The Serine/Threonine Kinase AP2-Associated Kinase 1 Plays an Important Role in Rabies Virus Entry
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Cells and Virus
2.3. RNA Interference (RNAi)
2.4. qRT-PCR
2.5. Western Blotting
2.6. Cell Viability Assay
2.7. Virus Titration
2.8. Cell plasma Membrane Protein Isolation
2.9. Pharmacological Inhibition
2.10. Multiplex Immunofluorescence
2.11. Mice Challenge Study
3. Results
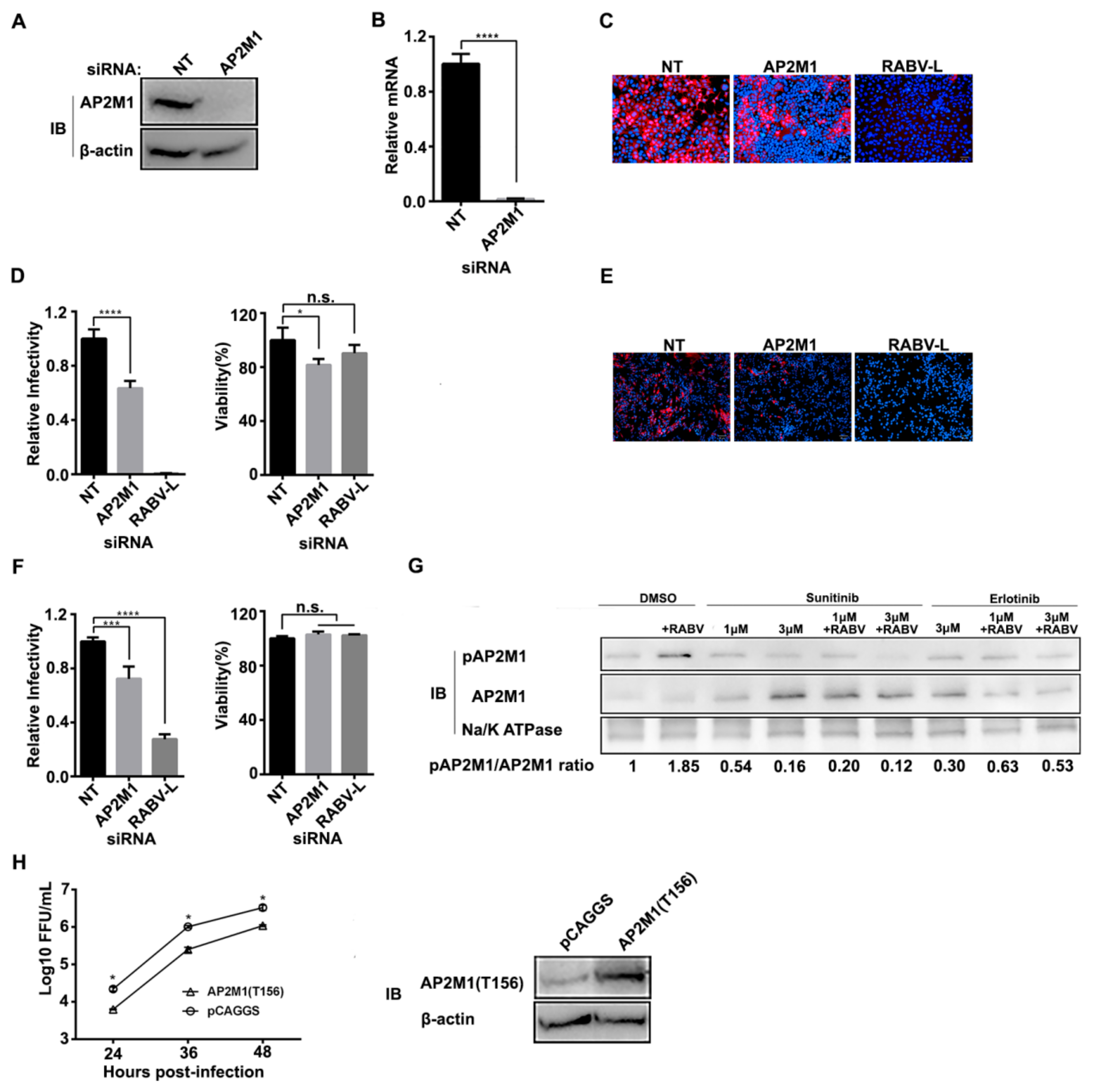
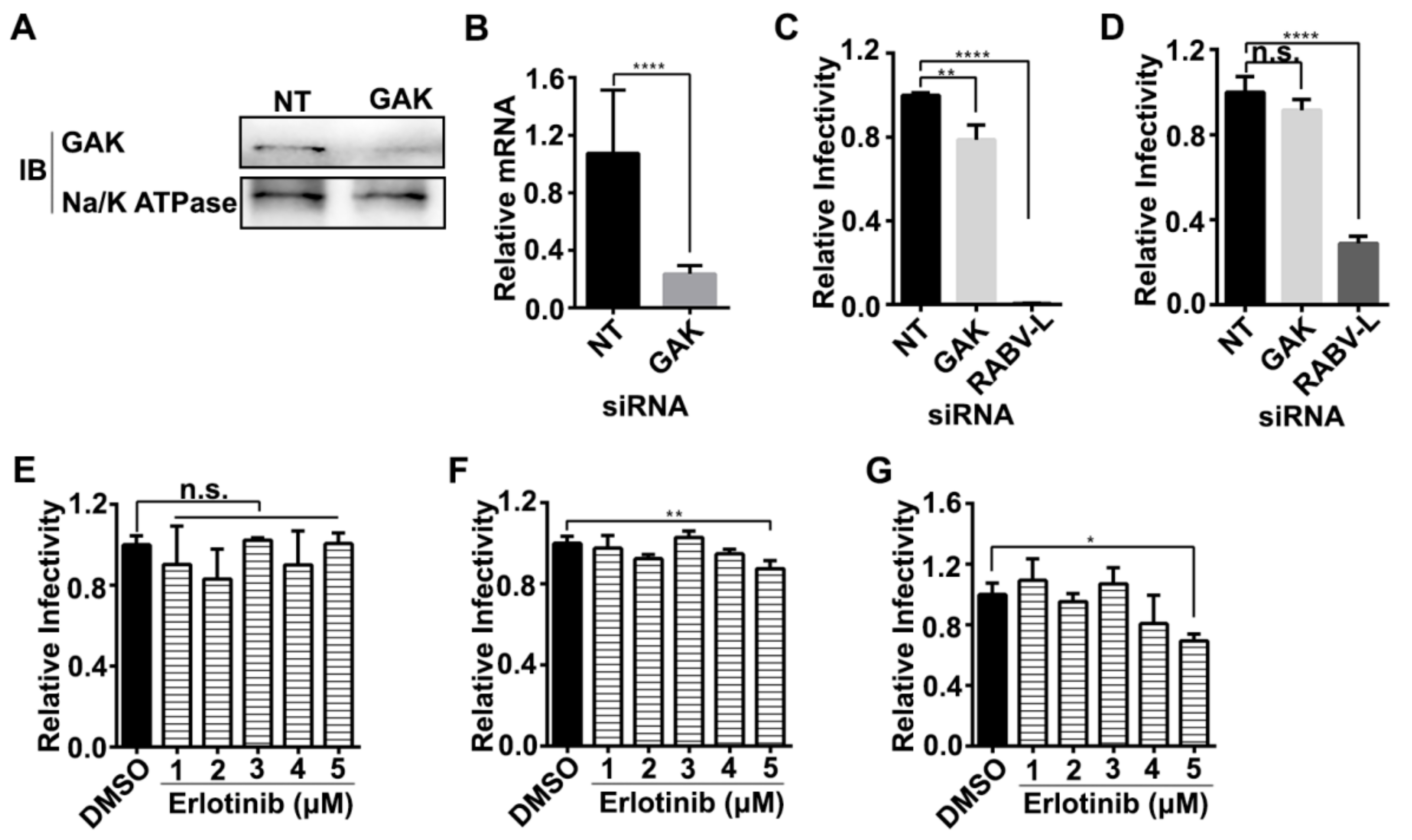
3.1. AAK1 Facilitates RABV Infection
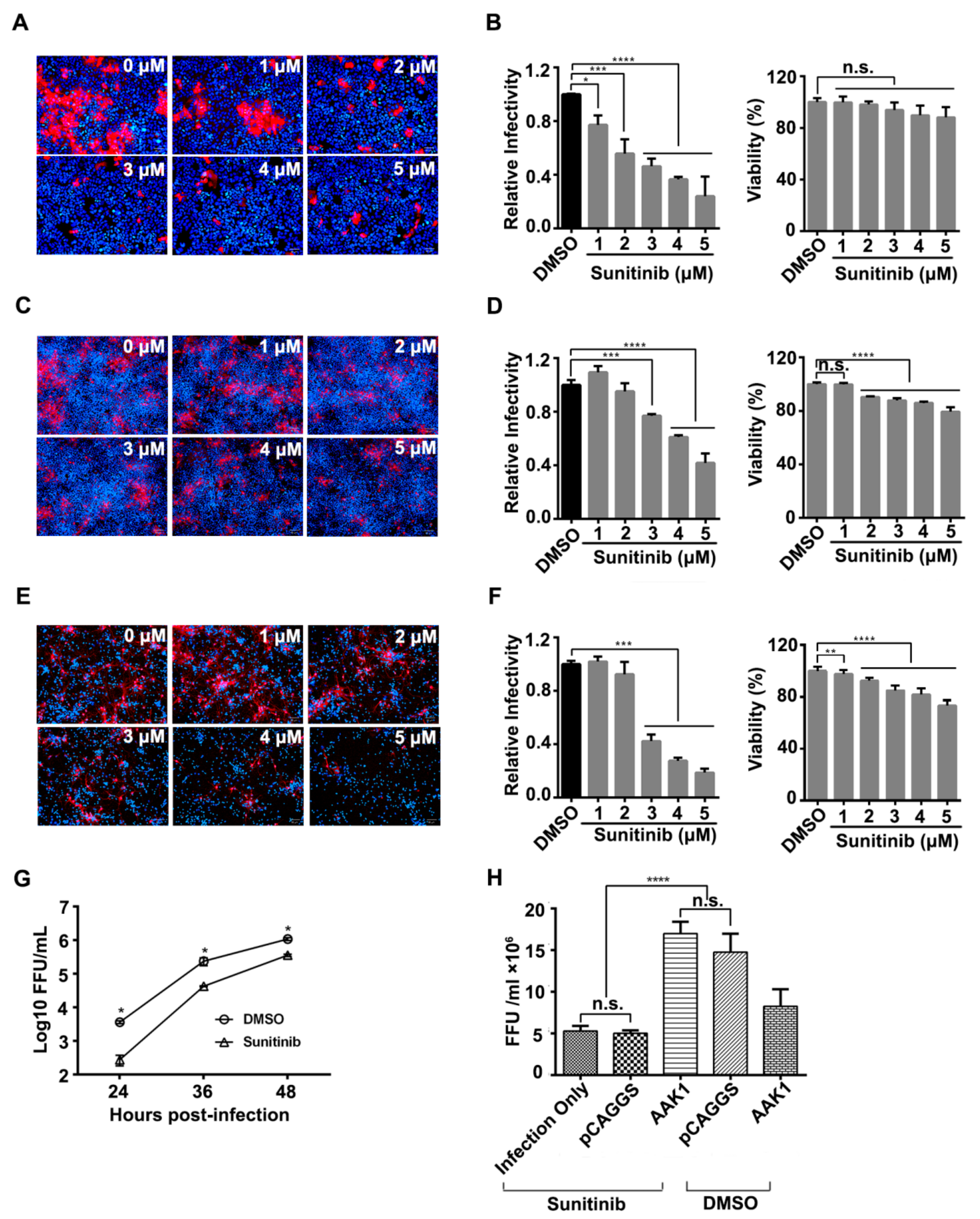
3.2. AAK1 Kinase Activity Facilitates RABV Infection
3.3. Phosphorylation of AP2M1 by AAK1 Is Required for RABV Infection
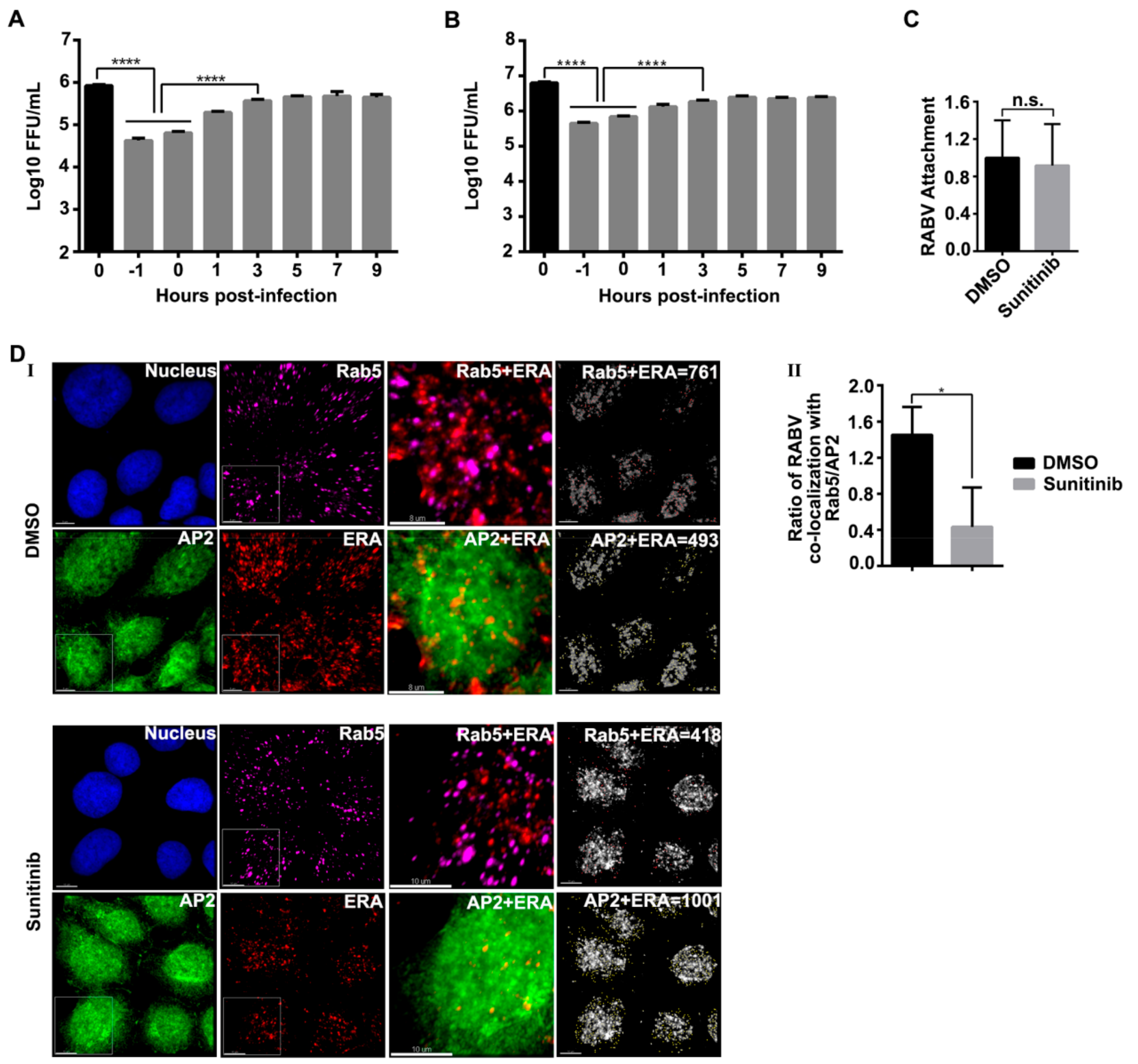
3.4. AAK1 Functions at the Endocytosis of RABV Infection
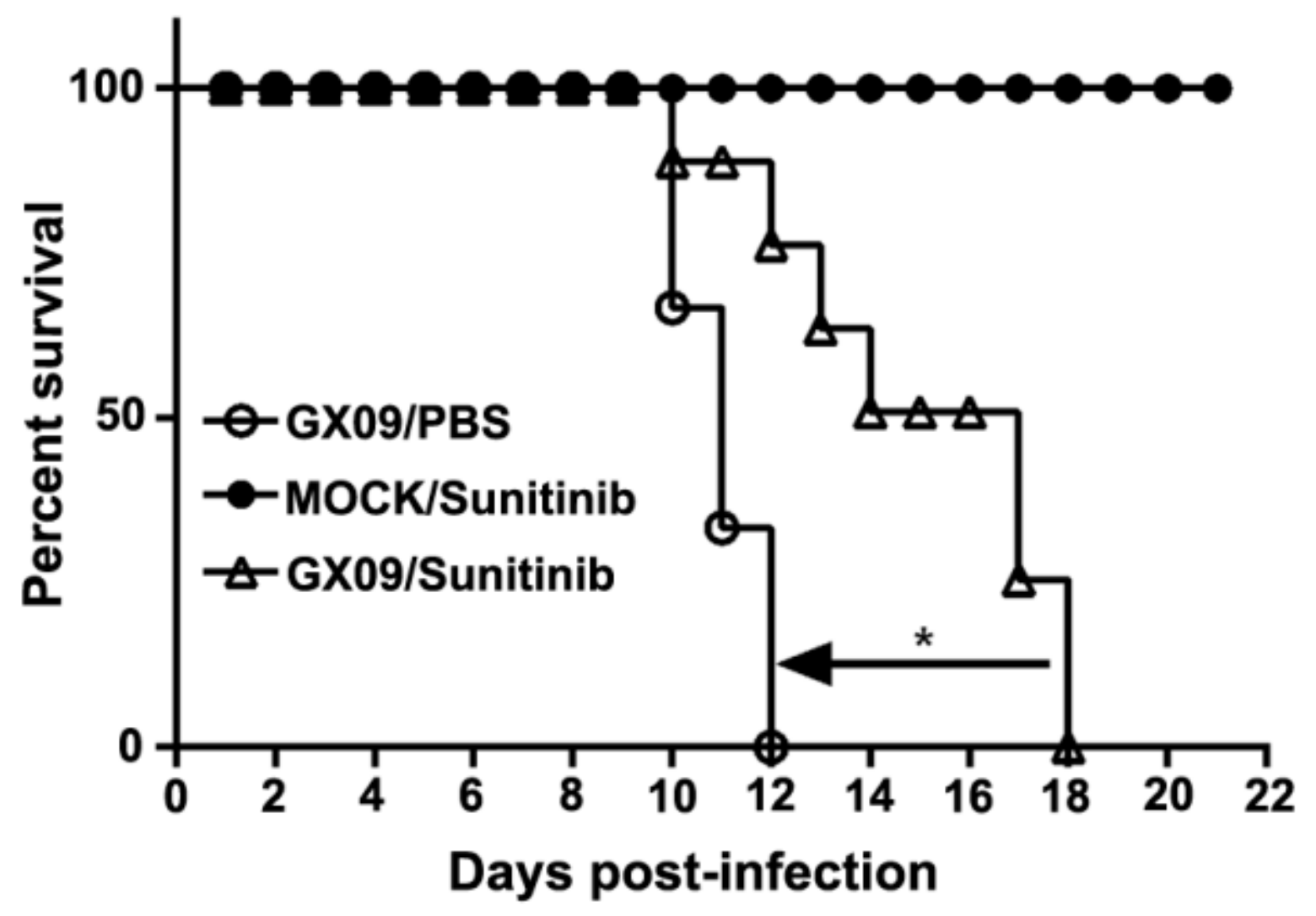
3.5. Sunitinib Prolongs the Survival but Not Prevents the Death of Mice Challenged with RABV Street Virus
4. Discussion
Author Contributions
Acknowledgments
Conflicts of Interest
References
- WHO. World Health Organization. Available online: http://www.who.int/news-room/feature-stories/detail/education-is-vital-to-prevent-rabies-deaths (accessed on 26 September 2018).
- Jackson, A.C. Human Disease. In Rabies, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2013; pp. 269–298. [Google Scholar] [CrossRef]
- Belot, L.; Albertini, A.; Gaudin, Y. Structural and cellular biology of rhabdovirus entry. Adv. Virus Res. 2019, 104, 147–183. [Google Scholar] [CrossRef]
- Guo, Y.; Duan, M.; Wang, X.; Gao, J.; Guan, Z.; Zhang, M. Early events in rabies virus infection-Attachment, entry, and intracellular trafficking. Virus Res. 2019, 263, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Piccinotti, S.; Kirchhausen, T.; Whelan, S.P.J. Uptake of Rabies Virus into Epithelial Cells by Clathrin-Mediated Endocytosis Depends upon Actin. J. Virol. 2013, 87, 11637–11647. [Google Scholar] [CrossRef] [PubMed]
- Gaudin, Y. Rabies Virus-Induced Membrane Fusion Pathway. J. Cell Boil. 2000, 150, 601–612. [Google Scholar] [CrossRef] [PubMed]
- Roche, S.; Gaudin, Y. Evidence that Rabies Virus Forms Different Kinds of Fusion Machines with Different pH Thresholds for Fusion. J. Virol. 2004, 78, 8746–8752. [Google Scholar] [CrossRef]
- Owen, D.J.; Collins, B.M.; Evans, P.R. Adaptors For Clathrin Coats: Structure and Function. Annu. Rev. Cell Dev. Boil. 2004, 20, 153–191. [Google Scholar] [CrossRef]
- Ferguson, S.M.; De Camilli, P. Dynamin, a membrane-remodelling GTPase. Nat. Rev. Mol. Cell Boil. 2012, 13, 75–88. [Google Scholar] [CrossRef] [PubMed]
- Schmid, S.L.; Frolov, V.A. Dynamin: Functional Design of a Membrane Fission Catalyst. Annu. Rev. Cell Dev. Boil. 2011, 27, 79–105. [Google Scholar] [CrossRef]
- Shuai, L.; Feng, N.; Wang, X.; Ge, J.; Wen, Z.; Chen, W.; Qin, L.; Xia, X.; Bu, Z. Genetically modified rabies virus ERA strain is safe and induces long-lasting protective immune response in dogs after oral vaccination. Antivir. Res. 2015, 121, 9–15. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Z.; Liu, R.; Shuai, L.; Wang, X.; Luo, J.; Wang, C.; Chen, W.; Wang, X.; Ge, J.; et al. Metabotropic glutamate receptor subtype 2 is a cellular receptor for rabies virus. PLoS Pathog. 2018, 14, e1007189. [Google Scholar] [CrossRef]
- Conner, S.D.; Schmid, S.L. Identification of an adaptor-associated kinase, AAK1, as a regulator of clathrin-mediated endocytosis. J. Cell Boil. 2002, 156, 921–929. [Google Scholar] [CrossRef]
- Goodman, V.L.; Rock, E.P.; Dagher, R.; Ramchandani, R.P.; Abraham, S.; Gobburu, J.V.; Booth, B.P.; Verbois, S.L.; Morse, D.E.; Liang, C.Y.; et al. Approval Summary: Sunitinib for the Treatment of Imatinib Refractory or Intolerant Gastrointestinal Stromal Tumors and Advanced Renal Cell Carcinoma. Clin. Cancer Res. 2007, 13, 1367–1373. [Google Scholar] [CrossRef]
- Karaman, M.W.; Herrgard, S.; Treiber, D.K.; Gallant, P.; Atteridge, C.E.; Campbell, B.T.; Chan, K.W.; Ciceri, P.; Davis, M.I.; Edeen, P.T.; et al. A quantitative analysis of kinase inhibitor selectivity. Nat. Biotechnol. 2008, 26, 127–132. [Google Scholar] [CrossRef]
- Conner, S.D.; Schröter, T.; Schmid, S.L. AAK1-mediated micro2 phosphorylation is stimulated by assembled clathrin. Traffic 2003, 4, 885–890. [Google Scholar] [CrossRef]
- Ricotta, D.; Conner, S.D.; Schmid, S.L.; Von Figura, K.; Höning, S. Phosphorylation of the AP2 μ subunit by AAK1 mediates high affinity binding to membrane protein sorting signals. J. Cell Boil. 2002, 156, 791–795. [Google Scholar] [CrossRef]
- Fingerhut, A.; von Figura, K.; Honing, S. Binding of AP2 to sorting signals is modulated by AP2 phosphorylation. J. Biol. Chem. 2001, 276, 5476–5482. [Google Scholar] [CrossRef]
- Zhang, C.X.; Engqvist-Goldstein Åsa, E.Y.; Carreno, S.; Owen, D.J.; Smythe, E.; Drubin, D.G. Multiple Roles for Cyclin G-Associated Kinase in Clathrin-Mediated Sorting Events. Traffic 2005, 6, 1103–1113. [Google Scholar] [CrossRef]
- Olusanya, O.; Andrews, P.D.; Swedlow, J.R.; Smythe, E. Phosphorylation of threonine 156 of the mu2 subunit of the AP2 complex is essential for endocytosis in vitro and in vivo. Curr. Boil. 2001, 11, 896–900. [Google Scholar] [CrossRef]
- Ge, J.; Wang, X.; Tao, L.; Wen, Z.; Feng, N.; Yang, S.; Xia, X.; Yang, C.; Chen, H.; Bu, Z. Newcastle Disease Virus-Vectored Rabies Vaccine Is Safe, Highly Immunogenic, and Provides Long-Lasting Protection in Dogs and Cats. J. Virol. 2011, 85, 8241–8252. [Google Scholar] [CrossRef]
- McMahon, H.T.; Boucrot, E. Molecular mechanism and physiological functions of clathrin-mediated endocytosis. Nat. Rev. Mol. Cell Boil. 2011, 12, 517–533. [Google Scholar] [CrossRef]
- Cureton, D.K.; Massol, R.H.; Saffarian, S.; Kirchhausen, T.L.; Whelan, S.P.J. Vesicular Stomatitis Virus Enters Cells through Vesicles Incompletely Coated with Clathrin That Depend upon Actin for Internalization. PLoS Pathog. 2009, 5, e1000394. [Google Scholar] [CrossRef]
- Sun, X.; Whittaker, G.R. Entry of influenza virus. Adv. Exp. Med. Biol. 2013, 790, 72–82. [Google Scholar] [CrossRef]
- Meier, O.; Boucke, K.; Hammer, S.V.; Keller, S.; Stidwill, R.P.; Hemmi, S.; Greber, U.F. Adenovirus triggers macropinocytosis and endosomal leakage together with its clathrin-mediated uptake. J. Cell Boil. 2002, 158, 1119–1131. [Google Scholar] [CrossRef]
- Carvajal-Gonzalez, J.M.; Gravotta, D.; Mattera, R.; Diaz, F.; Bay, A.P.; Roman, A.C.; Schreiner, R.P.; Thuenauer, R.; Bonifacino, J.S.; Rodriguez-Boulan, E. Basolateral sorting of the coxsackie and adenovirus receptor through interaction of a canonical YXXΦ motif with the clathrin adaptors AP-1A and AP-1B. Proc. Natl. Acad. Sci. USA 2012, 109, 3820–3825. [Google Scholar] [CrossRef]
- Martín-Acebes, M.A.; González-Magaldi, M.; Sandvig, K.; Sobrino, F.; Armas-Portela, R. Productive entry of type C foot-and-mouth disease virus into susceptible cultured cells requires clathrin and is dependent on the presence of plasma membrane cholesterol. Virology 2007, 369, 105–118. [Google Scholar] [CrossRef]
- Lentz, T.; Burrage, T.; Smith, A.; Crick, J.; Tignor, G. Is the acetylcholine receptor a rabies virus receptor? Science 1982, 215, 182–184. [Google Scholar] [CrossRef]
- Thoulouze, M.-I.; Lafage, M.; Schachner, M.; Hartmann, U.; Cremer, H.; Lafon, M. The Neural Cell Adhesion Molecule Is a Receptor for Rabies Virus. J. Virol. 1998, 72, 7181–7190. [Google Scholar]
- Tuffereau, C.; Benejean, J.; Blondel, D.; Kieffer, B.; Flamand, A. Low-affinity nerve-growth factor receptor (P75NTR) can serve as a receptor for rabies virus. EMBO J. 1998, 17, 7250–7259. [Google Scholar] [CrossRef]
- Deinhardt, K.; Reversi, A.; Berninghausen, O.; Hopkins, C.R.; Schiavo, G. Neurotrophins Redirect p75NTRfrom a Clathrin-Independent to a Clathrin-Dependent Endocytic Pathway Coupled to Axonal Transport. Traffic 2007, 8, 1736–1749. [Google Scholar] [CrossRef]
- Miñana, R.; Duran, J.M.; Tomas, M.; Renau-Piqueras, J.; Guerri, C. Neural cell adhesion molecule is endocytosed via a clathrin-dependent pathway. Eur. J. Neurosci. 2001, 13, 749–756. [Google Scholar] [CrossRef]
- Piccinotti, S.; Whelan, S.P.J. Rabies Internalizes into Primary Peripheral Neurons via Clathrin Coated Pits and Requires Fusion at the Cell Body. PLoS Pathog. 2016, 12, e1005753. [Google Scholar] [CrossRef]
- Mercer, J.; Schelhaas, M.; Helenius, A. Virus Entry by Endocytosis. Annu. Rev. Biochem. 2010, 79, 803–833. [Google Scholar] [CrossRef]
- Johannsdottir, H.K.; Mancini, R.; Kartenbeck, J.; Amato, L.; Helenius, A. Host cell factors and functions involved in vesicular stomatitis virus entry. J. Virol. 2009, 83, 440–453. [Google Scholar] [CrossRef]
- Conner, S.D.; Schmid, S.L. Differential requirements for AP-2 in clathrin-mediated endocytosis. J. Cell Boil. 2003, 162, 773–780. [Google Scholar] [CrossRef]
- Neveu, G.; Ziv-Av, A.; Barouch-Bentov, R.; Berkerman, E.; Mulholland, J.; Einav, S. AP-2-Associated Protein Kinase 1 and Cyclin G-Associated Kinase Regulate Hepatitis C Virus Entry and Are Potential Drug Targets. J. Virol. 2015, 89, 4387–4404. [Google Scholar] [CrossRef]
- Neveu, G.; Barouch-Bentov, R.; Ziv-Av, A.; Gerber, R.; Jacob, Y.; Einav, S. Identification and Targeting of an Interaction between a Tyrosine Motif within Hepatitis C Virus Core Protein and AP2M1 Essential for Viral Assembly. PLoS Pathog. 2012, 8, e1002845. [Google Scholar] [CrossRef]
- Xiao, F.; Wang, S.; Barouch-Bentov, R.; Neveu, G.; Pu, S.; Beer, M.; Schor, S.; Kumar, S.; Nicolaescu, V.; Lindenbach, B.D.; et al. Interactions between the Hepatitis C Virus Nonstructural 2 Protein and Host Adaptor Proteins 1 and 4 Orchestrate Virus Release. mBio 2018, 9, e02233-17. [Google Scholar] [CrossRef]
- Bekerman, E.; Neveu, G.; Shulla, A.; Brannan, J.; Pu, S.-Y.; Wang, S.; Xiao, F.; Barouch-Bentov, R.; Bakken, R.R.; Mateo, R.; et al. Anticancer kinase inhibitors impair intracellular viral trafficking and exert broad-spectrum antiviral effects. J. Clin. Investig. 2017, 127, 1338–1352. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.; Wang, J.; Shuai, L.; Ma, X.; Zhang, H.; Liu, R.; Chen, W.; Wang, X.; Ge, J.; Wen, Z.; et al. The Serine/Threonine Kinase AP2-Associated Kinase 1 Plays an Important Role in Rabies Virus Entry. Viruses 2020, 12, 45. https://doi.org/10.3390/v12010045
Wang C, Wang J, Shuai L, Ma X, Zhang H, Liu R, Chen W, Wang X, Ge J, Wen Z, et al. The Serine/Threonine Kinase AP2-Associated Kinase 1 Plays an Important Role in Rabies Virus Entry. Viruses. 2020; 12(1):45. https://doi.org/10.3390/v12010045
Chicago/Turabian StyleWang, Chong, Jinliang Wang, Lei Shuai, Xiao Ma, Hailin Zhang, Renqiang Liu, Weiye Chen, Xijun Wang, Jinying Ge, Zhiyuan Wen, and et al. 2020. "The Serine/Threonine Kinase AP2-Associated Kinase 1 Plays an Important Role in Rabies Virus Entry" Viruses 12, no. 1: 45. https://doi.org/10.3390/v12010045
APA StyleWang, C., Wang, J., Shuai, L., Ma, X., Zhang, H., Liu, R., Chen, W., Wang, X., Ge, J., Wen, Z., & Bu, Z. (2020). The Serine/Threonine Kinase AP2-Associated Kinase 1 Plays an Important Role in Rabies Virus Entry. Viruses, 12(1), 45. https://doi.org/10.3390/v12010045




