J.P.; Ancuta, P. HIV-1 selectively targets gut-homing CCR6+CD4+ T cells via mTOR-dependent mechanisms. JCI Insight 2017, 2, e93230. [Google Scholar] [CrossRef] [PubMed]
- Kader, M.; Wang, X.; Piatak, M.; Lifson, J.; Roederer, M.; Veazey, R.; Mattapallil, J.J. Alpha4(+)beta7(hi)CD4(+) memory T cells harbor most Th-17 cells and are preferentially infected during acute SIV infection. Mucosal Immunol. 2009, 2, 439–449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cicala, C.; Martinelli, E.; McNally, J.P.; Goode, D.J.; Gopaul, R.; Hiatt, J.; Jelicic, K.; Kottilil, S.; Macleod, K.; O’Shea, A.; et al. The integrin alpha4beta7 forms a complex with cell-surface CD4 and defines a T-cell subset that is highly susceptible to infection by HIV-1. Proc. Natl. Acad. Sci. USA 2009, 106, 20877–20882. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sivro, A.; Schuetz, A.; Sheward, D.; Joag, V.; Yegorov, S.; Liebenberg, L.J.; Yende-Zuma, N.; Stalker, A.; Mwatelah, R.S.; Selhorst, P.; et al. Integrin alpha4beta7 expression on peripheral blood CD4(+) T cells predicts HIV acquisition and disease progression outcomes. Sci Transl Med. 2018, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tokarev, A.; McKinnon, L.R.; Pagliuzza, A.; Sivro, A.; Omole, T.E.; Kroon, E.; Chomchey, N.; Phanuphak, N.; Schuetz, A.; Robb, M.L.; et al. Preferential infection of alpha4beta7+ memory CD4+ T cells during early acute HIV-1 infection. Clin. Infect. Dis. 2020. [Google Scholar] [CrossRef]
- Hellmuth, J.; Slike, B.M.; Sacdalan, C.; Best, J.; Kroon, E.; Phanuphak, N.; Fletcher, J.L.; Prueksakaew, P.; Jagodzinski, L.L.; Valcour, V.; et al. Very Early Initiation of Antiretroviral Therapy During Acute HIV Infection Is Associated with Normalized Levels of Immune Activation Markers in Cerebrospinal Fluid but Not in Plasma. J. Infect. Dis. 2019, 220, 1885–1891. [Google Scholar] [CrossRef] [Green Version]
- Liang, S.C.; Tan, X.Y.; Luxenberg, D.P.; Karim, R.; Dunussi-Joannopoulos, K.; Collins, M.; Fouser, L.A. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J. Exp. Med. 2006, 203, 2271–2279. [Google Scholar] [CrossRef]
- Huang, W.; Na, L.; Fidel, P.L.; Schwarzenberger, P. Requirement of Interleukin-17A for Systemic Anti–Candida albicansHost Defense in Mice. J. Infect. Dis. 2004, 190, 624–631. [Google Scholar] [CrossRef] [Green Version]
- Raffatellu, M.; Santos, R.L.; Verhoeven, D.E.; George, M.D.; Wilson, R.P.; Winter, S.E.; Godinez, I.; Sankaran, S.; Paixao, T.A.; Gordon, M.A.; et al. Simian immunodeficiency virus-induced mucosal interleukin-17 deficiency promotes Salmonella dissemination from the gut. Nat. Med. 2008, 14, 421–428. [Google Scholar] [CrossRef] [Green Version]
- Weaver, C.T.; Elson, C.O.; Fouser, L.A.; Kolls, J.K. The Th17 Pathway and Inflammatory Diseases of the Intestines, Lungs, and Skin. Annu. Rev. Pathol. 2013, 8, 477–512. [Google Scholar] [CrossRef] [Green Version]
- Kim, T.W.; Lee, S.-E.; Lim, J.; Ryu, D.; Jeon, Y.-W.; Yoon, J.-H.; Cho, B.; Eom, K.; Min, W. Clinical significance of pre-transplant circulating CD3(+)CD4(+)CD161(+) cell frequency on the occurrence of neutropenic infections after allogeneic stem cell transplantation. Transpl. Infect. Dis. 2017, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.E.; Lim, J.Y.; Ryu, D.-B.; Kim, T.W.; Park, S.S.; Jeon, Y.W.; Yoon, J.H.; Cho, B.S.; Eom, K.S.; Kim, Y.J.; et al. Low frequency of CD3+CD4+CD161+ T cells correlates with the occurrence of infections in refractory/relapsed multiple myeloma patients receiving lenalidomide plus low-dose dexamethasone treatment. Ann. Hematol. 2018, 97, 2163–2171. [Google Scholar] [CrossRef] [PubMed]
- Kawabe, T.; Jankovic, D.; Kawabe, S.; Huang, Y.; Lee, P.-H.; Yamane, H.; Zhu, J.; Sher, A.; Germain, R.N.; Paul, W.E. Memory-phenotype CD4+T cells spontaneously generated under steady-state conditions exert innate TH1-like effector function. Sci. Immunol. 2017, 2, eaam9304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huson, M.A.M.; Grobusch, M.P.; Van Der Poll, T. The effect of HIV infection on the host response to bacterial sepsis. Lancet Infect. Dis. 2015, 15, 95–108. [Google Scholar] [CrossRef]
- Perbost, I.; Malafronte, B.; Pradier, C.; Santo, L.; Dunais, B.; Counillon, E.; Vinti, H.; Enel, P.; Fuzibet, J.; Cassuto, J.; et al. In the era of highly active antiretroviral therapy, why are HIV-infected patients still admitted to hospital for an inaugural opportunistic infection? HIV Med. 2005, 6, 232–239. [Google Scholar] [CrossRef]
- Gupta, A.; Wood, R.; Kaplan, R.; Bekker, L.-G.; Lawn, S.D. Tuberculosis Incidence Rates during 8 Years of Follow-Up of an Antiretroviral Treatment Cohort in South Africa: Comparison with Rates in the Community. PLoS ONE 2012, 7, e34156. [Google Scholar] [CrossRef] [Green Version]





| Characteristics | Acute HIV-Infected (n = 22) | HIV-Uninfected (n = 20) |
|---|---|---|
| Median age (years) | 23 (18, 35) A | 25 (18, 45) A |
| Gender, Male/Female/TGW | 8:9:5 | 2:17:1 |
| Country, n (%) | ||
| Uganda | 6 (27.4) | 5 (20) |
| Tanzania | 3 (13.6) | 5 (20) |
| Kenya | 2 (9) | 5 (20) |
| Thailand | 11 (50) | 5 (20) |
| Median CD4+ T cell nadir (cells/mL) | 483 (286, 866) A | NA |
| Median time to peak VL (days) | 14 (6, 19) A | NA |
| Median peak VL (log10 copies/mL) | 6.68 (5.49, 7.94) A | NA |
| Median set point VL (log10 copies/mL) | 4.46 (3.52, 5.96) A | NA |
| Characteristics | Acute HIV-Infected at Time of Diagnosis (n = 23) | Acute HIV-Infected Post-ART-Initiation (n = 40) | HIV-Uninfected (n = 28) |
|---|---|---|---|
| Median age (years) | 29 (19, 46) A | 27 (18, 54) A | 34 (20–43) A |
| Gender, Male:Female:TGW | 21:2:0 | 39:1:0 | 14:6:4 |
| Risk behavior, n (%) | |||
| MSM | 19 (82.6) | 34 (85) | 14 (50) |
| Bisexual male | 2 (8.7) | 1 (2.5) | - |
| Heterosexual male | - | 4 (10.0) | 4 (14.3) |
| Heterosexual female | 2 (8.7) | 1 (2.5) | 6 (21.4) |
| TGW | - | - | 4 (14.3) |
| Fiebig Stage, n | |||
| I/II | 12 (7 I, 5 II) | 24 (11 I, 13 II) | NA |
| III | 11 | 16 | NA |
| Mean (SD) days since HIV exposure to enrollment | 16.3 (5.7) | 17.0 (7.2) | NA |
| Mean (SD) time to ART initiation following diagnosis (days) | NA | 4 (1.7) | NA |
| Median duration of ART (weeks) | NA | 12 (12–96) A | NA |
| Median plasma HIV RNA (log10 copies/mL) | 5.4 (2.8, 7.7) A | 1.6 (1.3, 2.6) A | NA |
| Median sigmoid colon HIV RNA (log10 copies/mg tissue) | 3.1 (1.3, 6.1) A | 1.7 (1.7, 1.7) A | NA |
| Median CD4+ T cell count (cell/mm3) | 532 (132, 1127) A | 890 (452, 1,266) A | 1005 (738–2059) A |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lal, K.G.; Phuang-Ngern, Y.; Suhkumvittaya, S.; Leeansyah, E.; Alrubayyi, A.; Dias, J.; Waickman, A.; Kim, D.; Kroon, E.; Pinyakorn, S.; et al. Longitudinal Analysis of Peripheral and Colonic CD161+ CD4+ T Cell Dysfunction in Acute HIV-1 Infection and Effects of Early Treatment Initiation. Viruses 2020, 12, 1426. https://doi.org/10.3390/v12121426
Lal KG, Phuang-Ngern Y, Suhkumvittaya S, Leeansyah E, Alrubayyi A, Dias J, Waickman A, Kim D, Kroon E, Pinyakorn S, et al. Longitudinal Analysis of Peripheral and Colonic CD161+ CD4+ T Cell Dysfunction in Acute HIV-1 Infection and Effects of Early Treatment Initiation. Viruses. 2020; 12(12):1426. https://doi.org/10.3390/v12121426
Chicago/Turabian StyleLal, Kerri G., Yuwadee Phuang-Ngern, Suchada Suhkumvittaya, Edwin Leeansyah, Aljawharah Alrubayyi, Joana Dias, Adam Waickman, Dohoon Kim, Eugène Kroon, Suteeraporn Pinyakorn, and et al. 2020. "Longitudinal Analysis of Peripheral and Colonic CD161+ CD4+ T Cell Dysfunction in Acute HIV-1 Infection and Effects of Early Treatment Initiation" Viruses 12, no. 12: 1426. https://doi.org/10.3390/v12121426
APA StyleLal, K. G., Phuang-Ngern, Y., Suhkumvittaya, S., Leeansyah, E., Alrubayyi, A., Dias, J., Waickman, A., Kim, D., Kroon, E., Pinyakorn, S., Eller, L. A., Maciel Jr., M., Rerknimitr, R., Chomchey, N., Phanuphak, N., de Souza, M. S., Nitayaphan, S., Ake, J. A., Vasan, S., ... on behalf of the RV217, RV254/SEARCH010, RV304/SEARCH Study Groups. (2020). Longitudinal Analysis of Peripheral and Colonic CD161+ CD4+ T Cell Dysfunction in Acute HIV-1 Infection and Effects of Early Treatment Initiation. Viruses, 12(12), 1426. https://doi.org/10.3390/v12121426






