Diversity of Noroviruses throughout Outbreaks in Germany 2018
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Sample Collection
2.3. PCR and Sequence Analysis
2.4. Surveillance Data
3. Results
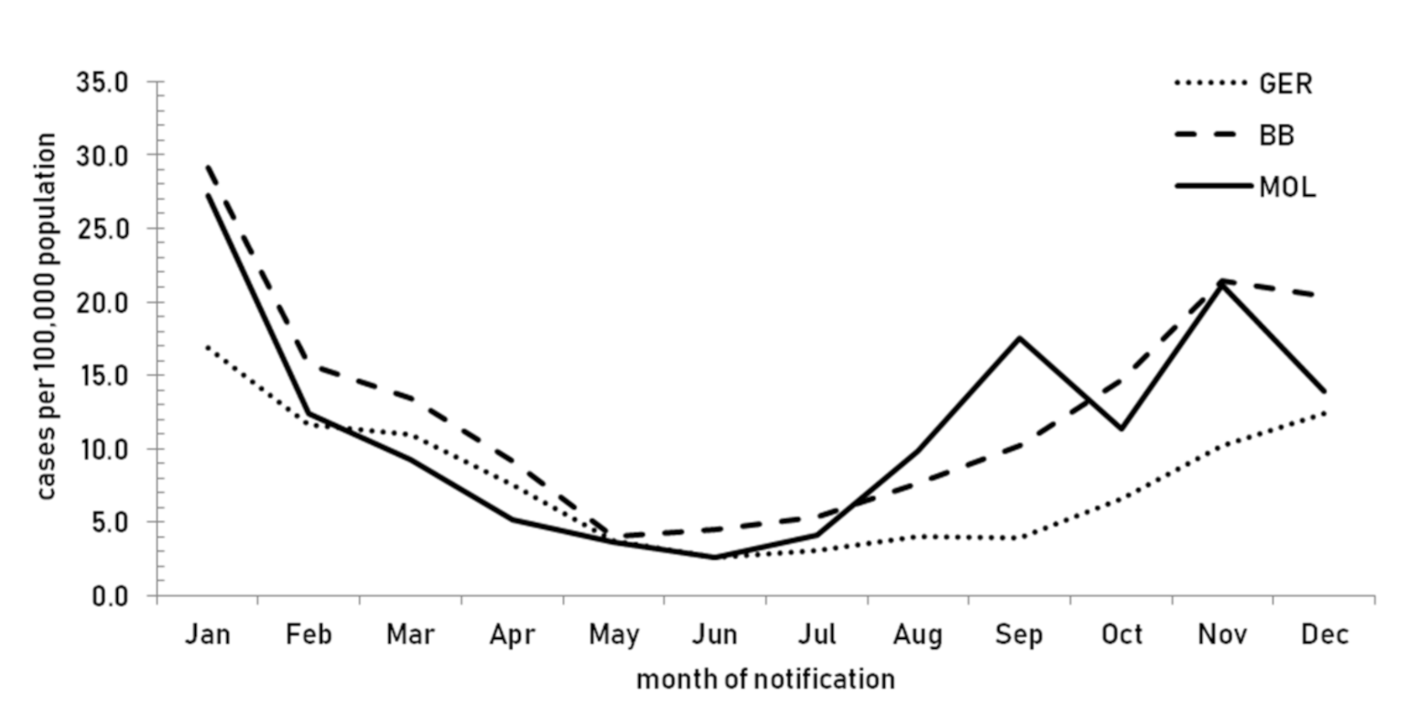
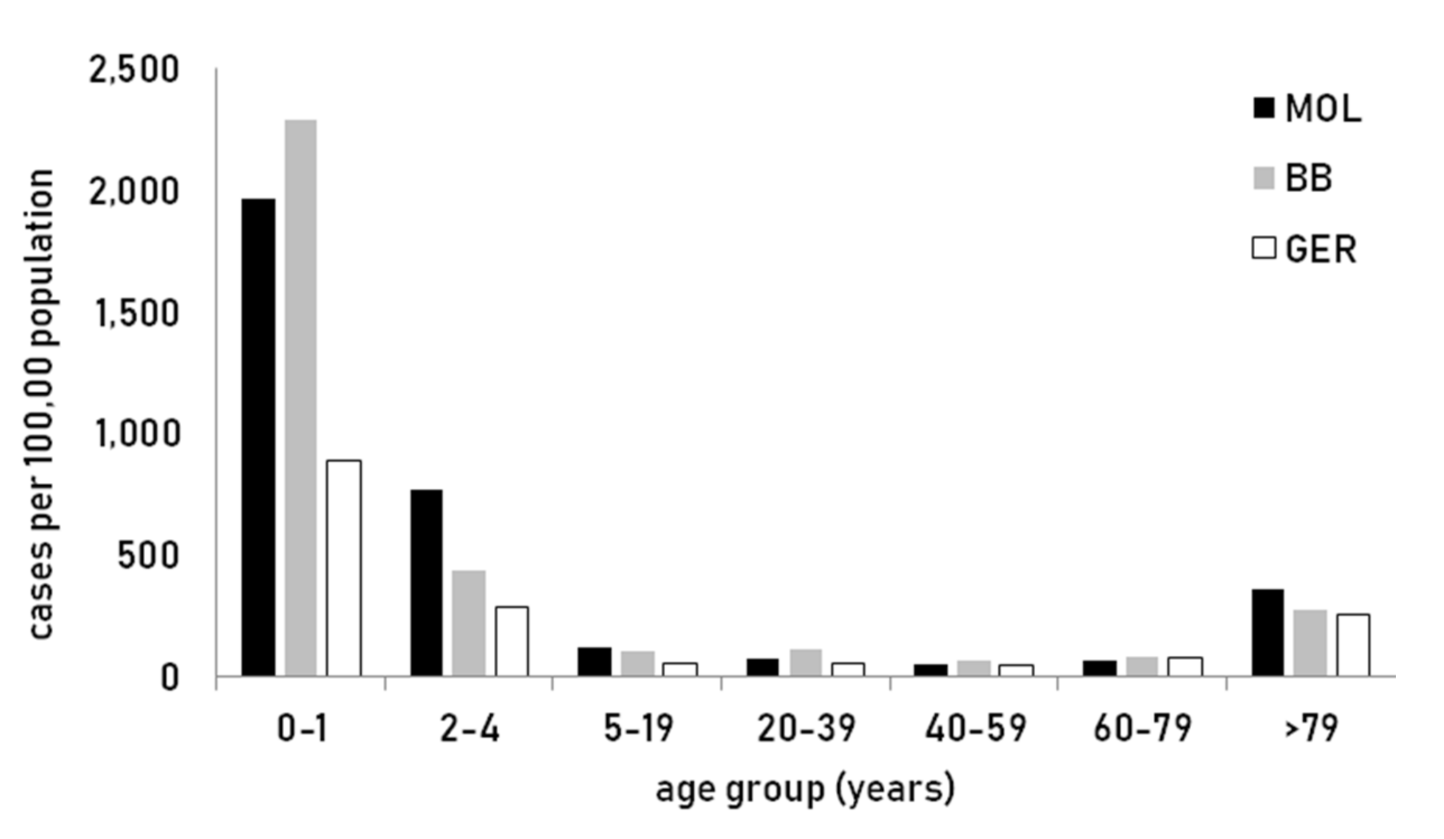
3.1. Epidemiology
3.2. Distribution of Norovirus Genotypes in Germany and Brandenburg
3.3. Correlation of Norovirus GII.4 Surveillance Data and Phylogenetic Analyses in Märkisch-Oderland (Brandenburg) and Germany in 2018
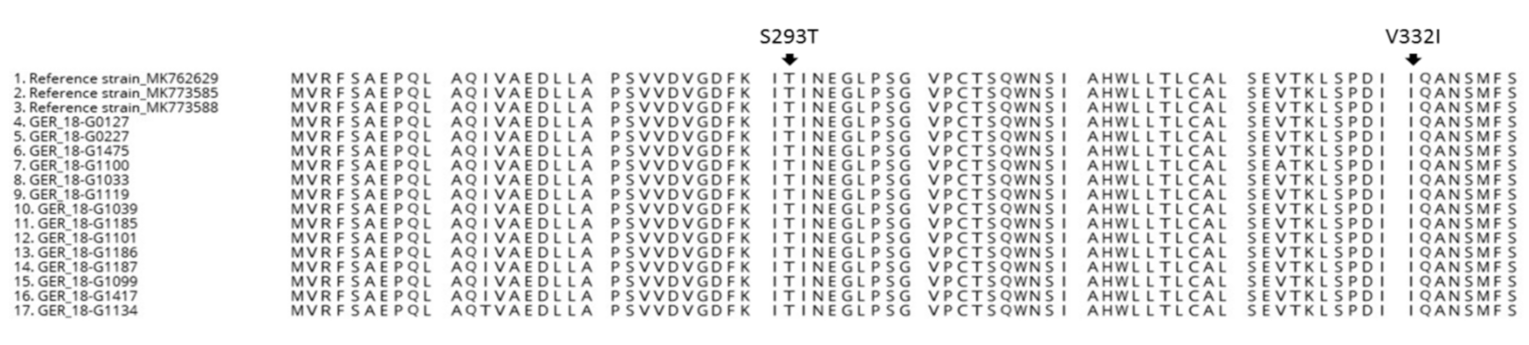
3.4. Amino Acid Changes in GII.P16 Variants
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ahmed, S.M.; Hall, A.J.; Robinson, A.E.; Verhoef, L.; Premkumar, P.; Parashar, U.D.; Koopmans, M.; Lopman, B.A. Global prevalence of norovirus in cases of gastroenteritis: A systematic review and meta-analysis. Lancet Infect. Dis. 2014, 14, 725–730. [Google Scholar] [CrossRef]
- Chhabra, P.; de Graaf, M.; Parra, G.I.; Chan, M.C.; Green, K.; Martella, V.; Wang, Q.; White, P.A.; Katayama, K.; Vennema, H.; et al. Updated classification of norovirus genogroups and genotypes. J. Gen. Virol. 2019, 100, 1393–1406. [Google Scholar] [CrossRef] [PubMed]
- Green, K. Caliciviridae: The noroviruses. In Fields Virology, 6th ed.; Knipe, D.M., Ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2013; Volume 1. [Google Scholar]
- Petronella, N.; Ronholm, J.; Suresh, M.; Harlow, J.; Mykytczuk, O.; Corneau, N.; Bidawid, S.; Nasheri, N. Genetic characterization of norovirus GII.4 variants circulating in Canada using a metagenomic technique. BMC Infect. Dis. 2018, 18, 521. [Google Scholar] [CrossRef] [PubMed]
- Parra, G.I.; Abente, E.J.; Sandoval-Jaime, C.; Sosnovtsev, S.V.; Bok, K.; Green, K.Y. Multiple antigenic sites are involved in blocking the interaction of GII.4 norovirus capsid with ABH histo-blood group antigens. J. Virol. 2012, 86, 7414–7426. [Google Scholar] [CrossRef] [PubMed]
- Lindesmith, L.C.; Costantini, V.; Swanstrom, J.; Debbink, K.; Donaldson, E.F.; Vinje, J.; Baric, R.S. Emergence of a norovirus GII.4 strain correlates with changes in evolving blockade epitopes. J. Virol. 2013, 87, 2803–2813. [Google Scholar] [CrossRef] [PubMed]
- Lindesmith, L.C.; Donaldson, E.F.; Lobue, A.D.; Cannon, J.L.; Zheng, D.P.; Vinje, J.; Baric, R.S. Mechanisms of GII.4 norovirus persistence in human populations. PLoS Med. 2008, 5, e31. [Google Scholar] [CrossRef]
- Tohma, K.; Lepore, C.J.; Gao, Y.; Ford-Siltz, L.A.; Parra, G.I. Population genomics of GII.4 noroviruses reveal complex diversification and new antigenic sites involved in the emergence of pandemic strains. mBio 2019, 10. [Google Scholar] [CrossRef]
- De Graaf, M.; van Beek, J.; Vennema, H.; Podkolzin, A.T.; Hewitt, J.; Bucardo, F.; Templeton, K.; Mans, J.; Nordgren, J.; Reuter, G.; et al. Emergence of a novel GII.17 norovirus—End of the GII.4 era? Eurosurveillance 2015, 20, 21178. [Google Scholar] [CrossRef]
- Parra, G.I.; Squires, R.B.; Karangwa, C.K.; Johnson, J.A.; Lepore, C.J.; Sosnovtsev, S.V.; Green, K.Y. Static and evolving norovirus genotypes: Implications for epidemiology and immunity. PLoS Pathog. 2017, 13, e1006136. [Google Scholar] [CrossRef]
- Barclay, L.; Cannon, J.L.; Wikswo, M.E.; Phillips, A.R.; Browne, H.; Montmayeur, A.M.; Tatusov, R.L.; Burke, R.M.; Hall, A.J.; Vinje, J. Emerging novel GII.P16 noroviruses associated with multiple capsid genotypes. Viruses 2019, 11, 535. [Google Scholar] [CrossRef]
- Niendorf, S.; Jacobsen, S.; Faber, M.; Eis-Hubinger, A.M.; Hofmann, J.; Zimmermann, O.; Hohne, M.; Bock, C.T. Steep rise in norovirus cases and emergence of a new recombinant strain GII.P16-GII.2, Germany, winter 2016. Eurosurveillance 2017, 22. [Google Scholar] [CrossRef] [PubMed]
- Hoehne, M.; Schreier, E. Detection of Norovirus genogroup I and II by multiplex real-time RT-PCR using a 3’-minor groove binder-DNA probe. BMC Infect. Dis. 2006, 6, 69. [Google Scholar] [CrossRef]
- Dreier, J.; Stormer, M.; Kleesiek, K. Use of bacteriophage MS2 as an internal control in viral reverse transcription-PCR assays. J. Clin. Microbiol. 2005, 43, 4551–4557. [Google Scholar] [CrossRef] [PubMed]
- Schreier, E.; Doring, F.; Kunkel, U. Molecular epidemiology of outbreaks of gastroenteritis associated with small round structured viruses in Germany in 1997/98. Arch. Virol. 2000, 145, 443–453. [Google Scholar] [CrossRef]
- Hohne, M.; Niendorf, S.; Mas Marques, A.; Bock, C.T. Use of sequence analysis of the P2 domain for characterization of norovirus strains causing a large multistate outbreak of norovirus gastroenteritis in Germany 2012. Int. J. Med. Microbiol. 2015, 305, 612–618. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Ruis, C.; Roy, S.; Brown, J.R.; Allen, D.J.; Goldstein, R.A.; Breuer, J. The emerging GII.P16-GII.4 Sydney 2012 norovirus lineage is circulating worldwide, arose by late-2014 and contains polymerase changes that may increase virus transmission. PloS ONE 2017, 12, e0179572. [Google Scholar] [CrossRef]
- Matsushima, Y.; Shimizu, T.; Ishikawa, M.; Komane, A.; Okabe, N.; Ryo, A.; Kimura, H.; Katayama, K.; Shimizu, H. Complete genome sequence of a recombinant GII.P16-GII.4 norovirus detected in Kawasaki City, Japan, in 2016. Genome Announc. 2016, 4. [Google Scholar] [CrossRef]
- Cannon, J.L.; Barclay, L.; Collins, N.R.; Wikswo, M.E.; Castro, C.J.; Magana, L.C.; Gregoricus, N.; Marine, R.L.; Chhabra, P.; Vinje, J. Genetic and epidemiologic trends of norovirus outbreaks in the United States from 2013 to 2016 demonstrated emergence of novel GII.4 recombinant viruses. J. Clin. Microbiol. 2017, 55, 2208–2221. [Google Scholar] [CrossRef]
- Xue, L.; Cai, W.; Gao, J.; Zhang, L.; Dong, R.; Li, Y.; Wu, H.; Chen, M.; Zhang, J.; Wang, J.; et al. The resurgence of the norovirus GII.4 variant associated with sporadic gastroenteritis in the post-GII.17 period in South China, 2015 to 2017. BMC Infect. Dis. 2019, 19, 1–8. [Google Scholar] [CrossRef]
- Kuang, X.; Teng, Z.; Zhang, X. Genotypic prevalence of norovirus GII in gastroenteritis outpatients in Shanghai from 2016 to 2018. Gut Pathog. 2019, 11, 40. [Google Scholar] [CrossRef]
- Guarines, K.M.; Mendes, R.P.G.; de Magalhaes, J.J.F.; Pena, L. Norovirus-associated gastroenteritis, Pernambuco, Northeast Brazil, 2014–2017. J. Med Virol. 2020, 92, 1093–1101. [Google Scholar] [CrossRef] [PubMed]
- Parra, G.I.; Green, K.Y. Genome of Emerging norovirus GII.17, United States, 2014. Emerg. Infect. Dis. 2015, 21, 1477–1479. [Google Scholar] [CrossRef]
- Tohma, K.; Lepore, C.J.; Ford-Siltz, L.A.; Parra, G.I.; Chan, M.; Bull, R.; Vinje, J. Phylogenetic analyses suggest that factors other than the capsid protein play a role in the epidemic potential of GII.2 norovirus. mSphere 2017, 2. [Google Scholar] [CrossRef] [PubMed]
- Bull, R.A.; Eden, J.S.; Rawlinson, W.D.; White, P.A. Rapid evolution of pandemic noroviruses of the GII.4 lineage. PLoS Pathog. 2010, 6, e1000831. [Google Scholar] [CrossRef]
- Sakon, N.; Yamazaki, K.; Nakata, K.; Kanbayashi, D.; Yoda, T.; Mantani, M.; Kase, T.; Takahashi, K.; Komano, J. Impact of genotype-specific herd immunity on the circulatory dynamism of norovirus: A 10-year longitudinal study of viral acute gastroenteritis. J. Infect. Dis. 2015, 211, 879–888. [Google Scholar] [CrossRef]
- Parra, G.I. Emergence of norovirus strains: A tale of two genes. Virus Evol. 2019, 5, vez048. [Google Scholar] [CrossRef]
- Qi, R.; Huang, Y.T.; Liu, J.W.; Sun, Y.; Sun, X.F.; Han, H.J.; Qin, X.R.; Zhao, M.; Wang, L.J.; Li, W.; et al. Global prevalence of asymptomatic norovirus infection: A meta-analysis. eClinicalMedicine 2018, 50–58. [Google Scholar] [CrossRef] [PubMed]

| Genogroup | Primer/Probe | Sequence | Localization a |
|---|---|---|---|
| RT-qPCR | |||
| I | 192 (sense) | 5’-GCYATGTTCCGCTGGATGC | 5321–5340 |
| 192a (sense) | 5´-GCAATGTTYCGCTGGATGC | 5321–5340 | |
| 193 (antisense) | 5´-CGTCCTTAGACGCCATCATCA | 5593–5574 | |
| TM9-MGB probe (sense) | 5´-VIC-TGGACAGGAGATCGC-MGB-NFQ | 5345–5359 | |
| II | NV107e (sense) | 5´-AACCAATGTTYAGMTGGATGAG | 5007–5026 |
| NV107f (sense) | 5´-AACCCATGTTCAGATGGATGAG | 5007–5026 | |
| NV107g (sense) | 5′-AGGCCATGTTYAGRTGGATGAG | 5007–5026 | |
| NV107h (sense) | 5′-AGCCAATGTTCAGATGGATGAG | 5007–5026 | |
| NV359 (antisense) | 5′-TCGACGCCATCTTCATTCACA | 5100–5080 | |
| TM15-MGB probe (antisense) | 5′-FAM-TCGATCGCCCTCCCA-MGB-NFQ | 5048–5062 | |
| MS-2 phage | MS2-fwd_KL (sense) | 5′-GGCTGCTCGCGGATAC | 3166–3181 |
| MS2-rev_KL (antisense) | 5′-AACTTGCGTTCTCGAGCGAT | 3210–3229 | |
| MS2 probe (sense) | 5′-Cy5-ACCTCGGGTTTCCGTCTTGCTCGT-BQH2 | 3186–3209 | |
| ORF1 genotyping RT-PCR | |||
| Genogroup | Primer/Probe | Sequence | localization a |
| I and II | NV1c (sense) | 5′-ATG AAC ATG AAT GAG GAT GG | 4499–4518 |
| NV1d (sense) | 5′-ATG AAT ATG AAT GAR GAT GG | 4499–4518 | |
| NV1e (sense) | 5′-ATG AAT TCA ATT GAG GAT GG | 4499–4518 | |
| NV1f (sense) | 5′-ATG AAT GCA ATT GAA GAT GG | 4499–4518 | |
| NV7b (antisense) | 5′-GGD CCH TCA STY TTA TC | 4977–4961 | |
| NV7c (antisense) | 5′-GGR CCY TCR CTY TTG TC | 4977–4961 | |
| NV7d (antisense) | 5′-GGT CCT TCT GAT TTG TC | 4977–4961 | |
| NV7e (antisense) | 5′-GGC CCC TCR GTT TTG TC | 4977–4961 | |
| NV7f (antisense) | 5′-GGY CCT TCA GTY TTG TC | 4977–4961 | |
| NV6e (sense) | 5′-ACC AYT WTG ATG CAG ACT A | 4554–4572 | |
| NV6f (sense) | 5′-ACC AYT ATG ATG CTG ATT A | 4554–4572 | |
| NV6g (sense) | 5′-ATC AYT ATG ATG CWG AYT A | 4554–4572 | |
| NV4d (antisense) | 5′-ACY ATC TCA TCA TCA CCA | 4884–4866 | |
| NV4e (antisense) | 5′-ACG ATC TCG TCR TCA CCG | 4884–4866 | |
| NV4f (antisense) | 5′-ACT ATY TCA TCA TCA CCA | 4884–4866 | |
| NV4g (antisense) | 5′-ACG ATC TCA TCG TCC CCA | 4884–4866 | |
| ORF2 genotyping RT-PCR | |||
| Genogroup | Primer/Probe | Sequence | Localization a |
| GI | NV351 a (sense) | CCI CAT GTI ATT GCT GAT GT | 5793–5812 |
| NV351 b (sense) | CCI CAC GTI ATM GCA GAT GT | 5793–5812 | |
| NV352 a (antisense) | TTC CCA CAG GCT TIA AYT G | 6909–6891 | |
| NV352 b (antisense) | TTC CCA CAG GCT TIA GYT G | 6909–6891 | |
| NV354 (sense) | ATG ATG ATG GCG TCT AAG GAC | 5358–5378 | |
| GII | NV347 a (sense) | GAI GAT GTC TTC ACA GTY TCT T | 5661–5682 |
| NV347 b (sense) | GAT GAT GTK TTC ACW GTI TCT T | 5661–5682 | |
| NV347 c (sense) | GAT GAY GTI TTC ACI GTI TCM T | 5661–5682 | |
| NV348 a (antisense) | GGT TRA CCC ARG AAT CAA A | 6648–6630 | |
| NV348 b (antisense) | GRT TMA CCC AAG AIT CAA A | 6648-6630 | |
| NV348 c (antisense) | GRT TRA CCC AIA CTT CAA A | 6648-6630 |
| Detected Genotype | Number of Outbreaks in Germany 2018 | % of Outbreaks in Germany | Number of Outbreaks in Märkisch-Oderland 2018 | % of Outbreaks in Märkisch-Oderland |
|---|---|---|---|---|
| GI.P1-GI.1 | 8 | 3.2 | ||
| GI.P2-GI.2 | 8 | 3.2 | 1 | 2.6 |
| GI.P2 | 2 | 0.8 | ||
| GI.P3-GI.3 | 3 | 1.2 | ||
| GI.P3 | 1 | 0.4 | ||
| GI.P4-GI.4 | 10 | 4.0 | 1 | 2.6 |
| GI.P4 | 1 | 0.4 | ||
| GI.P6-GI.6 | 2 | 0.8 | 1 | 2.6 |
| GI.P6 | 1 | 0.4 | ||
| GI.P6-GI.2 | 1 | 0.4 | ||
| GI.P7-GI.7 | 2 | 0.8 | ||
| GI.P9-GI.7 | 1 | 0.4 | ||
| GI.P11-GI.6 | 7 | 0.4 | ||
| GI.P11 | 1 | 1.6 | 1 | 2.6 |
| GI.P13-GI.3 | 1 | 1.2 | ||
| GI.P13 | 1 | 0.8 | ||
| GII.P4-GII.4 Sydney | 2 | 0.8 | ||
| GII.P4 2009-GII.4 Sydney | 7 | 2.8 | ||
| GII.P6-GII.6 | 4 | 0.4 | ||
| GII.P7-GII.6 | 24 | 9.6 | 1 | 2.6 |
| GII.P7-GII.7 | 3 | 2.8 | 1 | 2.6 |
| GII.P7-GII.14 | 5 | 2.0 | ||
| GII.P7 | 5 | 2.0 | ||
| GII.P8-GII.8 | 2 | 0.4 | ||
| GII.P12-GII.3 | 1 | 0.4 | ||
| GII.P16-GII.12 | 3 | 1.2 | ||
| GII.P16-GII.2 | 16 | 6.4 | 10 | 25.6 |
| GII.P16-GII.3 | 1 | 0.4 | 1 | 2.6 |
| GII.P16-GII.4 Sydney | 72 | 28.8 | 3 | 7.7 |
| GII.P16 | 10 | 4.0 | ||
| GII.P17-GII.17 | 1 | 0.4 | ||
| GII.P21-GII.3 | 10 | 4.0 | 6 | 15.4 |
| GII.P30-GII.3 | 1 | 0.4 | ||
| GII.P31-GII.4 Sydney | 32 | 12.8 | 13 | 33.3 |
| GII.P31 | 1 | 0.4 | ||
| total | 250 | 39 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niendorf, S.; Faber, M.; Tröger, A.; Hackler, J.; Jacobsen, S. Diversity of Noroviruses throughout Outbreaks in Germany 2018. Viruses 2020, 12, 1157. https://doi.org/10.3390/v12101157
Niendorf S, Faber M, Tröger A, Hackler J, Jacobsen S. Diversity of Noroviruses throughout Outbreaks in Germany 2018. Viruses. 2020; 12(10):1157. https://doi.org/10.3390/v12101157
Chicago/Turabian StyleNiendorf, Sandra, Mirko Faber, Andrea Tröger, Julian Hackler, and Sonja Jacobsen. 2020. "Diversity of Noroviruses throughout Outbreaks in Germany 2018" Viruses 12, no. 10: 1157. https://doi.org/10.3390/v12101157
APA StyleNiendorf, S., Faber, M., Tröger, A., Hackler, J., & Jacobsen, S. (2020). Diversity of Noroviruses throughout Outbreaks in Germany 2018. Viruses, 12(10), 1157. https://doi.org/10.3390/v12101157





