A Parallel Phenotypic Versus Target-Based Screening Strategy for RNA-Dependent RNA Polymerase Inhibitors of the Influenza A Virus
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Lines and Viruses
2.2. Compound Library and Control
2.3. Cell-Based Influenza RdRp Assay
2.4. Reporter Influenza Virus (PR8-Gluc) Infection Assay
2.5. Cell Viability
2.6. Titer Reduction Assay
2.7. Statistical Analysis
3. Results
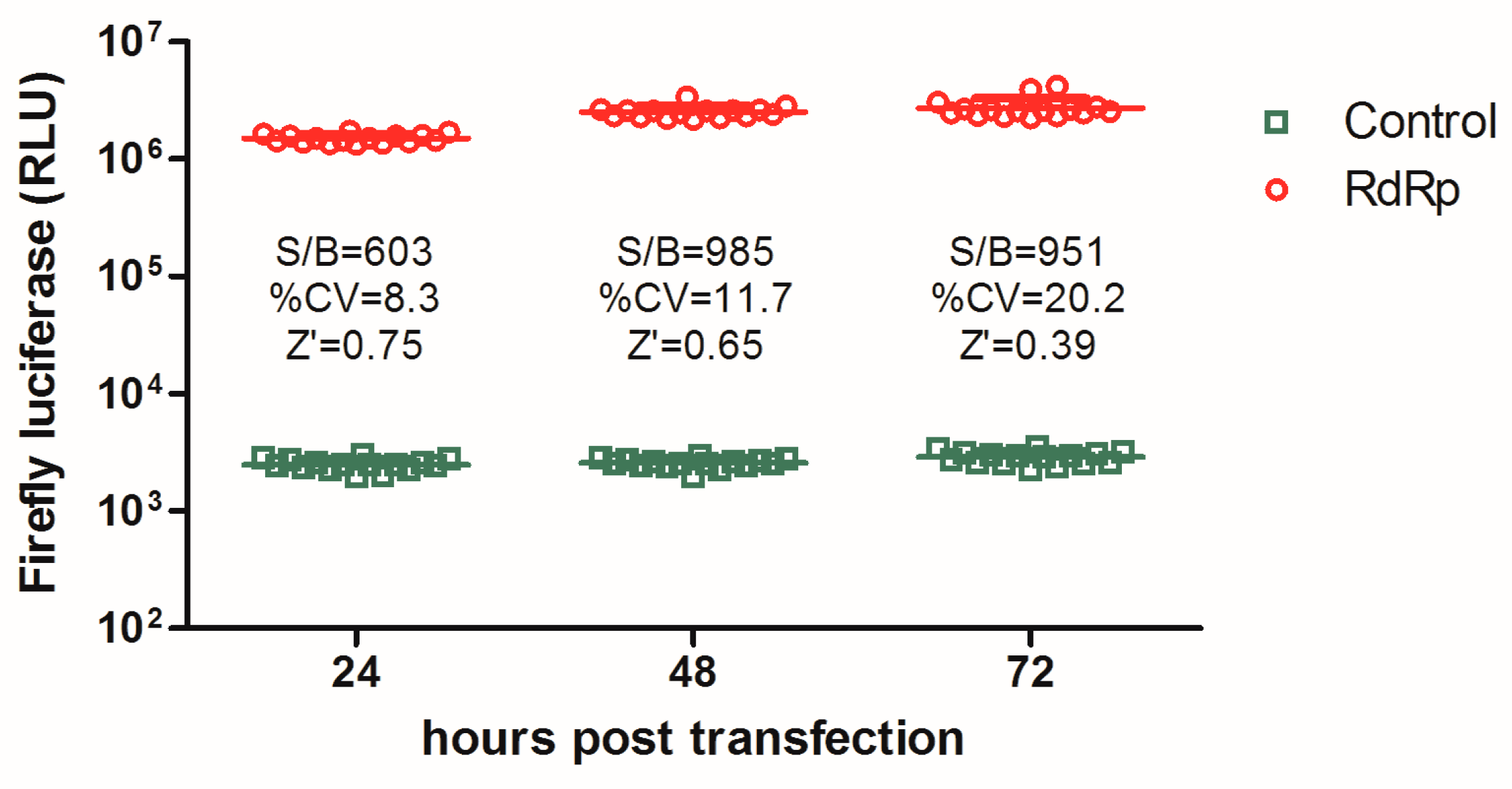
3.1. Establishment of an Influenza a Virus RdRp-Targeted HTS Assay
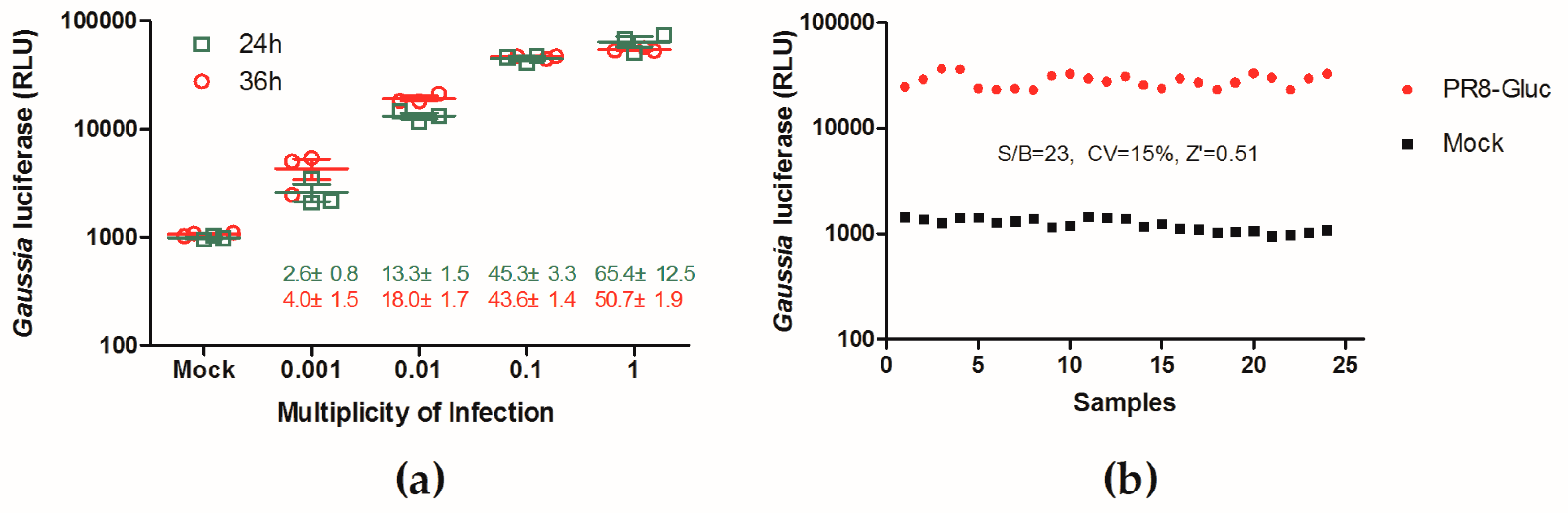
3.2. Evaluation of a Replication-Competent Reporter Influenza PR8-Gluc Virus for HTS
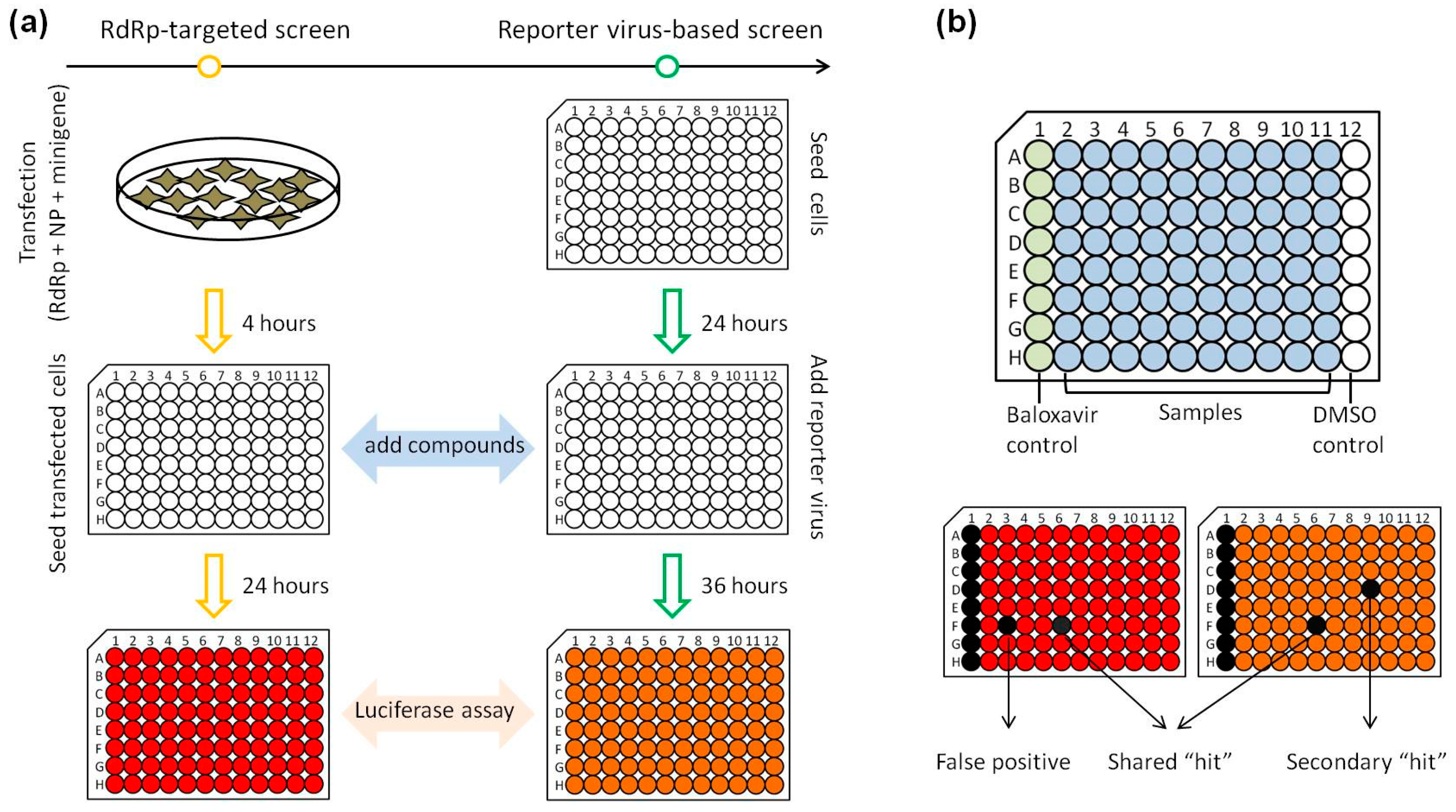
3.3. Development of a Parallel HTS Assay
3.4. Pilot Screen of a Compound Library of Natural Products
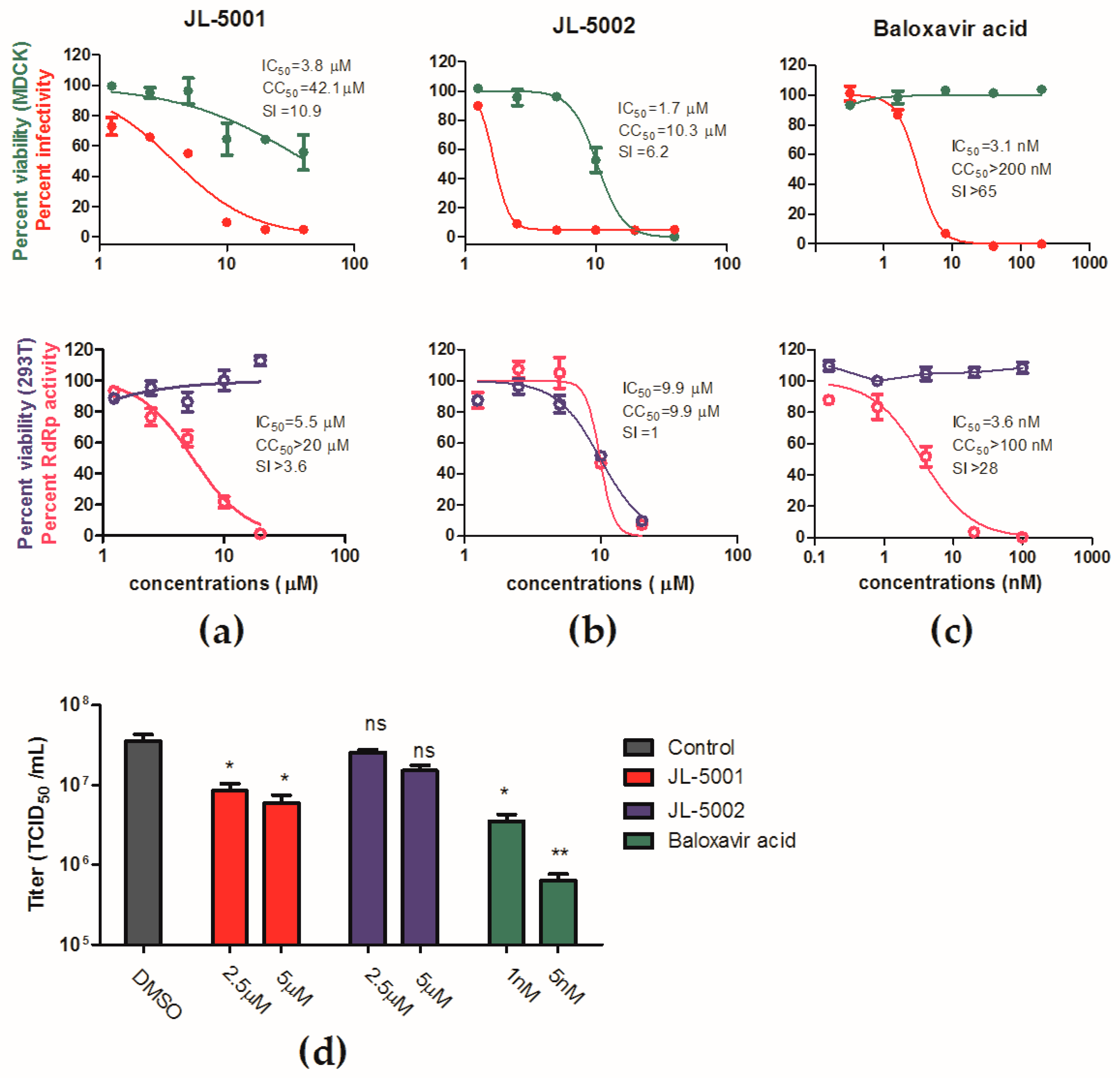
3.5. Identification of JL-5001 as an RdRp Inhibitor
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- WHO. 2018 Influenza (Seasonal) Fact Sheet; WHO: Geneva, Switzerland, 2018. [Google Scholar]
- Jiang, H.; Wu, P.; Uyeki, T.M.; He, J.; Deng, Z.; Xu, W.; Lv, Q.; Zhang, J.; Wu, Y.; Tsang, T.K.; et al. Preliminary Epidemiologic Assessment of Human Infections with Highly Pathogenic Avian Influenza A(H5N6) Virus, China. Clin. Infect. Dis. 2017, 65, 383–388. [Google Scholar] [CrossRef]
- Zhou, L.; Tan, Y.; Kang, M.; Liu, F.; Ren, R.; Wang, Y.; Chen, T.; Yang, Y.; Li, C.; Wu, J.; et al. Preliminary Epidemiology of Human Infections with Highly Pathogenic Avian Influenza A(H7N9) Virus, China, 2017. Emerg. Infect. Dis. 2017, 23, 1355–1359. [Google Scholar] [CrossRef]
- Ikematsu, H.; Kawai, N. Laninamivir octanoate: A new long-acting neuraminidase inhibitor for the treatment of influenza. Expert Rev. Anti Infect. Ther. 2011, 9, 851–857. [Google Scholar] [CrossRef]
- Alame, M.M.; Massaad, E.; Zaraket, H. Peramivir: A Novel Intravenous Neuraminidase Inhibitor for Treatment of Acute Influenza Infections. Front. Microbiol. 2016, 7, 647. [Google Scholar] [CrossRef]
- Lackenby, A.; Besselaar, T.G.; Daniels, R.S.; Fry, A.; Gregory, V.; Gubareva, L.V.; Huang, W.; Hurt, A.C.; Leang, S.-K.; Lee, R.T.; et al. Global update on the susceptibility of human influenza viruses to neuraminidase inhibitors and status of novel antivirals, 2016–2017. Antivir. Res. 2018, 157, 38–46. [Google Scholar] [CrossRef]
- Hata, M.; Tsuzuki, M.; Goto, Y.; Kumagai, N.; Harada, M.; Hashimoto, M.; Tanaka, S.; Sakae, K.; Kimura, T.; Minagawa, H.; et al. High frequency of amantadine-resistant influenza A (H3N2) viruses in the 2005-2006 season and rapid detection of amantadine-resistant influenza A (H3N2) viruses by MAMA-PCR. Jpn. J. Infect. Dis. 2007, 60, 202–204. [Google Scholar]
- Van Der Vries, E.; Schutten, M.; Fraaij, P.; Boucher, C.; Osterhaus, A. Influenza Virus Resistance to Antiviral Therapy. Adv. Pharmacol. 2013, 67, 217–246. [Google Scholar]
- Koszalka, P.; Tilmanis, D.; Hurt, A.C. Influenza antivirals currently in late-phase clinical trial. Influ. Other Respir. Viruses 2017, 11, 240–246. [Google Scholar] [CrossRef]
- Han, J.; Perez, J.; Schafer, A.; Cheng, H.; Peet, N.; Rong, L.; Manicassamy, B. Influenza Virus: Small Molecule Therapeutics and Mechanisms of Antiviral Resistance. Curr. Med. Chem. 2018, 25, 5115–5127. [Google Scholar] [CrossRef]
- Dias, A.; Bouvier, D.; Crepin, T.; McCarthy, A.A.; Hart, D.J.; Baudin, F.; Cusack, S.; Ruigrok, R.W.H. The cap-snatching endonuclease of influenza virus polymerase resides in the PA subunit. Nature 2009, 458, 914–918. [Google Scholar] [CrossRef]
- Yuan, P.; Bartlam, M.; Lou, Z.; Chen, S.; Zhou, J.; He, X.; Lv, Z.; Ge, R.; Li, X.; Deng, T.; et al. Crystal structure of an avian influenza polymerase PA(N) reveals an endonuclease active site. Nature 2009, 458, 909–913. [Google Scholar] [CrossRef]
- Fechter, P.; Mingay, L.; Sharps, J.; Fodor, E.; Brownlee, G.G.; Chambers, A. Two Aromatic Residues in the PB2 Subunit of Influenza A RNA Polymerase Are Crucial for Cap Binding. J. Boil. Chem. 2003, 278, 20381–20388. [Google Scholar] [CrossRef]
- Ma, X.; Xie, L.; Wartchow, C.; Warne, R.; Xu, Y.; Rivkin, A.; Tully, D.; Shia, S.; Uehara, K.; Baldwin, D.M.; et al. Structural basis for therapeutic inhibition of influenza A polymerase PB2 subunit. Sci. Rep. 2017, 7, 9385. [Google Scholar] [CrossRef]
- Xie, L.; Wartchow, C.; Shia, S.; Uehara, K.; Steffek, M.; Warne, R.; Sutton, J.; Muiru, G.T.; Leonard, V.H.; Bussiere, D.E.; et al. Molecular Basis of mRNA Cap Recognition by Influenza B Polymerase PB2 Subunit. J. Boil. Chem. 2016, 291, 363–370. [Google Scholar] [CrossRef]
- González, S.; Ortín, J. Characterization of Influenza Virus PB1 Protein Binding to Viral RNA: Two Separate Regions of the Protein Contribute to the Interaction Domain. J. Virol. 1999, 73, 631–637. [Google Scholar]
- Clark, M.P.; Ledeboer, M.W.; Davies, I.; Byrn, R.A.; Jones, S.M.; Perola, E.; Tsai, A.; Jacobs, M.; Nti-Addae, K.; Bandarage, U.K.; et al. Discovery of a novel, first-in-class, orally bioavailable azaindole inhibitor (VX-787) of influenza PB2. J. Med. Chem. 2014, 57, 6668–6678. [Google Scholar] [CrossRef]
- Furuta, Y.; Gowen, B.B.; Takahashi, K.; Shiraki, K.; Smee, D.F.; Barnard, D.L. Favipiravir (T-705), a novel viral RNA polymerase inhibitor. Antivir. Res. 2013, 100, 446–454. [Google Scholar] [CrossRef]
- Jones, J.C.; Marathe, B.M.; Vogel, P.; Gasser, R.; Najera, I.; Govorkova, E.A. The PA Endonuclease Inhibitor RO-7 Protects Mice from Lethal Challenge with Influenza A or B Viruses. Antimicrob. Agents Chemother. 2017, 61, e02460-16. [Google Scholar] [CrossRef]
- Noshi, T.; Kitano, M.; Taniguchi, K.; Yamamoto, A.; Omoto, S.; Baba, K.; Hashimoto, T.; Ishida, K.; Kushima, Y.; Hattori, K.; et al. In vitro characterization of baloxavir acid, a first-in-class cap-dependent endonuclease inhibitor of the influenza virus polymerase PA subunit. Antivir. Res. 2018, 160, 109–117. [Google Scholar] [CrossRef]
- Yuan, S.; Chu, H.; Singh, K.; Zhao, H.; Zhang, K.; Kao, R.Y.T.; Chow, B.K.C.; Zhou, J.; Zheng, B.-J. A novel small-molecule inhibitor of influenza A virus acts by suppressing PA endonuclease activity of the viral polymerase. Sci. Rep. 2016, 6, 22880. [Google Scholar] [CrossRef]
- Yuan, S.; Chu, H.; Zhang, K.; Ye, J.; Singh, K.; Kao, R.Y.T.; Chow, B.K.C.; Zhou, J.; Zheng, B.-J. A novel small-molecule compound disrupts influenza A virus PB2 cap-binding and inhibits viral replication. J. Antimicrob. Chemother. 2016, 71, 2489–2497. [Google Scholar] [CrossRef]
- Massari, S.; Nannetti, G.; DeSantis, J.; Muratore, G.; Sabatini, S.; Manfroni, G.; Mercorelli, B.; Cecchetti, V.; Palù, G.; Cruciani, G.; et al. A Broad Anti-influenza Hybrid Small Molecule That Potently Disrupts the Interaction of Polymerase Acidic Protein–Basic Protein 1 (PA-PB1) Subunits. J. Med. Chem. 2015, 58, 3830–3842. [Google Scholar] [CrossRef]
- Yuan, S.; Chu, H.; Zhao, H.; Zhang, K.; Singh, K.; Chow, B.K.; Kao, R.Y.; Zhou, J.; Zheng, B.-J. Identification of a small-molecule inhibitor of influenza virus via disrupting the subunits interaction of the viral polymerase. Antivir. Res. 2016, 125, 34–42. [Google Scholar] [CrossRef]
- Arivajiagane, A.; Varadharajulu, N.R.; Seerangan, K.; Rattinam, R.; Narendrakumar, R.V.; Kumar, S. In silico structure-based design of enhanced peptide inhibitors targeting RNA polymerase PAN-PB1C interaction. Comput. Boil. Chem. 2019, 78, 273–281. [Google Scholar] [CrossRef]
- He, X.; Zhou, J.; Bartlam, M.; Zhang, R.; Ma, J.; Lou, Z.; Li, X.; Li, J.; Joachimiak, A.; Zeng, Z.; et al. Crystal structure of the polymerase PA(C)-PB1(N) complex from an avian influenza H5N1 virus. Nature 2008, 454, 1123–1126. [Google Scholar] [CrossRef]
- Chase, G.; Wunderlich, K.; Reuther, P.; Schwemmle, M. Identification of influenza virus inhibitors which disrupt of viral polymerase protein–protein interactions. Methods 2011, 55, 188–191. [Google Scholar] [CrossRef]
- Massari, S.; Goracci, L.; DeSantis, J.; Tabarrini, O. Polymerase Acidic Protein–Basic Protein 1 (PA–PB1) Protein–Protein Interaction as a Target for Next-Generation Anti-influenza Therapeutics. J. Med. Chem. 2016, 59, 7699–7718. [Google Scholar] [CrossRef]
- Muratore, G.; Goracci, L.; Mercorelli, B.; Foeglein, A.; Digard, P.; Cruciani, G.; Palu, G.; Loregian, A. Small molecule inhibitors of influenza A and B viruses that act by disrupting subunit interactions of the viral polymerase. Proc. Natl. Acad. Sci. USA 2012, 109, 6247–6252. [Google Scholar] [CrossRef]
- Roche Announces FDA Approval of Xofluza (Baloxavir Marboxil) for Influenza. Available online: https://www.roche.com/media/releases/med-cor-2018-10-24.htm (accessed on 5 September 2019).
- Delang, L.; Abdelnabi, R.; Neyts, J. Favipiravir as a potential countermeasure against neglected and emerging RNA viruses. Antivir. Res. 2018, 153, 85–94. [Google Scholar] [CrossRef]
- Principi, N.; Camilloni, B.; Alunno, A.; Polinori, I.; Argentiero, A.; Esposito, S. Drugs for Influenza Treatment: Is There Significant News? Front. Med. 2019, 6, 109. [Google Scholar] [CrossRef]
- Wang, Z.; Zhao, F.; Gao, Q.; Liu, Z.; Zhang, Y.; Li, X.; Li, Y.; Ma, W.; Deng, T.; Zhang, Z.; et al. Establishment of a High-Throughput Assay to Monitor Influenza A Virus RNA Transcription and Replication. PLoS ONE 2015, 10, e0133558. [Google Scholar] [CrossRef]
- Ozawa, M.; Shimojima, M.; Goto, H.; Watanabe, S.; Hatta, Y.; Kiso, M.; Furuta, Y.; Horimoto, T.; Peters, N.R.; Hoffmann, F.M.; et al. A cell-based screening system for influenza A viral RNA transcription/replication inhibitors. Sci. Rep. 2013, 3, 1106. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, L.; Cui, Q.; Li, P.; Wang, Y.; Zhang, Y.; Yang, Y.; Rong, L.; Du, R. A Mechanism Underlying Attenuation of Recombinant Influenza A Viruses Carrying Reporter Genes. Viruses 2018, 10, 679. [Google Scholar] [CrossRef]
- HTS Assay Validation. Available online: https://www.ncbi.nlm.nih.gov/books/NBK83783/ (accessed on 5 September 2019).
- Heaton, N.S.; Leyva-Grado, V.H.; Tan, G.S.; Eggink, D.; Hai, R.; Palese, P. In Vivo Bioluminescent Imaging of Influenza A Virus Infection and Characterization of Novel Cross-Protective Monoclonal Antibodies. J. Virol. 2013, 87, 8272–8281. [Google Scholar] [CrossRef]
- Gao, Q.; Wang, Z.; Liu, Z.; Li, X.; Zhang, Y.; Zhang, Z.; Cen, S. A cell-based high-throughput approach to identify inhibitors of influenza A virus. Acta Pharm. Sin. B 2014, 4, 301–306. [Google Scholar] [CrossRef][Green Version]
- Cheng, H.; Koning, K.; O’Hearn, A.; Wang, M.; Rumschlag-Booms, E.; Varhegyi, E.; Rong, L. A parallel genome-wide RNAi screening strategy to identify host proteins important for entry of Marburg virus and H5N1 influenza virus. Virol. J. 2015, 12, 665. [Google Scholar] [CrossRef]
- Cui, Q.; Cheng, H.; Xiong, R.; Zhang, G.; Du, R.; Anantpadma, M.; Davey, R.A.; Rong, L. Identification of Diaryl-Quinoline Compounds as Entry Inhibitors of Ebola Virus. Viruses 2018, 10, 678. [Google Scholar] [CrossRef]
- Li, P.; Cui, Q.; Wang, L.; Zhao, X.; Zhang, Y.; Manicassamy, B.; Yang, Y.; Rong, L.; Du, R. A Simple and Robust Approach for Evaluation of Antivirals Using a Recombinant Influenza Virus Expressing Gaussia Luciferase. Viruses 2018, 10, 325. [Google Scholar] [CrossRef]
- Newman, D. Screening and identification of novel biologically active natural compounds. F1000Research 2017, 6, 783. [Google Scholar] [CrossRef]
- Tulloch, L.B.; Menzies, S.K.; Coron, R.P.; Roberts, M.D.; Florence, G.J.; Smith, T.K. Direct and indirect approaches to identify drug modes of action. IUBMB Life 2018, 70, 9–22. [Google Scholar] [CrossRef]
- Su, C.Y.; Cheng, T.J.; Lin, M.I.; Wang, S.Y.; Huang, W.I.; Lin-Chu, S.Y.; Chen, Y.H.; Wu, C.Y.; Lai, M.M.; Cheng, W.C.; et al. High-throughput identification of compounds targeting influenza RNA-dependent RNA polymerase activity. Proc. Natl. Acad. Sci. USA 2010, 107, 19151–19156. [Google Scholar] [CrossRef]
- Weisshaar, M.; Cox, R.; Morehouse, Z.; Kyasa, S.K.; Yan, D.; Oberacker, P.; Mao, S.; Golden, J.E.; Lowen, A.C.; Natchus, M.G.; et al. Identification and Characterization of Influenza Virus Entry Inhibitors through Dual Myxovirus High-Throughput Screening. J. Virol. 2016, 90, 7368–7387. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, X.; Wang, Y.; Cui, Q.; Li, P.; Wang, L.; Chen, Z.; Rong, L.; Du, R. A Parallel Phenotypic Versus Target-Based Screening Strategy for RNA-Dependent RNA Polymerase Inhibitors of the Influenza A Virus. Viruses 2019, 11, 826. https://doi.org/10.3390/v11090826
Zhao X, Wang Y, Cui Q, Li P, Wang L, Chen Z, Rong L, Du R. A Parallel Phenotypic Versus Target-Based Screening Strategy for RNA-Dependent RNA Polymerase Inhibitors of the Influenza A Virus. Viruses. 2019; 11(9):826. https://doi.org/10.3390/v11090826
Chicago/Turabian StyleZhao, Xiujuan, Yanyan Wang, Qinghua Cui, Ping Li, Lin Wang, Zinuo Chen, Lijun Rong, and Ruikun Du. 2019. "A Parallel Phenotypic Versus Target-Based Screening Strategy for RNA-Dependent RNA Polymerase Inhibitors of the Influenza A Virus" Viruses 11, no. 9: 826. https://doi.org/10.3390/v11090826
APA StyleZhao, X., Wang, Y., Cui, Q., Li, P., Wang, L., Chen, Z., Rong, L., & Du, R. (2019). A Parallel Phenotypic Versus Target-Based Screening Strategy for RNA-Dependent RNA Polymerase Inhibitors of the Influenza A Virus. Viruses, 11(9), 826. https://doi.org/10.3390/v11090826





