Characterization of the Immune Response of MERS-CoV Vaccine Candidates Derived from Two Different Vectors in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Cells and Virus
2.3. Production of the RV/MERS in BHK-21 Cells
2.4. Production of the MERS Bacterium-Like Particles
2.5. Size of the Particles
2.6. Animal Immunization and Sera Collection
2.7. ELISA for MERS-CoV Specific Antibody Test
2.8. Virus Neutralizing Assay
2.9. Cytokines Analysis in Splenocytes Culture Supernatants
2.10. Statistics
3. Results
3.1. The Particle Size of RV/MERS and MERS BLP
3.2. Neutralizing Antibody Response by Pseudovirions Neutralization Assay
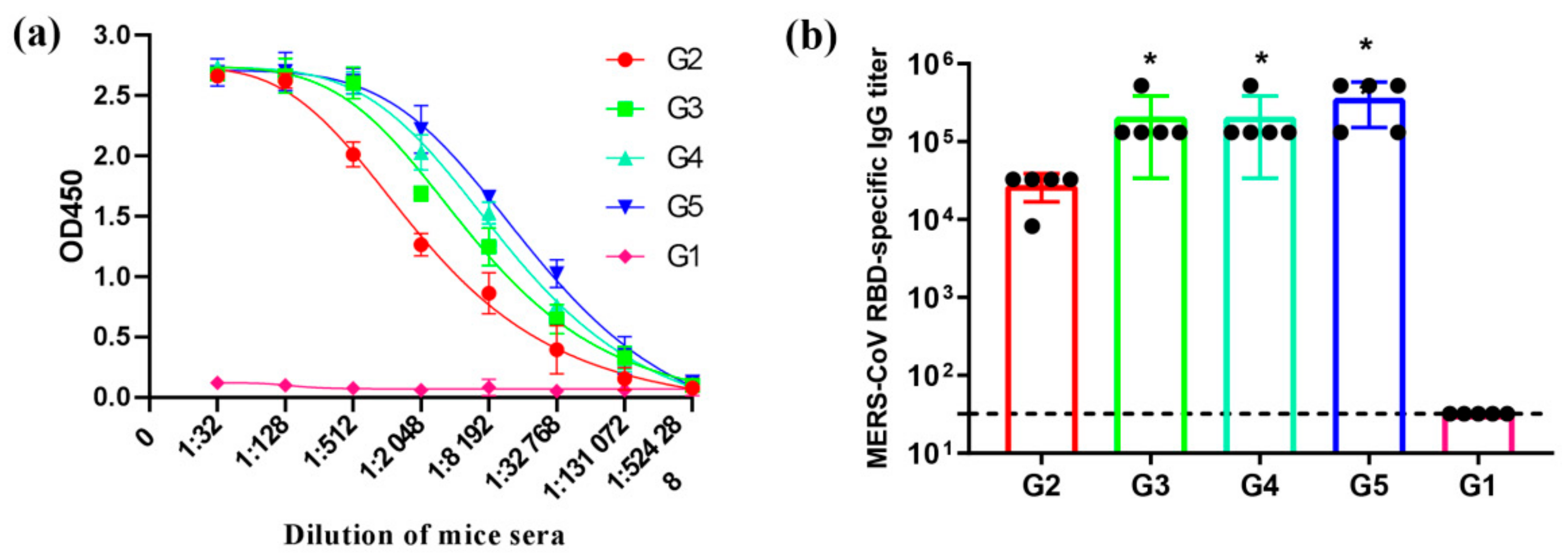
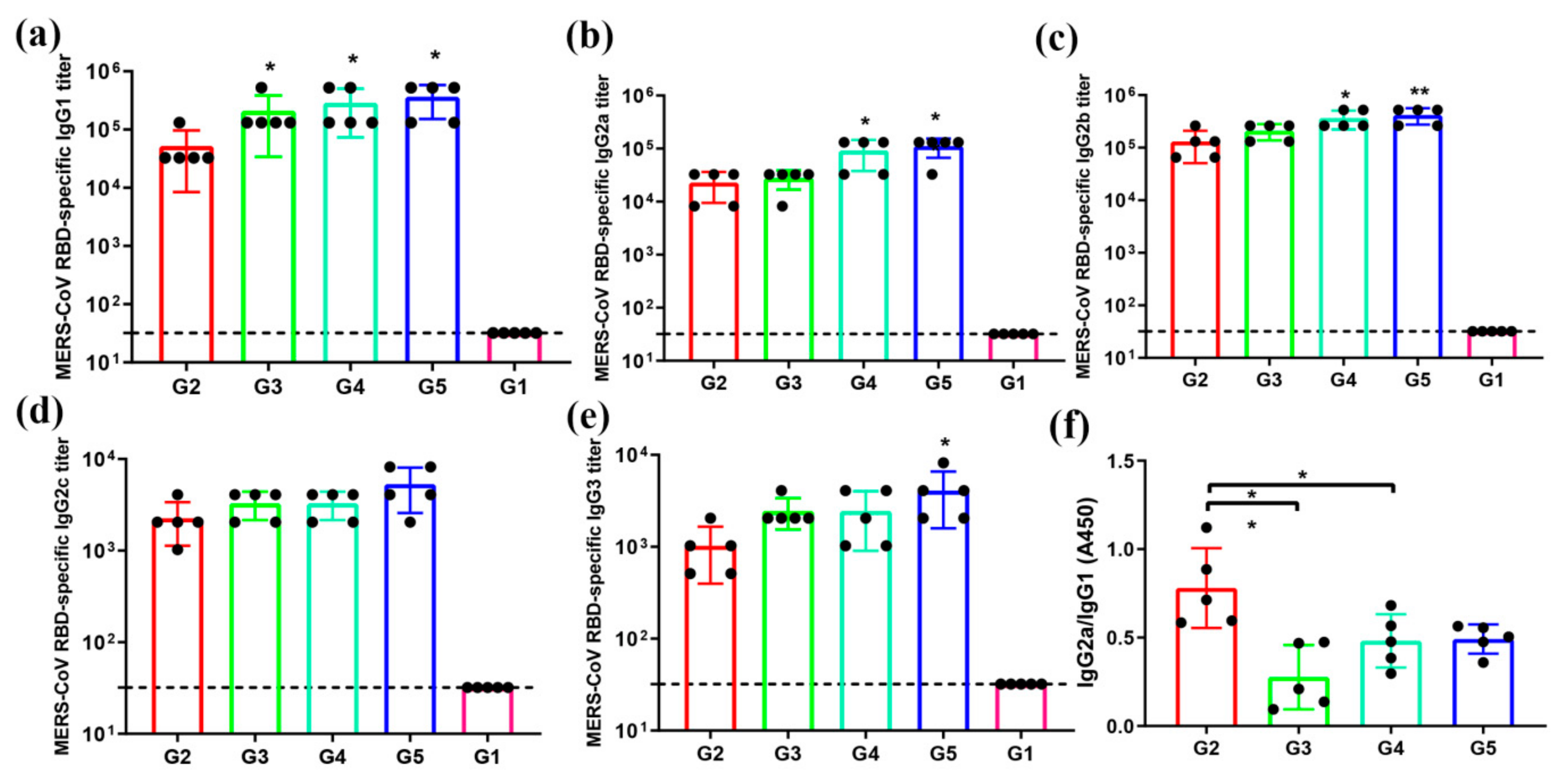
3.3. MERS-CoV RBD-Specific IgG Subtypes Responses by Indirect ELISA
3.4. Antigen-Specific T-Cell Immune Responses
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Zaki, A.M.; van Boheemen, S.; Bestebroer, T.M.; Osterhaus, A.D.; Fouchier, R.A. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N. Engl. J. Med. 2012, 367, 1814–1820. [Google Scholar] [CrossRef]
- Ki, M. 2015 MERS outbreak in Korea: Hospital-to-hospital transmission. Epidemiol. Health 2015, 37, e2015033. [Google Scholar] [CrossRef]
- Su, S.; Wong, G.; Shi, W.; Liu, J.; Lai, A.C.K.; Zhou, J.; Liu, W.; Bi, Y.; Gao, G.F. Epidemiology, Genetic Recombination, and Pathogenesis of Coronaviruses. Trends Microbiol. 2016, 24, 490–502. [Google Scholar] [CrossRef]
- Jia, W.; Channappanavar, R.; Zhang, C.; Li, M.; Zhou, H.; Zhang, S.; Zhou, P.; Xu, J.; Shan, S.; Shi, X.; et al. Single intranasal immunization with chimpanzee adenovirus-based vaccine induces sustained and protective immunity against MERS-CoV infection. Emerg. Microbes Infect. 2019, 8, 760–772. [Google Scholar] [CrossRef]
- Haagmans, B.L.; van den Brand, J.M.; Raj, V.S.; Volz, A.; Wohlsein, P.; Smits, S.L.; Schipper, D.; Bestebroer, T.M.; Okba, N.; Fux, R.; et al. An orthopoxvirus-based vaccine reduces virus excretion after MERS-CoV infection in dromedary camels. Science 2016, 351, 77–81. [Google Scholar] [CrossRef]
- Volz, A.; Kupke, A.; Song, F.; Jany, S.; Fux, R.; Shams-Eldin, H.; Schmidt, J.; Becker, C.; Eickmann, M.; Becker, S.; et al. Protective Efficacy of Recombinant Modified Vaccinia Virus Ankara Delivering Middle East Respiratory Syndrome Coronavirus Spike Glycoprotein. J. Virol. 2015, 89, 8651–8656. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Deng, Y.; Chen, H.; Lan, J.; Wang, W.; Zou, X.; Hung, T.; Lu, Z.; Tan, W. Systemic and mucosal immunity in mice elicited by a single immunization with human adenovirus type 5 or 41 vector-based vaccines carrying the spike protein of Middle East respiratory syndrome coronavirus. Immunology 2015, 145, 476–484. [Google Scholar] [CrossRef]
- Liu, R.Q.; Ge, J.Y.; Wang, J.L.; Shao, Y.; Zhang, H.L.; Wang, J.L.; Wen, Z.Y.; Bu, Z.G. Newcastle disease virus-based MERS-CoV candidate vaccine elicits high-level and lasting neutralizing antibodies in Bactrian camels. J. Integr. Agric. 2017, 16, 2264–2273. [Google Scholar] [CrossRef]
- Malczyk, A.H.; Kupke, A.; Prufer, S.; Scheuplein, V.A.; Hutzler, S.; Kreuz, D.; Beissert, T.; Bauer, S.; Hubich-Rau, S.; Tondera, C.; et al. A Highly Immunogenic and Protective Middle East Respiratory Syndrome Coronavirus Vaccine Based on a Recombinant Measles Virus Vaccine Platform. J. Virol. 2015, 89, 11654–11667. [Google Scholar] [CrossRef] [PubMed]
- Wirblich, C.; Coleman, C.M.; Kurup, D.; Abraham, T.S.; Bernbaum, J.G.; Jahrling, P.B.; Hensley, L.E.; Johnson, R.F.; Frieman, M.B.; Schnell, M.J. One-Health: A Safe, Efficient, Dual-Use Vaccine for Humans and Animals against Middle East Respiratory Syndrome Coronavirus and Rabies Virus. J. Virol. 2017, 91, e02040. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zheng, X.X.; Gai, W.W.; Wong, G.; Wang, H.L.; Jin, H.L.; Feng, N.; Zhao, Y.K.; Zhang, W.J.; Li, N.; et al. Novel chimeric virus-like particles vaccine displaying MERS-CoV receptor-binding domain induce specific humoral and cellular immune response in mice. Antivir. Res. 2017, 140, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zheng, X.X.; Gai, W.W.; Zhao, Y.K.; Wang, H.L.; Wang, H.J.; Feng, N.; Chi, H.; Qiu, B.N.; Li, N.; et al. MERS-CoV virus-like particles produced in insect cells induce specific humoural and cellular imminity in rhesus macaques. Oncotarget 2017, 8, 12686–12694. [Google Scholar] [CrossRef] [PubMed]
- Li, E.; Chi, H.; Huang, P.; Yan, F.; Zhang, Y.; Liu, C.; Wang, Z.; Li, G.; Zhang, S.; Mo, R.; et al. A Novel Bacterium-Like Particle Vaccine Displaying the MERS-CoV Receptor-Binding Domain Induces Specific Mucosal and Systemic Immune Responses in Mice. Viruses 2019, 11, 799. [Google Scholar] [CrossRef] [PubMed]
- Chi, H.; Zheng, X.; Wang, X.; Wang, C.; Wang, H.; Gai, W.; Perlman, S.; Yang, S.; Zhao, J.; Xia, X. DNA vaccine encoding Middle East respiratory syndrome coronavirus S1 protein induces protective immune responses in mice. Vaccine 2017, 35, 2069–2075. [Google Scholar] [CrossRef] [PubMed]
- Al-Amri, S.S.; Abbas, A.T.; Siddiq, L.A.; Alghamdi, A.; Sanki, M.A.; Al-Muhanna, M.K.; Alhabbab, R.Y.; Azhar, E.I.; Li, X.; Hashem, A.M. Immunogenicity of Candidate MERS-CoV DNA Vaccines Based on the Spike Protein. Sci. Rep. 2017, 7, 44875. [Google Scholar] [CrossRef] [PubMed]
- Muthumani, K.; Falzarano, D.; Reuschel, E.L.; Tingey, C.; Flingai, S.; Villarreal, D.O.; Wise, M.; Patel, A.; Izmirly, A.; Aljuaid, A.; et al. A synthetic consensus anti-spike protein DNA vaccine induces protective immunity against Middle East respiratory syndrome coronavirus in nonhuman primates. Sci. Transl. Med. 2015, 7, 301ra132. [Google Scholar] [CrossRef]
- Du, L.; Yang, Y.; Zhou, Y.; Lu, L.; Li, F.; Jiang, S. MERS-CoV spike protein: A key target for antivirals. Expert Opin. Ther. Targets 2017, 21, 131–143. [Google Scholar] [CrossRef]
- Lu, G.W.; Hu, Y.W.; Wang, Q.H.; Qi, J.X.; Gao, F.; Li, Y.; Zhang, Y.F.; Zhang, W.; Yuan, Y.; Bao, J.K.; et al. Molecular basis of binding between novel human coronavirus MERS-CoV and its receptor CD26. Nature 2013, 500, 227. [Google Scholar] [CrossRef]
- Chen, Y.; Rajashankar, K.R.; Yang, Y.; Agnihothram, S.S.; Liu, C.; Lin, Y.L.; Baric, R.S.; Li, F. Crystal structure of the receptor-binding domain from newly emerged Middle East respiratory syndrome coronavirus. J. Virol. 2013, 87, 10777–10783. [Google Scholar] [CrossRef]
- Wang, N.S.; Shi, X.L.; Jiang, L.W.; Zhang, S.Y.; Wang, D.L.; Tong, P.; Guo, D.X.; Fu, L.L.; Cui, Y.; Liu, X.; et al. Structure of MERS-CoV spike receptor-binding domain complexed with human receptor DPP4. Cell Res. 2013, 23, 986–993. [Google Scholar] [CrossRef]
- McGettigan, J.P.; Pomerantz, R.J.; Siler, C.A.; McKenna, P.M.; Foley, H.D.; Dietzschold, B.; Schnell, M.J. Second-generation rabies virus-based vaccine vectors expressing human immunodeficiency virus type 1 Gag have greatly reduced pathogenicity but are highly immunogenic. J. Virol. 2003, 77, 237–244. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wang, H.L.; Jin, H.L.; Feng, N.; Zheng, X.X.; Li, L.; Qi, Y.L.; Liang, M.; Zhao, Y.K.; Wang, T.C.; Gao, Y.W.; et al. Using rabies virus vaccine strain SRV9 as viral vector to express exogenous gene. Virus Genes 2015, 50, 299–302. [Google Scholar] [CrossRef] [PubMed]
- Barefoot, B.; Thornburg, N.J.; Barouch, D.H.; Yu, J.S.; Sample, C.; Johnston, R.E.; Liao, H.X.; Kepler, T.B.; Haynes, B.F.; Ramsburg, E. Comparison of multiple vaccine vectors in a single heterologous prime-boost trial. Vaccine 2008, 26, 6108–6118. [Google Scholar] [CrossRef] [PubMed]
- Xue, X.H.; Zheng, X.X.; Wang, H.L.; Ma, J.Z.; Li, L.; Gai, W.W.; Wang, T.C.; Yang, S.T.; Xia, X.Z. An inactivated recombinant rabies CVS-11 virus expressing two copies of the glycoprotein elicits a higher level of neutralizing antibodies and provides better protection in mice. Virus Genes 2014, 48, 411–420. [Google Scholar] [CrossRef]
- Zhao, J.C.; Li, K.; Wohlford-Lenane, C.; Agnihothram, S.S.; Fett, C.; Zhao, J.X.; Gale, M.J.; Baric, R.S.; Enjuanes, L.; Gallagher, T.; et al. Rapid generation of a mouse model for Middle East respiratory syndrome. Proc. Natl. Acad. Sci. USA 2014, 111, 4970–4975. [Google Scholar] [CrossRef]
- Kawasaki, Y.; Suzuki, J.; Sakai, N.; Isome, M.; Nozawa, R.; Tanji, M.; Suzuki, H. Evaluation of T helper-1/-2 balance on the basis of IgG subclasses and serum cytokines in children with glomerulonephritis. Am. J. Kidney Dis. Off. J. Natl. Kidney Found. 2004, 44, 42–49. [Google Scholar] [CrossRef]
- Blaney, J.E.; Wirblich, C.; Papaneri, A.B.; Johnson, R.F.; Myers, C.J.; Juelich, T.L.; Holbrook, M.R.; Freiberg, A.N.; Bernbaum, J.G.; Jahrling, P.B.; et al. Inactivated or live-attenuated bivalent vaccines that confer protection against rabies and Ebola viruses. J. Virol. 2011, 85, 10605–10616. [Google Scholar] [CrossRef]
- Abreu-Mota, T.; Hagen, K.R.; Cooper, K.; Jahrling, P.B.; Tan, G.; Wirblich, C.; Johnson, R.F.; Schnell, M.J. Non-neutralizing antibodies elicited by recombinant Lassa-Rabies vaccine are critical for protection against Lassa fever. Nat. Commun. 2018, 9, 4223. [Google Scholar] [CrossRef]
- Willet, M.; Kurup, D.; Papaneri, A.; Wirblich, C.; Hooper, J.W.; Kwilas, S.A.; Keshwara, R.; Hudacek, A.; Beilfuss, S.; Rudolph, G.; et al. Preclinical Development of Inactivated Rabies Virus-Based Polyvalent Vaccine Against Rabies and Filoviruses. J. Infect. Dis. 2015, 212, S414–S424. [Google Scholar] [CrossRef][Green Version]
- McGettigan, J.P.; Foley, H.D.; Belyakov, I.M.; Berzofsky, J.A.; Pomerantz, R.J.; Schnell, M.J. Rabies virus-based vectors expressing human immunodeficiency virus type 1 (HIV-1) envelope protein induce a strong, cross-reactive cytotoxic T-lymphocyte response against envelope proteins from different HIV-1 isolates. J. Virol. 2001, 75, 4430–4434. [Google Scholar] [CrossRef]
- Takayama-Ito, M.; Lim, C.K.; Yamaguchi, Y.; Posadas-Herrera, G.; Kato, H.; Iizuka, I.; Islam, M.T.; Morimoto, K.; Saijo, M. Replication-incompetent rabies virus vector harboring glycoprotein gene of lymphocytic choriomeningitis virus (LCMV) protects mice from LCMV challenge. PLoS Negl. Trop. Dis. 2018, 12, e0006398. [Google Scholar] [CrossRef] [PubMed]
- Cliquet, F.; Aubert, M. Elimination of terrestrial rabies in western European countries. Dev. Biol. 2004, 119, 185–204. [Google Scholar]
- Bosma, T.; Kanninga, R.; Neef, J.; Audouy, S.A.L.; van Roosmalen, M.L.; Steen, A.; Buist, G.; Kok, J.; Kuipers, O.P.; Robillard, G.; et al. Novel surface display system for proteins on non-genetically modified gram-positive bacteria. Appl. Environ. Microbiol. 2006, 72, 880–889. [Google Scholar] [CrossRef] [PubMed]
- Van Roosmalen, M.L.; Kanninga, R.; El Khattabi, M.; Neef, J.; Audouy, S.; Bosma, T.; Kuipers, A.; Post, E.; Steen, A.; Kok, J.; et al. Mucosal vaccine delivery of antigens tightly bound to an adjuvant particle made from food-grade bacteria. Methods 2006, 38, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Salminen, S.; von Wright, A.; Morelli, L.; Marteau, P.; Brassart, D.; de Vos, W.M.; Fonden, R.; Saxelin, M.; Collins, K.; Mogensen, G.; et al. Demonstration of safety of probiotics—A review. Int. J. Food Microbiol. 1998, 44, 93–106. [Google Scholar] [CrossRef]
- Ramasamy, R.; Yasawardena, S.; Zomer, A.; Venema, G.; Kok, J.; Leenhouts, K. Immunogenicity of a malaria parasite antigen displayed by Lactococcus lactis in oral immunisations. Vaccine 2006, 24, 3900–3908. [Google Scholar] [CrossRef]
- Audouy, S.A.L.; van Selm, S.; van Roosmalen, M.L.; Post, E.; Kanninga, R.; Neef, J.; Estevao, S.; Nieuwenhuis, E.E.S.; Adrian, P.V.; Leenhouts, K.; et al. Development of lactococcal GEM-based pneumococcal vaccines. Vaccine 2007, 25, 2497–2506. [Google Scholar] [CrossRef]
- Ramirez, K.; Ditamo, Y.; Rodriguez, L.; Picking, W.L.; van Roosmalen, M.L.; Leenhouts, K.; Pasetti, M.F. Neonatal mucosal immunization with a non-living, non-genetically modified Lactococcus lactis vaccine carrier induces systemic and local Th1-type immunity and protects against lethal bacterial infection. Mucosal Immunol. 2010, 3, 159–171. [Google Scholar] [CrossRef]
- Saluja, V.; Visser, M.R.; van Roosmalen, M.L.; Leenhouts, K.; Huckriede, A.; Hinrichs, W.L.J.; Frijlink, H.W. Gastro-intestinal delivery of influenza subunit vaccine formulation adjuvanted with Gram-positive enhancer matrix (GEM) particles. Eur. J. Pharm. Biopharm. 2010, 76, 470–474. [Google Scholar] [CrossRef]
- Imanishi, T.; Hara, H.; Suzuki, S.; Suzuki, N.; Akira, S.; Saito, T. Cutting edge: TLR2 directly triggers Th1 effector functions. J. Immunol. 2007, 178, 6715–6719. [Google Scholar] [CrossRef]
- Zeng, G.C.; Chen, J.B.; Zhong, L.Y.; Wang, R.; Jiang, L.F.; Cai, J.Y.; Yan, L.; Huang, D.; Chen, C.Y.; Chen, Z.W. NSOM- and AFM-based nanotechnology elucidates nano-structural and atomic-force features of a Y-pestis V immunogen-containing particle vaccine capable of eliciting robust response. Proteomics 2009, 9, 1538–1547. [Google Scholar] [CrossRef] [PubMed]
- Van Braeckel-Budimir, N.; Haijema, B.J.; Leenhouts, K. Bacterium-like particles for efficient immune stimulation of existing vaccines and new subunit vaccines in mucosal applications. Front. Immunol. 2013, 4, 282. [Google Scholar] [CrossRef]
- Rigter, A.; Widjaja, I.; Versantvoort, H.; Coenjaerts, F.E.; van Roosmalen, M.; Leenhouts, K.; Rottier, P.J.; Haijema, B.J.; de Haan, C.A. A protective and safe intranasal RSV vaccine based on a recombinant prefusion-like form of the F protein bound to bacterium-like particles. PLoS ONE 2013, 8, e71072. [Google Scholar] [CrossRef] [PubMed]
- Li, P.C.; Qiao, X.W.; Zheng, Q.S.; Hou, J.B. Immunogenicity and immunoprotection of porcine circovirus type 2 (PCV2) Cap protein displayed by Lactococcus lactis. Vaccine 2016, 34, 696–702. [Google Scholar] [CrossRef] [PubMed]
- Heine, S.J.; Franco-Mahecha, O.L.; Chen, X.; Choudhari, S.; Blackwelder, W.C.; van Roosmalen, M.L.; Leenhouts, K.; Picking, W.L.; Pasetti, M.F. Shigella IpaB and IpaD displayed on L. lactis bacterium-like particles induce protective immunity in adult and infant mice. Immunol. Cell Biol. 2015, 93, 641–652. [Google Scholar] [CrossRef] [PubMed]
- Nganou-Makamdop, K.; van Roosmalen, M.L.; Audouy, S.A.; van Gemert, G.J.; Leenhouts, K.; Hermsen, C.C.; Sauerwein, R.W. Bacterium-like particles as multi-epitope delivery platform for Plasmodium berghei circumsporozoite protein induce complete protection against malaria in mice. Malar. J. 2012, 11, 50. [Google Scholar] [CrossRef]
- Lopez-Sagaseta, J.; Malito, E.; Rappuoli, R.; Bottomley, M.J. Self-assembling protein nanoparticles in the design of vaccines. Comput. Struct. Biotechnol. J. 2016, 14, 58–68. [Google Scholar] [CrossRef]
- Kanchan, V.; Panda, A.K. Interactions of antigen-loaded polylactide particles with macrophages and their correlation with the immune response. Biomaterials 2007, 28, 5344–5357. [Google Scholar] [CrossRef]
- Caputo, A.; Sparnacci, K.; Ensoli, B.; Tondelli, L. Functional polymeric nano/microparticles for surface adsorption and delivery of protein and DNA vaccines. Curr. Drug Deliv. 2008, 5, 230–242. [Google Scholar] [CrossRef]
- Petrovsky, N.; Aguilar, J.C. Vaccine adjuvants: Current state and future trends. Immunol. Cell Biol. 2004, 82, 488–496. [Google Scholar] [CrossRef]
- Miyaji, E.N.; Carvalho, E.; Oliveira, M.L.; Raw, I.; Ho, P.L. Trends in adjuvant development for vaccines: DAMPs and PAMPs as potential new adjuvants. Braz. J. Med Biol. Res. Rev. Bras. Pesqui. Med. Biol. 2011, 44, 500–513. [Google Scholar] [CrossRef]
- Brull, F.; Mensink, R.P.; van den Hurk, K.; Duijvestijn, A.; Plat, J. TLR2 activation is essential to induce a Th1 shift in human peripheral blood mononuclear cells by plant stanols and plant sterols. J. Biol. Chem. 2010, 285, 2951–2958. [Google Scholar] [CrossRef] [PubMed]
- Gaudin, Y. Rabies virus-induced membrane fusion pathway. J. Cell Biol. 2000, 150, 601–612. [Google Scholar] [CrossRef] [PubMed]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, E.; Yan, F.; Huang, P.; Chi, H.; Xu, S.; Li, G.; Liu, C.; Feng, N.; Wang, H.; Zhao, Y.; et al. Characterization of the Immune Response of MERS-CoV Vaccine Candidates Derived from Two Different Vectors in Mice. Viruses 2020, 12, 125. https://doi.org/10.3390/v12010125
Li E, Yan F, Huang P, Chi H, Xu S, Li G, Liu C, Feng N, Wang H, Zhao Y, et al. Characterization of the Immune Response of MERS-CoV Vaccine Candidates Derived from Two Different Vectors in Mice. Viruses. 2020; 12(1):125. https://doi.org/10.3390/v12010125
Chicago/Turabian StyleLi, Entao, Feihu Yan, Pei Huang, Hang Chi, Shengnan Xu, Guohua Li, Chuanyu Liu, Na Feng, Hualei Wang, Yongkun Zhao, and et al. 2020. "Characterization of the Immune Response of MERS-CoV Vaccine Candidates Derived from Two Different Vectors in Mice" Viruses 12, no. 1: 125. https://doi.org/10.3390/v12010125
APA StyleLi, E., Yan, F., Huang, P., Chi, H., Xu, S., Li, G., Liu, C., Feng, N., Wang, H., Zhao, Y., Yang, S., & Xia, X. (2020). Characterization of the Immune Response of MERS-CoV Vaccine Candidates Derived from Two Different Vectors in Mice. Viruses, 12(1), 125. https://doi.org/10.3390/v12010125