A Glimmer of Hope: Recent Updates and Future Challenges in Zika Vaccine Development
Abstract
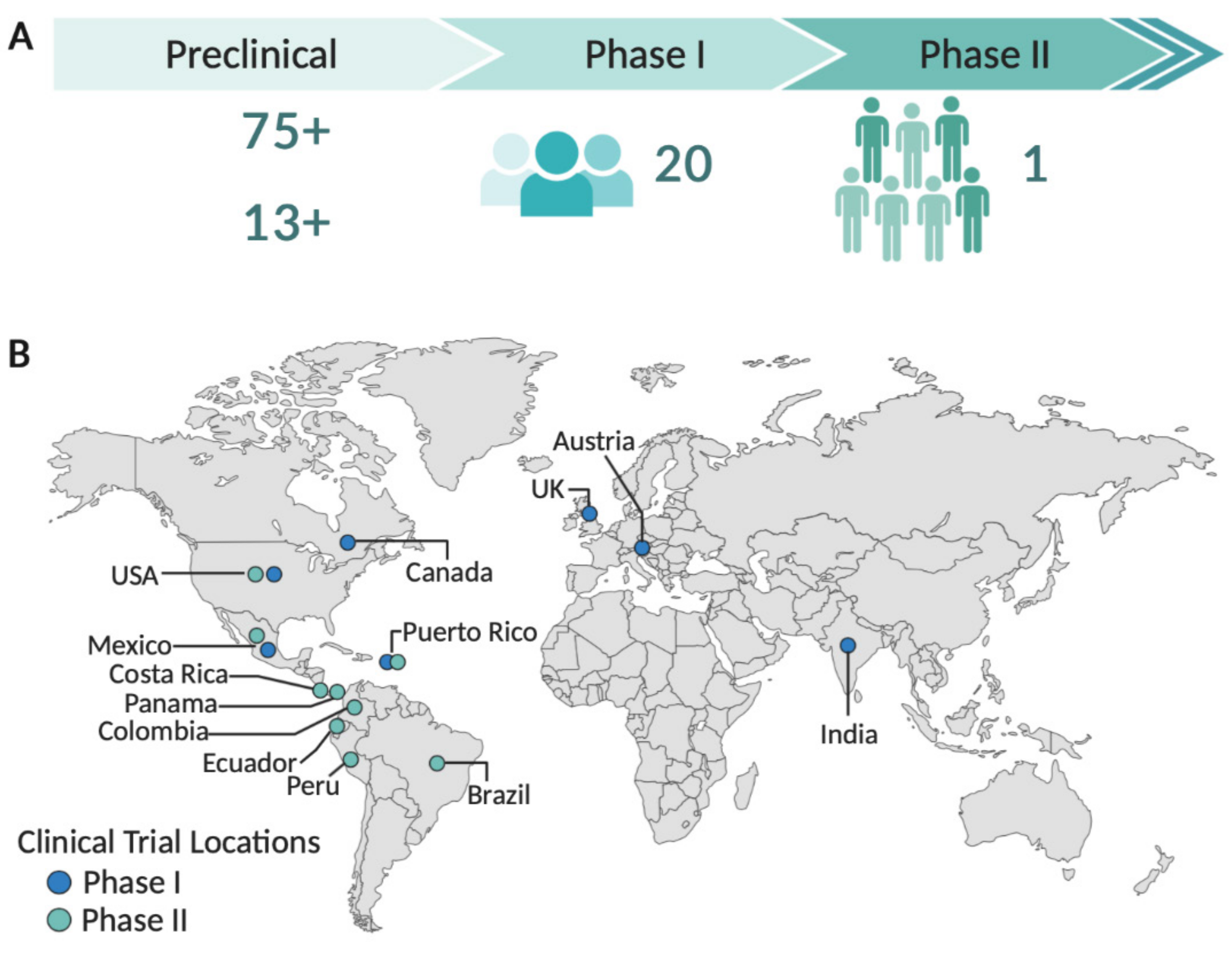
1. Introduction
2. Zika Virus Vaccine Development
3. Zika Virus Vaccines Candidates in Clinical Trials
3.1. DNA Vaccines
3.2. Purified, Inactivated Virus Vaccines
3.3. mRNA Vaccines
3.4. Live Attenuated Vaccines
3.5. Viral-Vectored Vaccines
4. Challenges in Late Stage Development of Zika Vaccines and Future Perspectives
5. Concluding Remarks
Author Contributions
Funding
Conflicts of Interest
References
- Dick, G.W.; Kitchen, S.; Haddow, A. Zika Virus (I). Isolations and serological specificity. Trans. R. Soc. Trop. Med. Hyg. 1952, 46, 509–520. [Google Scholar] [CrossRef]
- Vasilakis, N.; Weaver, S.C. Flavivirus transmission focusing on Zika. Curr. Opin. Virol. 2017, 22, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Filipe, A.R.; Martins, C.M.V.; Rocha, H. Laboratory infection with Zika virus after vaccination against yellow fever. Arch. Gesamte Virusforsch. 1973, 43, 315–319. [Google Scholar] [CrossRef] [PubMed]
- Simpson, D. Zika virus infection in man. Trans. R. Soc. Trop. Med. Hyg. 1964, 58, 335–338. [Google Scholar] [CrossRef]
- Kindhauser, M.K.; Allen, T.; Frank, V.; Santhana, R.S.; Dye, C. Zika: The origin and spread of a mosquito-borne virus. Bull. World Health Organ. 2016, 94, 675–686C. [Google Scholar] [CrossRef] [PubMed]
- Duffy, M.R.; Chen, T.-H.; Hancock, W.T.; Powers, A.M.; Kool, J.L.; Lanciotti, R.S.; Pretrick, M.; Marfel, M.; Holzbauer, S.; Dubray, C.; et al. Zika Virus Outbreak on Yap Island, Federated States of Micronesia. N. Engl. J. Med. 2009, 360, 2536–2543. [Google Scholar] [CrossRef] [PubMed]
- Musso, D.; Nilles, E.J.; Cao-Lormeau, V.-M. Rapid spread of emerging Zika virus in the Pacific area. Clin. Microbiol. Infect. 2014, 20, O595–O596. [Google Scholar] [CrossRef] [PubMed]
- Musso, D.; Nhan, T.; Robin, E.; Roche, C.; Bierlaire, D.; Zisou, K.; Shan Yan, A.; Cao-Lormeau, V.; Broult, J. Potential for Zika virus transmission through blood transfusion demonstrated during an outbreak in French Polynesia, November 2013 to February 2014. Eurosurveillance 2014, 19, 20761. [Google Scholar] [CrossRef]
- Cao-Lormeau, V.-M.; Musso, D. Emerging arboviruses in the Pacific. Lancet 2014, 384, 1571–1572. [Google Scholar] [CrossRef]
- Campos, G.S.; Bandeira, A.C.; Sardi, S.I. Zika Virus Outbreak, Bahia, Brazil. Emerg. Infect. Dis. 2015, 21, 1885–1886. [Google Scholar] [CrossRef]
- Zanluca, C.; De Melo, V.C.A.; Mosimann, A.L.P.; Dos Santos, G.I.V.; Dos Santos, C.N.D.; Luz, K. First report of autochthonous transmission of Zika virus in Brazil. Mem. Inst. Oswaldo Cruz 2015, 110, 569–572. [Google Scholar] [CrossRef] [PubMed]
- Lowe, R.; Barcellos, C.; Brasil, P.; Cruz, O.; Honório, N.; Kuper, H.; Carvalho, M. The Zika Virus Epidemic in Brazil: From Discovery to Future Implications. Int. J. Environ. Res. Public Health 2018, 15, 96. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Sun, K.; Chinazzi, M.; Pastore y Piontti, A.; Dean, N.E.; Rojas, D.P.; Merler, S.; Mistry, D.; Poletti, P.; Rossi, L.; et al. Spread of Zika virus in the Americas. Proc. Natl. Acad. Sci. USA 2017, 114, E4334–E4343. [Google Scholar] [CrossRef] [PubMed]
- Faria, N.R.; Quick, J.; Claro, I.M.; Thézé, J.; De Jesus, J.G.; Giovanetti, M.; Kraemer, M.U.G.; Hill, S.C.; Black, A.; Da Costa, A.C.; et al. Establishment and cryptic transmission of Zika virus in Brazil and the Americas. Nature 2017, 546, 406–410. [Google Scholar] [CrossRef]
- Teixeira, M.G.; Da Conceição, N.; Costa, M.; De Oliveira, W.K.; Nunes, M.L.; Rodrigues, L.C. The Epidemic of Zika Virus–Related Microcephaly in Brazil: Detection, Control, Etiology, and Future Scenarios. Am. J. Public Health 2016, 106, 601–605. [Google Scholar] [CrossRef]
- De Araújo, T.V.B.; Rodrigues, L.C.; De Alencar Ximenes, R.A.; De Barros Miranda-Filho, D.; Montarroyos, U.R.; De Melo, A.P.L.; Valongueiro, S.; De Albuquerque, M.D.F.P.M.; Souza, W.V.; Braga, C.; et al. Association between Zika virus infection and microcephaly in Brazil, January to May, 2016: Preliminary report of a case-control study. Lancet Infect. Dis. 2016, 16, 1356–1363. [Google Scholar] [CrossRef]
- De Araújo, T.V.B.; Ximenes, R.A.D.A.; Miranda-Filho, D.D.B.; Souza, W.V.; Montarroyos, U.R.; De Melo, A.P.L.; Valongueiro, S.; De Albuquerque, M.D.F.P.M.; Braga, C.; Filho, S.P.B.; et al. Association between microcephaly, Zika virus infection, and other risk factors in Brazil: Final report of a case-control study. Lancet. Infect. Dis. 2018, 18, 328–336. [Google Scholar] [CrossRef]
- World Health Organization WHO. Director-General summarizes the outcome of the Emergency Committee Regarding Clusters of Microcephaly and Guillain-Barré Syndrome. Available online: https://www.who.int/en/news-room/detail/01-02-2016-who-director-general-summarizes-the-outcome-of-the-emergency-committee-regarding-clusters-of-microcephaly-and-guillain-barré-syndrome (accessed on 12 September 2020).
- Magalhaes, T.; Morais, C.N.L.; Jacques, I.J.A.A.; Azevedo, E.A.N.; Brito, A.M.; Lima, P.V.; Carvalho, G.M.M.; Lima, A.R.S.; Castanha, P.M.S.; Cordeiro, M.T.; et al. Follow-up household serosurvey in Northeast Brazil for Zika virus: Sexual contacts of index patients have the highest risk for seropositivity. J. Infect. Dis. 2020. [Google Scholar] [CrossRef]
- Brooks, R.B.; Carlos, M.P.; Myers, R.A.; White, M.G.; Bobo-Lenoci, T.; Aplan, D.; Blythe, D.; Feldman, K.A. Likely Sexual Transmission of Zika Virus from a Man with No Symptoms of Infection—Maryland, 2016. MMWR. Morb. Mortal. Wkly. Rep. 2016, 65, 915–916. [Google Scholar] [CrossRef]
- Fréour, T.; Mirallié, S.; Hubert, B.; Splingart, C.; Barrière, P.; Maquart, M.; Leparc-Goffart, I. Sexual transmission of Zika virus in an entirely asymptomatic couple returning from a Zika epidemic area, France, April 2016. Eurosurveillance 2016, 21, 30254. [Google Scholar] [CrossRef]
- Davidson, A.; Slavinski, S.; Komoto, K.; Rakeman, J.; Weiss, D. Suspected Female-to-Male Sexual Transmission of Zika Virus—New York City, 2016. MMWR Morb. Mortal. Wkly. Rep. 2016, 65, 716–717. [Google Scholar] [CrossRef] [PubMed]
- Deckard, D.T.; Chung, W.M.; Brooks, J.T.; Smith, J.C.; Woldai, S.; Hennessey, M.; Kwit, N.; Mead, P. Male-to-Male Sexual Transmission of Zika Virus—Texas, January 2016. MMWR Morb. Mortal. Wkly. Rep. 2016, 65, 372–374. [Google Scholar] [CrossRef] [PubMed]
- Musso, D.; Roche, C.; Robin, E.; Nhan, T.; Teissier, A.; Cao-Lormeau, V.-M. Potential Sexual Transmission of Zika Virus. Emerg. Infect. Dis. 2015, 21, 359–361. [Google Scholar] [CrossRef] [PubMed]
- Bhatnagar, J.; Rabeneck, D.B.; Martines, R.B.; Reagan-Steiner, S.; Ermias, Y.; Estetter, L.B.C.; Suzuki, T.; Ritter, J.; Keating, M.K.; Hale, G.; et al. Zika Virus RNA Replication and Persistence in Brain and Placental Tissue. Emerg. Infect. Dis. 2017, 23, 405–414. [Google Scholar] [CrossRef]
- Miner, J.J.; Cao, B.; Govero, J.; Smith, A.M.; Fernandez, E.; Cabrera, O.H.; Garber, C.; Noll, M.; Klein, R.S.; Noguchi, K.K.; et al. Zika Virus Infection during Pregnancy in Mice Causes Placental Damage and Fetal Demise. Cell 2016, 165, 1081–1091. [Google Scholar] [CrossRef]
- Quicke, K.M.; Bowen, J.R.; Johnson, E.L.; McDonald, C.E.; Ma, H.; O’Neal, J.T.; Rajakumar, A.; Wrammert, J.; Rimawi, B.H.; Pulendran, B.; et al. Zika Virus Infects Human Placental Macrophages. Cell Host Microbe 2016, 20, 83–90. [Google Scholar] [CrossRef]
- Teixeira, F.M.E.; Pietrobon, A.J.; Oliveira, L.D.M.; Oliveira, L.M.D.S.; Sato, M.N. Maternal-Fetal Interplay in Zika Virus Infection and Adverse Perinatal Outcomes. Front. Immunol. 2020, 11. [Google Scholar] [CrossRef]
- Microcephaly Epidemic Research Group. Microcephaly in Infants, Pernambuco State, Brazil, 2015. Emerg. Infect. Dis. 2016, 22, 1090–1093. [Google Scholar] [CrossRef]
- Miranda-Filho, D.D.B.; Martelli, C.M.T.; Ximenes, R.A.d.A.; Araújo, T.V.B.; Rocha, M.A.W.; Ramos, R.C.F.; Dhalia, R.; França, R.F.d.O.; Marques Júnior, E.T.d.A.; Rodrigues, L.C. Initial Description of the Presumed Congenital Zika Syndrome. Am. J. Public Health 2016, 106, 598–600. [Google Scholar] [CrossRef]
- Brasil, P.; Pereira, J.P.; Moreira, M.E.; Ribeiro Nogueira, R.M.; Damasceno, L.; Wakimoto, M.; Rabello, R.S.; Valderramos, S.G.; Halai, U.-A.; Salles, T.S.; et al. Zika Virus Infection in Pregnant Women in Rio de Janeiro. N. Engl. J. Med. 2016, 375, 2321–2334. [Google Scholar] [CrossRef]
- Barjas-Castro, M.L.; Angerami, R.N.; Cunha, M.S.; Suzuki, A.; Nogueira, J.S.; Rocco, I.M.; Maeda, A.Y.; Vasami, F.G.S.; Katz, G.; Boin, I.F.S.F.; et al. Probable transfusion-transmitted Zika virus in Brazil. Transfusion 2016, 56, 1684–1688. [Google Scholar] [CrossRef] [PubMed]
- Motta, I.J.F.; Spencer, B.R.; Cordeiro da Silva, S.G.; Arruda, M.B.; Dobbin, J.A.; Gonzaga, Y.B.M.; Arcuri, I.P.; Tavares, R.C.B.S.; Atta, E.H.; Fernandes, R.F.M.; et al. Evidence for Transmission of Zika Virus by Platelet Transfusion. N. Engl. J. Med. 2016, 375, 1101–1103. [Google Scholar] [CrossRef] [PubMed]
- Méndez, N.; Oviedo-Pastrana, M.; Mattar, S.; Caicedo-Castro, I.; Arrieta, G. Zika virus disease, microcephaly and Guillain-Barré syndrome in Colombia: Epidemiological situation during 21 months of the Zika virus outbreak, 2015–2017. Arch. Public Heal. 2017, 75, 65. [Google Scholar] [CrossRef] [PubMed]
- Barbi, L.; Coelho, A.V.C.; Alencar, L.C.A.d.; Crovella, S. Prevalence of Guillain-Barré syndrome among Zika virus infected cases: A systematic review and meta-analysis. Braz. J. Infect. Dis. 2018, 22, 137–141. [Google Scholar] [CrossRef]
- Styczynski, A.R.; Malta, J.M.A.S.; Krow-Lucal, E.R.; Percio, J.; Nóbrega, M.E.; Vargas, A.; Lanzieri, T.M.; Leite, P.L.; Staples, J.E.; Fischer, M.X.; et al. Increased rates of Guillain-Barré syndrome associated with Zika virus outbreak in the Salvador metropolitan area, Brazil. PLoS Negl. Trop. Dis. 2017, 11, e0005869. [Google Scholar] [CrossRef]
- Rozé, B.; Najioullah, F.; Fergé, J.-L.; Apetse, K.; Brouste, Y.; Cesaire, R.; Fagour, C.; Fagour, L.; Hochedez, P.; Jeannin, S.; et al. Zika virus detection in urine from patients with Guillain-Barré syndrome on Martinique, January 2016. Eurosurveillance 2016, 21, 30154. [Google Scholar] [CrossRef]
- Cao-Lormeau, V.-M.; Blake, A.; Mons, S.; Lastère, S.; Roche, C.; Vanhomwegen, J.; Dub, T.; Baudouin, L.; Teissier, A.; Larre, P.; et al. Guillain-Barré Syndrome outbreak associated with Zika virus infection in French Polynesia: A case-control study. Lancet 2016, 387, 1531–1539. [Google Scholar] [CrossRef]
- De Siqueira, I.C.; Rodrigues, S.G.; Martins, L.C.; Vasilakis, N.; Novaes, M.A.C.; Alcântara, L.C.J.; Farias, D.S.; Do Rosário, M.S.; De Jesus, P.A.P.; Ko, A.I.; et al. Guillain–Barré Syndrome After Zika Virus Infection in Brazil. Am. J. Trop. Med. Hyg. 2016, 95, 1157–1160. [Google Scholar] [CrossRef]
- Soto-Hernández, J.L.; Ponce de León Rosales, S.; Vargas Cañas, E.S.; Cárdenas, G.; Carrillo Loza, K.; Díaz-Quiñonez, J.A.; López-Martínez, I.; Jiménez-Corona, M.-E.; Ruiz-Matus, C.; Kuri Morales, P. Guillain–Barré Syndrome Associated With Zika Virus Infection: A Prospective Case Series From Mexico. Front. Neurol. 2019, 10. [Google Scholar] [CrossRef]
- Cauchemez, S.; Besnard, M.; Bompard, P.; Dub, T.; Guillemette-Artur, P.; Eyrolle-Guignot, D.; Salje, H.; Van Kerkhove, M.D.; Abadie, V.; Garel, C.; et al. Association between Zika virus and microcephaly in French Polynesia, 2013–2015: A retrospective study. Lancet 2016, 387, 2125–2132. [Google Scholar] [CrossRef]
- Cuevas, E.L.; Tong, V.T.; Rozo, N.; Valencia, D.; Pacheco, O.; Gilboa, S.M.; Mercado, M.; Renquist, C.M.; González, M.; Ailes, E.C.; et al. Preliminary Report of Microcephaly Potentially Associated with Zika Virus Infection During Pregnancy—Colombia, January–November 2016. MMWR Morb. Mortal. Wkly. Rep. 2016, 65, 1409–1413. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, B.; Boyle, C.; Honein, M.A. Birth Defects Potentially Related to Zika Virus Infection During Pregnancy in the United States. JAMA 2018, 319, 1195. [Google Scholar] [CrossRef] [PubMed]
- Collins, M.H.; Zepeda, O.; Blette, B.; Jadi, R.; Morales, M.; Pérez, R.; Liou, G.-J.A.; Montoya-Cruz, M.; Harris, E.; Becker-Dreps, S.; et al. Serologic surveillance of maternal Zika infection in a prospective cohort in Leon, Nicaragua during the peak of the Zika epidemic. PLoS ONE 2020, 15, e0230692. [Google Scholar] [CrossRef] [PubMed]
- Hoen, B.; Schaub, B.; Funk, A.L.; Ardillon, V.; Boullard, M.; Cabié, A.; Callier, C.; Carles, G.; Cassadou, S.; Césaire, R.; et al. Pregnancy Outcomes after ZIKV Infection in French Territories in the Americas. N. Engl. J. Med. 2018, 378, 985–994. [Google Scholar] [CrossRef] [PubMed]
- Honein, M.A.; Dawson, A.L.; Petersen, E.E.; Jones, A.M.; Lee, E.H.; Yazdy, M.M.; Ahmad, N.; Macdonald, J.; Evert, N.; Bingham, A.; et al. Birth Defects Among Fetuses and Infants of US Women With Evidence of Possible Zika Virus Infection During Pregnancy. JAMA 2017, 317, 59. [Google Scholar] [CrossRef] [PubMed]
- Leal, M.C.; Muniz, L.F.; Ferreira, T.S.A.; Santos, C.M.; Almeida, L.C.; Van Der Linden, V.; Ramos, R.C.F.; Rodrigues, L.C.; Neto, S.S.C. Hearing Loss in Infants with Microcephaly and Evidence of Congenital Zika Virus Infection—Brazil, November 2015–May 2016. MMWR Morb. Mortal. Wkly. Rep. 2016, 65, 917–919. [Google Scholar] [CrossRef]
- De Paula Freitas, B.; De Oliveira Dias, J.R.; Prazeres, J.; Sacramento, G.A.; Ko, A.I.; Maia, M.; Belfort, R. Ocular Findings in Infants With Microcephaly Associated With Presumed Zika Virus Congenital Infection in Salvador, Brazil. JAMA Ophthalmol. 2016, 134, 529. [Google Scholar] [CrossRef]
- Mulkey, S.B.; Arroyave-Wessel, M.; Peyton, C.; Bulas, D.I.; Fourzali, Y.; Jiang, J.; Russo, S.; McCarter, R.; Msall, M.E.; Du Plessis, A.J.; et al. Neurodevelopmental Abnormalities in Children with in Utero Zika Virus Exposure Without Congenital Zika Syndrome. JAMA Pediatr. 2020, 174, 269. [Google Scholar] [CrossRef]
- Einspieler, C.; Utsch, F.; Brasil, P.; Panvequio Aizawa, C.Y.; Peyton, C.; Hydee Hasue, R.; Françoso Genovesi, F.; Damasceno, L.; Moreira, M.E.; Adachi, K.; et al. Association of Infants Exposed to Prenatal Zika Virus Infection with Their Clinical, Neurologic, and Developmental Status Evaluated via the General Movement Assessment Tool. JAMA Netw. Open 2019, 2, e187235. [Google Scholar] [CrossRef]
- Morrison, T.E.; Diamond, M.S. Animal Models of Zika Virus Infection, Pathogenesis, and Immunity. J. Virol. 2017, 91. [Google Scholar] [CrossRef]
- El Costa, H.; Gouilly, J.; Mansuy, J.-M.; Chen, Q.; Levy, C.; Cartron, G.; Veas, F.; Al-Daccak, R.; Izopet, J.; Jabrane-Ferrat, N. ZIKA virus reveals broad tissue and cell tropism during the first trimester of pregnancy. Sci. Rep. 2016, 6, 35296. [Google Scholar] [CrossRef] [PubMed]
- Retallack, H.; Di Lullo, E.; Arias, C.; Knopp, K.A.; Laurie, M.T.; Sandoval-Espinosa, C.; Mancia Leon, W.R.; Krencik, R.; Ullian, E.M.; Spatazza, J.; et al. Zika virus cell tropism in the developing human brain and inhibition by azithromycin. Proc. Natl. Acad. Sci. USA 2016, 113, 14408–14413. [Google Scholar] [CrossRef] [PubMed]
- Leda, A.R.; Bertrand, L.; Andras, I.E.; El-Hage, N.; Nair, M.; Toborek, M. Selective Disruption of the Blood–Brain Barrier by Zika Virus. Front. Microbiol. 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Brito, C.A.A.; Henriques-Souza, A.; Soares, C.R.P.; Castanha, P.M.S.; Machado, L.C.; Pereira, M.R.; Sobral, M.C.M.; Lucena-Araujo, A.R.; Wallau, G.L.; Franca, R.F.O. Persistent detection of Zika virus RNA from an infant with severe microcephaly—A case report. BMC Infect. Dis. 2018, 18, 388. [Google Scholar] [CrossRef] [PubMed]
- Paz-Bailey, G.; Rosenberg, E.S.; Doyle, K.; Munoz-Jordan, J.; Santiago, G.A.; Klein, L.; Perez-Padilla, J.; Medina, F.A.; Waterman, S.H.; Adams, L.E.; et al. Persistence of Zika Virus in Body Fluids—Final Report. N. Engl. J. Med. 2018, 379, 1234–1243. [Google Scholar] [CrossRef]
- Katzelnick, L.C.; Narvaez, C.; Arguello, S.; Lopez Mercado, B.; Collado, D.; Ampie, O.; Elizondo, D.; Miranda, T.; Bustos Carillo, F.; Mercado, J.C.; et al. Zika virus infection enhances future risk of severe dengue disease. Science 2020, 369, 1123–1128. [Google Scholar] [CrossRef]
- Rodriguez-Barraquer, I.; Costa, F.; Nascimento, E.J.M.; Nery, N.; Castanha, P.M.S.; Sacramento, G.A.; Cruz, J.; Carvalho, M.; De Olivera, D.; Hagan, J.E.; et al. Impact of preexisting dengue immunity on Zika virus emergence in a dengue endemic region. Science 2019, 363, 607–610. [Google Scholar] [CrossRef]
- Castanha, P.M.S.; Erdos, G.; Watkins, S.C.; Falo, L.D.; Marques, E.T.A.; Barratt-Boyes, S.M. Reciprocal immune enhancement of dengue and Zika virus infection in human skin. JCI Insight 2020, 5. [Google Scholar] [CrossRef]
- George, J.; Valiant, W.G.; Mattapallil, M.J.; Walker, M.; Huang, Y.-J.S.; Vanlandingham, D.L.; Misamore, J.; Greenhouse, J.; Weiss, D.E.; Verthelyi, D.; et al. Prior Exposure to Zika Virus Significantly Enhances Peak Dengue-2 Viremia in Rhesus Macaques. Sci. Rep. 2017, 7, 10498. [Google Scholar] [CrossRef]
- Kawiecki, A.B.; Christofferson, R.C. Zika Virus-Induced Antibody Response Enhances Dengue Virus Serotype 2 Replication in vitro. J. Infect. Dis. 2016, 214, 1357–1360. [Google Scholar] [CrossRef]
- Castanha, P.M.S.; Nascimento, E.J.M.; Braga, C.; Cordeiro, M.T.; De Carvalho, O.V.; De Mendonça, L.R.; Azevedo, E.A.N.; França, R.F.O.; Dhalia, R.; Marques, E.T.A. Dengue Virus-Specific Antibodies Enhance Brazilian Zika Virus Infection. J. Infect. Dis. 2017, 215, 781–785. [Google Scholar] [CrossRef] [PubMed]
- Bardina, S.V.; Bunduc, P.; Tripathi, S.; Duehr, J.; Frere, J.J.; Brown, J.A.; Nachbagauer, R.; Foster, G.A.; Krysztof, D.; Tortorella, D.; et al. Enhancement of Zika virus pathogenesis by preexisting antiflavivirus immunity. Science 2017, 356, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Fowler, A.M.; Tang, W.W.; Young, M.P.; Mamidi, A.; Viramontes, K.M.; McCauley, M.D.; Carlin, A.F.; Schooley, R.T.; Swanstrom, J.; Baric, R.S.; et al. Maternally Acquired Zika Antibodies Enhance Dengue Disease Severity in Mice. Cell Host Microbe 2018, 24, 743–750.e5. [Google Scholar] [CrossRef] [PubMed]
- Priyamvada, L.; Quicke, K.M.; Hudson, W.H.; Onlamoon, N.; Sewatanon, J.; Edupuganti, S.; Pattanapanyasat, K.; Chokephaibulkit, K.; Mulligan, M.J.; Wilson, P.C.; et al. Human antibody responses after dengue virus infection are highly cross-reactive to Zika virus. Proc. Natl. Acad. Sci. USA 2016, 113, 7852–7857. [Google Scholar] [CrossRef] [PubMed]
- Collins, M.H.; Metz, S.W. Progress and Works in Progress: Update on Flavivirus Vaccine Development. Clin. Ther. 2017, 39, 1519–1536. [Google Scholar] [CrossRef]
- Deng, S.-Q.; Yang, X.; Wei, Y.; Chen, J.-T.; Wang, X.-J.; Peng, H.-J. A Review on Dengue Vaccine Development. Vaccines 2020, 8, 63. [Google Scholar] [CrossRef]
- Sridhar, S.; Luedtke, A.; Langevin, E.; Zhu, M.; Bonaparte, M.; Machabert, T.; Savarino, S.; Zambrano, B.; Moureau, A.; Khromava, A.; et al. Effect of Dengue Serostatus on Dengue Vaccine Safety and Efficacy. N. Engl. J. Med. 2018, 379, 327–340. [Google Scholar] [CrossRef]
- World Health Organization. Zika Vaccine Development Technology Roadmap. Available online: https://www.who.int/immunization/research/development/Zika_Vaccine_Development_Technology_Roadmap_after_consultation_April_2019.pdf?ua=1 (accessed on 14 September 2020).
- Vartak, A.; Sucheck, S. Recent Advances in Subunit Vaccine Carriers. Vaccines 2016, 4, 12. [Google Scholar] [CrossRef]
- Wallis, J.; Shenton, D.P.; Carlisle, R.C. Novel approaches for the design, delivery and administration of vaccine technologies. Clin. Exp. Immunol. 2019, cei.13287. [Google Scholar] [CrossRef]
- Cabral-Miranda, G.; Lim, S.M.; Mohsen, M.O.; Pobelov, I.V.; Roesti, E.S.; Heath, M.D.; Skinner, M.A.; Kramer, M.F.; Martina, B.E.E.; Bachmann, M.F. Zika Virus-Derived E-DIII Protein Displayed on Immunologically Optimized VLPs Induces Neutralizing Antibodies without Causing Enhancement of Dengue Virus Infection. Vaccines 2019, 7, 72. [Google Scholar] [CrossRef]
- Yang, M.; Lai, H.; Sun, H.; Chen, Q. Virus-like particles that display Zika virus envelope protein domain III induce potent neutralizing immune responses in mice. Sci. Rep. 2017, 7, 7679. [Google Scholar] [CrossRef] [PubMed]
- Garg, H.; Mehmetoglu-Gurbuz, T.; Joshi, A. Virus Like Particles (VLP) as multivalent vaccine candidate against Chikungunya, Japanese Encephalitis, Yellow Fever and Zika Virus. Sci. Rep. 2020, 10, 4017. [Google Scholar] [CrossRef] [PubMed]
- Liu, A. Comparison of Plasmid DNA and mRNA as Vaccine Technologies. Vaccines 2019, 7, 37. [Google Scholar] [CrossRef] [PubMed]
- Marques, E.T.A.; Chikhlikar, P.; De Arruda, L.B.; Leao, I.C.; Lu, Y.; Wong, J.; Chen, J.-S.; Byrne, B.; August, J.T. HIV-1 p55Gag encoded in the lysosome-associated membrane protein-1 as a DNA plasmid vaccine chimera is highly expressed, traffics to the major histocompatibility class II compartment, and elicits enhanced immune responses. J. Biol. Chem. 2003, 278, 37926–37936. [Google Scholar] [CrossRef]
- Arruda, L.B.; Sim, D.; Chikhlikar, P.R.; Maciel, M.; Akasaki, K.; August, J.T.; Marques, E.T.A. Dendritic cell-lysosomal-associated membrane protein (LAMP) and LAMP-1-HIV-1 gag chimeras have distinct cellular trafficking pathways and prime T and B cell responses to a diverse repertoire of epitopes. J. Immunol. 2006, 177, 2265–2275. [Google Scholar] [CrossRef]
- Han, H.-H.; Diaz, C.; Acosta, C.; Liu, M.; Borkowski, A. Safety and immunogenicity of a purified inactivated Zika virus vaccine (PIZV) candidate in healthy adults; A randomised, observer-blind phase 1 clinical trial. Lancet Infect. Dis. in press.
- Stephenson, K.E.; Tan, C.S.; Walsh, S.R.; Hale, A.; Ansel, J.L.; Kanjilal, D.G.; Jaegle, K.; Peter, L.; Borducchi, E.N.; Nkolola, J.P.; et al. Safety and immunogenicity of a Zika purified inactivated virus vaccine given via standard, accelerated, or shortened schedules: A single-centre, double-blind, sequential-group, randomised, placebo-controlled, phase 1 trial. Lancet Infect. Dis. 2020, 20, 1061–1070. [Google Scholar] [CrossRef]
- Modjarrad, K.; Lin, L.; George, S.L.; Stephenson, K.E.; Eckels, K.H.; De La Barrera, R.A.; Jarman, R.G.; Sondergaard, E.; Tennant, J.; Ansel, J.L.; et al. Preliminary aggregate safety and immunogenicity results from three trials of a purified inactivated Zika virus vaccine candidate: Phase 1, randomised, double-blind, placebo-controlled clinical trials. Lancet 2018, 391, 563–571. [Google Scholar] [CrossRef]
- Gaudinski, M.R.; Houser, K.V.; Morabito, K.M.; Hu, Z.; Yamshchikov, G.; Rothwell, R.S.; Berkowitz, N.; Mendoza, F.; Saunders, J.G.; Novik, L.; et al. Safety, tolerability, and immunogenicity of two Zika virus DNA vaccine candidates in healthy adults: Randomised, open-label, phase 1 clinical trials. Lancet 2018, 391, 552–562. [Google Scholar] [CrossRef]
- Tebas, P.; Roberts, C.C.; Muthumani, K.; Reuschel, E.; White, S.; Khan, A.S.; Racine, T.; Choi, H.; Zaidi, F.; Boyer, J.; et al. ZIKA-001: Safety and Immunogenicity of an Engineered DNA Vaccine Against ZIKA virus infection. Open Forum Infect. Dis. 2017, 4, S300–S301. [Google Scholar] [CrossRef]
- Richner, J.M.; Himansu, S.; Dowd, K.A.; Butler, S.L.; Salazar, V.; Fox, J.M.; Julander, J.G.; Tang, W.W.; Shresta, S.; Pierson, T.C.; et al. Modified mRNA Vaccines Protect against Zika Virus Infection. Cell 2017, 168, 1114–1125.e10. [Google Scholar] [CrossRef]
- Moderna, T.X. Moderna Highlights Opportunity of mRNA Vaccines at Its First Vaccines Day. Available online: https://investors.modernatx.com/news-releases/news-release-details/moderna-highlights-opportunity-mrna-vaccines-its-first-vaccines (accessed on 10 September 2020).
- Annamalai, A.S.; Pattnaik, A.; Sahoo, B.R.; Muthukrishnan, E.; Natarajan, S.K.; Steffen, D.; Vu, H.L.X.; Delhon, G.; Osorio, F.A.; Petro, T.M.; et al. Zika Virus Encoding Nonglycosylated Envelope Protein Is Attenuated and Defective in Neuroinvasion. J. Virol. 2017, 91. [Google Scholar] [CrossRef] [PubMed]
- Annamalai, A.S.; Pattnaik, A.; Sahoo, B.R.; Guinn, Z.P.; Bullard, B.L.; Weaver, E.A.; Steffen, D.; Natarajan, S.K.; Petro, T.M.; Pattnaik, A.K. An Attenuated Zika Virus Encoding Non-Glycosylated Envelope (E) and Non-Structural Protein 1 (NS1) Confers Complete Protection against Lethal Challenge in a Mouse Model. Vaccines 2019, 7, 112. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Kum, D.B.; Xia, H.; Luo, H.; Shan, C.; Zou, J.; Muruato, A.E.; Medeiros, D.B.A.; Nunes, B.T.D.; Dallmeier, K.; et al. A Single-Dose Live-Attenuated Zika Virus Vaccine with Controlled Infection Rounds that Protects against Vertical Transmission. Cell Host Microbe 2018, 24, 487–499.e5. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-F.; Dong, H.-L.; Wang, H.-J.; Huang, X.-Y.; Qiu, Y.-F.; Ji, X.; Ye, Q.; Li, C.; Liu, Y.; Deng, Y.-Q.; et al. Development of a chimeric Zika vaccine using a licensed live-attenuated flavivirus vaccine as backbone. Nat. Commun. 2018, 9, 673. [Google Scholar] [CrossRef]
- Shan, C.; Muruato, A.E.; Jagger, B.W.; Richner, J.; Nunes, B.T.D.; Medeiros, D.B.A.; Xie, X.; Nunes, J.G.C.; Morabito, K.M.; Kong, W.-P.; et al. A single-dose live-attenuated vaccine prevents Zika virus pregnancy transmission and testis damage. Nat. Commun. 2017, 8, 676. [Google Scholar] [CrossRef]
- Shan, C.; Muruato, A.E.; Nunes, B.T.D.; Luo, H.; Xie, X.; Medeiros, D.B.A.; Wakamiya, M.; Tesh, R.B.; Barrett, A.D.; Wang, T.; et al. A live-attenuated Zika virus vaccine candidate induces sterilizing immunity in mouse models. Nat. Med. 2017, 23, 763–767. [Google Scholar] [CrossRef]
- Nürnberger, C.; Bodmer, B.S.; Fiedler, A.H.; Gabriel, G.; Mühlebach, M.D. A Measles Virus-Based Vaccine Candidate Mediates Protection against Zika Virus in an Allogeneic Mouse Pregnancy. Model. J. Virol. 2018, 93. [Google Scholar] [CrossRef]
- López-Camacho, C.; Abbink, P.; Larocca, R.A.; Dejnirattisai, W.; Boyd, M.; Badamchi-Zadeh, A.; Wallace, Z.R.; Doig, J.; Velazquez, R.S.; Neto, R.D.L.; et al. Rational Zika vaccine design via the modulation of antigen membrane anchors in chimpanzee adenoviral vectors. Nat. Commun. 2018, 9, 2441. [Google Scholar] [CrossRef]
- Cox, F.; Van Der Fits, L.; Abbink, P.; Larocca, R.A.; Van Huizen, E.; Saeland, E.; Verhagen, J.; Peterson, R.; Tolboom, J.; Kaufmann, B.; et al. Adenoviral vector type 26 encoding Zika virus (ZIKV) M-Env antigen induces humoral and cellular immune responses and protects mice and nonhuman primates against ZIKV challenge. PLoS ONE 2018, 13, e0202820. [Google Scholar] [CrossRef]
- Vannice, K.S.; Cassetti, M.C.; Eisinger, R.W.; Hombach, J.; Knezevic, I.; Marston, H.D.; Wilder-Smith, A.; Cavaleri, M.; Krause, P.R. Demonstrating vaccine effectiveness during a waning epidemic: A WHO/NIH meeting report on approaches to development and licensure of Zika vaccine candidates. Vaccine 2019, 37, 863–868. [Google Scholar] [CrossRef] [PubMed]
- Katzelnick, L.C.; Gresh, L.; Halloran, M.E.; Mercado, J.C.; Kuan, G.; Gordon, A.; Balmaseda, A.; Harris, E. Antibody-dependent enhancement of severe dengue disease in humans. Science 2017, 358, 929–932. [Google Scholar] [CrossRef] [PubMed]
| Vaccine Platform | Vaccine Name | Developer(s) | Study Description/Current Status | Clinical Trial |
|---|---|---|---|---|
| Purified Inactivated Vaccines | Zika Virus Purified Inactivated Vaccine (ZPIV) | NIAID, WRAIR, BIDMC | Phase I trial on the safety, reactogenicity and immunogenicity, and comparison of different doses and schedules of ZPIV in healthy, flavivirus-naïve adult individuals—Completed | NCT02937233 |
| Phase I trial on the safety, reactogenicity and immunogenicity of a higher ZPIV dose in healthy, flavivirus-naïve adult individuals—Completed | NCT02952833 | |||
| Phase I trial on the safety, reactogenicity and immunogenicity of two doses of ZPIV and a late ZPIV boost in healthy, flavivirus-naïve adult individuals—Completed | NCT02963909 | |||
| Phase I trial on the safety, reactogenicity and immunogenicity of two doses of ZPIV in healthy adults residing in a flavivirus endemic area—Active, not recruiting | NCT03008122 | |||
| Purified Inactivated Zika Virus Vaccine (PIZV) | Takeda | Phase I trial on the safety, immunogenicity and dose ranging of PIZV in flavivirus-naïve and primed healthy adults—Active, not recruiting | NCT03343626 | |
| VLA1601 | Valneva Austria GmbH, Emergent BioSolutions | Phase I trial on the safety and immunogenicity of two dose levels of the VLA1601 vaccine in healthy, flavivirus-naïve adults—Completed | NCT03425149 | |
| BBV121 | Bharat Biotech International Limited | Phase I trial to evaluate two doses of three sequentially escalating cohort of BBV121 in healthy adult dengue seronegative and dengue seropositive volunteers—Completed | NCT04478656 | |
| DNA Vaccines | VRC5288 (Zika virus and Japanese encephalitis virus chimera) VRC5283 (wild-type Zika virus) | NIAID, VRC | Phase I trial on the safety and immunogenicity of VRC5283 administered via needle and syringe or needle-free injector in healthy adults—Completed | NCT02996461 |
| Phase I/Ib trial on the safety, tolerability, and immunogenicity of VRC5288 administered via needle and syringe in healthy adults—Completed | NCT02840487 | |||
| VRC5283 (wild-type Zika virus) | NIAID, VRC, Emmes Company, Leidos Biomedical Research, FHI Clinical, PPD | Phase II/IIb trial on the safety, immunogenicity, and efficacy of a three-dose vaccination regimen of the VRC5283 administered via needle-free device in healthy adults and adolescents residing in flavivirus endemic and nonendemic areas—Completed | NCT03110770 | |
| GLS-5700 | GeneOne Life Science, Inc., Inovio Pharmaceuticals | Phase I trial, dose-ranging study to evaluate the safety, tolerability, and immunogenicity of GLS-5700 in healthy, dengue-naïve adults—Completed | NCT02809443 | |
| Phase I trial to evaluate the safety, tolerability, and immunogenicity of GLS-5700 in dengue-seropositive adults—Completed | NCT02887482 | |||
| mRNA Vaccines | mRNA-1893 | ModernaTX, Inc., Biomedical Advanced Research and Development Authority | Phase I dose-ranging study to evaluate the safety, tolerability, and immunogenicity of mRNA-1893 in healthy flavivirus-seropositive and seronegative adults—Active, not recruiting | NCT04064905 |
| mRNA 1325 | Phase I dose-ranging study to evaluate the safety and immunogenicity of mRNA 1325 in healthy adults in a nonendemic Zika region—Completed | NCT03014089 | ||
| Live-Attenuated Vaccines | rZIKV/D4Δ30-713 | NIAID | Phase I trial to evaluate the safety, reactogenicity, and immunogenicity of a single dose of the rZIKV/D4Δ30-713 vaccine in healthy, flavivirus-naïve adults—Completed | NCT03611946 |
| Viral Vectored Vaccines | MV-ZIKA-RSP (Measles virus-based) | Themis Bioscience GmbH | Phase I trial comparing different dose levels of the MV-ZIKA-RSP vaccine to evaluate the safety, tolerability, and immunogenicity in healthy adults—Recruiting | NCT04033068 |
| MV-ZIKA | Phase I dose-finding study to evaluate the optimal dose of MV-ZIKA and to asses immunogenicity, safety, and tolerability in healthy adult volunteers—Completed | NCT02996890 | ||
| ChAdOx1 Zika (Chimpanzee Adenovirus-based) | University of Oxford | Phase I trial to determine the safety and immunogenicity of ChAdOx1 Zika as a standalone vaccine or coadministered with a CHIKV vaccine (ChAdOx1 Chik) in healthy adults—Recruiting | NCT04015648 | |
| Phase Ib trial to evaluate the safety and immunogenicity of the ChAdOx1 Zika vaccine as a standalone vaccine or coadministered with ChAdOx1 Chik in healthy adults in Mexico—Not yet recruiting | NCT04440774 | |||
| Ad26.ZIKV.001 (Adenovirus serotype 26-based) | Janssen Vaccines and Prevention B.V. | Phase I trial to evaluate the safety, reactogenicity and immunogenicity of Ad26.ZIKV.001 in healthy adult volunteers—Completed | NCT03356561 |
| Vaccine Platform | Name/Sponsor | Antigen | Regimen (Dosage, Intervals, Route) | Study Design | Subject Characteristics | Clinical Trial/Reference |
|---|---|---|---|---|---|---|
| Purified Inactivated Virus | PIZV or TAK-426 Takeda Pharmaceuticals | Whole Virus, PRVABC59 strain/Aluminum hydroxide adjuvant |
| Multicenter, randomized, observer-blind, placebo-controlled |
| NCT03343626 [78] |
| ZPIV NIAID, WRAIR, BIDMC | Whole Virus, PRVABC59 strain/Aluminum hydroxide adjuvant |
| Single-center, randomized, double-blind, placebo-controlled |
| NCT02937233 [79] | |
| Single-center (three independent trials), randomized, double-blind, placebo-controlled |
| NCT02963909 NCT02952833 NCT02937233 [80] | |||
| DNA Vaccine | VRC5288 and VRC5283 NIAID/VRC | prM and E VRC5288 (ZIKV and JEV chimera) VRC5283 (wild-type ZIKV) |
| Two independent trials (VRC 319, multicenter; and VRC320, single-center), randomized, open-label |
| NCT02840487 NCT02996461 [81] |
| GLS-5700 GeneOne Life Science, Inc., Inovio Pharmaceuticals | prM and E |
| Multicenter, nonrandomized, open-label, placebo-controlled |
| NCT02809443 [82] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castanha, P.M.S.; Marques, E.T.A. A Glimmer of Hope: Recent Updates and Future Challenges in Zika Vaccine Development. Viruses 2020, 12, 1371. https://doi.org/10.3390/v12121371
Castanha PMS, Marques ETA. A Glimmer of Hope: Recent Updates and Future Challenges in Zika Vaccine Development. Viruses. 2020; 12(12):1371. https://doi.org/10.3390/v12121371
Chicago/Turabian StyleCastanha, Priscila M. S., and Ernesto T. A. Marques. 2020. "A Glimmer of Hope: Recent Updates and Future Challenges in Zika Vaccine Development" Viruses 12, no. 12: 1371. https://doi.org/10.3390/v12121371
APA StyleCastanha, P. M. S., & Marques, E. T. A. (2020). A Glimmer of Hope: Recent Updates and Future Challenges in Zika Vaccine Development. Viruses, 12(12), 1371. https://doi.org/10.3390/v12121371






