A Decade in Review: A Systematic Review of Universal Influenza Vaccines in Clinical Trials during the 2010 Decade
Abstract
1. Introduction
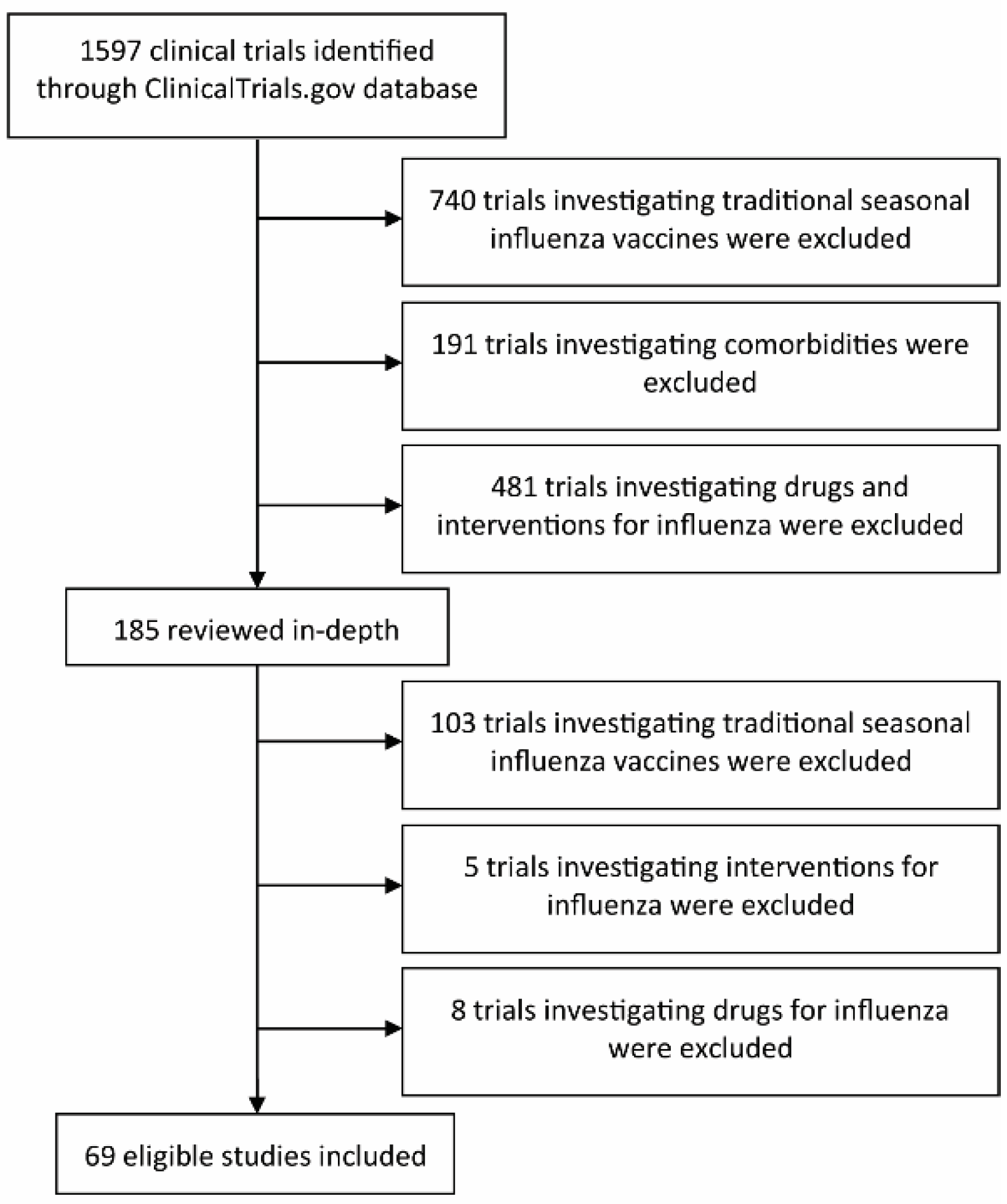
2. Materials and Methods
3. Results
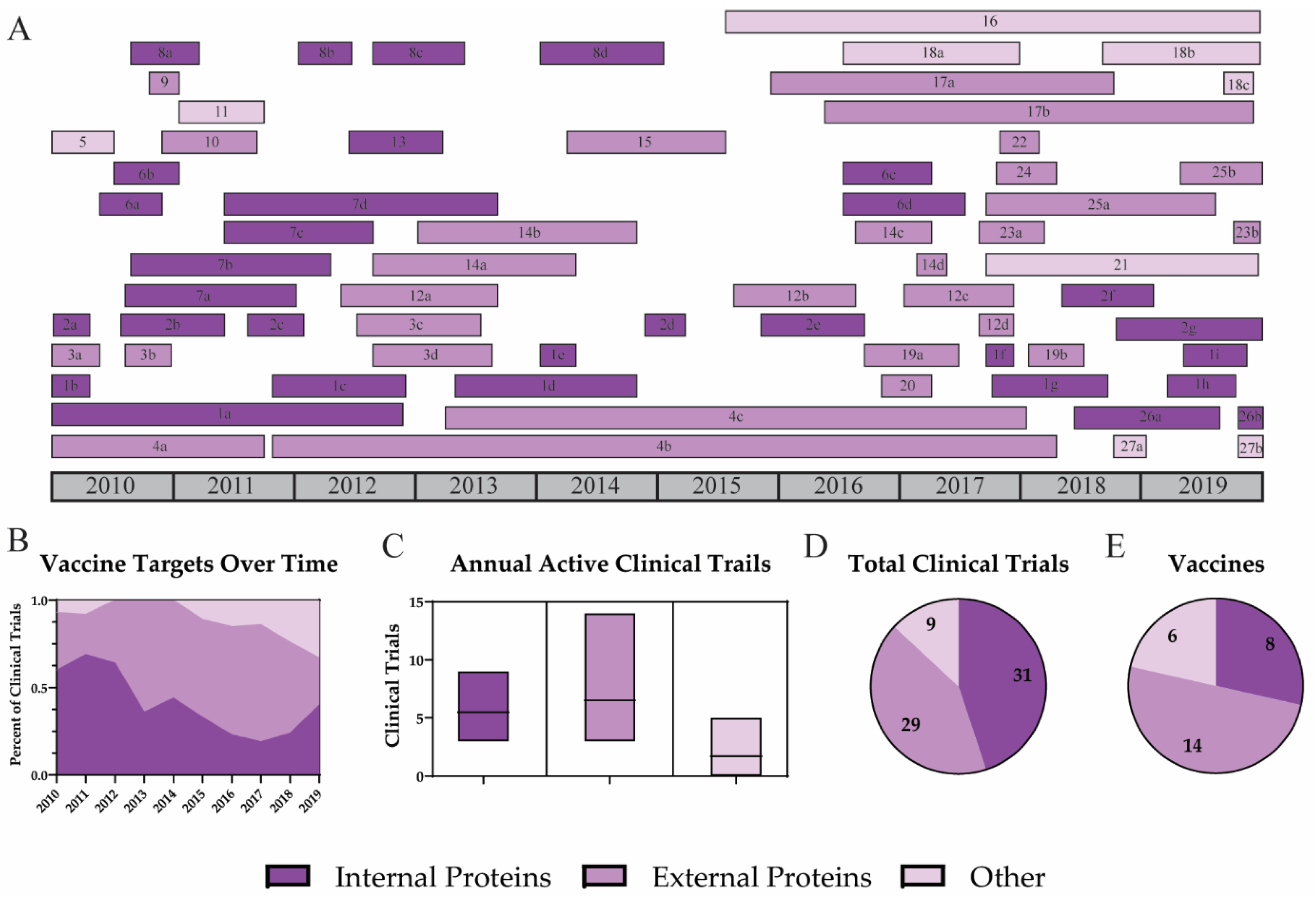
3.1. Vaccine Targets
3.1.1. Internal Proteins
3.1.2. External Proteins
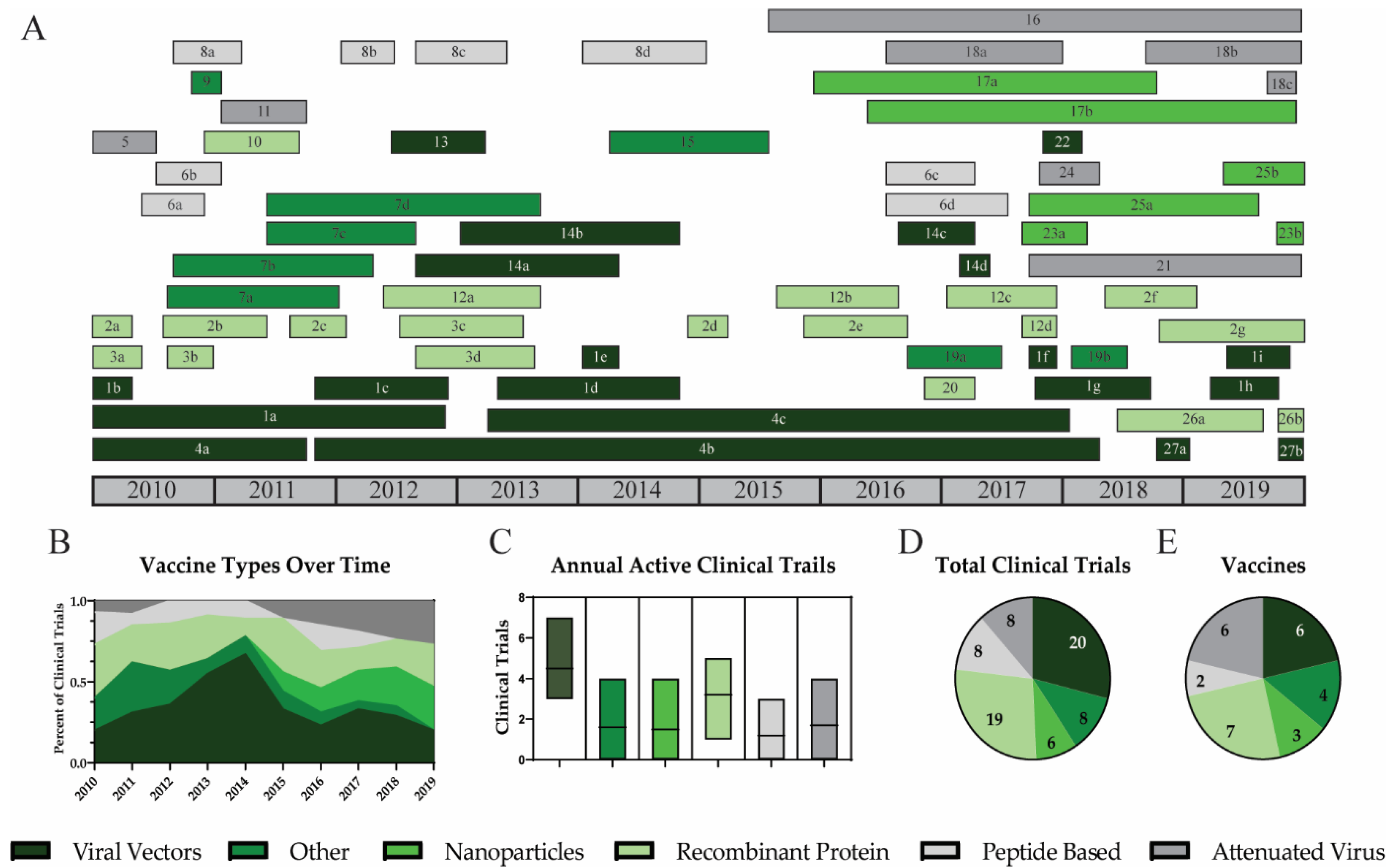
3.2. Vaccine Platforms
3.2.1. Viral Vectors
3.2.2. Nanoparticles
3.2.3. Recombinant Protein
3.2.4. Peptide Based Vaccines
3.2.5. Attenuated Virus
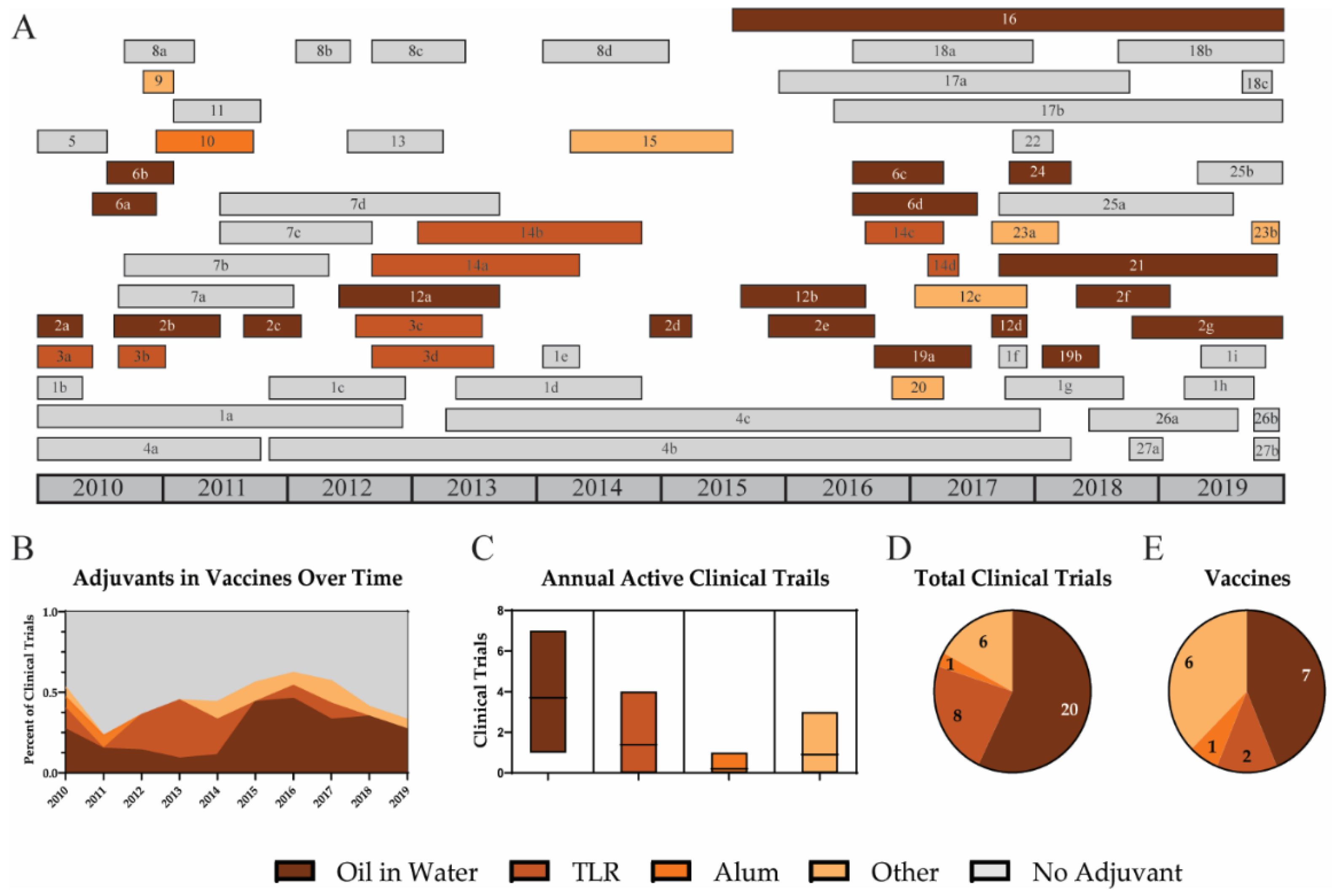
3.3. Adjuvants
3.3.1. Oil-in-Water Emulsions
3.3.2. TLR Agonists
3.3.3. Alum
3.4. Clinical Trial Phases
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Erbelding, E.J.; Post, D.J.; Stemmy, E.J.; Roberts, P.C.; Augustine, A.D.; Ferguson, S.; Paules, C.I.; Graham, B.S.; Fauci, A.S. A Universal Influenza Vaccine: The Strategic Plan for the National Institute of Allergy and Infectious Diseases. J. Infect. Dis. 2018, 218, 347–354. [Google Scholar] [CrossRef] [PubMed]
- WHO. Influenza (Seasonal). Available online: https://www.who.int/news-room/fact-sheets/detail/influenza-(seasonal) (accessed on 12 November 2018).
- Iuliano, A.D.; Roguski, K.M.; Chang, H.H.; Muscatello, D.J.; Palekar, R.; Tempia, S.; Cohen, C.; Gran, J.M.; Schanzer, D.; Cowling, B.J.; et al. Estimates of global seasonal influenza-associated respiratory mortality: A modelling study. Lancet 2018, 391, 1285–1300. [Google Scholar] [CrossRef]
- Lafond, K.E.; Nair, H.; Rasooly, M.H.; Valente, F.; Booy, R.; Rahman, M.; Kitsutani, P.; Yu, H.; Guzman, G.; Coulibaly, D.; et al. Global Role and Burden of Influenza in Pediatric Respiratory Hospitalizations, 1982–2012: A Systematic Analysis. PLoS Med. 2016, 13, e1001977. [Google Scholar] [CrossRef] [PubMed]
- Rolfes, M.A.; Foppa, I.M.; Garg, S.; Flannery, B.; Brammer, L.; Singleton, J.A.; Burns, E.; Jernigan, D.; Olsen, S.J.; Bresee, J.; et al. Annual estimates of the burden of seasonal influenza in the United States: A tool for strengthening influenza surveillance and preparedness. Influenza Other Respir. Viruses 2018, 12, 132–137. [Google Scholar] [CrossRef] [PubMed]
- Molinari, N.A.; Ortega-Sanchez, I.R.; Messonnier, M.L.; Thompson, W.W.; Wortley, P.M.; Weintraub, E.; Bridges, C.B. The annual impact of seasonal influenza in the US: Measuring disease burden and costs. Vaccine 2007, 25, 5086–5096. [Google Scholar] [CrossRef]
- Van Kerkhove, M.D.; Hirve, S.; Koukounari, A.; Mounts, A.W. Estimating age-specific cumulative incidence for the 2009 influenza pandemic: A meta-analysis of A(H1N1)pdm09 serological studies from 19 countries. Influenza Other Respir. Viruses 2013, 7, 872–886. [Google Scholar] [CrossRef]
- Grohskopf, L.A.; Alyanak, E.; Broder, K.R.; Walter, E.B.; Fry, A.M.; Jernigan, D.B. Prevention and Control of Seasonal Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices—United States, 2018–2019 Influenza Season. Mmwr Recomm. Rep. 2018, 67, 1–20. [Google Scholar] [CrossRef]
- Foppa, I.M.; Cheng, P.Y.; Reynolds, S.B.; Shay, D.K.; Carias, C.; Bresee, J.S.; Kim, I.K.; Gambhir, M.; Fry, A.M. Deaths averted by influenza vaccination in the U.S. during the seasons 2005/06 through 2013/14. Vaccine 2015, 33, 3003–3009. [Google Scholar] [CrossRef]
- CDC. CDC Seasonal Flu Vaccine Effectiveness Studies. Available online: https://www.cdc.gov/flu/vaccines-work/effectiveness-studies.htm (accessed on 31 August 2020).
- Lewnard, J.A.; Cobey, S. Immune History and Influenza Vaccine Effectiveness. Vaccines 2018, 6, 28. [Google Scholar] [CrossRef]
- University of Oxford; Wellcome Trust. A Study to Assess the Safety and Immunogenicity of a New Influenza Vaccine Candidate MVA-NP+M1 in Healthy Adults. Available online: https://ClinicalTrials.gov/show/NCT00942071 (accessed on 13 January 2020).
- Berthoud, T.K.; Hamill, M.; Lillie, P.J.; Hwenda, L.; Collins, K.A.; Ewer, K.J.; Milicic, A.; Poyntz, H.C.; Lambe, T.; Fletcher, H.A.; et al. Potent CD8+ T-cell immunogenicity in humans of a novel heterosubtypic influenza A vaccine, MVA-NP+M1. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2011, 52, 1–7. [Google Scholar] [CrossRef]
- Antrobus, R.D.; Berthoud, T.K.; Mullarkey, C.E.; Hoschler, K.; Coughlan, L.; Zambon, M.; Hill, A.V.; Gilbert, S.C. Coadministration of seasonal influenza vaccine and MVA-NP+M1 simultaneously achieves potent humoral and cell-mediated responses. Mol. Ther. J. Am. Soc. Gene Ther. 2014, 22, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Antrobus, R.D.; Lillie, P.J.; Berthoud, T.K.; Spencer, A.J.; McLaren, J.E.; Ladell, K.; Lambe, T.; Milicic, A.; Price, D.A.; Hill, A.V.; et al. A T cell-inducing influenza vaccine for the elderly: Safety and immunogenicity of MVA-NP+M1 in adults aged over 50 years. PLoS ONE 2012, 7, e48322. [Google Scholar] [CrossRef]
- Lillie, P.J.; Berthoud, T.K.; Powell, T.J.; Lambe, T.; Mullarkey, C.; Spencer, A.J.; Hamill, M.; Peng, Y.; Blais, M.E.; Duncan, C.J.; et al. Preliminary assessment of the efficacy of a T-cell-based influenza vaccine, MVA-NP+M1, in humans. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2012, 55, 19–25. [Google Scholar] [CrossRef] [PubMed]
- University of Oxford; Wellcome Trust. A Study to Assess the Safety and Efficacy of a New Influenza Candidate Vaccine MVA-NP+M1 In Healthy Adults. Available online: https://ClinicalTrials.gov/show/NCT00993083 (accessed on 13 January 2020).
- Powell, T.J.; Peng, Y.; Berthoud, T.K.; Blais, M.E.; Lillie, P.J.; Hill, A.V.; Rowland-Jones, S.L.; McMichael, A.J.; Gilbert, S.C.; Dong, T. Examination of influenza specific T cell responses after influenza virus challenge in individuals vaccinated with MVA-NP+M1 vaccine. PLoS ONE 2013, 8, e62778. [Google Scholar] [CrossRef] [PubMed]
- University of Oxford. A Study to Determine the Safety and Immunogenicity of Co-administration of the Candidate Influenza Vaccine MVA-NP+M1 and Seasonal Influenza Vaccine. Available online: https://ClinicalTrials.gov/show/NCT01465035 (accessed on 13 January 2020).
- University of Oxford. A Phase I Study of Candidate Influenza Vaccines MVA-NP+M1 and ChAdOx1 NP+M1. Available online: https://ClinicalTrials.gov/show/NCT01818362 (accessed on 13 January 2020).
- Coughlan, L.; Sridhar, S.; Payne, R.; Edmans, M.; Milicic, A.; Venkatraman, N.; Lugonja, B.; Clifton, L.; Qi, C.; Folegatti, P.M.; et al. Heterologous Two-Dose Vaccination with Simian Adenovirus and Poxvirus Vectors Elicits Long-Lasting Cellular Immunity to Influenza Virus A in Healthy Adults. EBioMedicine 2018, 29, 146–154. [Google Scholar] [CrossRef]
- University of Oxford. Safety and Immunogenicity of Co-administration of Candidate Influenza Vaccine MVA-NP+M1 and Viroflu® Seasonal Influenza Vaccine. Available online: https://ClinicalTrials.gov/show/NCT02014168 (accessed on 13 January 2020).
- Vaccitech Limited; University of Oxford. A Study to Determine the Safety and Immunogenicity of the Candidate Influenza Vaccine MVA-NP+M1. Available online: https://ClinicalTrials.gov/show/NCT03277456 (accessed on 13 January 2020).
- Folegatti, P.M.; Bellamy, D.; Flaxman, A.; Mair, C.; Ellis, C.; Ramon, R.L.; Ramos Lopez, F.; Mitton, C.; Baker, M.; Poulton, I.; et al. Safety and Immunogenicity of the Heterosubtypic Influenza A Vaccine MVA-NP+M1 Manufactured on the AGE1.CR.pIX Avian Cell Line. Vaccines 2019, 7, 33. [Google Scholar] [CrossRef]
- Vaccitech Limited; University of Oxford. Improved Novel VaccIne CombinaTion InflUenza Study. Available online: https://ClinicalTrials.gov/show/NCT03300362 (accessed on 13 January 2020).
- Swayze, H.; Allen, J.; Folegatti, P.; Yu, L.; Gilbert, S.; Hill, A.; Ellis, C.; Butler, C. A phase IIb study to determine the safety and efficacy of candidate INfluenza Vaccine MVA-NP+M1 in combination with licensed InaCTivated inflUenza vaccine in adultS aged 65 years and above (INVICTUS): A study protocol [version 1; peer review: 1 approved with reservations]. F1000Research 2019, 8, 719. [Google Scholar] [CrossRef]
- Vaccitech Limited; Clinical Network Services (CNS) Pty Ltd. Efficacy of Candidate Influenza Vaccine MVA-NP+M1 in Adults. Available online: https://ClinicalTrials.gov/show/NCT03880474 (accessed on 13 January 2020).
- Townsend, A. Learning lessons from MVA85A, a failed booster vaccine for BCG. BMJ (Clin. Res. Ed.).
- Vaccitech Limited. Efficacy of MVA-NP+M1 in the Influenza H3N2 Human Challenge Model. Available online: https://ClinicalTrials.gov/show/NCT03883113 (accessed on 13 January 2020).
- BiondVax Pharmaceuticals Ltd. A Double-Dose Safety Study of An Influenza Vaccine (Multimeric-001) Injected to Elderly Volunteers. Available online: https://ClinicalTrials.gov/show/NCT01010737 (accessed on 13 January 2020).
- Atsmon, J.; Caraco, Y.; Ziv-Sefer, S.; Shaikevich, D.; Abramov, E.; Volokhov, I.; Bruzil, S.; Haima, K.Y.; Gottlieb, T.; Ben-Yedidia, T. Priming by a novel universal influenza vaccine (Multimeric-001)-a gateway for improving immune response in the elderly population. Vaccine 2014, 32, 5816–5823. [Google Scholar] [CrossRef]
- BiondVax Pharmaceuticals Ltd. Further Investigation of an Intramuscular Influenza Vaccine (Multimeric-001). Available online: https://ClinicalTrials.gov/show/NCT01146119 (accessed on 13 January 2020).
- Lowell, G.H.; Ziv, S.; Bruzil, S.; Babecoff, R.; Ben-Yedidia, T. Back to the future: Immunization with M-001 prior to trivalent influenza vaccine in 2011/12 enhanced protective immune responses against 2014/15 epidemic strain. Vaccine 2017, 35, 713–715. [Google Scholar] [CrossRef]
- BiondVax Pharmaceuticals Ltd. A Study to Assess the Safety and Immunogenicity of M-001 Influenza Vaccine as a Primer to TIV in Elderly Volunteers. Available online: https://ClinicalTrials.gov/show/NCT01419925 (accessed on 13 January 2020).
- BiondVax Pharmaceuticals Ltd. Phase II Study to Assess Safety & Immunogenicity of Multimeric-001 Influenza Vaccine, Followed by TIV. Available online: https://ClinicalTrials.gov/show/NCT02293317 (accessed on 13 January 2020).
- BiondVax Pharmaceuticals Ltd.; Seventh Framework Programme. Assess the Safety and Immunogenicity of M-001 as a Standalone Influenza Vaccine and as a H5N1 Vaccine Primer in Adults. Available online: https://ClinicalTrials.gov/show/NCT02691130 (accessed on 13 January 2020).
- National Institute of Allergy and Infectious Diseases (NIAID). Two Doses of Multimeric-001 (M-001) Followed by Influenza Vaccine. Available online: https://ClinicalTrials.gov/show/NCT03058692 (accessed on 13 January 2020).
- BiondVax Pharmaceuticals Ltd. A Pivotal Trial to Assess the Safety and Clinical Efficacy of the M-001 as a Standalone Universal Flu Vaccine. Available online: https://ClinicalTrials.gov/show/NCT03450915 (accessed on 13 January 2020).
- VaxInnate Corporation. Safety and Immunogenicity of VAX125 Influenza Vaccine in Community-living Adults >= 65 Years of Age. Available online: https://ClinicalTrials.gov/show/NCT00966238 (accessed on 13 January 2020).
- Taylor, D.N.; Treanor, J.J.; Strout, C.; Johnson, C.; Fitzgerald, T.; Kavita, U.; Ozer, K.; Tussey, L.; Shaw, A. Induction of a potent immune response in the elderly using the TLR-5 agonist, flagellin, with a recombinant hemagglutinin influenza-flagellin fusion vaccine (VAX125, STF2.HA1 SI). Vaccine 2011, 29, 4897–4902. [Google Scholar] [CrossRef] [PubMed]
- VaxInnate Corporation. Comparative Safety and Immunogenicity of VAX128A, VAC128B and VAX128C Novel H1N1 Influenza Vaccine in Healthy Adults. Available online: https://ClinicalTrials.gov/show/NCT01172054 (accessed on 13 January 2020).
- Taylor, D.N.; Treanor, J.J.; Sheldon, E.A.; Johnson, C.; Umlauf, S.; Song, L.; Kavita, U.; Liu, G.; Tussey, L.; Ozer, K.; et al. Development of VAX128, a recombinant hemagglutinin (HA) influenza-flagellin fusion vaccine with improved safety and immune response. Vaccine 2012, 30, 5761–5769. [Google Scholar] [CrossRef] [PubMed]
- VaxInnate Corporation. Safety and Immunogenicity of a Novel H5N1 Influenza Vaccine in Healthy Adults Age 18–49 Years. Available online: https://ClinicalTrials.gov/show/NCT01560793 (accessed on 13 January 2020).
- Song, L.; Zhang, Y.; Yun, N.E.; Poussard, A.L.; Smith, J.N.; Smith, J.K.; Borisevich, V.; Linde, J.J.; Zacks, M.A.; Li, H.; et al. Superior efficacy of a recombinant flagellin:H5N1 HA globular head vaccine is determined by the placement of the globular head within flagellin. Vaccine 2009, 27, 5875–5884. [Google Scholar] [CrossRef]
- VaxInnate Corporation. Study of the Safety and Immunogenicity of a Novel H5N1 Influenza Vaccine in Healthy Adults Age 18–49. Available online: https://ClinicalTrials.gov/show/NCT01658800 (accessed on 13 January 2020).
- Gurwith, M.; Lock, M.; Taylor, E.M.; Ishioka, G.; Alexander, J.; Mayall, T.; Ervin, J.E.; Greenberg, R.N.; Strout, C.; Treanor, J.J.; et al. Safety and immunogenicity of an oral, replicating adenovirus serotype 4 vector vaccine for H5N1 influenza: A randomised, double-blind, placebo-controlled, phase 1 study. Lancet. Infect. Dis. 2013, 13, 238–250. [Google Scholar] [CrossRef]
- Emergent BioSolutions. Safety and Immunogenicity of Replication-Competent Adenovirus 4-vectored Vaccine for Avian Influenza H5N1. Available online: https://ClinicalTrials.gov/show/NCT01006798 (accessed on 13 January 2020).
- National Institute of Allergy and Infectious Diseases (NIAID); National Institutes of Health Clinical Center (CC). Experimental AD4-H5-VTN Vaccine in Healthy Volunteers. Available online: https://ClinicalTrials.gov/show/NCT01443936 (accessed on 13 January 2020).
- National Institute of Allergy and Infectious Diseases (NIAID); National Institutes of Health Clinical Center (CC). Intranasal AD4-H5-VTN as an Adenovirus Vaccine. Available online: https://ClinicalTrials.gov/show/NCT01806909 (accessed on 13 January 2020).
- AVIR Green Hills Biotechnology AG. Dose Finding Study of Single Dose GHB11L1 in Healthy Adults. Available online: https://ClinicalTrials.gov/show/NCT01078701 (accessed on 13 January 2020).
- PepTcell Limited. Phase 1b Influenza Vaccine Study in Healthy Subjects. Available online: https://ClinicalTrials.gov/show/NCT01181336 (accessed on 13 January 2020).
- Pleguezuelos, O.; Robinson, S.; Stoloff, G.A.; Caparros-Wanderley, W. Synthetic Influenza vaccine (FLU-v) stimulates cell mediated immunity in a double-blind, randomised, placebo-controlled Phase I trial. Vaccine 2012, 30, 4655–4660. [Google Scholar] [CrossRef] [PubMed]
- PepTcell Limited. Influenza Vaccine Challenge Study in Healthy Subjects. Available online: https://ClinicalTrials.gov/show/NCT01226758 (accessed on 13 January 2020).
- Pleguezuelos, O.; Robinson, S.; Fernandez, A.; Stoloff, G.A.; Caparros-Wanderley, W. Meta-Analysis and Potential Role of Preexisting Heterosubtypic Cellular Immunity Based on Variations in Disease Severity Outcomes for Influenza Live Viral Challenges in Humans. Clin. Vaccine Immunol. CVI 2015, 22, 949–956. [Google Scholar] [CrossRef] [PubMed]
- Pleguezuelos, O.; Robinson, S.; Fernandez, A.; Stoloff, G.A.; Mann, A.; Gilbert, A.; Balaratnam, G.; Wilkinson, T.; Lambkin-Williams, R.; Oxford, J.; et al. A Synthetic Influenza Virus Vaccine Induces a Cellular Immune Response That Correlates with Reduction in Symptomatology and Virus Shedding in a Randomized Phase Ib Live-Virus Challenge in Humans. Clin. Vaccine Immunol. CVI 2015, 22, 828–835. [Google Scholar] [CrossRef] [PubMed]
- PepTcell Limited; National Institute of Allergy and Infectious Diseases (NIAID). Efficacy of FLU-v in an H1N1 Influenza Human Challenge Model. Available online: https://ClinicalTrials.gov/show/NCT03180801 (accessed on 13 January 2020).
- PepTcell Limited; Seventh Framework Programme; University of Groningen; University Medical Center Groningen; Robert Koch Institut; Norwegian Institute of Public Health. A Randomised, Double-blind, Placebo-controlled Phase IIb Trial to Test FLU-v Vaccine. Available online: https://ClinicalTrials.gov/show/NCT02962908 (accessed on 13 January 2020).
- van Doorn, E.; Pleguezuelos, O.; Liu, H.; Fernandez, A.; Bannister, R.; Stoloff, G.; Oftung, F.; Norley, S.; Huckriede, A.; Frijlink, H.W.; et al. Evaluation of the immunogenicity and safety of different doses and formulations of a broad spectrum influenza vaccine (FLU-v) developed by SEEK: Study protocol for a single-center, randomized, double-blind and placebo-controlled clinical phase IIb trial. BMC Infect. Dis. 2017, 17, 241. [Google Scholar] [CrossRef] [PubMed]
- Inovio Pharmaceuticals. Study of VGX-3400X, H5N1 Avian Influenza Virus DNA Plasmid + Electroporation in Healthy Adults. Available online: https://ClinicalTrials.gov/show/NCT01142362 (accessed on 13 January 2020).
- Inovio. Inovio Biomedical H5N1 Avian Influenza DNA Vaccine Receives Korean Approval to Begin Clinical Trials. In First Component of Inovio’s SynCon(TM) Universal Flu Vaccine to Be Tested in Healthy Volunteers; Richardson, J., Ed.; Richardson & Associates: Omaha, NE, USA, 2010. [Google Scholar]
- GeneOne Life Science, Inc.; Inovio Pharmaceuticals. Study Of VGX-3400, H5N1 Avian Flu Virus Plasmid DNA With Electroporation Device In Healthy Adult Males. Available online: https://ClinicalTrials.gov/show/NCT01184976 (accessed on 13 January 2020).
- Inovio Pharmaceuticals. A Follow-On Study With an H5 Influenza Vaccine for Subjects Who Participated in Study FLU-001. Available online: https://ClinicalTrials.gov/show/NCT01403155 (accessed on 13 January 2020).
- Inovio Pharmaceuticals. A Study of DNA Vaccine With Electroporation for the Prevention of Disease Caused by H1 and H5 Influenza Virus. Available online: https://ClinicalTrials.gov/show/NCT01405885 (accessed on 13 January 2020).
- Immune Targeting Systems Ltd.; Hammersmith Medicines Research. A Study to Evaluate the Safety, Tolerability and Immunogenicity of a Universal Influenza A Vaccine. Available online: https://ClinicalTrials.gov/show/NCT01265914 (accessed on 13 January 2020).
- Francis, J.N.; Bunce, C.J.; Horlock, C.; Watson, J.M.; Warrington, S.J.; Georges, B.; Brown, C.B. A novel peptide-based pan-influenza A vaccine: A double blind, randomised clinical trial of immunogenicity and safety. Vaccine 2015, 33, 396–402. [Google Scholar] [CrossRef]
- Immune Targeting Systems Ltd.; Limited, I.A.P. Safety, Tolerability and Immunogenicity of Two Different Formulations of an Influenza A Vaccine (FP-01.1). Available online: https://ClinicalTrials.gov/show/NCT01677676 (accessed on 13 January 2020).
- Immune Targeting Systems Ltd. Influenza A Vaccine (FP-01.1) Formulated With and Without Adjuvant, in the Presence or Absence of a Single Administration of a Trivalent Inactivated Influenza Virus Vaccine in Older Adults. Available online: https://ClinicalTrials.gov/show/NCT01701752 (accessed on 13 January 2020).
- Immune Targeting Systems Ltd. Safety, Tolerability, Efficacy and Immunogenicity of an Influenza A Vaccine (FP-01.1) in Healthy Volunteers Following Virus Challenge. Available online: https://ClinicalTrials.gov/show/NCT02071329 (accessed on 13 January 2020).
- Crucell Holland BV. Immunogenicity and Safety of a Single 0.5 mL Dose of Inflexal V With a 0.25 mL 2-dose Regimen of Inflexal V. Available online: https://ClinicalTrials.gov/show/NCT01229397 (accessed on 13 January 2020).
- Mischler, R.; Metcalfe, I.C. Inflexal V a trivalent virosome subunit influenza vaccine: Production. Vaccine 2002, 20 (Suppl. S5), B17–B23. [Google Scholar] [CrossRef]
- Fraunhofer, Center for Molecular Biotechnology. Safety and Immunogenicity Of A Recombinant H5N1 Vaccine In Adults. Available online: https://ClinicalTrials.gov/show/NCT01250795 (accessed on 13 January 2020).
- Chichester, J.A.; Jones, R.M.; Green, B.J.; Stow, M.; Miao, F.; Moonsammy, G.; Streatfield, S.J.; Yusibov, V. Safety and immunogenicity of a plant-produced recombinant hemagglutinin-based influenza vaccine (HAI-05) derived from A/Indonesia/05/2005 (H5N1) influenza virus: A phase 1 randomized, double-blind, placebo-controlled, dose-escalation study in healthy adults. Viruses 2012, 4, 3227–3244. [Google Scholar] [CrossRef] [PubMed]
- AVIR Green Hills Biotechnology AG. Study of Single Dose GHB16L2 Trivalent Influenza Vaccine in Healthy Adults. Available online: https://ClinicalTrials.gov/show/NCT01369862 (accessed on 13 January 2020).
- Mossler, C.; Groiss, F.; Wolzt, M.; Wolschek, M.; Seipelt, J.; Muster, T. Phase I/II trial of a replication-deficient trivalent influenza virus vaccine lacking NS1. Vaccine 2013, 31, 6194–6200. [Google Scholar] [CrossRef] [PubMed]
- Protein Sciences Corporation. Safety and Immunogenicity of PanBlok Influenza Vaccine in Healthy Adults. Available online: https://ClinicalTrials.gov/show/NCT01612000 (accessed on 13 January 2020).
- Treanor, J.J.; Chu, L.; Essink, B.; Muse, D.; El Sahly, H.M.; Izikson, R.; Goldenthal, K.L.; Patriarca, P.; Dunkle, L.M. Stable emulsion (SE) alone is an effective adjuvant for a recombinant, baculovirus-expressed H5 influenza vaccine in healthy adults: A Phase 2 trial. Vaccine 2017, 35, 923–928. [Google Scholar] [CrossRef] [PubMed]
- Protein Sciences Corporation. Trial to Evaluate the Immunogenicity and Safety of Panblok® (H7 rHA) in Healthy Adults Aged 18 and Older. Available online: https://ClinicalTrials.gov/show/NCT02464163 (accessed on 13 January 2020).
- Stadlbauer, D.; Rajabhathor, A.; Amanat, F.; Kaplan, D.; Masud, A.; Treanor, J.J.; Izikson, R.; Cox, M.M.; Nachbagauer, R.; Krammer, F. Vaccination with a Recombinant H7 Hemagglutinin-Based Influenza Virus Vaccine Induces Broadly Reactive Antibodies in Humans. mSphere 2017, 2, 6. [Google Scholar] [CrossRef] [PubMed]
- Vaxine Pty Ltd.; Australian Respiratory and Sleep Medicine Institute. Recombinant H7 Hemagglutinin Influenza Vaccine Trial. Available online: https://ClinicalTrials.gov/show/NCT03038776 (accessed on 13 January 2020).
- Honda-Okubo, Y.; Saade, F.; Petrovsky, N. Advax, a polysaccharide adjuvant derived from delta inulin, provides improved influenza vaccine protection through broad-based enhancement of adaptive immune responses. Vaccine 2012, 30, 5373–5381. [Google Scholar] [CrossRef] [PubMed]
- Biomedical Advanced Research and Development Authority; Rho, Inc. Panblok H7 Vaccine Adjuvanted With AS03 or MF59. Available online: https://ClinicalTrials.gov/show/NCT03283319 (accessed on 13 January 2020).
- University of Oxford. A Phase I Study to Determine the Safety and Immunogenicity of the Candidate Influenza Vaccine ChAdOx1-NP+M1. Available online: https://ClinicalTrials.gov/show/NCT01623518 (accessed on 13 January 2020).
- Antrobus, R.D.; Coughlan, L.; Berthoud, T.K.; Dicks, M.D.; Hill, A.V.; Lambe, T.; Gilbert, S.C. Clinical assessment of a novel recombinant simian adenovirus ChAdOx1 as a vectored vaccine expressing conserved Influenza A antigens. Mol. Ther. J. Am. Soc. Gene Ther. 2014, 22, 668–674. [Google Scholar] [CrossRef] [PubMed]
- Liebowitz, D.; Lindbloom, J.D.; Brandl, J.R.; Garg, S.J.; Tucker, S.N. High titre neutralising antibodies to influenza after oral tablet immunisation: A phase 1, randomised, placebo-controlled trial. Lancet. Infect. Dis. 2015, 15, 1041–1048. [Google Scholar] [CrossRef]
- Vaxart. Safety Study of an Oral Vaccine to Prevent Seasonal Influenza. Available online: https://ClinicalTrials.gov/show/NCT01688297 (accessed on 13 January 2020).
- Vaxart. Immunogenicity of Seasonal Influenza by Delivery Directly to Ileum. Available online: https://ClinicalTrials.gov/show/NCT01761123 (accessed on 13 January 2020).
- Kolhatkar, N.; Gottlieb, K.; Kasparek, K.; Hodgson, K.; Tucker, S.; Liebowitz, D. 1947. Influenza Vaccination via Oral Tablet is Protective and Induces a Unique Mucosal Immune Response. Open Forum Infect. Dis. 2018, 5, S561–S562. [Google Scholar] [CrossRef]
- Biomedical Advanced Research and Development Authority. A Phase 2 Influenza A Challenge Study Following Oral Administration of an H1N1 HA Ad-Vector Seasonal Flu Vaccine. Available online: https://ClinicalTrials.gov/show/NCT02918006 (accessed on 13 January 2020).
- Vaxart. Pharmacodynamic Open-Label Trial With VXA-A1.1 Oral H1 Vaccine in Healthy Adults. Available online: https://ClinicalTrials.gov/show/NCT03121339 (accessed on 13 January 2020).
- Novavax; Department of Health and Human Services. A(H7N9) VLP Antigen Dose-Ranging Study With Matrix-M1™ Adjuvant. Available online: https://ClinicalTrials.gov/show/NCT02078674 (accessed on 13 January 2020).
- Nova Immunotherapeutics Limited; Mercia Pharma Inc.; Nova Laboratories Limited; The Emmes Company, LLC. The Safety, Tolerance, and Immunogenicity of MAS-1-Adjuvanted Seasonal Inactivated Influenza Vaccine (MER4101). Available online: https://ClinicalTrials.gov/show/NCT02500680 (accessed on 13 January 2020).
- Feldman, R.A.; Fuhr, R.; Smolenov, I.; Mick Ribeiro, A.; Panther, L.; Watson, M.; Senn, J.J.; Smith, M.; Almarsson, Ö.; Pujar, H.S.; et al. mRNA vaccines against H10N8 and H7N9 influenza viruses of pandemic potential are immunogenic and well tolerated in healthy adults in phase 1 randomized clinical trials. Vaccine 2019, 37, 3326–3334. [Google Scholar] [CrossRef]
- ModernaTX, Inc. Safety, Tolerability, and Immunogenicity of VAL-506440 in Healthy Adult Subjects. Available online: https://ClinicalTrials.gov/show/NCT03076385 (accessed on 13 January 2020).
- ModernaTX, Inc. Safety, Tolerability, and Immunogenicity of VAL-339851 in Healthy Adult Subjects. Available online: https://ClinicalTrials.gov/show/NCT03345043 (accessed on 13 January 2020).
- FluGen Inc. Safety and Immunogenicity Study of H3N2 M2SR Monovalent Influenza Vaccine in Healthy Volunteers. Available online: https://ClinicalTrials.gov/show/NCT02822105 (accessed on 13 January 2020).
- National Institute of Allergy and Infectious Diseases (NIAID). Safety and Immunogenicity Study of an Influenza Vaccination Strategy Including a H3N2 M2SR Prime Followed by a Seasonal Quadrivalent Inactivated Vaccine Boost in a Pediatric Population 9–17 Years Old. Available online: https://ClinicalTrials.gov/show/NCT03553940 (accessed on 13 January 2020).
- FluGen Inc. Safety and Immunogenicity of the Bris10 M2SR and Sing2016 M2SR H3N2 Monovalent Influenza Vaccines. Available online: https://ClinicalTrials.gov/show/NCT03999554 (accessed on 13 January 2020).
- Hatta, Y.; Boltz, D.; Sarawar, S.; Kawaoka, Y.; Neumann, G.; Bilsel, P. Novel influenza vaccine M2SR protects against drifted H1N1 and H3N2 influenza virus challenge in ferrets with pre-existing immunity. Vaccine 2018, 36, 5097–5103. [Google Scholar] [CrossRef]
- Eurocine Vaccines AB. Study to Assess the Safety, Tolerability and Immune Response Following Vaccination With Immunose™ FLU. Available online: https://ClinicalTrials.gov/show/NCT02998996 (accessed on 13 January 2020).
- Eurocine Vaccines AB. Study to Assess the Safety, Tolerability and Immune Response Following Vaccination With Immunose™ FLU in Older Adults. Available online: https://ClinicalTrials.gov/show/NCT03437304 (accessed on 13 January 2020).
- Nitto Denko Corporation. Evaluation of the Safety and Immunogenicity of a Sublingual Influenza Vaccine NSV0001 in Healthy Male Volunteers. Available online: https://ClinicalTrials.gov/show/NCT02955030 (accessed on 13 January 2020).
- GlaxoSmithKline. A Study to Evaluate the Reactogenicity, Safety and Immunogenicity of GlaxoSmithKline (GSK) Biologicals’ Investigational Supra-Seasonal Universal Influenza Vaccines—Inactivated (SUIVs) (GSK3816302A) in Healthy Adults Aged 18 to 39 Years. Available online: https://ClinicalTrials.gov/show/NCT03275389 (accessed on 13 January 2020).
- Altimmune, Inc. Single-Ascending-Dose Study of the Safety and Immunogenicity of NasoVAX. Available online: https://ClinicalTrials.gov/show/NCT03232567 (accessed on 13 January 2020).
- Tasker, S.; Krishnan, V.; Bart, S.; Suyundikov, A.; Booth, P.-G.; Wight O’Rourke, A.; Zhang, J.; Georges, B.; Roberts, S. 2554. Safety and Immunogenicity of NasoVAX, a Novel Intranasal Influenza Vaccine. Open Forum Infect. Dis. 2018, 5, S68. [Google Scholar] [CrossRef]
- Altimmune, Inc. Dr. Sybil Tasker to Present on April 16 at 12:55 p.m. Eastern Time; Robinson, A.R., Ed.; GlobeNewswire: Los Angeles, CA, USA, 2019. [Google Scholar]
- Novavax. Evaluation of the Safety and Immunogenicity of a Recombinant Trivalent Nanoparticle Influenza Vaccine With Matrix M-1 Adjuvant (NanoFlu). Available online: https://ClinicalTrials.gov/show/NCT03293498 (accessed on 13 January 2020).
- Novavax. Novavax Announces Positive Phase 2 NanoFlu Results in Older Adults. In Sets the Stage for Phase 3 Clinical Trial in 2019; Cohen, A., Ed.; Sam Brown Inc.: Wayne, PA, USA, 2019. [Google Scholar]
- Novavax. Phase 3 Pivotal Trial of NanoFlu™ in Older Adults. Available online: https://ClinicalTrials.gov/show/NCT04120194 (accessed on 13 January 2020).
- Bernstein, D.I.; Guptill, J.; Naficy, A.; Nachbagauer, R.; Berlanda-Scorza, F.; Feser, J.; Wilson, P.C.; Solorzano, A.; Van der Wielen, M.; Walter, E.B.; et al. Immunogenicity of chimeric haemagglutinin-based, universal influenza virus vaccine candidates: Interim results of a randomised, placebo-controlled, phase 1 clinical trial. Lancet. Infect. Dis. 2019, 20, 80–91. [Google Scholar] [CrossRef]
- PATHI.; Icahn School of Medicine at Mount Sinai; Children’s Hospital Medical Center, Cincinnati; Duke University; The Emmes Company, LLC.; GlaxoSmithKline. Safety and Immunogenicity of a Live-Attenuated Universal Flu Vaccine Followed by an Inactivated Universal Flu Vaccine. Available online: https://ClinicalTrials.gov/show/NCT03300050 (accessed on 13 January 2020).
- National Institute of Allergy and Infectious Diseases (NIAID); National Institutes of Health Clinical Center (CC). Influenza HA Ferritin Vaccine, Alone or in Prime-Boost Regimens With an Influenza DNA Vaccine in Healthy Adults. Available online: https://ClinicalTrials.gov/show/NCT03186781 (accessed on 13 January 2020).
- Kanekiyo, M.; Wei, C.J.; Yassine, H.M.; McTamney, P.M.; Boyington, J.C.; Whittle, J.R.; Rao, S.S.; Kong, W.P.; Wang, L.; Nabel, G.J. Self-assembling influenza nanoparticle vaccines elicit broadly neutralizing H1N1 antibodies. Nature 2013, 499, 102–106. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, I.; Iwahori, K.; Kumagai, S. Ferritin in the field of nanodevices. Biochim. Biophys. Acta 2010, 1800, 846–857. [Google Scholar] [CrossRef] [PubMed]
- Nabel, G.J.; Wei, C.J.; Ledgerwood, J.E. Vaccinate for the next H2N2 pandemic now. Nature 2011, 471, 157–158. [Google Scholar] [CrossRef] [PubMed]
- National Institute of Allergy and Infectious Diseases (NIAID); National Institutes of Health Clinical Center (CC). Dose, Safety, Tolerability and Immunogenicity of an Influenza H1 Stabilized Stem Ferritin Vaccine, VRCFLUNPF099-00-VP, in Healthy Adults. Available online: https://ClinicalTrials.gov/show/NCT03814720 (accessed on 13 January 2020).
- Yassine, H.M.; Boyington, J.C.; McTamney, P.M.; Wei, C.J.; Kanekiyo, M.; Kong, W.P.; Gallagher, J.R.; Wang, L.; Zhang, Y.; Joyce, M.G.; et al. Hemagglutinin-stem nanoparticles generate heterosubtypic influenza protection. Nat. Med. 2015, 21, 1065–1070. [Google Scholar] [CrossRef] [PubMed]
- Osivax SAS.; Aepodia. Safety and Immune Response of Increasing Doses of OVX836 After Intramuscular or Intranasal Administrations in Healthy Subjects. Available online: https://ClinicalTrials.gov/show/NCT03594890 (accessed on 13 January 2020).
- Del Campo, J.; Pizzorno, A.; Djebali, S.; Bouley, J.; Haller, M.; Perez-Vargas, J.; Lina, B.; Boivin, G.; Hamelin, M.E.; Nicolas, F.; et al. OVX836 a recombinant nucleoprotein vaccine inducing cellular responses and protective efficacy against multiple influenza A subtypes. NPJ Vaccines 2019, 4, 4. [Google Scholar] [CrossRef]
- Osivax SAS. Safety and Immune Response of One Dose of OVX836 at Two Dose Levels, in Comparison to Influvac TetraTM, After Intramuscular Administration in Healthy Subjects Aged 18–65 Years. Available online: https://clinicaltrials.gov/ct2/show/record/NCT04192500 (accessed on 13 January 2020).
- Gamaleya Research Institute of Epidemiology and Microbiology, Health Ministry of the Russian Federation. The Study of the Safety, Reactogenicity and Immunogenicity of the GamFluVac. Available online: https://ClinicalTrials.gov/show/NCT03651544 (accessed on 13 January 2020).
- Gamaleya Research Institute of Epidemiology and Microbiology, Health Ministry of the Russian Federation; Main military clinical hospital named after academician N. N. Burdenko. A Double-blind Randomized Placebo-controlled Study Study of the Safety, Reactogenicity and Immunogenicity of the GamFluVac. Available online: https://ClinicalTrials.gov/show/NCT04034290 (accessed on 13 January 2020).
- Spitaels, J.; Roose, K.; Saelens, X. Influenza and Memory T Cells: How to Awake the Force. Vaccines 2016, 4, 33. [Google Scholar] [CrossRef]
- Chen, X.; Liu, S.; Goraya, M.U.; Maarouf, M.; Huang, S.; Chen, J.-L. Host Immune Response to Influenza A Virus Infection. Front. Immunol. 2018, 9, 320. [Google Scholar] [CrossRef]
- Grant, E.; Wu, C.; Chan, K.F.; Eckle, S.; Bharadwaj, M.; Zou, Q.M.; Kedzierska, K.; Chen, W. Nucleoprotein of influenza A virus is a major target of immunodominant CD8+ T-cell responses. Immunol. Cell Biol. 2013, 91, 184–194. [Google Scholar] [CrossRef]
- Krammer, F. Emerging influenza viruses and the prospect of a universal influenza virus vaccine. Biotechnol. J. 2015, 10, 690–701. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-Q.; Wohlbold, T.J.; Zheng, N.-Y.; Huang, M.; Huang, Y.; Neu, K.E.; Lee, J.; Wan, H.; Rojas, K.T.; Kirkpatrick, E.; et al. Influenza Infection in Humans Induces Broadly Cross-Reactive and Protective Neuraminidase-Reactive Antibodies. Cell 2018, 173, 417–429.e10. [Google Scholar] [CrossRef] [PubMed]
- Hoft, D.F.; Lottenbach, K.R.; Blazevic, A.; Turan, A.; Blevins, T.P.; Pacatte, T.P.; Yu, Y.; Mitchell, M.C.; Hoft, S.G.; Belshe, R.B. Comparisons of the Humoral and Cellular Immune Responses Induced by Live Attenuated Influenza Vaccine and Inactivated Influenza Vaccine in Adults. Clin. Vaccine Immunol. CVI 2017, 24, 1. [Google Scholar] [CrossRef] [PubMed]
- Barberis, I.; Myles, P.; Ault, S.K.; Bragazzi, N.L.; Martini, M. History and evolution of influenza control through vaccination: From the first monovalent vaccine to universal vaccines. J. Prev. Med. Hyg. 2016, 57, E115–E120. [Google Scholar]
- Lundstrom, K. Viral Vectors in Gene Therapy. Diseases 2018, 6, 42. [Google Scholar] [CrossRef]
- Pati, R.; Shevtsov, M.; Sonawane, A. Nanoparticle Vaccines Against Infectious Diseases. Front. Immunol. 2018, 9, 2224. [Google Scholar] [CrossRef]
- Nascimento, I.P.; Leite, L.C.C. Recombinant vaccines and the development of new vaccine strategies. Braz. J. Med. Biol. Res. 2012, 45, 1102–1111. [Google Scholar] [CrossRef]
- Del Giudice, G.; Rappuoli, R.; Didierlaurent, A.M. Correlates of adjuvanticity: A review on adjuvants in licensed vaccines. Semin. Immunol. 2018, 39, 14–21. [Google Scholar] [CrossRef]
- Krug, R.M. Functions of the influenza A virus NS1 protein in antiviral defense. Curr. Opin. Virol. 2015, 12, 1–6. [Google Scholar] [CrossRef]
- Awate, S.; Babiuk, L.A.; Mutwiri, G. Mechanisms of action of adjuvants. Front. Immunol. 2013, 4, 114. [Google Scholar] [CrossRef]
- Gnjatic, S.; Sawhney, N.B.; Bhardwaj, N. Toll-like receptor agonists: Are they good adjuvants? Cancer J. 2010, 16, 382–391. [Google Scholar] [CrossRef] [PubMed]
- CDC. Adjuvants Help Vaccines Work Better. Available online: https://www.cdc.gov/vaccinesafety/concerns/adjuvants.html (accessed on 13 January 2020).
- Atsmon, J.; Kate-Ilovitz, E.; Shaikevich, D.; Singer, Y.; Volokhov, I.; Haim, K.Y.; Ben-Yedidia, T. Safety and Immunogenicity of Multimeric-001—A Novel Universal Influenza Vaccine. J. Clin. Immunol. 2012, 32, 595–603. [Google Scholar] [CrossRef]
- Chattopadhyay, S.; Sen, G.C. dsRNA-activation of TLR3 and RLR signaling: Gene induction-dependent and independent effects. J. Interferon Cytokine Res. 2014, 34, 427–436. [Google Scholar] [CrossRef]
- NIH. What Are Clinical Trials and Studies? Available online: https://www.nia.nih.gov/health/what-are-clinical-trials-and-studies#four (accessed on 13 January 2020).
- Valkenburg, S.A.; Leung, N.H.L.; Bull, M.B.; Yan, L.M.; Li, A.P.Y.; Poon, L.L.M.; Cowling, B.J. The Hurdles From Bench to Bedside in the Realization and Implementation of a Universal Influenza Vaccine. Front. Immunol. 2018, 9, 1479. [Google Scholar] [CrossRef]
- Nachbagauer, R.; Krammer, F. Universal influenza virus vaccines and therapeutic antibodies. Clin. Microbiol. Infect. 2017, 23, 222–228. [Google Scholar] [CrossRef] [PubMed]
- CDC. Weekly U.S. Influenza Surveillance Report. Available online: https://www.cdc.gov/flu/weekly/index.htm (accessed on 27 August 2020).
- Heikkinen, T.; Ikonen, N.; Ziegler, T. Impact of influenza B lineage-level mismatch between trivalent seasonal influenza vaccines and circulating viruses, 1999–2012. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2014, 59, 1519–1524. [Google Scholar] [CrossRef] [PubMed]
- Fleminger, J.; Goldacre, B. Prevalence of clinical trial status discrepancies: A cross-sectional study of 10,492 trials registered on both ClinicalTrials.gov and the European Union Clinical Trials Register. PLoS ONE 2018, 13, e0193088. [Google Scholar] [CrossRef] [PubMed]
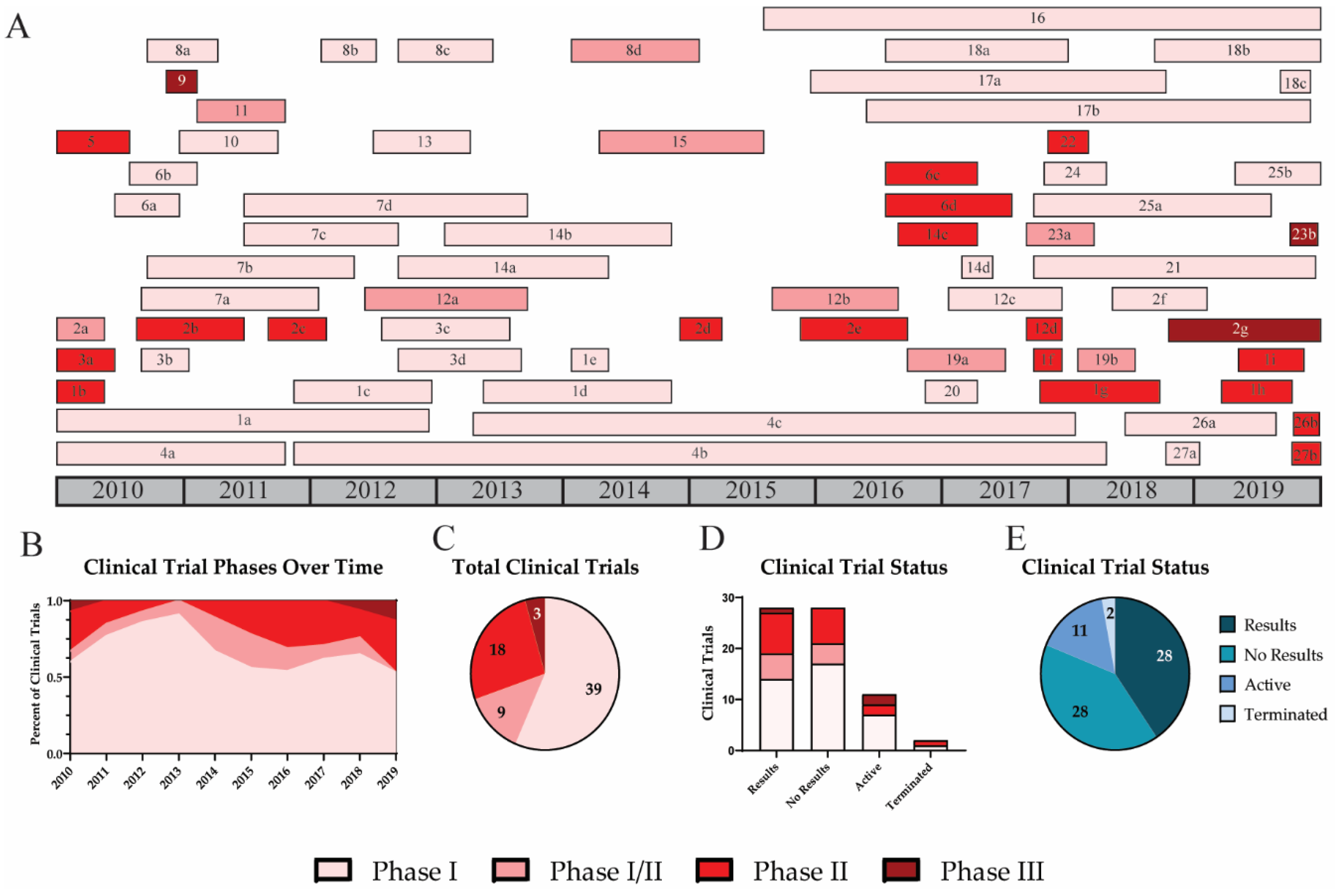
| ID | Vaccine Name | Identifier | Phase | Target | Platform | Adjuvant | Adjuvant Type | Prime Boost | Results/Status | References |
|---|---|---|---|---|---|---|---|---|---|---|
| 1a | MVA-NP+M1 | NCT00942071 | 1 | NP, M1 | Viral Vector | Prime | Yes | [12,13,14,15] | ||
| 1b | MVA-NP+M1 | NCT00993083 | 2 | NP, M1 | Viral Vector | Prime | Yes | [16,17,18] | ||
| 1c | MVA-NP+M1 | NCT01465035 | 1 | NP, M1 | Viral Vector | Prime-Boost | No | [19] | ||
| 1d | MVA-NP+M1 | NCT01818362 | 1 | NP, M1 | Viral Vector | Prime-Boost | Yes | [20,21] | ||
| 1e | MVA-NP+M1 | NCT02014168 | 1 | NP, M1 | Viral Vector | Prime-Boost | Terminated | [22] | ||
| 1f | MVA-NP+M1 | NCT03277456 | 1 | NP, M1 | Viral Vector | Prime | Yes | [23,24] | ||
| 1g | MVA-NP+M1 | NCT03300362 | 2 | NP, M1 | Viral Vector | Prime-Boost | Terminated | [25,26] | ||
| 1h | MVA-NP+M1 | NCT03880474 | 2 | NP, M1 | Viral Vector | Prime | No | [27,28] | ||
| 1i | MVA-NP+M1 | NCT03883113 | 2 | NP, M1 | Viral Vector | Prime | No | [28,29] | ||
| 2a | M-001 | NCT01010737 | 1/2 | HA, NP, M1 | Recombinant Protein | Montanide ISA-51 | Oil in Water | Prime-Boost | Yes | [30,31] |
| 2b | M-001 | NCT01146119 | 2 | HA, NP, M1 | Recombinant Protein | Montanide ISA-51 | Oil in Water | Prime-Boost | No | [32,33] |
| 2c | M-001 | NCT01419925 | 2 | HA, NP, M1 | Recombinant Protein | Montanide ISA-51 | Oil in Water | Prime-Boost | Yes | [31,34] |
| 2d | M-001 | NCT02293317 | 2 | HA, NP, M1 | Recombinant Protein | Montanide ISA-51 | Oil in Water | Prime-Boost-Boost | No | [33,35] |
| 2e | M-001 | NCT02691130 | 2 | HA, NP, M1 | Recombinant Protein | Montanide ISA-51 | Oil in Water | Prime-Boost-Boost | No | [33,36] |
| 2f | M-001 | NCT03058692 | 2 | HA, NP, M1 | Recombinant Protein | Montanide ISA-51 | Oil in Water | Prime-Boost | No | [33,37] |
| 2g | M-001 | NCT03450915 | 3 | HA, NP, M1 | Recombinant Protein | Montanide ISA-51 | Oil in Water | Prime-Boost | Active | [33,38] |
| 3a | VAX125 | NCT00966238 | 2 | HA | Recombinant Protein | TLR5 + Flagellin | TLR Agonist | Prime | Yes | [39,40] |
| 3b | VAX128 | NCT01172054 | 1 | HA | Recombinant Protein | TLR5 + Flagellin | TLR Agonist | Prime | Yes | [41,42] |
| 3c | VAX161 | NCT01560793 | 1 | HA | Recombinant Protein | TLR5 + Flagellin | TLR Agonist | Prime-Boost | No | [43,44] |
| 3d | VAX 161 | NCT01658800 | 1 | HA | Recombinant Protein | TLR5 + Flagellin | TLR Agonist | Prime-Boost | No | [45] |
| 4a | Ad4-H5-Vtn | NCT01006798 | 1 | HA | Viral Vector | Prime-Boost-Boost | Yes | [46,47] | ||
| 4b | Ad4-H5-Vtn | NCT01443936 | 1 | HA | Viral Vector | Prime | No | [48] | ||
| 4c | Ad4-H5-Vtn | NCT01806909 | 1 | HA | Viral Vector | Prime | No | [49] | ||
| 5 | GHB11L1 | NCT01078701 | 2 | ΔNS1 | Attenuated Virus | Prime | No | [50] | ||
| 6a | FLU-v | NCT01181336 | 1 | NP, M1, M2 | Peptide Based | Montanide ISA-51 | Oil in Water | Prime | Yes | [51,52] |
| 6b | FLU-v | NCT01226758 | 1 | NP, M1, M2 | Peptide Based | Montanide ISA-51 | Oil in Water | Prime | Yes | [53,54,55] |
| 6c | FLU-v | NCT03180801 | 2 | NP, M1, M2 | Peptide Based | Montanide ISA-51 | Oil in Water | Prime-Boost | Yes | [56] |
| 6d | FLU-v | NCT02962908 | 2 | NP, M1, M2 | Peptide Based | Montanide ISA-51 | Oil in Water | Prime-Boost | Yes | [57,58] |
| 7a | VGX-3400X | NCT01142362 | 1 | HA, NA, NP | DNA | Prime-Boost | No | [59,60] | ||
| 7b | VGX-3400X | NCT01184976 | 1 | HA, NA, NP | DNA | Prime-Boost | No | [60,61] | ||
| 7c | VGX-3400X | NCT01403155 | 1 | HA, NA, NP | DNA | Prime-Boost | No | [60,62] | ||
| 7d | VGX-3400X | NCT01405885 | 1 | HA, NA, NP | DNA | Prime-Boost-Boost | No | [60,63] | ||
| 8a | FP-01.1 | NCT01265914 | 1 | NP, M1, P1, P2 | Peptide Based | Prime | Yes | [64,65] | ||
| 8b | FP-01.1 | NCT01677676 | 1 | NP, M1, P1, P2 | Peptide Based | Prime | No | [66] | ||
| 8c | FP-01.1 | NCT01701752 | 1 | NP, M1, P1, P2 | Peptide Based | Prime-Boost | No | [67] | ||
| 8d | FP-01.1 | NCT02071329 | 1/2 | NP, M1, P1, P2 | Peptide Based | Prime | No | [68] | ||
| 9 | Inflexal V | NCT01229397 | 3 | HA | Virosome | Virosome | Virosome | Prime-Boost | Yes | [69,70] |
| 10 | HAI-05 | NCT01250795 | 1 | HA | Recombinant Protein | Alhydrogel | Alum | Prime-Boost | Yes | [71,72] |
| 11 | GHB16L2 | NCT01369862 | 1/2 | ΔNS1 | Attenuated Virus | Prime | Yes | [73,74] | ||
| 12a | PanBlok | NCT01612000 | 1/2 | HA | Recombinant Protein | ASO3 | Oil in Water | Prime-Boost | Yes | [75,76] |
| 12b | PanBlok | NCT02464163 | 1/2 | HA | Recombinant Protein | ASO3 | Oil in Water | Prime-Boost | Yes | [77,78] |
| 12c | PanBlok-H7 | NCT03038776 | 1 | HA | Recombinant Protein | AdVax | Δinsulin | Prime-Boost | No | [79,80] |
| 12d | PanBlok-H7 | NCT03283319 | 2 | HA | Recombinant Protein | ASO3, MF59 | Oil in Water | Prime-Boost | Yes | [81] |
| 13 | ChAdOx1-NP+M1 | NCT01623518 | 1 | NP, M1 | Viral Vector | Prime-Boost-Boost | Yes | [82,83] | ||
| 14a | VXA-A1.1 | NCT01688297 | 1 | HA | Viral Vector | dsRNA | TLR Agonist | Prime-Boost | Yes | [84,85] |
| 14b | VXA-A1.1 | NCT01761123 | 1 | HA | Viral Vector | dsRNA | TLR Agonist | Prime | No | [86] |
| 14c | VXA-A1.1 | NCT02918006 | 2 | HA | Viral Vector | dsRNA | TLR Agonist | Prime | Yes | [87,88] |
| 14d | VXA-A1.1 | NCT03121339 | 1 | HA | Viral Vector | dsRNA | TLR Agonist | Prime | No | [89] |
| 15 | Avian Influenza VLP | NCT02078674 | 1/2 | HA, NA | VLP | Matrix M1 | Saponin Based | Prime-Boost | No | [90] |
| 16 | MER4101 | NCT02500680 | 1 | Whole Virus | Attenuated Virus | MAS-1 | Oil in Water | Prime | Active | [91] |
| 17a | VAL-506440 | NCT03076385 | 1 | HA | Lipid Nanoparticle | Prime | Yes | [92,93] | ||
| 17b | VAL-339851 | NCT03345043 | 1 | HA | Lipid Nanoparticle | Prime | Yes | [92,94] | ||
| 18a | M2SR | NCT02822105 | 1 | ΔM2 | Attenuated Virus | Prime | Active | [95] | ||
| 18b | M2SR | NCT03553940 | 1 | ΔM2 | Attenuated Virus | Prime-Boost | Active | [96] | ||
| 18c | M2SR | NCT03999554 | 1 | ΔM2 | Attenuated Virus | Prime-Boost | Active | [97,98] | ||
| 19a | Immunose Flu | NCT02998996 | 1/2 | HA, whole virus | Split Virion | Endocine | Oil in Water | Prime | Yes | [99] |
| 19b | Immunose FLU | NCT03437304 | 1/2 | HA, whole virus | Split Virion | Endocine | Oil in Water | Prime-Boost | No | [100] |
| 20 | NSV0001 | NCT02955030 | 1 | HA | Recombinant Protein | ND002 | Other | Prime-Boost | No | [101] |
| 21 | D-SUIV | NCT03275389 | 1 | Whole Virus | Attenuated Virus | ASO3 | Oil in Water | Prime-Boost-Boost | Active | [102] |
| 22 | NasoVax | NCT03232567 | 2 | HA | Viral Vector | Prime | Yes | [103,104,105] | ||
| 23a | NanoFlu | NCT03293498 | 1/2 | HA | Nanoparticle | Matrix M1 | Saponin Based | Prime-Boost | No | [106,107] |
| 23b | NanoFlu | NCT04120194 | 3 | HA | Nanoparticle | Matrix M1 | Saponin Based | Prime | Active | [108] |
| 24 | cH8/1N1, H5/1N1 | NCT03300050 | 1 | HA Stalk | Attenuated Virus | ASO3A | Oil in Water | Prime-Boost | Yes | [109,110] |
| 25a | VRCFLUDNA081-00-VP | NCT03186781 | 1 | HA | Ferritin Nanoparticle | Prime-Boost | Active | [111,112,113,114] | ||
| 25b | VRCFLUNPF099-00-VP | NCT03814720 | 1 | HA Stalk | Ferritin Nanoparticle | Prime-Boost | Active | [112,115,116] | ||
| 26a | OVX836 | NCT03594890 | 1 | NP | Recombinant Protein | Prime-Boost | No | [117,118] | ||
| 26b | OVX836 | NCT04192500 | 2 | NP | Recombinant Protein | Prime | Active | [118,119] | ||
| 27a | GamFluVac | NCT03651544 | 1 | Unknown | Viral Vector | Prime | No | [120] | ||
| 27b | GamFluVac | NCT04034290 | 2 | Unknown | Viral Vector | Prime | Active | [121] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Corder, B.N.; Bullard, B.L.; Poland, G.A.; Weaver, E.A. A Decade in Review: A Systematic Review of Universal Influenza Vaccines in Clinical Trials during the 2010 Decade. Viruses 2020, 12, 1186. https://doi.org/10.3390/v12101186
Corder BN, Bullard BL, Poland GA, Weaver EA. A Decade in Review: A Systematic Review of Universal Influenza Vaccines in Clinical Trials during the 2010 Decade. Viruses. 2020; 12(10):1186. https://doi.org/10.3390/v12101186
Chicago/Turabian StyleCorder, Brigette N., Brianna L. Bullard, Gregory A. Poland, and Eric A. Weaver. 2020. "A Decade in Review: A Systematic Review of Universal Influenza Vaccines in Clinical Trials during the 2010 Decade" Viruses 12, no. 10: 1186. https://doi.org/10.3390/v12101186
APA StyleCorder, B. N., Bullard, B. L., Poland, G. A., & Weaver, E. A. (2020). A Decade in Review: A Systematic Review of Universal Influenza Vaccines in Clinical Trials during the 2010 Decade. Viruses, 12(10), 1186. https://doi.org/10.3390/v12101186





