HBV Prevention and Treatment in Countries of Central Asia and the Caucasus
Abstract
1. Introduction
2. Prevalence
3. Blood Donor Screening
4. HBV Vaccination
5. Treatment for HBV
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Burke, J. Post-Soviet World: What You Need to Know about the 15 States. The Guardian. 2014. Available online: https://www.theguardian.com/world/2014/jun/09/-sp-profiles-post-soviet-states (accessed on 24 July 2020).
- Gvozdetsky, N.A.; Bruk, S.I.; Owen, L. Caucasus. Encyclopædia Britannica. 2019. Available online: https://www.britannica.com/place/Caucasus (accessed on 22 July 2020).
- Hermann, W.; Linn, J. (Eds.) Central Asia and the Caucasus: At the Crossroads of Eurasia in the 21st Century; SAGE Publications: New Delhi, India, 2011. [Google Scholar]
- Worldometer. Countries in the World by Population. 2020. Available online: https://www.worldometers.info/world-population/population-by-country/ (accessed on 20 June 2020).
- YourFreeTemplates.com. Free Central Asia and Caucasus Editable Map. 2017. Available online: https://yourfreetemplates.com/free-central-asia-caucasus-editable-map/ (accessed on 20 June 2020).
- Lazarus, J.V.; Shete, P.B.; Eramova, I.; Merkinaite, S.; Matic, S. HIV/hepatitis coinfection in eastern Europe and new pan-European approaches to hepatitis prevention and management. Int. J. Drug Policy 2007, 18, 426–432. Available online: https://www.sciencedirect.com/science/article/pii/S0955395907000126?casa_token=S0AA2MLiC2QAAAAA:HCRgbtBPWa2np3X_i1NTV8HVBpSf9pPQnUqbbu1ybPi0axUEtfWJfbWieYXJGHOTc0R_vBaP4Mg (accessed on 24 June 2020). [CrossRef] [PubMed]
- Demirchyan, A.; Mirzoyan, L.; Thompson, M.E. Synthesis of the Existing Data on Hepatitis B in Armenia; American University of Armenia: Yerevan, Armenia, 2000. [Google Scholar]
- Ghazinyan, H.; Asoyan, A.; Mkhitaryan, A.; Melik-Andreasyan, G. Updating HBV status in Armenia. In Proceedings of the EASL Special Conference: Optimal Management of HBV infection, Athens, Greece, 25–27 September 2014; Available online: https://livertree.easl.eu/easl/2014/athens/62141/hasmik.levon.ghazinyan.updating.hepatitis.b.virus.(hbv).status.in.armenia.html?f=p6m3e757 (accessed on 1 August 2020).
- Kravchenko, N.; Maistat, L.; Golovin, S.; Nikelsen, T.; Aliyev, A.; Harantyunyan, A.; Biryukov, S.; Gulov, K.; Jamolov, P.; Pashaev, E.; et al. Otchet “Gepatit V i S v regione Vostochnoĭ Evropy i T͡Sentralʹnoĭ Azii: Epidemii͡a i Otvetnye Mery”. 2017. Available online: http://mv.ecuo.org/download/otchet-gepatit-v-i-s-v-regione-vostochnoj-evropy-i-tsentralnoj-azii-epidemiya-i-otvetnye-mery/ (accessed on 1 August 2020).
- Mamedov, M.; Dadasheva, A.; Kadyrova, A.; Tagizade, R.; Mikhailov, M. Serologicheskie Markery Infekt͡siĭ, Vyzvannykh Virusami Gepatitov v i s, u Zhiteleĭ Azerbaĭdzhana iz Grupp s Vysokim Riskom Parenteral’nogo Infit͡sirovanii͡a [Serological Markers of Infections Caused by Hepatitis B and C Viruses in Residents of Azerbaijan from Groups with a High Risk of Parenteral Infection]. Ėpidemiologii͡a vakt͡sinoprofilaktika 2012, 2, 63. Available online: https://cyberleninka.ru/article/n/serologicheskie-markery-infektsiy-vyzvannyh-virusami-gepatitov-v-i-s-u-zhiteley-azerbaydzhana-iz-grupp-s-vysokim-riskom-parenteralnogo (accessed on 24 June 2020).
- Schweitzer, A.; Akmatov, M.K.; Krause, G. Hepatitis B vaccination timing: Results from demographic health surveys in 47 countries. Bull. World Health Organ. 2017, 95, 199. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5328113/ (accessed on 24 June 2020). [CrossRef] [PubMed]
- Butsashvili, M.; Tsertsvadze, T.; McNutt, L.; Kamkamidze, G.; Gvetadze, R.; Badridze, N. Prevalence of hepatitis B, hepatitis C, syphilis and HIV in Georgian blood donors. Eur. J. Epidemiol. 2001, 17, 693–695. Available online: https://link.springer.com/article/10.1023/A:1015566132757 (accessed on 24 June 2020). [CrossRef] [PubMed]
- Stvilia, K.; Meparidze, M.; Tsertsvadze, T.; Sharvadze, L.; Dzigua, L. Prevalence of HBV and HCV infections and high risk behavior for blood born infections among general population of Tbilisi, Georgia. Ann. Biomed. Res. Educ. 2005, 5, 289–298. Available online: http://citeseerx.ist.psu.edu/viewdoc/download? (accessed on 24 June 2020).
- Khochava, M.; Shalamberidze, I.; Jokhtaberidze, T. Problema B i C gepatitov i ikh Registrat͡sii v Gruzii [The Problem of B and C Hepatitis and Their Registration in Georgia]. 2013. Available online: http://elib.grsmu.by/handle/files/15779 (accessed on 24 June 2020).
- Badridze, N.; Chkhartishvili, N.; Abutidze, A.; Gatserelia, L.; Sharvadze, L. Prevalence of hepatitis B and C among HIV positive patients in Georgia and its associated risk factors. Georgian Med. News, 2008; 165, 54–60. Available online: https://pubmed.ncbi.nlm.nih.gov/19124918/ (accessed on 24 June 2020).
- Skorikova, S.V.; Burkitbaev, L.; Savchuk, T.; Zhiburt, E. Rasprostranennost’ VICH-, VGS-, VGV-infekt͡siĭ u donorov krovi g. Astany [Prevalence of HIV, HCV, HBV Infections among Blood Donors in Astana]. Voprosy Virusologii 2015, 60. Available online: https://cyberleninka.ru/article/n/rasprostranennost-vich-vgs-vgv-infektsiy-u-donorov-krovi-g-astany (accessed on 24 June 2020).
- Hope, V.; Eramova, I.; Capurro, D.; Donoghoe, M. Prevalence and estimation of hepatitis B and C infections in the WHO European Region: A review of data focusing on the countries outside the European Union and the European Free Trade Association. Epidemiol. Infect. 2014; 142, 270–286. Available online: https://pubmed.ncbi.nlm.nih.gov/23714072/ (accessed on 24 June 2020). [CrossRef] [PubMed]
- Savchuk, T.; Greenwald, E.; Ilyasova, N. Rezul’taty Avtomatizat͡sii Laboratornogo Skrininga Donorskoĭ Krovi na Gemotransmissivnye Infekt͡sii v Respublike Kazaхstan. Res. Prod. Cent. Transfus. 2015, 29. Available online: https://spct.kz/specialist/публикации (accessed on 24 June 2020).
- Nersesov, A.; Berkinbaev, S.; Dzhunusbekova, H.; Dzhumabayeva, A.; Novitskaya, M.; Kuanish, N. Rasprostranennost’ Virusnyх Gepatitov Sredi Zhiteleĭ I͡uzhno-Kazaхstanskoĭ Oblasti [Prevalence of Viral Hepatitis among Residents of the South Kazakhstan Region]. Medicine 2016, 9, 30–33. Available online: http://www.medzdrav.kz/index.php/журнал-медицина/94-2016/№-9-171-2016/1197-распрoстраненнoсть-вирусных-гепатитoв-среди-жителей-южнo-казахстанскoй-oбласти (accessed on 24 June 2020).
- Karabaev, B.B.; Beisheeva, N.J.; Satybaldieva, A.B.; Ismailova, A.D.; Pessler, F.; Akmatov, M.K. Seroprevalence of hepatitis B, hepatitis C, human immunodeficiency virus, Treponema pallidum, and co-infections among blood donors in Kyrgyzstan: A retrospective analysis (2013–2015). Infect. Dis. Poverty 2017, 6, 45. Available online: https://link.springer.com/article/10.1186/s40249-017-0255-9 (accessed on 24 June 2020). [CrossRef] [PubMed][Green Version]
- Mozalevskis, A.; Harmanci, H.; Bobrik, A. Assessment of the Viral Hepatitis Response in Kyrgyzstan, 11–15 July 2016; World Health Organization: Copenhagen, Denmark, 2017; Available online: https://www.euro.who.int/en/countries/kyrgyzstan/publications/assessment-of-the-viral-hepatitis-response-in-kyrgyzstan,-1115-july-2016-2017 (accessed on 24 June 2020).
- Klushkina, V.V.; Kyuregyan, K.K.; Kozhanova, T.V.; Popova, O.E.; Dubrovina, P.G.; Isaeva, O.V.; Gordeychuk, I.V.; Mikhailov, M.I. Impact of universal hepatitis B vaccination on prevalence, infection-associated morbidity and mortality, and circulation of immune escape variants in Russia. PLoS ONE 2016, 11, e0157161. Available online: https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0157161 (accessed on 24 June 2020). [CrossRef] [PubMed]
- Alsalikh, N.; Sychev, D.; Potemkin, I.; Kyureghian, K.; Mikhailov, M. Rasprostranennostʹ serologicheskikh markerov virusnykh gepatitov sredi trudovykh migrantov, pribyvai͡ushchikh v Rossiĭskui͡u Federat͡sii͡u [Prevalence of serological markers of viral hepatitis among labor migrants arriving in the Russian Federation]. Zhurnal Infektologii 2017, 9, 80–85. Available online: https://journal.niidi.ru/jofin/article/view/604 (accessed on 24 June 2020). [CrossRef][Green Version]
- Shukurov, A.; Begendjova, M.; Atamuradova, L.; Shayimov, B.; Ibragimov, M. Epidemiological characteristics of the spread of hepatitis C in Turkmenistan. Young Sci. 2018, 40, 115–119. Available online: https://elibrary.ru/item.asp?id=36066614 (accessed on 24 June 2020).
- European Centre for Disease Prevention and Control. Monitoring the Responses to Hepatitis B and C Epidemics in EU/EEA Member States, 2019 Stockholm: ECDC. 2020. Available online: https://www.ecdc.europa.eu/en/publications-data/monitoring-responses-hepatitis-b-and-c-epidemics-eueea-member-states-2019 (accessed on 24 August 2020).
- Zeynalova, K. Virusnye gepatity V i S: ėpidemiologicheskai͡a situat͡sii͡a v Azerbaĭdzhane v poslednie gody [Viral hepatitis B and C: Epidemiological situation in Azerbaijan in recent years]. Ėpidemiol. Vakt͡sinoprofil. 2010, 4. Available online: https://cyberleninka.ru/article/n/virusnye-gepatity-v-i-s-epidemiologicheskaya-situatsiya-v-azerbaydzhane-v-poslednie-gody (accessed on 24 June 2020).
- Postanovlenie Pravitel’stva Respubliki Tadzhikistan ot 2 ii͡uli͡a 2015 Goda No. 422. O Nat͡sional’noĭ Programme po Razvitii͡u Donorstva Krovi i eë Komponentov v Respublike Tadzhikistan na 2015–2019 Gody. 2016. Available online: https://online.zakon.kz/Document/?doc_id=35408765 (accessed on 24 June 2020).
- Vershinina, N.; Golosova, S.; Daykhes, N.; Dorunova, N.; Stefanyuk, Y.; Eykhler, O. Opyt Zarubezhnih Stran v Reshenii Voprosov Donorstva Krovi. Informat͡sionno-Metodicheskoe Posobie v Pomoshch’ Organizatoram Donorskogo Dvizhenii͡a [Informational-Methodical Manual to Help the Organizers of the Donor Movement]. 2016. Available online: http://spasibodonor.ru/wp-content/uploads/2016/11/Zarubezh_donorstvo_preview.pdf (accessed on 24 June 2020).
- Asadov, C. Present and Future of Transfusion Medicine in the Countries of Far-Eastern Europe and Central Asia. 2011. Available online: https://www.researchgate.net/publication/239979407_PRESENT_AND_FUTURE_OF_TRANSFUSION_MEDICINE_IN_THE_COUNTRIES_OF_FAR-EASTERN_EUROPE_AND_CENTRAL_ASIA_Chingiz_Asadov_Baku_Azerbaijan (accessed on 24 June 2020).
- Ob utverzhdenii Trebovaniĭ k Medit͡sinskomu Osvidetel’Stvovanii͡u Donorov, Bezopasnosti i Kachestvu pri Proizvodstve Produktov Krovi Dli͡a Medit͡sinskogo Primenenii͡a. Prikaz Ministra Zdravookhranenii͡a Respubliki Kazakhstan ot 15 Apreli͡a 2019 Goda № ҚR DSM-34. Zaregistrirovan v Ministerstve Iustit͡sii Respubliki Kazakhstan 16 Apreli͡a 2019 Goda № 18524. 2019. Available online: http://adilet.zan.kz/rus/docs/V1900018524 (accessed on 24 June 2020).
- Postanovlenie Pravitel’stva RF ot 31 Dekabri͡a 2010 g. N 1230 “Ob Utverzhdenii Pravil i Metodov Issledovaniĭ i Pravil Otbora Obrazt͡sov Donorskoĭ Krovi, Neobkhodimykh dli͡a Primenenii͡a i Ispolnenii͡a Tekhnicheskogo Reglamenta o Trebovanii͡akh Bezopasnosti Krovi, ee Produktov, Krovezameshchai͡ushchikh Rastvorov i Tekhnicheskikh Sredstv, Ispol’zuemykh v Transfuzionno-Infuzionnoĭ terapii”. 2011. Available online: http://www.garant.ru/products/ipo/prime/doc/12081836/ (accessed on 24 June 2020).
- Zakon Turkmenistana o Donorstve Krovi. 2017. Available online: https://www.parahat.info/law/parahat-info-law-01zs (accessed on 24 June 2020).
- Prikazom Ministra Zdravookhranenii͡a (Zaregistrirovan MI͡U 15.01.2014 g. № 2556) Utverzhdeno Polozhenie o Pori͡adke Sdachi Krovi i ee Komponentov. 2014. Available online: https://minzdrav.uz/m/docs/detail/36281/ (accessed on 24 June 2020).
- World Health Organization. Immunization, Vaccines and Biologicals. Data, Statistics and Graphics. [updated 7 June 2020]. 2020. Available online: https://www.who.int/immunization/monitoring_surveillance/data/en/ (accessed on 24 June 2020).
- Kalendar’ Privivok v Gruzii Sputnik Georgia. 2017. Available online: https://sputnik-georgia.ru/infographics/20171010/237693326/kalendar-privivok-v-gruzii.html (accessed on 20 July 2020).
- Ob Utverzhdenii Perechnia Zabolevanii, Protiv Kotorykh Provodiatsia Profilakticheskie Privivki, Pravil ikh Provedeniia i Grupp Naseleniia, Podlezhashchikh Planovym Privivkam. 2009. Available online: http://adilet.zan.kz/rus/docs/P090002295_#z12 (accessed on 13 July 2020).
- Prikaz Ministerstva Zdravookhranenii͡a RF ot 21 Marta 2014 g. N 125n “Ob Utverzhdenii Nat͡sional’nogo Kalendari͡a Profilakticheskikh Privivok i Kalendari͡a Profilakticheskikh Privivok po Epidemicheskim Pokazanii͡am” (s Izmenenii͡ami i Dopolnenii͡ami). 2014. Available online: https://base.garant.ru/70647158/ (accessed on 13 July 2020).
- Yagudina, T. Turkmenistan Rasshiri͡aet Kalendar’ Privivok, Soglasno Rekomendat͡sii͡am VOZ. 2020. Available online: https://arzuw.news/turkmenistan-rasshirjaet-kalendar-privivok-soglasno-rekomendacijam-voz (accessed on 19 July 2020).
- Saydaliev, S.; Tursunova, Y.; Khalilova, G.; Mullaeva, L.; Mirzabaev, D.; Kim, L. Sanitarnye Pravila, Normy, Gigienicheskie Normativy, Immunoprofilaktika, Infekt͡sionnykh Zabolevaniĭ v Respublike Uzbekistan. 2015. Available online: https://www.minzdrav.uz/documentation/detail.php?ID=45175 (accessed on 19 July 2020).
- World Health Organization. Guidelines for the Prevention Care and Treatment of Persons with Chronic Hepatitis B Infection: Mar-15; World Health Organization: Geneva, Switzerland, 2015. [Google Scholar]
- World Health Organization. Global policy report on the prevention and control of viral hepatitis in WHO Member States. 2013. Available online: https://www.who.int/hepatitis/publications/global_report/en/ (accessed on 19 July 2020).
- Republican Center for Healthcare Development of the Ministry of Health of the Republic of Kazakhstan. Chronic Hepatitis B in Adults. 2019 [Updated 19 November 2019]. Available online: https://diseases.medelement.com/disease/хрoнический-гепатит-в-у-взрoслых-2019/16388 (accessed on 19 July 2020).
- Ivashkin, V.; Yushchuk, N.; Mayevskaya, M.; Znojko, O.; Dudin, K.; Karetkina, G.; Klimova, S.L.; Maksimov, Y.V.; Martynov, I.V.; Maev, H.S.; et al. Mezhdunarodnai͡a Koalit͡sii͡a po Gotovnosti k Lechenii͡u Vostochnai͡a Evropa i T͡Sentral’nai͡a Azii͡a. Klinicheskiĭ Protokol Respubliki Tadzhikistan “Gepatit V i VICH-infekt͡sii͡a: Taktika Vedenii͡a Pat͡sientov s Koinfekt͡sieĭ”. 2011. Available online: https://itpcru.org/2015/09/16/13495/ (accessed on 19 July 2020).
- Ministerstvo Zdravookhranenii͡a Respubliki Uzbekistan. Klinicheskoe Rukovodstvo po Diagnostike, Lechenii͡u i Profilaktike Khronicheskikh Gepatitov u Vzroslykh v Pervichnom Zvene Zdravookhranenii͡a. 2013. Available online: https://www.minzdrav.uz/documentation/detail.php?ID=41092 (accessed on 19 July 2020). (In Russian).
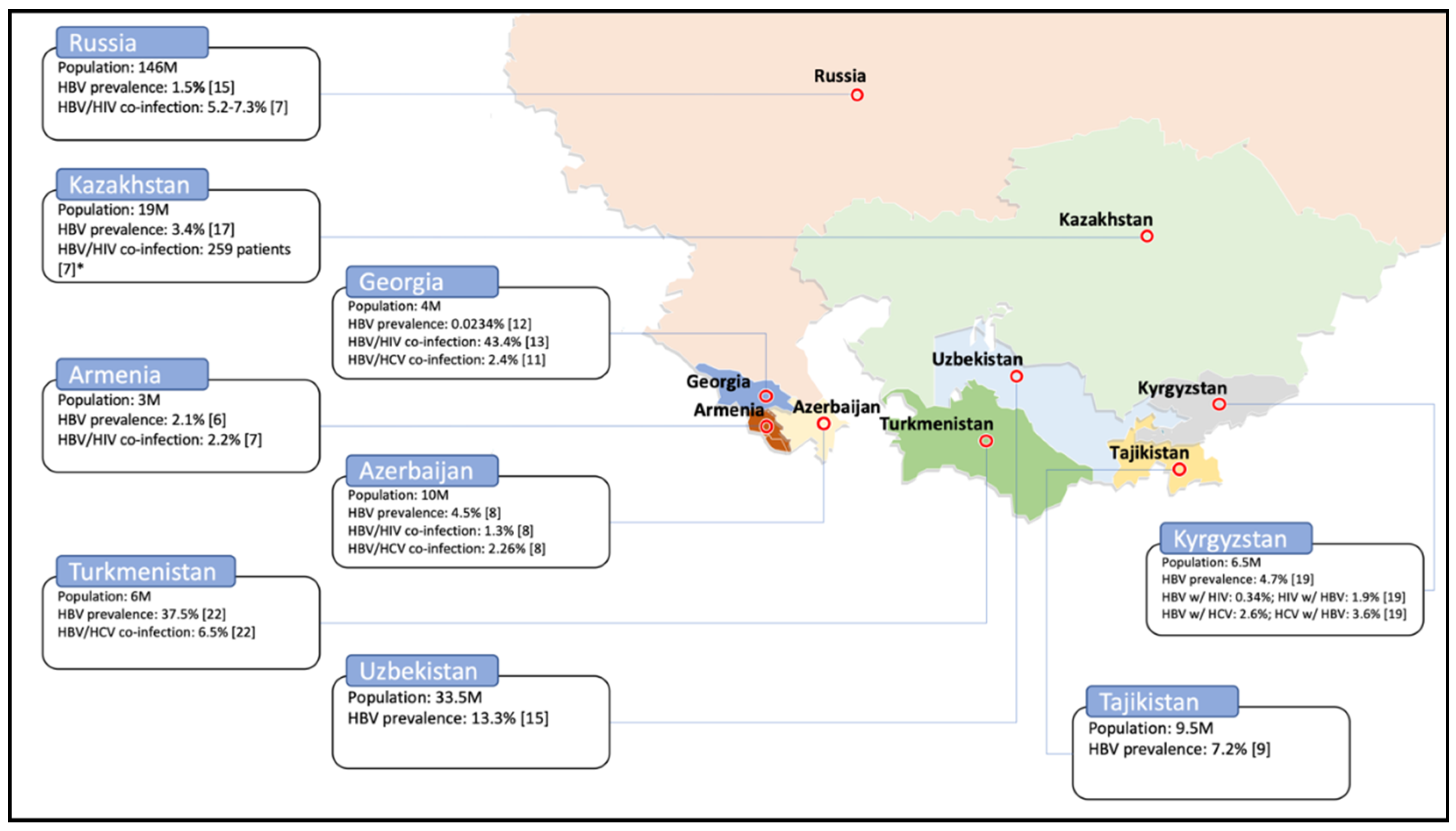
| Country | HBV | Co-Infection HBV/HIV | Co-Infection HBV/HCV | |||
|---|---|---|---|---|---|---|
| Prevalence | Year | Prevalence | Year | Prevalence | Year | |
| Armenia | 2.1% | 2012 [8] | 2.2% | 2017 [9] | n/a | n/a |
| 8% | 2007 [6] | |||||
| <2% | 2000 [7] | |||||
| Azerbaijan | 2.8% | 2017 [11] | 1.3% | 2012 [10] | 2.26% | 2012 [10] |
| 4.5% | 2012 [10] | |||||
| 0.02% | 2010 [26] | |||||
| 8% | 2007 [6] | |||||
| Georgia | 0.0234% | 2013 [14] | 43.42% * | 2008 [15] | 2.4% | 2005 [13] |
| 1.1% | 2005 [13] | |||||
| 6.86% ** | 2008 [15] | |||||
| 3.4% | 2001 [12] | |||||
| Kazakhstan | 3.4% | 2016 [19] | n = 259 *** | 2017 [9] | n/a | n/a |
| 1.12% | 2015 [18] | |||||
| 2.1% | 2015 [16] | |||||
| 1.8% | 2014 [17] | |||||
| 3.8% | ||||||
| 10% | 2007 [6] | |||||
| Kyrgyzstan | 4.7% | 2017 [21] | HBV w/HIV: 0.34% HIV w/HBV: 1.9% | 2017 [20] | HBV w/HCV: 2.6% HCV w/HBV: 3.6% | 2017 [20] |
| 3.6% | 2017 [20] | |||||
| 6.6% | 2017 [9] | |||||
| Russian Federation | 1.2–8.2% | 2016 [22] | 5.2–7.3% | 2017 [9] | n/a | n/a |
| 1.5% | 2014 [17] | |||||
| Tajikistan | 5.3% | 2017 [23] | n/a | n/a | n/a | n/a |
| 7.2% | 2017 [11] | |||||
| Turkmenistan | 37.5% | 2018 [24] | n/a | n/a | 6.5% | 2018 [24] |
| Uzbekistan | 4.1% | 2017 [23] | n/a | n/a | n/a | n/a |
| >10% | 2017 [9] | |||||
| 13.3% | 2014 [17] | |||||
| Country | Is Screening Performed? | Screening |
|---|---|---|
| Armenia | Yes [28] | Not specified. |
| Azerbaijan | Yes [29] | HBsAg screening. |
| Georgia | Yes [12] | Not specified. |
| Kazakhstan | Yes [30] |
|
| Kyrgyzstan | Yes [21] | HBsAg screening. |
| Russian Federation | Yes [31] |
|
| Tajikistan | Yes [27] | Not specified. National program is aimed to introduce both ELISA- and PCR-based test systems. |
| Turkmenistan | Yes [32] | n/a |
| Country | Year of Vaccine Introduced in Entire Country | Year of Birth Dose Introduced | Coverage of Birth Dose in 2018, % | Coverage of 3rd Dose in 2018, % | Type of Vaccine/Schedule in Weeks |
|---|---|---|---|---|---|
| Armenia | 1999 | 1999 | 97 | 92 | Monovalent vaccine given at 0, 6, 26 weeks [11]. |
| Azerbaijan | 2001 | 2001 | 99 | 95 | Monovalent vaccine given at 0, 9, 17 weeks [11]. |
| Georgia | 2001 | 2003 | 97 | 93 | Monovalent vaccine given at birth [35]; Pentavalent vaccine given at 8, 12, 16 weeks [35]. |
| Kazakhstan | 1998 | 1998 | 95 | 98 | Monovalent vaccine given at birth [36]; Tetravalent vaccine given at 8 and 16 weeks [36]. |
| Kyrgyzstan | 2001 | 1998 | 97 | 92 | Monovalent vaccine given at birth [21]; Pentavalent vaccine given at 8, 14, 24 weeks [21]. |
| Russian Federation | 2000 | 2000 | n/a | 97 | Monovalent vaccine given at 0, 4, 24 weeks OR at 0, 4, 8, 48 weeks [37]. |
| Tajikistan | 2002 | 1998 | 99 | 96 | Monovalent vaccine given at 0, 9, 17 weeks [11]. |
| Turkmenistan | 2002 | 2002 | 99 | 99 | Monovalent vaccine given at birth [38]; Pentavalent vaccine given at 8, 12, 16 weeks [38]. |
| Uzbekistan | 2001 | 1998 | 95 | 98 | Monovalent vaccine given at birth [39]; Pentavalent vaccine given at 8, 12, 16 weeks [39]. |
| Country | Implementation of Treatment Protocol | Antiviral Medications Available in the Country |
|---|---|---|
| Armenia | Yes [9] | Interferon alpha, pegylated interferon, lamivudine [41] |
| Azerbaijan | Yes [9] | Lamivudine, lamivudine generic, tenofovir [9] |
| Georgia | n/a | Interferon alpha, pegylated interferon, lamivudine, adefovir dipivoxil and tenofovir [41] |
| Kazakhstan | Yes [9] | Pegylated interferon alpha, tenofovir disoproxil fumarate, tenofovir alafenamide fumarate, entecavir [42] |
| Kyrgyzstan | Yes [9] | Lamivudine generic, entecavir generic, tenofovir, tenofovir generic, emtricitabine, emtricitabine generic [9] |
| Russian Federation | Yes [9] | Pegylated interferon alpha, lamivudine, entecavir, tenofovir, telbivudine [43] |
| Tajikistan | Yes [9] | Interferon alpha, pegylated interferon, adefovir, entecavir, emtricitabine, lamivudine, tenofovir, telbivudine [44] |
| Turkmenistan | n/a | n/a |
| Uzbekistan | Yes [9] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amerzhanov, D.; Suleimenova, I.; Davlidova, S.; Nugmanova, Z.; Ali, S. HBV Prevention and Treatment in Countries of Central Asia and the Caucasus. Viruses 2020, 12, 1112. https://doi.org/10.3390/v12101112
Amerzhanov D, Suleimenova I, Davlidova S, Nugmanova Z, Ali S. HBV Prevention and Treatment in Countries of Central Asia and the Caucasus. Viruses. 2020; 12(10):1112. https://doi.org/10.3390/v12101112
Chicago/Turabian StyleAmerzhanov, Daulet, Indira Suleimenova, Salima Davlidova, Zhamilya Nugmanova, and Syed Ali. 2020. "HBV Prevention and Treatment in Countries of Central Asia and the Caucasus" Viruses 12, no. 10: 1112. https://doi.org/10.3390/v12101112
APA StyleAmerzhanov, D., Suleimenova, I., Davlidova, S., Nugmanova, Z., & Ali, S. (2020). HBV Prevention and Treatment in Countries of Central Asia and the Caucasus. Viruses, 12(10), 1112. https://doi.org/10.3390/v12101112




