Reactivation of BK Polyomavirus in Urine Cytology Is Not Associated with Urothelial Cell Carcinoma
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Cytology
2.3. Histology
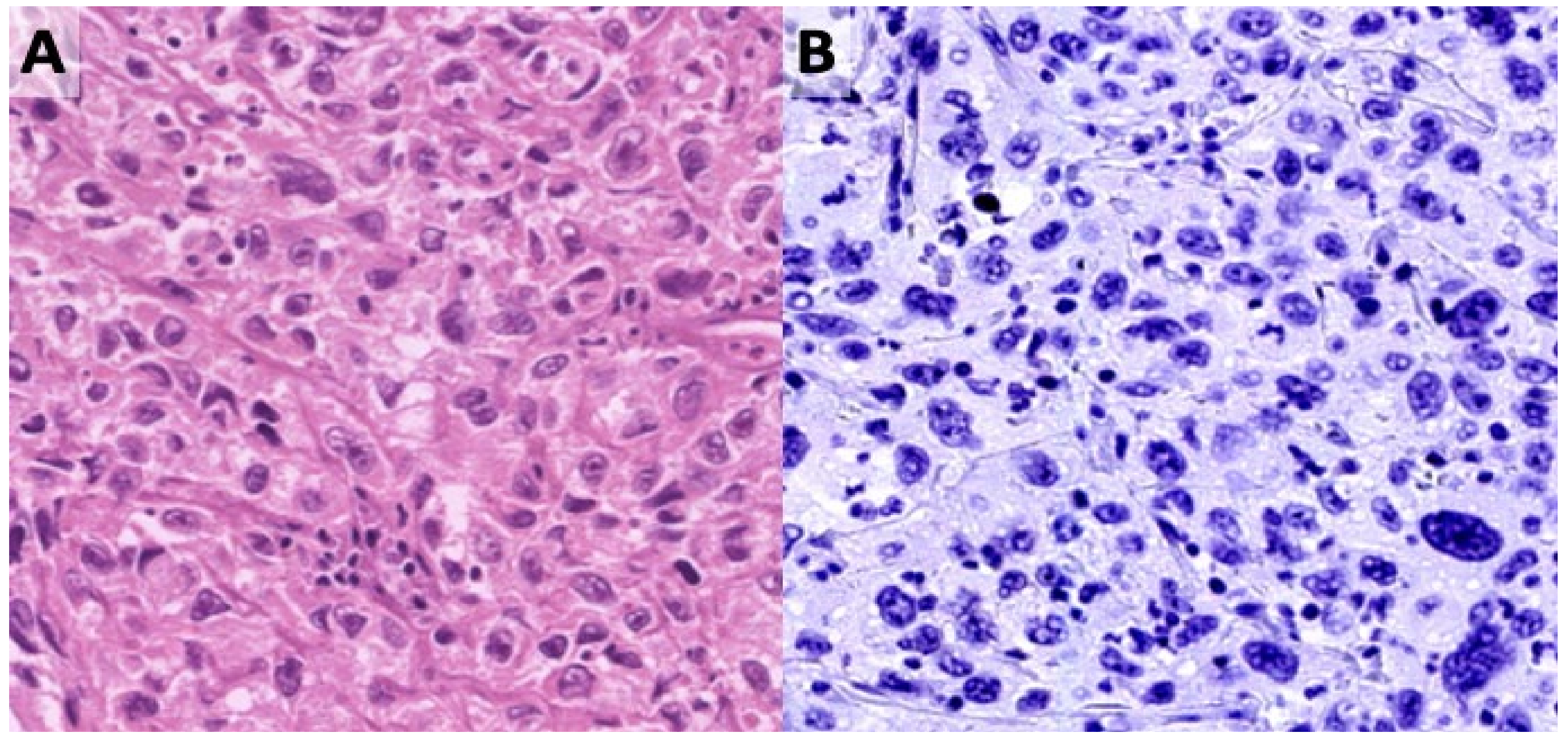
2.4. Immunohistochemistry
2.5. BKPyV PCR
3. Results
3.1. Study Population and Urine Cytology
3.2. BKPyV in Patients without a History of Urothelial Cell Carcinoma
3.3. Presence of BKPyV in Urothelial Cell Carcinoma
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Roberts, I.S.; Besarani, D.; Mason, P.; Turner, G.; Friend, P.J.; Newton, R. Polyoma virus infection and urothelial carcinoma of the bladder following renal transplantation. Br. J. Cancer 2008, 99, 1383–1386. [Google Scholar] [CrossRef] [PubMed]
- Bouvard, V.; Baan, R.A.; Grosse, Y.; Lauby-Secretan, B.; El Ghissassi, F.; Benbrahim-Tallaa, L.; Guha, N.; Straif, K. Carcinogenicity of malaria and of some polyomaviruses. Lancet Oncol. 2012, 13, 339–340. [Google Scholar] [CrossRef]
- Galed-Placed, I.; Valbuena-Ruvira, L. Decoy cells and malignant cells coexisting in the urine from a transplant recipient with BK virus nephropathy and bladder adenocarcinoma. Diagn. Cytopathol. 2011, 39, 933–937. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ahmed, H.; Haririan, A.; Ugarte, R.; Papadimitriou, J.C.; Drachenberg, C.B. Granulomatous inflammation in BK polyomavirus-associated nephropathy. Transpl. Infect. Dis. 2018, 20, e12939. [Google Scholar] [CrossRef]
- Liu, S.; Chaudhry, M.R.; Berrebi, A.A.; Papadimitriou, J.C.; Drachenberg, C.B.; Haririan, A.; Alexiev, B.A. Polyomavirus Replication and Smoking Are Independent Risk Factors for Bladder Cancer After Renal Transplantation. Transplantation 2017, 101, 1488–1494. [Google Scholar] [CrossRef] [PubMed]
- Papadimitriou, J.C.; Randhawa, P.; Rinaldo, C.H.; Drachenberg, C.B.; Alexiev, B.; Hirsch, H.H. BK Polyomavirus Infection and Renourinary Tumorigenesis. Am. J. Transplant. Off. J. Am. Soc. Transplant. Am. Soc. Transpl. Surg. 2016, 16, 398–406. [Google Scholar] [CrossRef]
- Alexiev, B.A.; Papadimitriou, J.C.; Drachenberg, C.B. BK polyomavirus-infected nephrogenic adenoma of the urinary bladder in a renal transplant recipient: A case report. Pathol. Res. Pract. 2015, 211, 697–701. [Google Scholar] [CrossRef]
- Alexiev, B.A.; Drachenberg, C.B.; Papadimitriou, J.C. Polyomavirus-cystitis associated with in situ and invasive urothelial carcinoma in a heart transplant recipient: Evidence suggesting sequential progression/evolution from infection to carcinoma. Transplantation 2015, 99, e3–e4. [Google Scholar] [CrossRef]
- Alexiev, B.A.; Papadimitriou, J.C.; Chai, T.C.; Ramos, E.; Staats, P.N.; Drachenberg, C.B. Polyomavirus (BK)-associated pleomorphic giant cell carcinoma of the urinary bladder: A case report. Pathol. Res. Pract. 2013, 209, 255–259. [Google Scholar] [CrossRef]
- Alexiev, B.A.; Randhawa, P.; Vazquez Martul, E.; Zeng, G.; Luo, C.; Ramos, E.; Drachenberg, C.B.; Papadimitriou, J.C. BK virus-associated urinary bladder carcinoma in transplant recipients: Report of 2 cases, review of the literature, and proposed pathogenetic model. Hum. Pathol. 2013, 44, 908–917. [Google Scholar] [CrossRef]
- Prado, J.C.M.; Monezi, T.A.; Amorim, A.T.; Lino, V.; Paladino, A.; Boccardo, E. Human polyomaviruses and cancer: An overview. Clinics 2018, 73 Suppl. S1, e558s. [Google Scholar] [CrossRef] [PubMed]
- Herawi, M.; Parwani, A.V.; Chan, T.; Ali, S.Z.; Epstein, J.I. Polyoma virus-associated cellular changes in the urine and bladder biopsy samples: A cytohistologic correlation. Am. J. Surg. Pathol. 2006, 30, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Morace, R.; Kumar, T.; Tantisattamo, E.; Gibson, J.; Britton, S.; Li, W.; Kanaan, H.D.; Cohn, S.R.; Samarapungavan, D.; Zhang, P.L.; et al. Feasibility of BK Virus Real-Time PCR Testing in Renal Graft Biopsies With Negative SV40 Staining. Transplant. Proc. 2017, 49, 1294–1300. [Google Scholar] [CrossRef]
- FEDERA. Human Tissue and Medical Research: Code of Conduct for Responsible Use; FEDERA: Nijmegen, The Netherlands, 2011. [Google Scholar]
- Qamar, I.; Rehman, S.; Mehdi, G.; Maheshwari, V.; Ansari, H.A.; Chauhan, S. Utility of Cytospin and Cell block Technology in Evaluation of Body Fluids and Urine Samples: A Comparative Study. J. Cytol. 2018, 35, 79–82. [Google Scholar] [CrossRef] [PubMed]
- Klufah, F.; Mobaraki, G.; Chteinberg, E.; Alharbi, R.A.; Winnepenninckx, V.; Speel, E.J.M.; Rennspiess, D.; Olde Damink, S.W.; Neumann, U.P.; Kurz, A.K.; et al. High Prevalence of Human Polyomavirus 7 in Cholangiocarcinomas and Adjacent Peritumoral Hepatocytes: Preliminary Findings. Microorganisms 2020, 8, 1125. [Google Scholar] [CrossRef] [PubMed]
- Van Dongen, J.J.; Langerak, A.W.; Bruggemann, M.; Evans, P.A.; Hummel, M.; Lavender, F.L.; Delabesse, E.; Davi, F.; Schuuring, E.; Garcia-Sanz, R.; et al. Design and standardization of PCR primers and protocols for detection of clonal immunoglobulin and T-cell receptor gene recombinations in suspect lymphoproliferations: Report of the BIOMED-2 Concerted Action BMH4-CT98-3936. Leukemia 2003, 17, 2257–2317. [Google Scholar] [CrossRef]
- Yamada, Y.; Tsuchiya, T.; Inagaki, I.; Seishima, M.; Deguchi, T. Prediction of Early BK Virus Infection in Kidney Transplant Recipients by the Number of Cells With Intranuclear Inclusion Bodies (Decoy Cells). Transplant. Direct 2018, 4, e340. [Google Scholar] [CrossRef]
- Wu, Z.B.; Lin, G.B.; Zeng, A.P.; Chen, Z.Q.; Chen, J.; Zheng, M.Q.; Tu, G.W.; Rong, R.M. Detection of BK virus infection in renal transplant recipients and clinical application. Zhonghua Shi Yan He Lin Chuang Bing Du Xue Za Zhi = Chin. J. Exp. Clin. Virol. 2010, 24, 367–369. [Google Scholar]
- Ranzi, A.D.; Prolla, J.C.; Keitel, E.; Brackmann, R.; Kist, R.; dos Santos, G.; Bica, C.G. The role of urine cytology for ‘decoy cells’ as a screening tool in renal transplant recipients. Acta Cytol. 2012, 56, 543–547. [Google Scholar] [CrossRef]
- Chakera, A.; Dyar, O.J.; Hughes, E.; Bennett, S.; Hughes, D.; Roberts, I.S. Detection of polyomavirus BK reactivation after renal transplantation using an intensive decoy cell surveillance program is cost-effective. Transplantation 2011, 92, 1018–1023. [Google Scholar] [CrossRef]
- Geramizadeh, B.; Roozbeh, J.; Malek-Hosseini, S.A.; Azarpira, N.; Ayatollahi, M.; Salahi, H.; Aghdaee, M.; Yaghoobi, R. Urine cytology as a useful screening method for polyoma virus nephropathy in renal transplant patients: A single-center experience. Transplant. Proc. 2006, 38, 2923–2925. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.W.; Lee, H.H.; Lee, D.S.; Kim, S.J.; Joh, J.W.; Oh, H.Y.; Kim, J.W.; Kim, Y.G.; Huh, W.S.; Kim, D.J.; et al. Polymerase chain reaction for the diagnosis of human polyomavirus-associated nephropathy in renal transplant recipients. Transplant. Proc. 2004, 36, 2116–2117. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Elsheikh, T.M.; Zhang, Y. Polyomavirus (BK) cytopathic effect in urine cytology is not associated with high risk of developing high-grade urothelial carcinoma. J. Am. Soc. Cytopathol. 2020, 9, 84–88. [Google Scholar] [CrossRef] [PubMed]
- Costa, C.; Cavallo, R. Polyomavirus-associated nephropathy. World J. Transpl. 2012, 2, 84–94. [Google Scholar] [CrossRef]
- Mert, D.; Batgi, H.; Merdin, A.; Çeken, S.; Dal, M.S.; Tekgündüz, E.; Altuntaş, F.; Ertek, M. BK Virus-associated Hemorrhagic Cystitis in Patients with Allogeneic Hematopoietic Cell Transplantation: Report of Three Cases. Hematol. Rep. 2017, 9, 7205. [Google Scholar] [CrossRef] [PubMed]
- Egli, A.; Infanti, L.; Dumoulin, A.; Buser, A.; Samaridis, J.; Stebler, C.; Gosert, R.; Hirsch, H.H. Prevalence of polyomavirus BK and JC infection and replication in 400 healthy blood donors. J. Infect. Dis. 2009, 199, 837–846. [Google Scholar] [CrossRef]
- Jamboti, J.S. BK virus nephropathy in renal transplant recipients. Nephrology 2016, 21, 647–654. [Google Scholar] [CrossRef]
- Csoma, E.; Bidiga, L.; Mehes, G.; Gergely, L. No Evidence of Human Polyomavirus 9, WU and KI DNA in Kidney and Urinary Bladder Tumour Tissue Samples. Pathobiol. J. Immunopathol. Mol. Cell. Biol. 2016, 83, 252–257. [Google Scholar] [CrossRef]
- Geetha, V.; Rao, L.; Monappa, V.; Susmitha, M.; Prabhu, R. Decoy cells in urine cytology: A useful clue to post-transplant polyoma virus infection. J. Cytol. 2012, 29, 133–134. [Google Scholar] [CrossRef]
- Reploeg, M.D.; Storch, G.A.; Clifford, D.B. BK Virus: A Clinical Review. Clin. Infect. Dis. 2001, 33, 191–202. [Google Scholar] [CrossRef]
- Winter, B.J.; O’Connell, H.E.; Bowden, S.; Carey, M.; Eisen, D.P. A Case Control Study Reveals That Polyomaviruria Is Significantly Associated with Interstitial Cystitis and Vesical Ulceration. PLoS ONE 2015, 10, e0137310. [Google Scholar] [CrossRef] [PubMed]
- Kumari, K.; Pradeep, I.; Kakkar, A.; Dinda, A.K.; Seth, A.; Nayak, B.; Singh, G. BK polyomavirus and urothelial carcinoma: Experience at a tertiary care centre in India with review of literature. Ann. Diagn. Pathol. 2019, 40, 77–80. [Google Scholar] [CrossRef] [PubMed]
- Yong, C.; Steinberg, R.L.; O’Donnell, M.A. Severe Infectious Complications of Intravesical Bacillus Calmette-Guérin: A Case Series of 10 Patients. Urology 2020, 137, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Prasanna, T.; Craft, P.; Balasingam, G.; Haxhimolla, H.; Pranavan, G. Intravesical Gemcitabine versus Intravesical Bacillus Calmette-Guérin for the Treatment of Non-Muscle Invasive Bladder Cancer: An Evaluation of Efficacy and Toxicity. Front. Oncol. 2017, 7, 260. [Google Scholar] [CrossRef] [PubMed]
- Luckenbaugh, A.N.; Marks, R.M.; Miller, D.C.; Weizer, A.Z.; Stoffel, J.T.; Montgomery, J.S. A Management Algorithm for Mitomycin C Induced Cystitis. Bladder Cancer 2017, 3, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, C.; Li, X.; Zhuang, H.; Tian, S.; Cui, H.; Jiang, R.; Liu, C.; Tao, R.; Lin, X. Evaluating the efficacy and safety of intravesical chemotherapies for non-muscle invasive bladder cancer: A network meta-analysis. Oncotarget 2016, 7, 82567–82579. [Google Scholar] [CrossRef] [PubMed][Green Version]

| Clinicopathological Characteristic | Patients (n = 30) |
|---|---|
| Age range (years) | 25–90 |
| Male | 25–90 |
| Female | 38–80 |
| BKPyV-IHC+ve in urine cytology | 27/30 |
| Non-UCC patients | 13/16 |
| UCC-patients | 14/14 |
| Non-UCC patients | 16 |
| Immunosuppressed | 10 |
| Transplantation | 6 |
| Renal | 5 |
| Stem cell | 1 |
| Chronic disease (IBD, AIH, CTD, other malignancy) | 4 |
| No chronic disease | 6 |
| UCC patients | 14 |
| LGUCC | 6 |
| HGUCC | 1 |
| INUCC | 1 |
| CIS | 2 |
| LGUCC and HGUCC | 2 |
| CIS and HGUCC | 1 |
| CIS and INUCC | 1 |
| UCC with treatment | 9 |
| Intravesical treatment | 7 |
| BCG | 4 |
| Mitomycin | 2 |
| Both BCG and mitomycin | 1 |
| Radiotherapy | 1 |
| Intravesical treatment and radiotherapy | 1 |
| UCC with unknown treatment | 5 |
| Patient ID. | Diagnosis | (Intravesical) Treatment | BKPyV IHC | BKPyV PCR | ||||
|---|---|---|---|---|---|---|---|---|
| Urine Cytology | FFPE Tissue | Urine Sediments | FFPE Tissue | |||||
| LTAg | VP1 | LTAg | VP1 | |||||
| I.7 | LGUCC | Mitomycin | + | n.a. | n.a. | n.a. | n.a. | n.a. |
| I.8 | LGUCC | Unknown | + | - | n.a. | n.a. | n.a. | n.a. |
| I.9 | LGUCC | Unknown | + | - | n.a. | n.a. | n.a. | n.a. |
| I.10 | LGUCC, HGUCC | BCG/Mitomycin | + | - | n.a. | n.a. | - | - |
| I.12 | INUCC | Unknown | + | n.a. | n.a. | n.a. | n.a. | n.a. |
| I.13 | CIS, INUCC | BCG/radiotherapy | + | - | n.a. | n.a. | - | - |
| I.15 | LGUCC | Unknown | + | - | n.a. | n.a. | - | - |
| I.17 | CIS | BCG | + | - | n.a. | n.a. | - | - |
| I.21 | HGUCC | Radiotherapy | + | - | n.a. | n.a. | - | - |
| I.22 | CIS | BCG | + | - | + | - | - | - |
| I.23 | LGUCC, HGUCC | BCG | + | - | + | - | - | - |
| I.26 | CIS and HGUCC | BCG | + | - | + | + | - | - |
| I.29 | LGUCC | Mitomycin | + | - | + | - | n.a. | n.a. |
| I.30 | LGUCC | Unknown | + | - | + | - | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klufah, F.; Mobaraki, G.; Hausen, A.z.; Samarska, I.V. Reactivation of BK Polyomavirus in Urine Cytology Is Not Associated with Urothelial Cell Carcinoma. Viruses 2020, 12, 1412. https://doi.org/10.3390/v12121412
Klufah F, Mobaraki G, Hausen Az, Samarska IV. Reactivation of BK Polyomavirus in Urine Cytology Is Not Associated with Urothelial Cell Carcinoma. Viruses. 2020; 12(12):1412. https://doi.org/10.3390/v12121412
Chicago/Turabian StyleKlufah, Faisal, Ghalib Mobaraki, Axel zur Hausen, and Iryna V. Samarska. 2020. "Reactivation of BK Polyomavirus in Urine Cytology Is Not Associated with Urothelial Cell Carcinoma" Viruses 12, no. 12: 1412. https://doi.org/10.3390/v12121412
APA StyleKlufah, F., Mobaraki, G., Hausen, A. z., & Samarska, I. V. (2020). Reactivation of BK Polyomavirus in Urine Cytology Is Not Associated with Urothelial Cell Carcinoma. Viruses, 12(12), 1412. https://doi.org/10.3390/v12121412







