CXCL10 Signaling Contributes to the Pathogenesis of Arthritogenic Alphaviruses
Abstract
1. Introduction
2. Materials and Methods
2.1. Mice
2.2. Cells and Viruses
2.3. Plaque Forming Assay
2.4. Mouse Infection and Disease Monitoring
2.5. Histology Studies
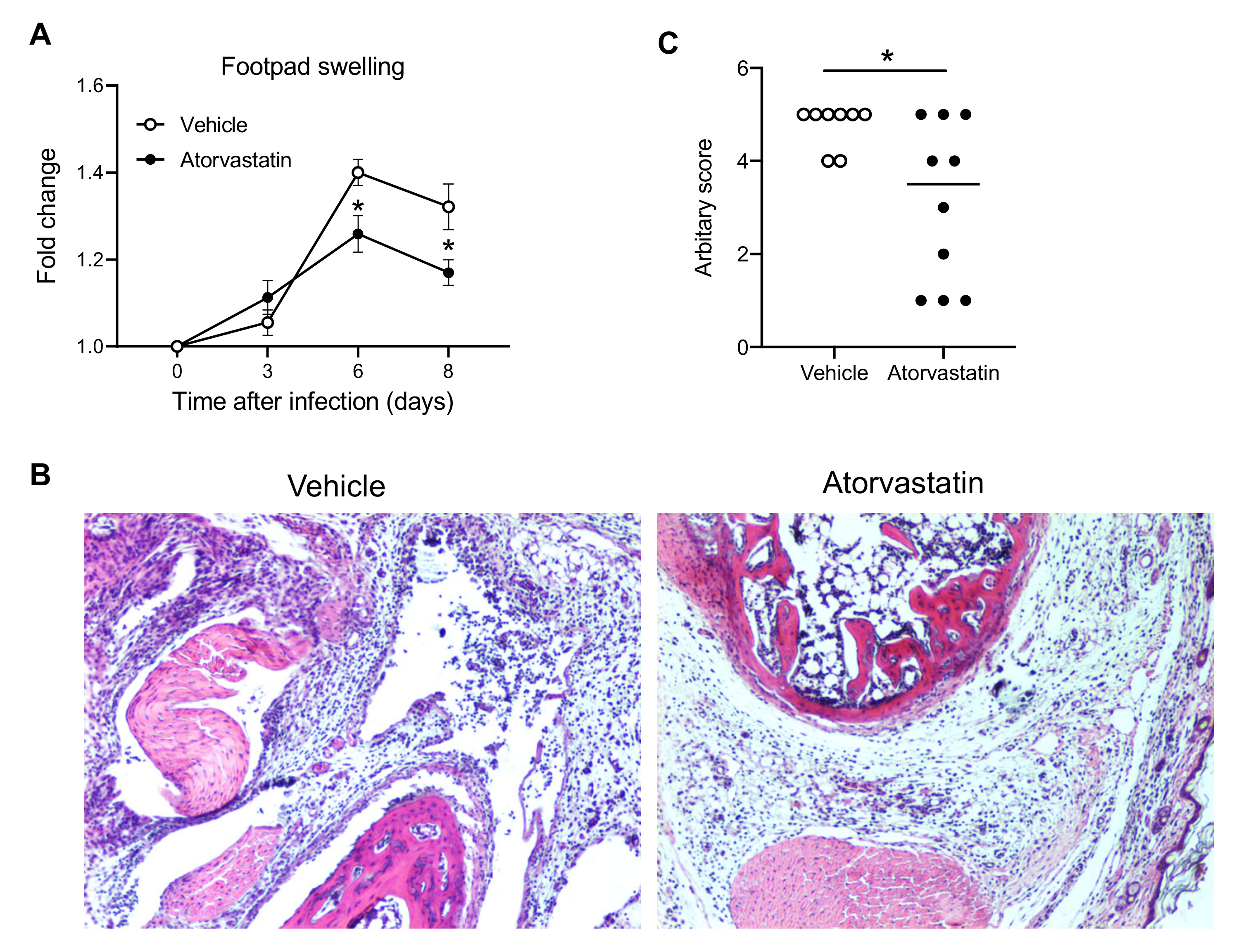
2.6. Treatment with Dimethyl Sulfoxide (Vehicle) and Atorvastatin In Vivo
2.7. Flow Cytometry and Florescence Activated Cell Sorting
2.8. Real-Time Quantitative RT-PCR
2.9. Statistical Analysis
3. Results
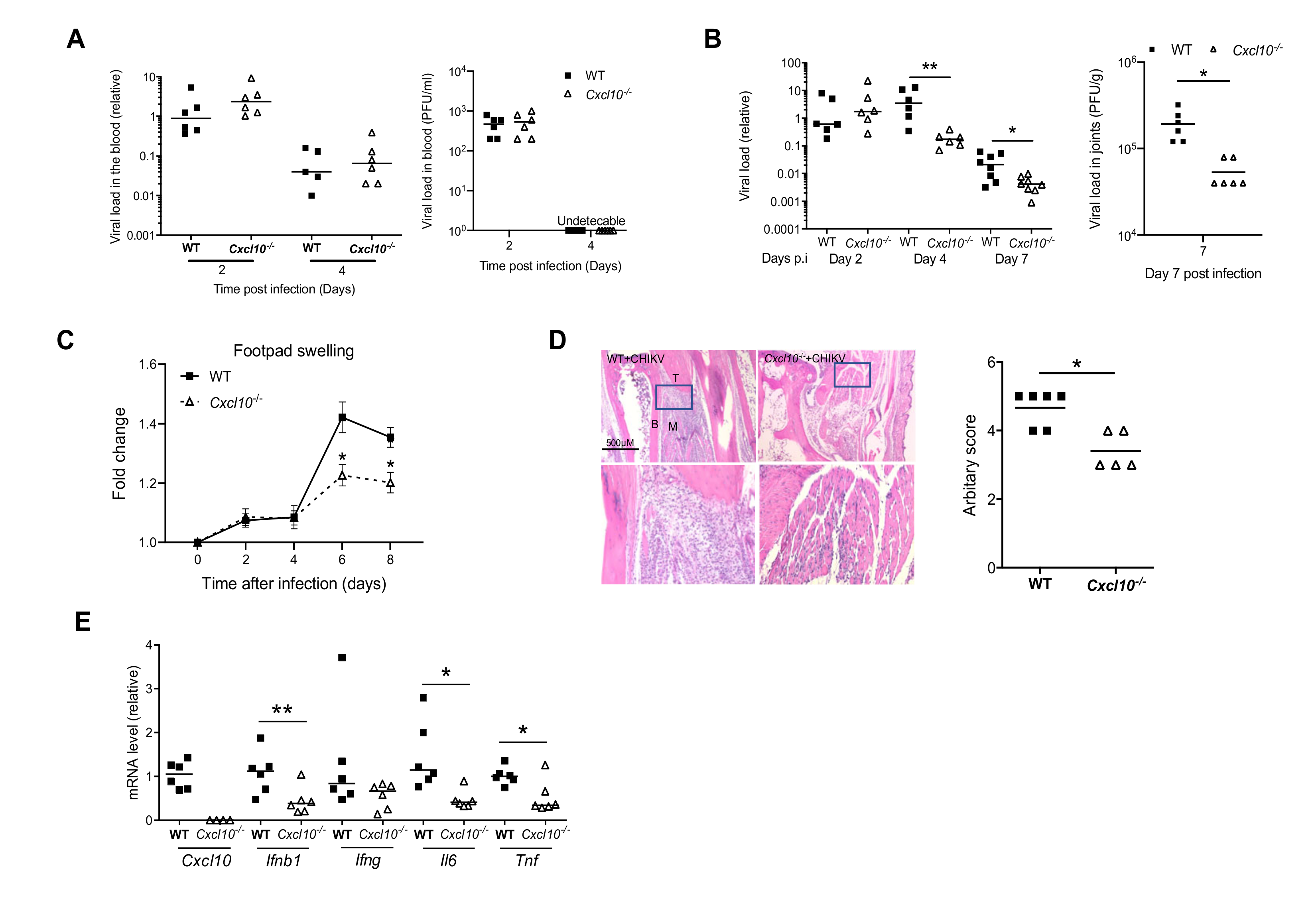
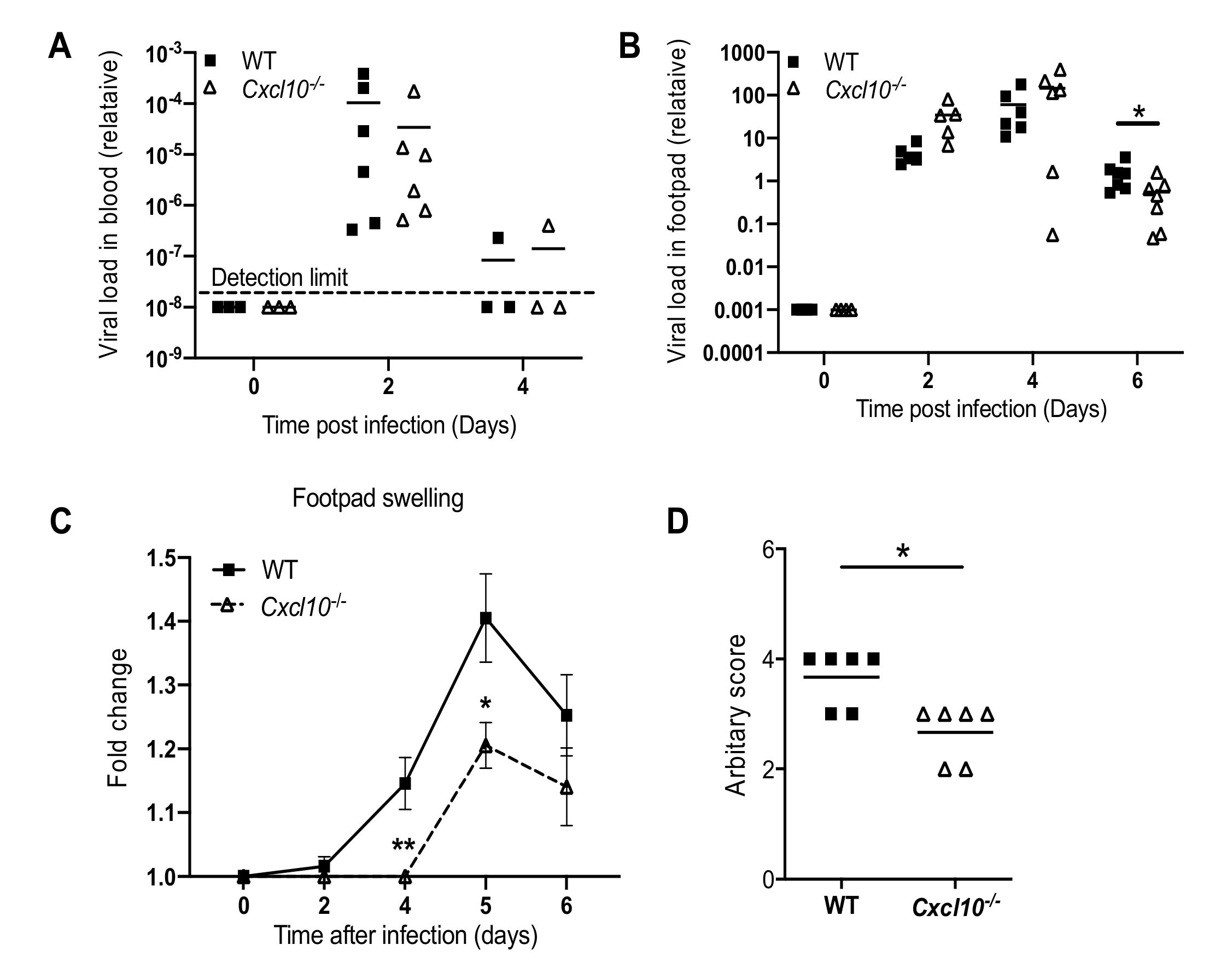
3.1. CXCL10 Signaling Contributes to Alphavirus Pathogenesis
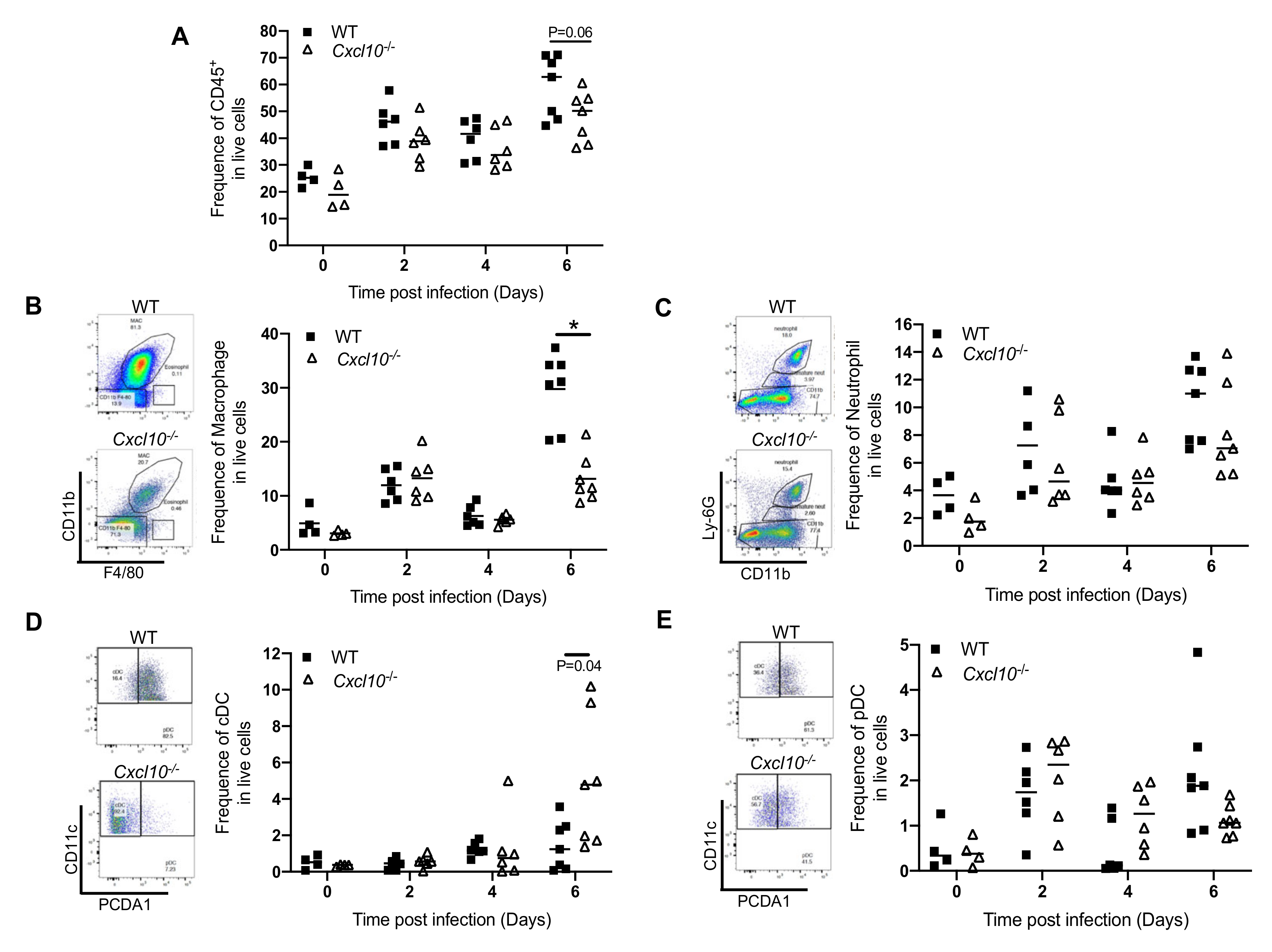
3.2. CXCL10 Signaling Promotes Macrophage Recruitment to Infected Feet
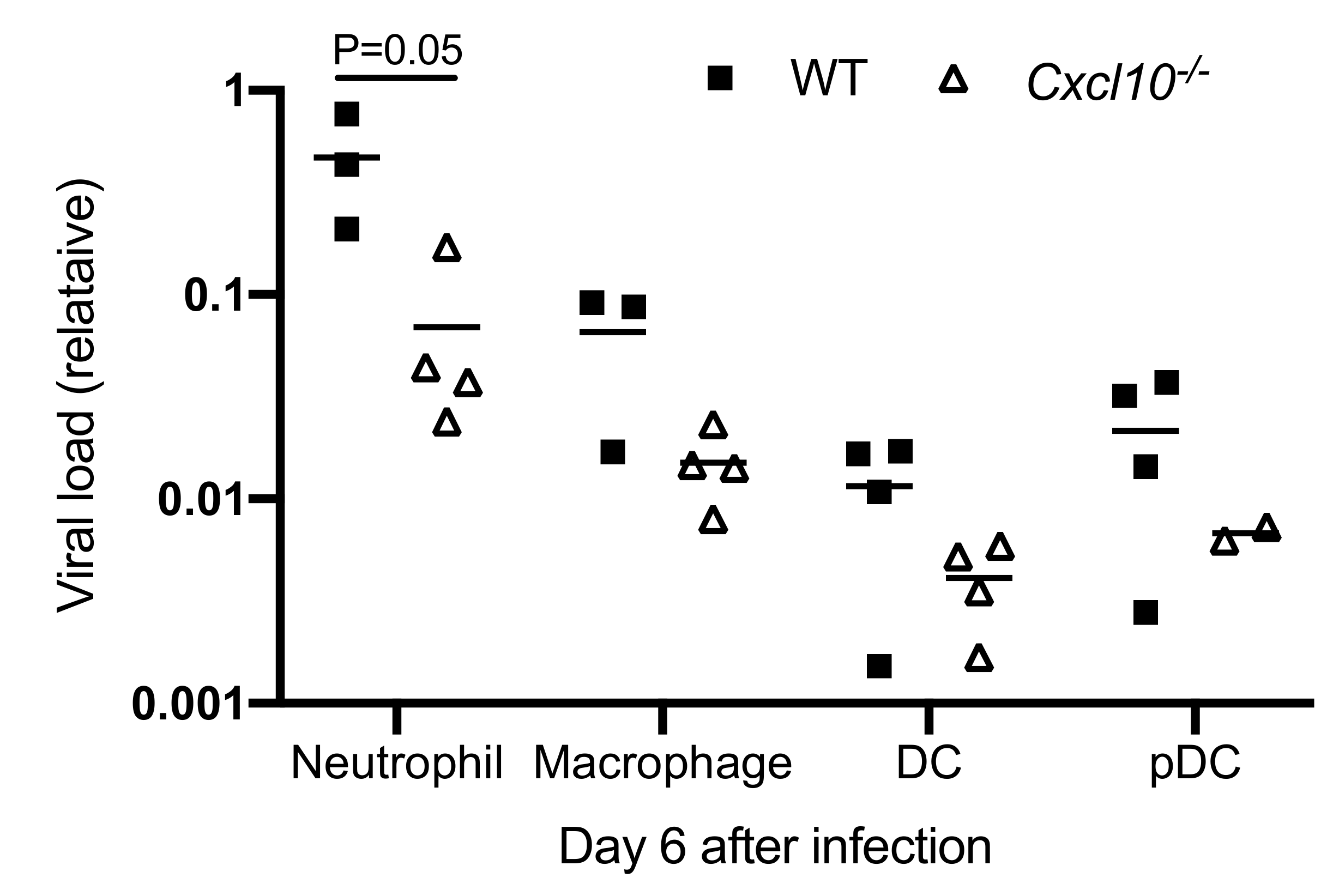
3.3. CXCL10 Signaling Promotes Alphavirus Persistence in Infiltrating Neutrophils and Macrophages
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Robinson, M.C. An epidemic of virus disease in Southern Province, Tanganyika territory, in 1952–1953. Trans. R. Soc. Trop. Med. Hyg. 1955, 49, 28–32. [Google Scholar] [CrossRef]
- Silva, L.A.; Dermody, T.S. Chikungunya virus: Epidemiology, replication, disease mechanisms, and prospective intervention strategies. J. Clin. Investig. 2017, 127, 737–749. [Google Scholar] [CrossRef] [PubMed]
- Weaver, S.C.; Lecuit, M. Chikungunya Virus and the Global Spread of a Mosquito-Borne Disease. N. Engl. J. Med. 2015, 372, 1231–1239. [Google Scholar] [CrossRef] [PubMed]
- Weaver, S.C.; Lecuit, M. Chikungunya Virus Infections. N. Engl. J. Med. 2015, 373, 94–95. [Google Scholar]
- Sourisseau, M.; Schilte, C.; Casartelli, N.; Trouillet, C.; Guivel-Benhassine, F.; Rudnicka, D.; Sol-Foulon, N.; Le Roux, K.; Prevost, M.-C.; Fsihi, H.; et al. Characterization of Reemerging Chikungunya Virus. PLoS Pathog. 2007, 3, e89. [Google Scholar] [CrossRef]
- Krejbich-Trotot, P.; Denizot, M.; Hoarau, J.; Jaffar-Bandjee, M.; Das, T.; Gasque, P. Chikungunya virus mobilizes the apoptotic machinery to invade host cell defenses. FASEB J. 2010, 25, 314–325. [Google Scholar] [CrossRef]
- Dhanwani, R.; Khan, M.; Alam, S.I.; Rao, P.V.L.; Parida, M. Differential proteome analysis of Chikungunya virus-infected new-born mice tissues reveal implication of stress, inflammatory and apoptotic pathways in disease pathogenesis. Proteomics 2011, 11, 1936–1951. [Google Scholar] [CrossRef] [PubMed]
- Teng, T.-S.; Kam, Y.-W.; Lee, B.; Hapuarachchi, H.C.; Wimal, A.; Ng, L.-C.; Ng, L.F.P. A Systematic Meta-analysis of Immune Signatures in Patients with Acute Chikungunya Virus Infection. J. Infect. Dis. 2015, 211, 1925–1935. [Google Scholar] [CrossRef]
- Silva, M.R.; van der Ende-Metselaar, H.; Mulder, H.L.; Smit, J.M.; Rodenhuis-Zybert, I.A. Mechanism and role of MCP-1 upregulation upon chikungunya virus infection in human peripheral blood mononuclear cells. Sci. Rep. 2016, 6, srep32288. [Google Scholar] [CrossRef]
- Her, Z.; Malleret, B.; Chan, M.; Ong, E.K.S.; Wong, S.-C.; Kwek, D.J.C.; Tolou, H.; Lin, R.T.P.; Tambyah, P.A.; Rénia, L.; et al. Active Infection of Human Blood Monocytes by Chikungunya Virus Triggers an Innate Immune Response. J. Immunol. 2010, 184, 5903–5913. [Google Scholar] [CrossRef]
- Schilte, C.; Couderc, T.; Chretien, F.; Sourisseau, M.; Gangneux, N.; Guivel-Benhassine, F.; Kraxner, A.; Tschopp, J.; Higgs, S.; Michault, A.; et al. Type I IFN controls chikungunya virus via its action on nonhematopoietic cells. J. Exp. Med. 2010, 207, 429–442. [Google Scholar] [CrossRef] [PubMed]
- Wauquier, N.; Becquart, P.; Nkoghé, D.; Padilla, C.; Ndjoyi-Mbiguino, A.; Leroy, E.M. The Acute Phase of Chikungunya Virus Infection in Humans Is Associated with Strong Innate Immunity and T CD8 Cell Activation. J. Infect. Dis. 2010, 204, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Chang, A.Y.; Martins, K.A.O.; Encinales, L.; Reid, S.P.; Acuña, M.; Encinales, C.; Matranga, C.B.; Pacheco, N.; Cure, C.; Shukla, B.; et al. Chikungunya Arthritis Mechanisms in the Americas. Arthr. Rheumatol. 2018, 70, 585–593. [Google Scholar] [CrossRef]
- Kelvin, A.A.; Banner, D.; Silvi, G.; Moro, M.L.; Spataro, N.; Gaibani, P.; Cavrini, F.; Pierro, A.; Rossini, G.; Cameron, M.J.; et al. Inflammatory Cytokine Expression Is Associated with Chikungunya Virus Resolution and Symptom Severity. PLoS Neg. Trop. Dis. 2011, 5, e1279. [Google Scholar] [CrossRef] [PubMed]
- Rudd, P.A.; Wilson, J.; Gardner, J.; Larcher, T.; Babarit, C.; Le, T.T.; Anraku, I.; Kumagai, Y.; Loo, Y.-M.; Gale, M.; et al. Interferon Response Factors 3 and 7 Protect against Chikungunya Virus Hemorrhagic Fever and Shock. J. Virol. 2012, 86, 9888–9898. [Google Scholar] [CrossRef] [PubMed]
- Poo, Y.S.; Rudd, P.A.; Gardner, J.; Wilson, J.A.C.; Larcher, T.; Colle, M.-A.; Le, T.T.; Nakaya, H.I.; Warrilow, D.; Allcock, R.; et al. Multiple Immune Factors Are Involved in Controlling Acute and Chronic Chikungunya Virus Infection. PLoS Neg. Trop. Dis. 2014, 8, e3354. [Google Scholar] [CrossRef] [PubMed]
- Couderc, T.; Chrétien, F.; Schilte, C.; Disson, O.; Brigitte, M.; Guivel-Benhassine, F.; Touret, Y.; Barau, G.; Cayet, N.; Schuffenecker, I.; et al. A Mouse Model for Chikungunya: Young Age and Inefficient Type-I Interferon Signaling Are Risk Factors for Severe Disease. PLoS Pathog. 2008, 4, e29. [Google Scholar] [CrossRef]
- Akahata, W.; Yang, Z.-Y.; Andersen, H.; Sun, S.; Holdaway, H.A.; Kong, W.-P.; Lewis, M.G.; Higgs, S.; Rossmann, M.G.; Rao, S.; et al. A virus-like particle vaccine for epidemic Chikungunya virus protects nonhuman primates against infection. Nat. Med. 2010, 16, 334–338. [Google Scholar] [CrossRef]
- Fric, J.; Bertin-Maghit, S.; Wang, C.-I.; Nardin, A.; Warter, L. Use of Human Monoclonal Antibodies to Treat Chikungunya Virus Infection. J. Infect. Dis. 2012, 207, 319–322. [Google Scholar] [CrossRef]
- Prow, T.W.; Chen, X.; Prow, N.A.; Fernando, G.J.P.; Tan, C.S.E.; Raphael, A.P.; Chang, D.; Ruutu, M.P.; Jenkins, D.W.K.; Pyke, A.; et al. Nanopatch-Targeted Skin Vaccination against West Nile Virus and Chikungunya Virus in Mice. Small 2010, 6, 1776–1784. [Google Scholar] [CrossRef]
- Wang, D.; Suhrbier, A.; Penn-Nicholson, A.; Woraratanadharm, J.; Gardner, J.; Luo, M.; Le, T.T.; Anraku, I.; Sakalian, M.; Einfeld, D.; et al. A complex adenovirus vaccine against chikungunya virus provides complete protection against viraemia and arthritis. Vaccine 2011, 29, 2803–2809. [Google Scholar] [CrossRef] [PubMed]
- Goh, L.Y.; Hobson-Peters, J.; Prow, N.A.; Gardner, J.; Bielefeldt-Ohmann, H.; Pyke, A.T.; Suhrbier, A.; Suhrbier, A. Neutralizing monoclonal antibodies to the E2 protein of chikungunya virus protects against disease in a mouse model. Clin. Immunol. 2013, 149, 487–497. [Google Scholar] [CrossRef]
- Metz, S.W.; Gardner, J.; Geertsema, C.; Le, T.T.; Goh, L.; Vlak, J.M.; Suhrbier, A.; Pijlman, G.P. Effective Chikungunya Virus-like Particle Vaccine Produced in Insect Cells. PLoS Neg. Trop. Dis. 2013, 7, e2124. [Google Scholar] [CrossRef]
- Selvarajah, S.; Sexton, N.R.; Kahle, K.M.; Fong, R.H.; Mattia, K.-A.; Gardner, J.; Lu, K.; Liss, N.M.; Salvador, B.; Tucker, D.F.; et al. A Neutralizing Monoclonal Antibody Targeting the Acid-Sensitive Region in Chikungunya Virus E2 Protects from Disease. PLoS Neg. Trop. Dis. 2013, 7, e2423. [Google Scholar] [CrossRef]
- Pal, P.; Dowd, K.A.; Brien, J.D.; Edeling, M.A.; Gorlatov, S.; Johnson, S.; Lee, I.; Akahata, W.; Nabel, G.J.; Richter, M.K.S.; et al. Development of a Highly Protective Combination Monoclonal Antibody Therapy against Chikungunya Virus. PLoS Pathog. 2013, 9, e1003312. [Google Scholar] [CrossRef]
- Fox, J.M.; Diamond, M.S. Immune-Mediated Protection and Pathogenesis of Chikungunya Virus. J. Immunol. 2016, 197, 4210–4218. [Google Scholar] [CrossRef]
- Teo, T.-H.; Lum, F.-M.; Lee, W.W.L.; Ng, L.F.P. Mouse models for Chikungunya virus: Deciphering immune mechanisms responsible for disease and pathology. Immunol. Res. 2012, 53, 136–147. [Google Scholar] [CrossRef]
- Teo, T.-H.; Lum, F.-M.; Claser, C.; Lulla, V.; Lulla, A.; Merits, A.; Rénia, L.; Hong, J.-S.; Ng, L.F.P.; Liao, J.; et al. A Pathogenic Role for CD4+T Cells during Chikungunya Virus Infection in Mice. J. Immunol. 2012, 190, 259–269. [Google Scholar] [CrossRef] [PubMed]
- Gasque, P.; Couderc, T.; Lecuit, M.; Roques, P.; Ng, L.F. Chikungunya Virus Pathogenesis and Immunity. Vect. Borne Zoon. Dis. 2015, 15, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Petitdemange, C.; Becquart, P.; Wauquier, N.; Béziat, V.; Debré, P.; Leroy, E.M.; Vieillard, V. Unconventional Repertoire Profile Is Imprinted during Acute Chikungunya Infection for Natural Killer Cells Polarization toward Cytotoxicity. PLoS Pathog. 2011, 7, e1002268. [Google Scholar] [CrossRef]
- Stoermer, K.A.; Burrack, A.; Oko, L.; Montgomery, S.A.; Borst, L.B.; Gill, R.G.; Morrison, T.E. Genetic Ablation of Arginase 1 in Macrophages and Neutrophils Enhances Clearance of an Arthritogenic Alphavirus. J. Immunol. 2012, 189, 4047–4059. [Google Scholar] [CrossRef] [PubMed]
- Abdelnabi, R.; Neyts, J.; Delang, L. Towards antivirals against chikungunya virus. Antivir. Res. 2015, 121, 59–68. [Google Scholar] [CrossRef]
- Rezza, G. Do we need a vaccine against chikungunya? Pathog. Glob. Health 2015, 109, 170–173. [Google Scholar] [CrossRef] [PubMed]
- Wang, P. Exploration of West Nile Virus Infection in Mouse Models. Adv. Struct. Safety Stud. 2016, 1435, 71–81. [Google Scholar] [CrossRef]
- Pal, U.; Wang, P.; Bao, F.; Yang, X.; Samanta, S.; Schoen, R.; Wormser, G.P.; Schwartz, I.; Fikrig, E. Borrelia burgdorferi basic membrane proteins A and B participate in the genesis of Lyme arthritis. J. Exp. Med. 2007, 205, 133–141. [Google Scholar] [CrossRef]
- Wilson, N.O.; Solomon, W.; Anderson, L.; Patrickson, J.; Pitts, S.; Bond, V.; Liu, M.; Stiles, J.K. Pharmacologic Inhibition of CXCL10 in Combination with Anti-malarial Therapy Eliminates Mortality Associated with Murine Model of Cerebral Malaria. PLoS ONE 2013, 8, e60898. [Google Scholar] [CrossRef]
- Grip, O.; Janciauskiene, S. Atorvastatin Reduces Plasma Levels of Chemokine (CXCL10) in Patients with Crohn’s Disease. PLoS ONE 2009, 4, e5263. [Google Scholar] [CrossRef]
- Labadie, K.; Larcher, T.; Joubert, C.; Mannioui, A.; Delache, B.; Brochard, P.; Guigand, L.; Dubreil, L.; Lebon, P.; Verrier, B.; et al. Chikungunya disease in nonhuman primates involves long-term viral persistence in macrophages. J. Clin. Investig. 2010, 120, 894–906. [Google Scholar] [CrossRef]
- Angiolillo, A.L.; Sgadari, C.; Taub, D.D.; Liao, F.; Farber, J.M.; Maheshwari, S.; Kleinman, H.K.; Reaman, G.H.; Tosato, G. Human interferon-inducible protein 10 is a potent inhibitor of angiogenesis in vivo. J. Exp. Med. 1995, 182, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Dufour, J.H.; Dziejman, M.; Liu, M.T.; Leung, J.H.; Lane, T.E.; Luster, A.D. IFN-γ-Inducible Protein 10 (IP-10; CXCL10)-Deficient Mice Reveal a Role for IP-10 in Effector T Cell Generation and Trafficking. J. Immunol. 2002, 168, 3195–3204. [Google Scholar] [CrossRef]
- Petrovic-Djergovic, D.; Popovic, M.; Chittiprol, S.; Cortado, H.; Ransom, R.F.; Partida-Sánchez, S. CXCL10 induces the recruitment of monocyte-derived macrophages into kidney, which aggravate puromycin aminonucleoside nephrosis. Clin. Exp. Immunol. 2015, 180, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Hoarau, J.-J.; Bandjee, M.-C.J.; Trotot, P.K.; Das, T.; Li-Pat-Yuen, G.; Dassa, B.; Denizot, M.; Guichard, E.; Ribera, A.; Henni, T.; et al. Persistent Chronic Inflammation and Infection by Chikungunya Arthritogenic Alphavirus in Spite of a Robust Host Immune Response. J. Immunol. 2010, 184, 5914–5927. [Google Scholar] [CrossRef] [PubMed]
- Gardner, J.; Anraku, I.; Le, T.T.; Larcher, T.; Major, L.; Roques, P.; Schroder, W.A.; Higgs, S.; Suhrbier, A. Chikungunya Virus Arthritis in Adult Wild-Type Mice. J. Virol. 2010, 84, 8021–8032. [Google Scholar] [CrossRef]
- Lane, B.R.; King, S.R.; Bock, P.J.; Strieter, R.M.; Coffey, M.J.; Markovitz, D.M. The C-X-C chemokine IP-10 stimulates HIV-1 replication. Virology 2003, 307, 122–134. [Google Scholar] [CrossRef]
- Xing, B.; Yin, Y.-F.; Zhao, L.-D.; Wang, L.; Zheng, W.-J.; Chen, H.; Wu, Q.-J.; Tang, F.-L.; Zhang, F.-C.; Shan, G.; et al. Effect of 3-Hydroxy-3-Methylglutaryl-Coenzyme A Reductase Inhibitor on Disease Activity in Patients with Rheumatoid Arthritis. Medicine 2015, 94, e572. [Google Scholar] [CrossRef]
- Shrivastava-Ranjan, P.; Flint, M.; Bergeron, E.; McElroy, A.; Chatterjee, P.; Albariño, C.G.; Nichol, S.T.; Spiropoulou, C.F. Statins Suppress Ebola Virus Infectivity by Interfering with Glycoprotein Processing. mBio 2018, 9, e00660. [Google Scholar] [CrossRef]
- Españo, E.; Nam, J.-H.; Song, E.-J.; Song, D.; Lee, C.-K.; Kim, J.-K. Lipophilic statins inhibit Zika virus production in Vero cells. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef]
- Episcopio, D.; Aminov, S.; Benjamin, S.; Germain, G.; Datan, E.; Landazuri, J.; Lockshin, R.A.; Zakeri, Z. Atorvastatin restricts the ability of influenza virus to generate lipid droplets and severely suppresses the replication of the virus. FASEB J. 2019, 33, 9516–9525. [Google Scholar] [CrossRef]
- Wagner, A.H.; Schwabe, O.; Hecker, M. Atorvastatin inhibition of cytokine-inducible nitric oxide synthase expression in native endothelial cells in situ. Br. J. Pharmacol. 2002, 136, 143–149. [Google Scholar] [CrossRef]
- Wickert, L.E.; Karta, M.R.; Audhya, A.; Gern, J.E.; Bertics, P.J. Simvastatin attenuates rhinovirus-induced interferon and CXCL10 secretion from monocytic cells in vitro. J. Leukoc. Biol. 2014, 95, 951–959. [Google Scholar] [CrossRef]
- Moreno, M.; Ramalho, L.N.; Sancho-Bru, P.; Ruiz-Ortega, M.; Ramalho, F.; Abraldes, J.G.; Colmenero, J.; Dominguez, M.; Egido, J.; Arroyo, V.; et al. Atorvastatin attenuates angiotensin II-induced inflammatory actions in the liver. Am. J. Physiol. Liver Physiol. 2009, 296, G147–G156. [Google Scholar] [CrossRef]
- Li, D.; Chen, H.; Romeo, F.; Sawamura, T.; Saldeen, T.; Mehta, J.L. Statins Modulate Oxidized Low-Density Lipoprotein-Mediated Adhesion Molecule Expression in Human Coronary Artery Endothelial Cells: Role of LOX-1. J. Pharmacol. Exp. Ther. 2002, 302, 601–605. [Google Scholar] [CrossRef] [PubMed]
- Teo, T.-H.; Chan, Y.-H.; Lee, W.W.L.; Lum, F.-M.; Amrun, S.N.; Her, Z.; Rajarethinam, R.; Merits, A.; Rötzschke, O.; Renia, L.; et al. Fingolimod treatment abrogates chikungunya virus–induced arthralgia. Sci. Transl. Med. 2017, 9, eaal1333. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, T.; Geng, T.; Harrison, A.G.; Yang, D.; Vella, A.T.; Fikrig, E.; Wang, P. CXCL10 Signaling Contributes to the Pathogenesis of Arthritogenic Alphaviruses. Viruses 2020, 12, 1252. https://doi.org/10.3390/v12111252
Lin T, Geng T, Harrison AG, Yang D, Vella AT, Fikrig E, Wang P. CXCL10 Signaling Contributes to the Pathogenesis of Arthritogenic Alphaviruses. Viruses. 2020; 12(11):1252. https://doi.org/10.3390/v12111252
Chicago/Turabian StyleLin, Tao, Tingting Geng, Andrew G. Harrison, Duomeng Yang, Anthony T. Vella, Erol Fikrig, and Penghua Wang. 2020. "CXCL10 Signaling Contributes to the Pathogenesis of Arthritogenic Alphaviruses" Viruses 12, no. 11: 1252. https://doi.org/10.3390/v12111252
APA StyleLin, T., Geng, T., Harrison, A. G., Yang, D., Vella, A. T., Fikrig, E., & Wang, P. (2020). CXCL10 Signaling Contributes to the Pathogenesis of Arthritogenic Alphaviruses. Viruses, 12(11), 1252. https://doi.org/10.3390/v12111252





