CD4 and CD8 Lymphocyte Counts as Surrogate Early Markers for Progression in SARS-CoV-2 Pneumonia: A Prospective Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Assessments
2.2. Lymphocyte Subsets Determination
2.3. Statistical Methods
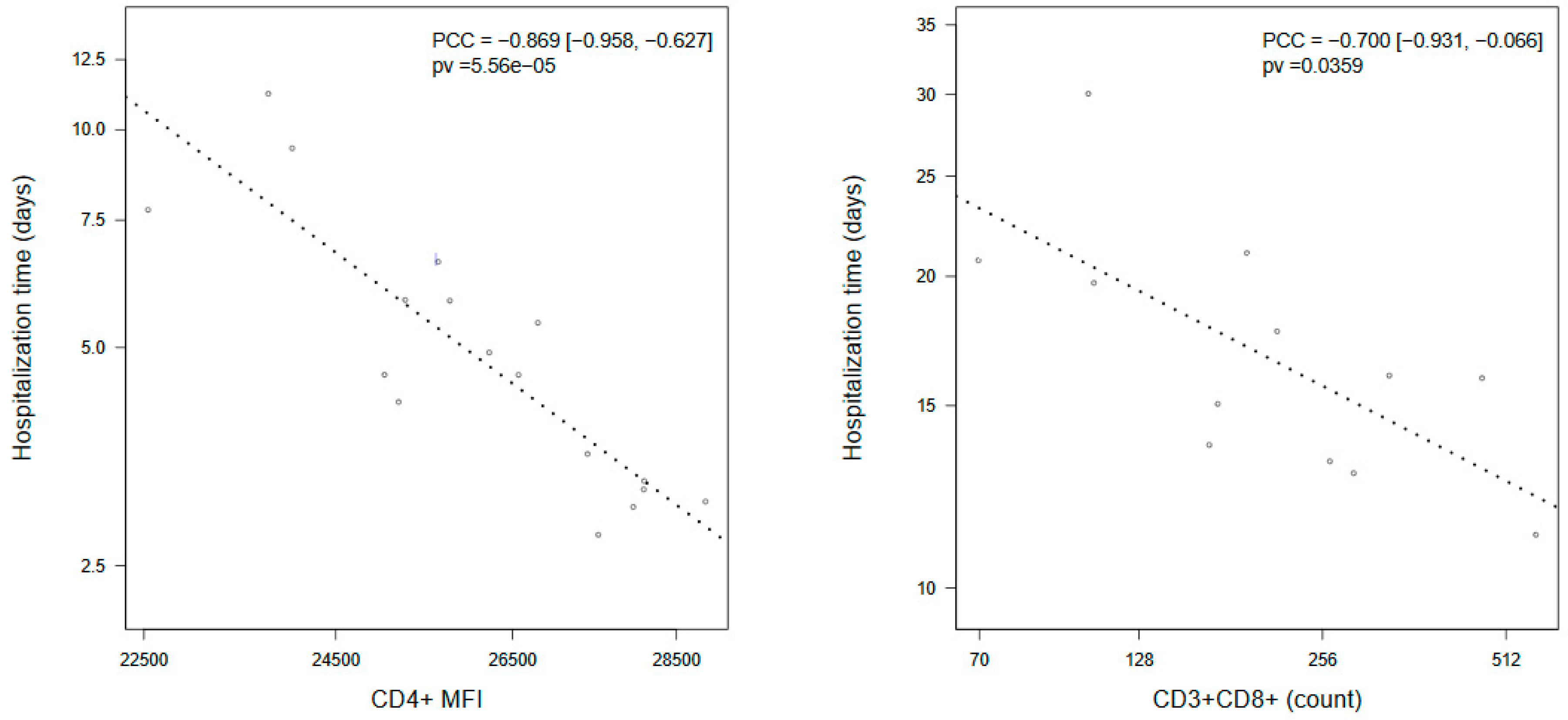
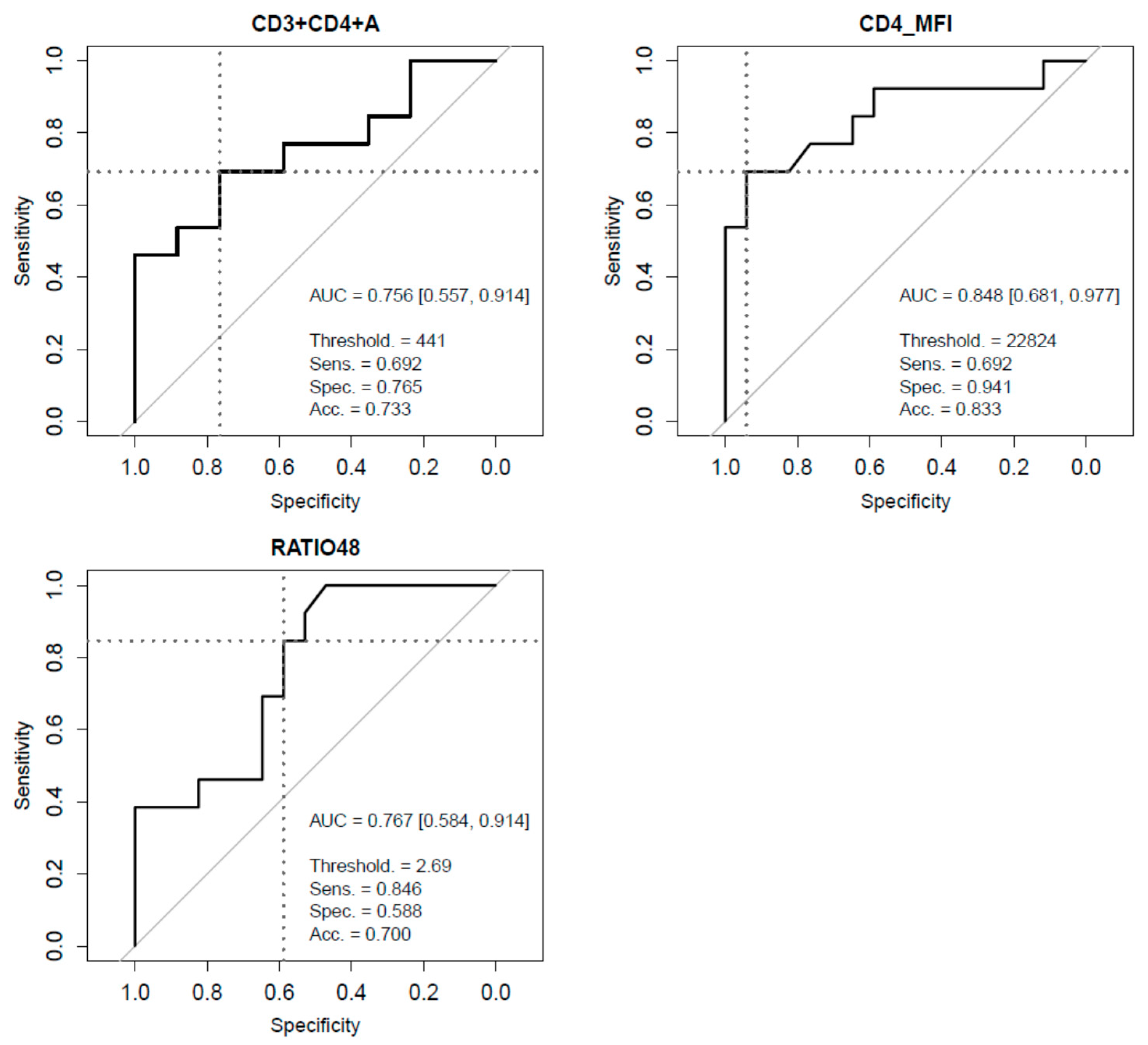
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef]
- Jin, Y.; Yang, H.; Ji, W.; Wu, W.; Chen, S.; Zhang, W.; Duan, G. Virology, Epidemiology, Pathogenesis, and Control of COVID-19. Viruses 2020, 12, 372. [Google Scholar] [CrossRef] [PubMed]
- Giamarellos-Bourboulis, E.J.; Netea, M.G.; Rovina, N.; Akinosoglou, K.; Antoniadou, A.; Antonakos, N.; Damoraki, G.; Gkavogianni, T.; Adami, M.-E.; Katsaounou, P.; et al. Complex Immune Dysregulation in COVID-19 Patients with Severe Respiratory Failure. Cell Host Microbe 2020, 27, 992–1000. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Hu, B.; Hu, C.; Zhu, F.; Liu, X.; Zhang, J.; Wang, B.; Xiang, H.; Cheng, Z.; Xiong, Y.; et al. Clinical Characteristics of 138 Hospitalized Patients with 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA 2020, 323, 1061. [Google Scholar] [CrossRef] [PubMed]
- Tay, M.Z.; Poh, C.M.; Rénia, L.; Macary, P.A.; Ng, L.F.P. The trinity of COVID-19: Immunity, inflammation and intervention. Nat. Rev. Immunol. 2020, 20, 363–374. [Google Scholar] [CrossRef]
- Li, T.; Qiu, Z.; Zhang, L.; Han, Y.; He, W.; Liu, Z.-Y.; Ma, X.; Fan, H.; Lu, W.; Xie, J.; et al. Significant Changes of Peripheral T Lymphocyte Subsets in Patients with Severe Acute Respiratory Syndrome. J. Infect. Dis. 2004, 189, 648–651. [Google Scholar] [CrossRef] [PubMed]
- Qin, C.; Zhou, L.; Hu, Z.; Zhang, S.; Yang, S.; Tao, Y.; Xie, C.; Ma, K.; Shang, K.; Wang, W.; et al. Dysregulation of immune response in patients with COVID-19 in Wuhan, China. Clin. Infect. Dis. 2020, 71, 762–768. [Google Scholar] [CrossRef]
- Janice, O.H.L.; Ken-En Gan, S.; Bertoletti, A.; Tan, Y.J. Understanding the T cell immune response in SARS coronavirus infection. Emerg. Microbes Infect. 2012, 1, e23. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wu, Z.; Li, J.-W.; Zhao, H.; Wang, G.-Q. Cytokine release syndrome in severe COVID-19: Interleukin-6 receptor antagonist tocilizumab may be the key to reduce mortality. Int. J. Antimicrob. Agents 2020, 55, 105954. [Google Scholar] [CrossRef]
- Martínez-Sanz, J.; Muriel, A.; Ron, R.; Herrera, S.; Pérez-Molina, J.A.; Moreno, S.; Serrano-Villar, S. Effects of Tocilizumab on Mortality in Hospitalized Patients with COVID-19: A Multicenter Cohort Study. Clin. Microbiol. Infect. 2020. [CrossRef]
- Tomazini, B.M.; Maia, I.S.; Cavalcanti, A.B.; Berwanger, O.; Rosa, R.G.; Veiga, V.C.; Avezum, A.; Lopes, R.D.; Bueno, F.R.; Silva, M.V.A.O.; et al. Effect of Dexamethasone on Days Alive and Ventilator-Free in Patients With Moderate or Severe Acute Respiratory Distress Syndrome and COVID-19: The CoDEX Randomized Clinical Trial. JAMA 2020, 324, 1307. [Google Scholar] [CrossRef]
- Cecconi, M.; Piovani, D.; Brunetta, E.; Aghemo, A.; Greco, M.; Ciccarelli, M.; Angelini, C.; Voza, A.; Omodei, P.; Vespa, E.; et al. Early Predictors of Clinical Deterioration in a Cohort of 239 Patients Hospitalized for Covid-19 Infection in Lombardy, Italy. J. Clin. Med. 2020, 9, 1548. [Google Scholar] [CrossRef] [PubMed]
- Wen, W.; Su, W.; Tang, H.; Le, W.; Zhang, X.; Zheng, Y.; Ding, W.; Xie, L.; Li, J.; Ye, J.; et al. Immune cell profiling of COVID-19 patients in the recovery stage by single-cell sequencing. Cell Discov. 2020, 6, 31. [Google Scholar] [CrossRef]
- Whitmire, J.K. Induction and function of virus-specific CD4+ T cell responses. Virology 2011, 411, 216–228. [Google Scholar] [CrossRef]
- Channappanavar, R.; Zhao, J.; Perlman, S. T cell-mediated immune response to respiratory coronaviruses. Immunol. Res. 2014, 59, 118–128. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.-W.; Zhang, D.; Tian, R.-H.; Li, Y.; Wang, Y.-S.; Cao, J.; Tang, Y.; Zhang, N.; Zan, T.; Gao, L.; et al. The underlying changes and predicting role of peripheral blood inflammatory cells in severe COVID-19 patients: A sentinel? Clin. Chim. Acta 2020, 508, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Nie, J.; Wang, H.; Zhao, Q.; Xiong, Y.; Deng, L.; Song, S.; Ma, Z.; Mo, P.; Zhang, Y. Characteristics of Peripheral Lymphocyte Subset Alteration in COVID-19 Pneumonia. J. Infect. Dis. 2020, 221, 1762–1769. [Google Scholar] [CrossRef]
- Zhao, J.; Zhao, J.; Perlman, S. T cell responses are required for protection from clinical disease and for virus clearance in severe acute respiratory syndrome coronavirus-infected mice. J. Virol. 2010, 84, 9318–9325. [Google Scholar] [CrossRef]
- Chen, J.; Lau, Y.F.; Lamirande, E.W.; Paddock, C.D.; Bartlett, J.H.; Zaki, S.R.; Subbarao, K. Cellular immune responses to severe acute respiratory syndrome coronavirus (SARS-CoV) infection in senescent BALB/c mice: CD4+ T cells are important in control of SARS-CoV infection. J. Virol. 2010, 84, 1289–1301. [Google Scholar] [CrossRef]
- Jiang, M.; Guo, Y.; Luo, Q.; Huang, Z.; Zhao, R.; Liu, S.; Le, A.; Li, J.; Wan, L. T cell subset counts in peripheral blood can be used as discriminatory biomarkers for diagnosis and severity prediction of COVID-19. J. Infect. Dis. 2020, 222, 198–202. [Google Scholar] [CrossRef]
- Urra, J.; Cabrera, C.; Porras, L.; Ródenas, I. Selective CD8 cell reduction by SARS-CoV-2 is associated with a worse prognosis and systemic inflammation in COVID-19 patients. Clin. Immunol. 2020, 217, 108486. [Google Scholar] [CrossRef]
- Sun, Y.; Dong, Y.; Wang, L.; Xie, H.; Li, B.; Chang, C.; Wang, F.-S. Characteristics and prognostic factors of disease severity in patients with COVID-19: The Beijing experience. J. Autoimmun. 2020, 112, 102473. [Google Scholar] [CrossRef]
- Zheng, H.-Y.; Zhang, M.; Yang, C.-X.; Zhang, N.; Wang, X.-C.; Yang, X.-P.; Dong, X.-Q.; Zheng, Y.-T. Elevated exhaustion levels and reduced functional diversity of T cells in peripheral blood may predict severe progression in COVID-19 patients. Cell. Mol. Immunol. 2020, 17, 541–543. [Google Scholar] [CrossRef]
- Hengeveld, P.J.; Khader, A.O.; De Bruin, L.H.A.; Geelen, I.G.P.; Van Baalen, E.A.; Jansen, E.; Bouwer, N.I.; Balak, Ö.; Riedl, J.A.; Langerak, A.W.; et al. Blood cell counts and lymphocyte subsets of patients admitted during the COVID-19 pandemic: A prospective cohort study. Br. J. Haematol. 2020, 190. [Google Scholar] [CrossRef]
- Hoffmann, H.-H.; Schneider, W.M.; Sánchez-Rivera, F.J.; Luna, J.M.; Ashbrook, A.W.; Soto-Feliciano, Y.M.; Leal, A.A.; Le Pen, J.; Ricardo-Lax, I.; Michailidis, E.; et al. Functional interrogation of a SARS-CoV-2 host protein interactome identifies unique and shared coronavirus host factors. bioRxiv 2020. [CrossRef]
- Ganji, A.; Farahani, I.; Khansarinejad, B.; Ghazavi, A.; Mosayebi, G. Increased expression of CD8 marker on T-cells in COVID-19 patients. Blood Cells Mol. Dis. 2020, 83, 102437. [Google Scholar] [CrossRef]
- Xu, Z.; Shi, L.; Wang, Y.; Zhang, J.; Huang, L.; Zhang, C.; Liu, S.; Zhao, P.; Liu, H.; Zhu, L.; et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir. Med. 2020, 8, 420–422. [Google Scholar] [CrossRef]
- Jurado, A.; Martin, M.C.; Abad-Molina, C.; Orduna, A.; Martinez, A.; Ocana, E.; Yarce, O.; Navas, A.M.; Trujillo, A.; Fernandez-Pereira, L.; et al. COVID-19: Age, Interleukin-6, C-Reactive Protein and lymphocytes as key clues from a multicentre retrospective study in Spain. medRxiv 2020. [Google Scholar] [CrossRef]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Cummings, M.J.; Baldwin, M.R.; Abrams, D.; Jacobson, S.D.; Meyer, B.J.; Balough, E.M.; Aaron, J.G.; Claassen, J.; Rabbani, L.E.; Hastie, J.; et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: A prospective cohort study. Lancet 2020, 395, 1763–1770. [Google Scholar] [CrossRef]
- Shang, W.; Dong, J.; Ren, Y.; Tian, M.; Li, W.; Hu, J.; Li, Y. The value of clinical parameters in predicting the severity of COVID-19. J. Med. Virol. 2020, 92, 2188–2192. [Google Scholar] [CrossRef] [PubMed]
- Du, R.-H.; Liang, L.-R.; Yang, C.-Q.; Wang, W.; Cao, T.-Z.; Li, M.; Guo, G.-Y.; Du, J.; Zheng, C.-L.; Zhu, Q.; et al. Predictors of mortality for patients with COVID-19 pneumonia caused by SARS-CoV-2: A prospective cohort study. Eur. Respir. J. 2020, 55, 2000524. [Google Scholar] [CrossRef]
- Tibshirani, R. Regression Shrinkage and Selection via the Lasso. J. R. Stat. Soc. Ser. B 1996, 58, 267–288. [Google Scholar] [CrossRef]
- Friedman, J.H.; Hastie, T.; Tibshirani, R. Regularization Paths for Generalized Linear Models via Coordinate Descent. J. Stat. Softw. 2008, 33, 1–22. [Google Scholar] [CrossRef]
- Efron, B. Annals of Statistics. Bootstrap Methods: Another Look at the Jackknife. Ann. Stat. 1979, 7, 1–26. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018. [Google Scholar]
- Re, S.L.; Lison, D.; Huaux, F. CD4+ T lymphocytes in lung fibrosis: Diverse subsets, diverse functions. J. Leukoc. Biol. 2013, 93, 499–510. [Google Scholar]
- Sun, Q.; Li, L.; Ji, S.; Chen, J.; Yin, G.; Tang, Z.; Liu, Z. Variation of CD4+ and CD8+ T lymphocytes as predictor of outcome in renal allograft recipients who developed acute respiratory distress syndrome caused by cytomegalovirus pneumonia. Transplant. Proc. 2005, 37, 2118–2121. [Google Scholar] [CrossRef]

| Patients Characteristics and Blood Measurements | All (n = 30) | Non-Critical (n = 17) | Critical (n = 13) | p-Value |
|---|---|---|---|---|
| Age | 60.6 (6.1, 63.3) | 60.1 (51.7, 74.9) | 61.1 (55.2, 64.5) | 0.9833 |
| Gender (Male) | 20 (66.7%) | 12 (70.6%) | 8 (61.5%) | 0.6030 |
| Days of symptoms onset | 7.000 (6.000, 10.000) | 7.000 (4.000, 11.000) | 6.000 (5.000, 10.000) | 0.6439 |
| Days to hospital discharge | 8.000 (5.000, 14.000) | 5.000 (4.000, 6.000) | 15.500 (12.000, 22.000) | <0.001 |
| HT | 6 (20.0%) | 3 (17.6%) | 3 (23.1%) | 0.7134 |
| DM | 1 (3.3%) | 1 (5.9%) | 0 (0.0%) | 0.2810 |
| DLP | 5 (16.7%) | 2 (11.8%) | 3 (23.1%) | 0.4119 |
| OBESITY | 1 (3.3%) | 0 (0.0%) | 1 (7.7%) | 0.1900 |
| Leucocyte count (cells × 109/L) | 6310 (5310, 8860) | 6550 (5310, 9440) | 5970 (5120, 11370) | 0.4512 |
| Neutrophyl count (cells × 109/L) | 4440 (3920, 6650) | 4570 (3950, 7030) | 4200 (2900, 9370) | 0.4388 |
| Lymphocyte count (cells × 109/L) | 1215 (1040, 1310) | 1260 (1040, 1440) | 1180 (920, 1840) | 0.5030 |
| Ratio N/L | 4.26 (3.05, 5.08) | 4.19 (2.90, 5.08) | 4.33 (1.58, 7.95) | 0.8835 |
| Ferritin (ng/mL) | 711.7 (382.6, 1136.2) | 639.7 (270.6, 1136.2) | 783.7 (354.5, 2390) | 0.2330 |
| CRP (mg/dL) | 8.80 (5.07, 11.25) | 8.54 (4.74, 11.25) | 9.50 (5.00, 15.64) | 0.3909 |
| D-Dimer (mg/mL) | 691 (443, 860) | 703 (443, 860) | 679 (269, 1722) | 0.7695 |
| LDH (U/L) | 282 (244, 365) | 267 (238, 387) | 356 (243, 446) | 0.1713 |
| T lymphocyte count | 714 (497, 823) | 725 (497, 1119) | 647 (375, 1113) | 0.4025 |
| CD3+CD4+ count | 467 (303, 574) | 545 (445, 767) | 278 (178, 663) | 0.0180 |
| CD3+CD8+ count | 245 (171, 319) | 253 (145, 319) | 237 (87, 586) | 0.7064 |
| CD3+CD4+CD8+ count | 13 (8, 21) | 16 (9, 24) | 11 (4, 35) | 0.295 |
| CD3+CD4−CD8− count | 18.000 (12.000, 23.000) | 19 (12, 27) | 12 (5, 23) | 0.2249 |
| B Lymphocyte count | 112 (78, 162) | 121 (86, 185) | 79 (46, 197) | 0.3254 |
| Natural Killer count | 196 (154, 253) | 192 (140, 278) | 234 (128, 327) | 0.8017 |
| Ratio CD4+/CD8+ | 1.91 (1.58, 3.12) | 3.12 (1.58, 3.99) | 1.72 (0.78, 2.52) | 0.0135 |
| CD4+ MFI | 24861 (22770, 26259) | 26259 (24683, 27939) | 21820 (20666, 25157) | 0.0013 |
| CD8+ MFI | 25856 (23819, 27476) | 25948 (23819, 27607) | 25337 (22878, 32176) | 0.7855 |
| Blood Determinations | Adjusted Means (95% CI) | F-Test | |
|---|---|---|---|
| Non-Critical | Critical | p-Value | |
| Leucocyte count (cells × 109/L) | 7292.5 (5851.2, 9088.9) | 6789.8 (5275.6, 8738.5) | 0.6665 |
| Neutrophyl count (cells × 109/L) | 5167.1 (3921.5, 6808.3) | 4871.5 (3551.3, 6682.3) | 0.7764 |
| Lymphocyte count (cells × 109/L) | 1281.7 (1081.5, 1519.0) | 1209.2 (995.3, 1468.9) | 0.6487 |
| Ratio N/L | 4.03 (2.89, 5.62) | 4.03 (2.75, 5.91) | 0.9980 |
| Ferritin (ng/mL) | 485.2 (290.9, 809.1) | 981.9 (546.5, 1764.2) | 0.0757 |
| CRP (mg/dL) | 7.37 (4.56, 10.84) | 8.93 (5.41, 13.33) | 0.5285 |
| D-Dimer (mg/mL) | 573.9 (426.2, 814.4) | 588.6 (418.3, 888.8) | 0.9175 |
| LDH (U/L) | 268.5 (229.0, 319.2) | 341.4 (280.2, 425.1) | 0.0776 |
| T lymphocyte count | 829.3 (606.8, 1086.5) | 683.3 (456.6, 955.6) | 0.3991 |
| CD3+CD4+ count | 597.8 (445.8, 801.6) | 331.5 (236.9, 464.0) | 0.0122 |
| CD3+CD8+ count | 214.8 (153.1, 301.5) | 217.2 (147.3, 320.3) | 0.9659 |
| CD3+CD4+CD8+ count | 15.4 (9.8, 24.3) | 11.5 (6.8, 19.5) | 0.4027 |
| CD3+CD4−CD8− count | 19.3 (12.4, 30.1) | 10.7 (6.4, 17.7) | 0.0840 |
| B Lymphocyte count | 129.7 (92.5, 173.3) | 101.9 (65.0, 147.0) | 0.3356 |
| Natural Killer count | 198.0 (148.9, 254.1) | 199.4 (143.5, 264.4) | 0.9725 |
| Ratio CD4+/CD8+ | 2.65 (2.01, 3.50) | 1.49 (1.08, 2.05) | 0.0010 |
| CD4+ MFI | 26128 (24878, 27441) | 22416 (21192, 23712) | 0.0003 |
| CD8+ MFI | 26076 (23953, 28386) | 25863 (23465, 28506) | 0.8980 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calvet, J.; Gratacós, J.; Amengual, M.J.; Llop, M.; Navarro, M.; Moreno, A.; Berenguer-Llergo, A.; Serrano, A.; Orellana, C.; Cervantes, M. CD4 and CD8 Lymphocyte Counts as Surrogate Early Markers for Progression in SARS-CoV-2 Pneumonia: A Prospective Study. Viruses 2020, 12, 1277. https://doi.org/10.3390/v12111277
Calvet J, Gratacós J, Amengual MJ, Llop M, Navarro M, Moreno A, Berenguer-Llergo A, Serrano A, Orellana C, Cervantes M. CD4 and CD8 Lymphocyte Counts as Surrogate Early Markers for Progression in SARS-CoV-2 Pneumonia: A Prospective Study. Viruses. 2020; 12(11):1277. https://doi.org/10.3390/v12111277
Chicago/Turabian StyleCalvet, Joan, Jordi Gratacós, María José Amengual, Maria Llop, Marta Navarro, Amàlia Moreno, Antoni Berenguer-Llergo, Alejandra Serrano, Cristóbal Orellana, and Manel Cervantes. 2020. "CD4 and CD8 Lymphocyte Counts as Surrogate Early Markers for Progression in SARS-CoV-2 Pneumonia: A Prospective Study" Viruses 12, no. 11: 1277. https://doi.org/10.3390/v12111277
APA StyleCalvet, J., Gratacós, J., Amengual, M. J., Llop, M., Navarro, M., Moreno, A., Berenguer-Llergo, A., Serrano, A., Orellana, C., & Cervantes, M. (2020). CD4 and CD8 Lymphocyte Counts as Surrogate Early Markers for Progression in SARS-CoV-2 Pneumonia: A Prospective Study. Viruses, 12(11), 1277. https://doi.org/10.3390/v12111277





