Detection of Pig Cells Harboring Porcine Endogenous Retroviruses in Non-Human Primate Bladder After Renal Xenotransplantation
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal and Tissues
2.2. Xenotransplantation
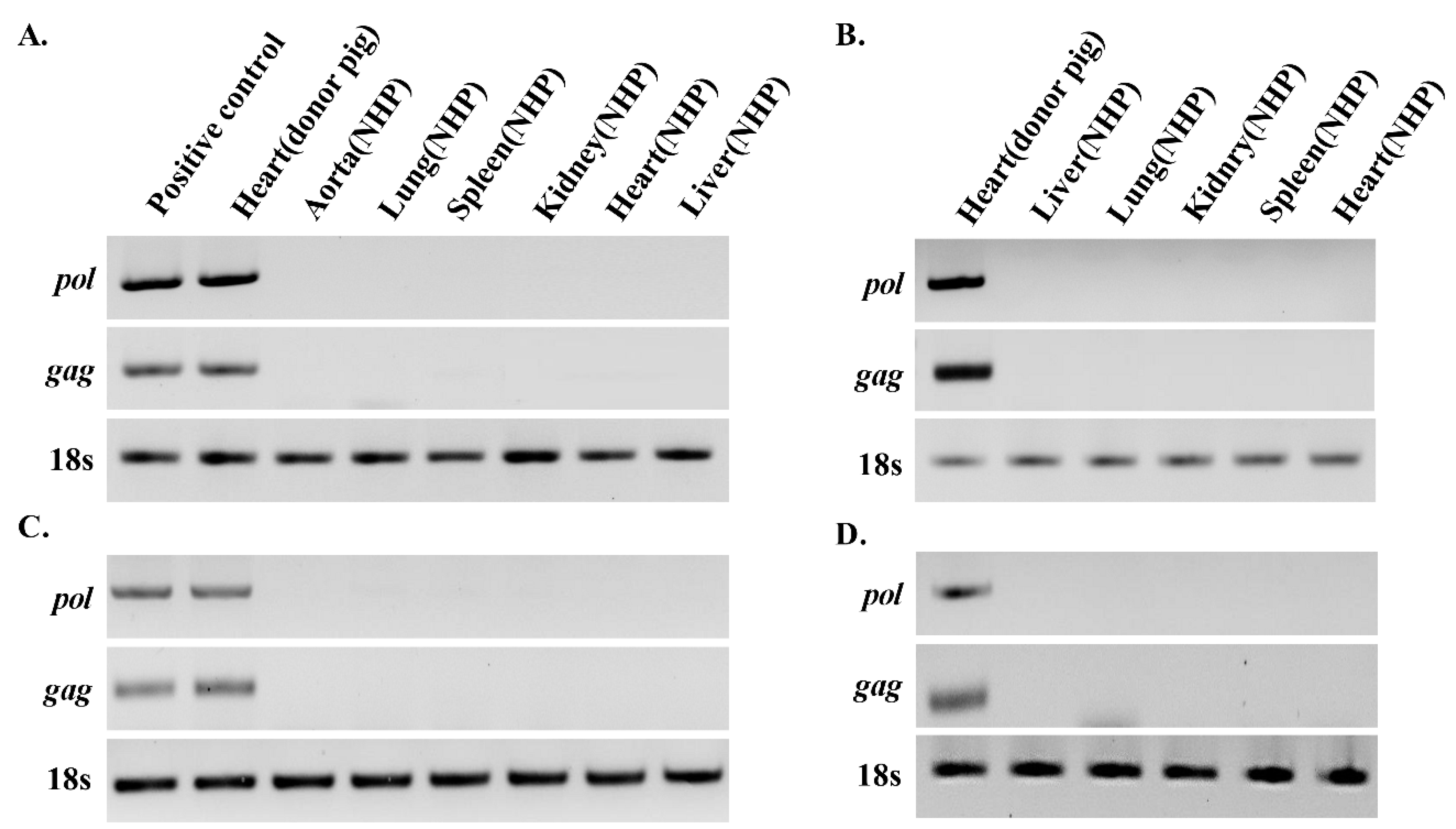
2.3. PCR and RT-PCR Detection of PERV Genes
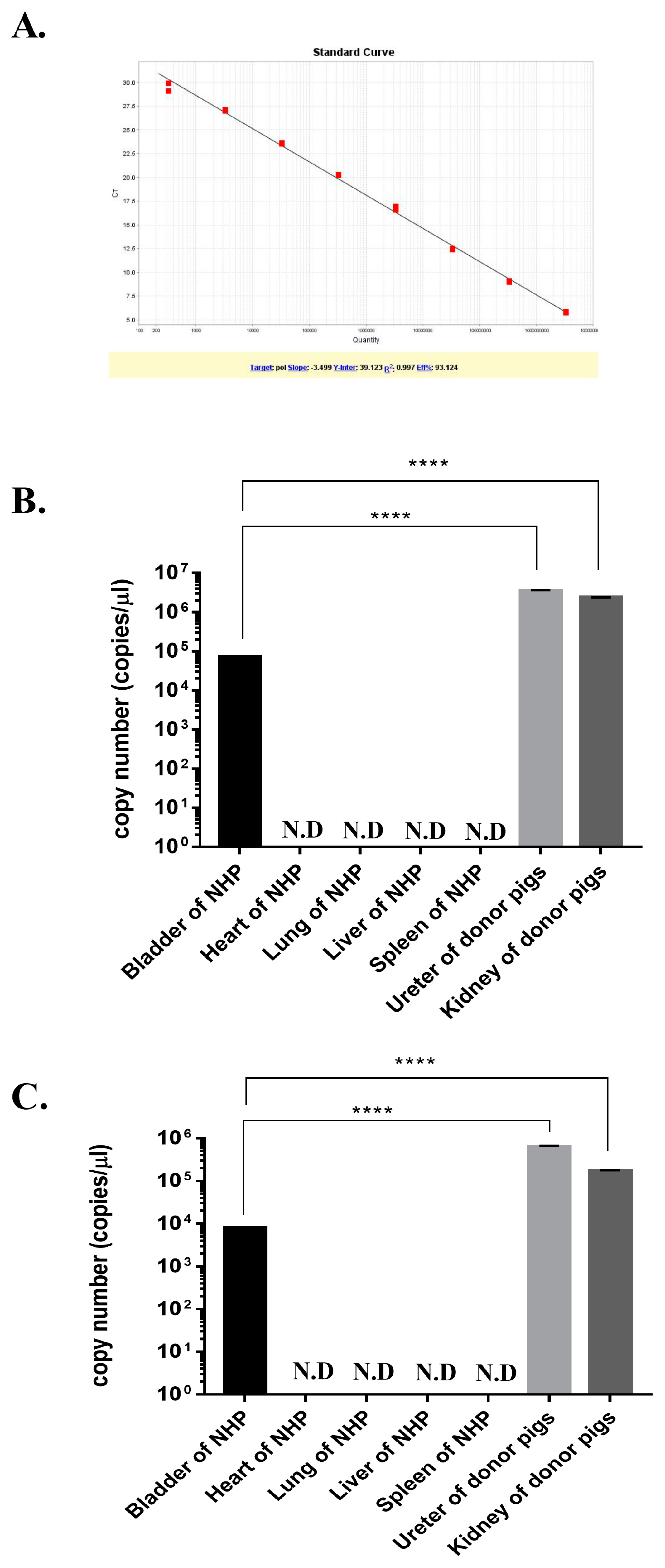
2.4. Quantitative Real-Time PCR Analysis of PERV Genes
2.5. Analysis of PERV Integration
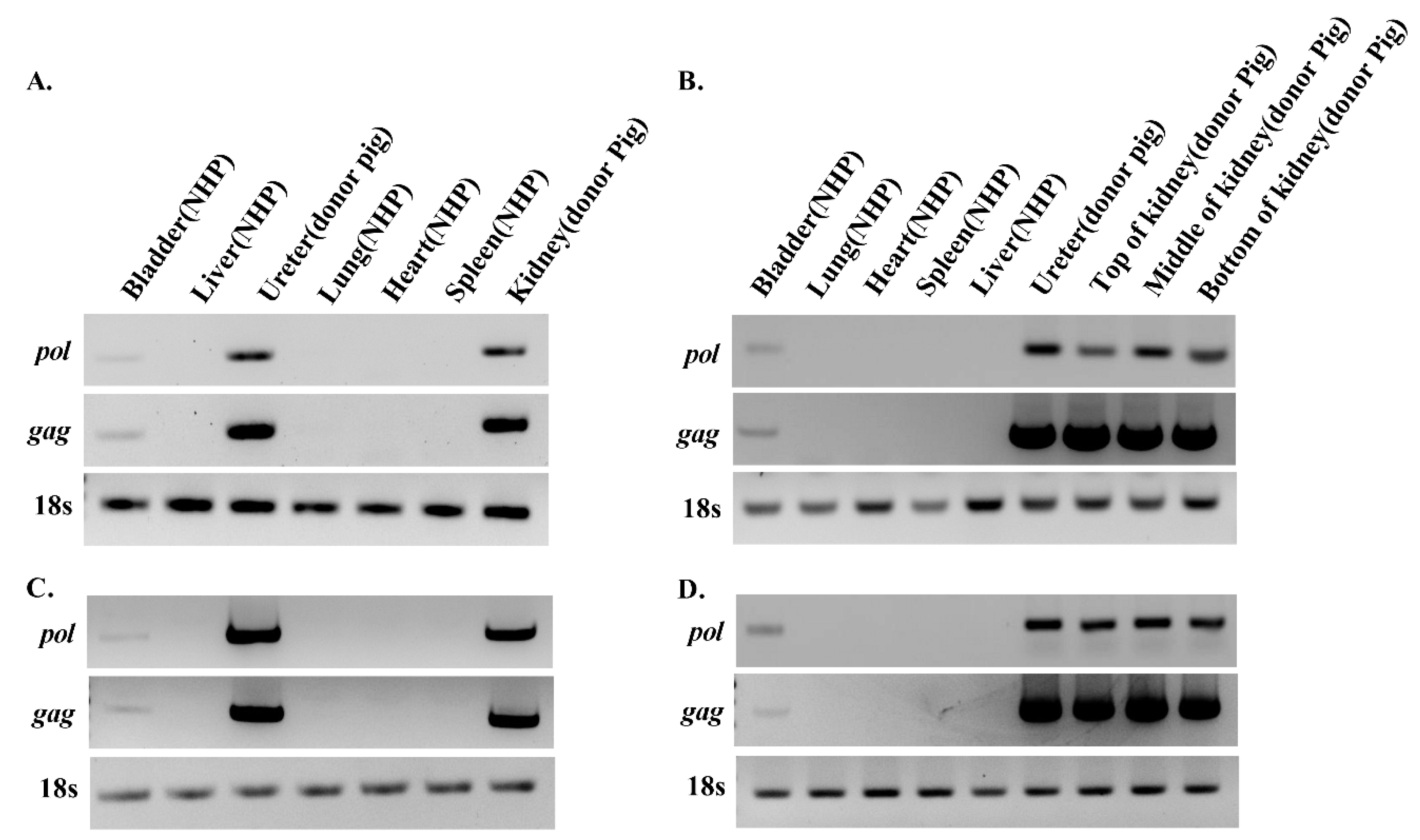
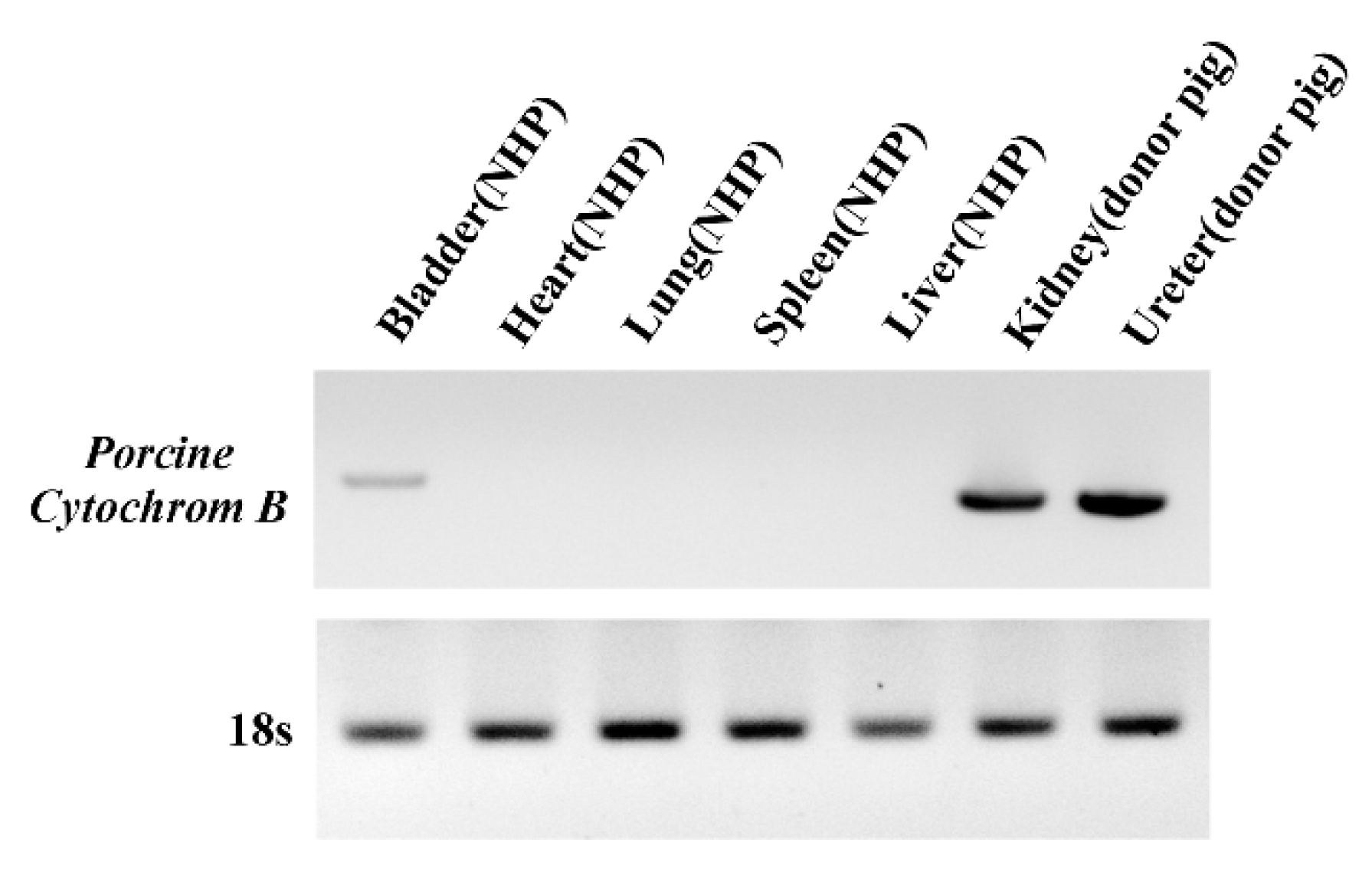
2.6. Analysis of Porcine Cell Microchimerism using PCR
3. Results
3.1. PERV Is not Detected in Recipient NHPs of Heart Xenotransplantation
3.2. PERV Is Detected in Recipient NHPs of Kidney Xenotransplantation
3.3. Quantitative Real-Time PCR Analysis of PERV
3.4. Detection of PERV Is Not Due to Integration into the NHP Chromosome but to the Presence of Porcine Cells
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Griesemer, A.D.; Hirakata, A.; Shimizu, A.; Moran, S.; Tena, A.; Iwaki, H.; Ishikawa, Y.; Schule, P.; Arn, J.S.; Robson, S.C.; et al. Results of Gal-Knockout Porcine Thymokidney Xenografts. Am. J. Transplant. 2009, 9, 2669–2678. [Google Scholar] [CrossRef] [PubMed]
- Yamada, K.; Yazawa, K.; Shimizu, A.; Iwanaga, T.; Hisashi, Y.; Nuhn, M.; O’Malley, P.; Nobori, S.; Vagefi, P.A.; Patience, C.; et al. Marked prolongation of porcine renal xenograft survival in baboons through the use of alpha 1,3-galactosyltransferase gene-knockout donors and the cotransplantation of vascularized thymic tissue. Nat. Med. 2005, 11, 32–34. [Google Scholar] [CrossRef] [PubMed]
- Michel, S.G.; Madariaga, M.L.L.; Villani, V.; Shanmugarajah, K. Current progress in xenotransplantation and organ bioengineering. Int. J. Surg. 2015, 13, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Mou, L.S.; Chen, F.J.; Dai, Y.F.; Cai, Z.M.; Cooper, D.K.C. Potential alternative approaches to xenotransplantation. Int. J. Surg. 2015, 23, 322–326. [Google Scholar] [CrossRef] [PubMed]
- Cooper, D.K.C.; Gaston, R.; Eckhoff, D.; Ladowski, J.; Yamamoto, T.; Wang, L.; Iwase, H.; Hara, H.; Tector, M.; Tector, A.J. Xenotransplantation-the current status and prospects. Br. Med. Bull. 2018, 125, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Hryhorowicz, M.; Zeyland, J.; Slomski, R.; Lipinski, D. Genetically Modified Pigs as Organ Donors for Xenotransplantation. Mol. Biotechnol. 2017, 59, 435–444. [Google Scholar] [CrossRef] [PubMed]
- Denner, J. How Active Are Porcine Endogenous Retroviruses (PERVs)? Viruses-Basel 2016, 8, 215. [Google Scholar] [CrossRef] [PubMed]
- Lopata, K.; Wojdas, E.; Nowak, R.; Lopata, P.; Mazurek, U. Porcine Endogenous Retrovirus (PERV) - Molecular Structure and Replication Strategy in the Context of Retroviral Infection Risk of Human Cells. Front. Microbiol. 2018, 9, 730. [Google Scholar] [CrossRef] [PubMed]
- Denner, J. Can Antiretroviral Drugs Be Used to Treat Porcine Endogenous Retrovirus (PERV) Infection after Xenotransplantation? Viruses-Basel 2017, 9, 213. [Google Scholar] [CrossRef]
- Karlas, A.; Irgang, M.; Votteler, J.; Specke, V.; Ozel, M.; Kurth, R.; Denner, J. Characterisation of a human cell-adapted porcine endogenous retrovirus PERV-A/C. Ann. Transplant 2010, 15, 45–54. [Google Scholar]
- Deng, Y.M.; Tuch, B.E.; Rawlinson, W.D. Transmission of porcine endogenous retroviruses in severe combined immunodeficient mice xenotransplanted with fetal porcine pancreatic cells. Transplantation 2000, 70, 1010–1016. [Google Scholar] [CrossRef] [PubMed]
- van der Laan, L.J.W.; Lockey, C.; Griffeth, B.C.; Frasier, F.S.; Wilson, C.A.; Onions, D.E.; Hering, B.J.; Long, Z.F.; Otto, E.; Torbett, B.E.; et al. Infection by porcine endogenous retrovirus after islet xenotransplantation in SCID mice. Nature 2000, 407, 90–94. [Google Scholar] [CrossRef] [PubMed]
- Martina, Y.; Marcucci, K.T.; Cherqui, S.; Szabo, A.; Drysdale, T.; Srinivisan, U.; Wilson, C.A.; Patience, C.; Salomon, D.R. Mice transgenic for a human porcine endogenous retrovirus receptor are susceptible to productive viral infection. J. Virol. 2006, 80, 5100. [Google Scholar] [CrossRef]
- Bittmann, I.; Mihica, D.; Plesker, R.; Denner, J. Expression of porcine endogenous retroviruses (PERV) in different organs of a pig. Virology 2012, 433, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Ericsson, T.A.; Takeuchi, Y.; Templin, C.; Quinn, G.; Farhadian, S.F.; Wood, J.C.; Oldmixon, B.A.; Suling, K.M.; Ishii, J.K.; Kitagawa, Y.; et al. Identification of receptors for pig endogenous retrovirus. Proc. Natl. Acad. Sci. USA 2003, 100, 6759–6764. [Google Scholar] [CrossRef]
- Pierson, R.N.; Dorling, A.; Ayares, D.; Rees, M.A.; Seebach, J.D.; Fishman, J.A.; Hering, B.J.; Cooper, D.K.C. Current status of xenotransplantation and prospects for clinical application. Xenotransplantation 2009, 16, 263–280. [Google Scholar] [CrossRef] [PubMed]
- Higginbotham, L.; Mathews, D.; Breeden, C.A.; Song, M.Q.; Farris, A.B.; Larsen, C.P.; Ford, M.L.; Lutz, A.J.; Tector, M.; Newell, K.A.; et al. Pre-transplant antibody screening and anti-CD154 costimulation blockade promote long-term xenograft survival in a pig-to-primate kidney transplant model. Xenotransplantation 2015, 22, 221–230. [Google Scholar] [CrossRef]
- Iwase, H.; Hara, H.; Ezzelarab, M.; Li, T.; Zhang, Z.Q.; Gao, B.S.; Liu, H.; Long, C.; Wang, Y.; Cassano, A.; et al. Immunological and physiological observations in baboons with life-supporting genetically engineered pig kidney grafts. Xenotransplantation 2017, 24, 1–13. [Google Scholar] [CrossRef]
- Shin, J.S.; Kim, J.M.; Kim, J.S.; Min, B.H.; Kim, Y.H.; Kim, H.J.; Jang, J.Y.; Yoon, I.H.; Kang, H.J.; Kim, J.; et al. Long-term control of diabetes in immunosuppressed nonhuman primates (NHP) by the transplantation of adult porcine islets. Am. J. Transplant 2015, 15, 2837–2850. [Google Scholar] [CrossRef]
- Mohiuddin, M.M.; Singh, A.K.; Corcoran, P.C.; Thomas, M.L.; Clark, T.; Lewis, B.G.; Hoyt, R.F.; Eckhaus, M.; Pierson, R.N.; Belli, A.J.; et al. Chimeric 2C10R4 anti-CD40 antibody therapy is critical for long-term survival of GTKO.hCD46.hTBM pig-to-primate cardiac xenograft. Nat. Commun. 2016, 7, 11138. [Google Scholar] [CrossRef]
- Kim, N.; Choi, J.; Kim, S.; Gwon, Y.D.; Cho, Y.; Yang, J.M.; Oh, Y.K.; Kim, Y.B. Transmission of Porcine Endogenous Retrovirus Produced from Different Recipient Cells In Vivo. Plos ONE 2016, 11, e0165156. [Google Scholar] [CrossRef] [PubMed]
- Sandrin, M.S.; McKenzie, I.F.C. Gal-alpha(1,3)gal, the major xenoantigen(s) recognized in pigs by human natural antibodies. Immunol. Rev. 1994, 141, 169–190. [Google Scholar] [CrossRef] [PubMed]
- Cooper, D.K.C.; Koren, E.; Oriol, R. Oligosaccharides and discordant xenotransplantation. Immunol. Rev. 1994, 141, 31–58. [Google Scholar] [CrossRef] [PubMed]
- Hisashi, Y.; Yamada, K.; Kuwaki, K.; Tseng, Y.L.; Dor, F.; Houser, S.L.; Robson, S.C.; Schuurman, H.J.; Cooper, D.K.C.; Sachs, D.H.; et al. Rejection of Cardiac Xenografts Transplanted from alpha 1,3-Galactosyltransferase Gene-Knockout (GalT-KO) Pigs to Baboons. Am. J. Transplant. 2008, 8, 2516–2526. [Google Scholar] [CrossRef] [PubMed]
- Dwyer, K.M.; Robson, S.C.; Nandurkar, H.H.; Campbell, D.J.; Gock, H.; Murray-Segal, L.J.; Fisicaro, N.; Mysore, T.B.; Kaczmarek, E.; Cowan, P.J.; et al. Thromboregulatory manifestations in human CD39 transgenic mice and the implications for thrombotic disease and transplantation. J. Clin. Invest. 2004, 113, 1440–1446. [Google Scholar] [CrossRef] [PubMed]
- Imai, M.; Takigami, K.; Guckelberger, O.; Kaczmarek, E.; Csizmadia, E.; Bach, F.H.; Robson, S.C. Recombinant adenoviral mediated CD39 gene transfer prolongs cardiac xenograft survival. Transplantation 2000, 70, 864–870. [Google Scholar] [CrossRef] [PubMed]
- Cai, M.; Huttinger, Z.M.; He, H.; Zhang, W.Z.; Li, F.; Goodman, L.A.; Wheeler, D.G.; Druhan, L.J.; Zweier, J.L.; Dwyer, K.M.; et al. Transgenic over expression of ectonucleotide triphosphate diphosphohydrolase-1 protects against murine myocardial ischemic injury. J. Mol. Cell. Cardiol. 2011, 51, 927–935. [Google Scholar] [CrossRef] [PubMed]
- Loveland, B.E.; Milland, J.; Kyriakou, P.; Thorley, B.R.; Christiansen, D.; Lanteri, M.B.; van Regensburg, M.; Duffield, M.; French, A.J.; Williams, L.; et al. Characterization of a CD46 transgenic pig and protection of transgenic kidneys against hyperacute rejection in non-immunosuppressed baboons. Xenotransplantation 2004, 11, 171–183. [Google Scholar] [CrossRef] [PubMed]
- Fischer, K.; Kraner-Scheiber, S.; Petersen, B.; Rieblinger, B.; Buermann, A.; Flisikowska, T.; Flisikowski, K.; Christan, S.; Edlinger, M.; Baars, W.; et al. Efficient production of multi-modified pigs for xenotransplantation by ‘combineering’, gene stacking and gene editing. Sci. Rep. 2016, 6, 29081. [Google Scholar] [CrossRef] [PubMed]
- Jensen, T.S.; Bjorge, L.; Wollen, A.L.; Ulstein, M. Identification of the complement regulatory proteins cd46, cd55, and cd59 in human fallopian-tube, endometrium, and cervical mucosa and secretion. Am. J. Reprod. Immunol. 1995, 34, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ko, N.; Lee, J.W.; Hwang, S.S.; Kim, B.; Ock, S.A.; Lee, S.S.; Im, G.S.; Kang, M.J.; Park, J.K.; Oh, S.J.; et al. Nucleofection-Mediated 1,3-galactosyltransferase Gene Inactivation and Membrane Cofactor Protein Expression for Pig-to-Primate Xenotransplantation. Anim. Biotechnol. 2013, 24, 253–267. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.; Oh, K.B.; Kwon, D.J.; Ock, S.A.; Lee, J.W.; Im, G.S.; Lee, S.S.; Lee, K.; Park, J.K. Improvement of Cloning Efficiency in Minipigs Using Post-thawed Donor Cells Treated with Roscovitine. Mol. Biotechnol. 2013, 55, 212–216. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.; Shim, J.; Ko, N.; Eom, H.; Kim, J.; Lee, J.W.; Jin, D.I.; Kim, H. Production of heterozygous alpha 1,3-galactosyltransferase (GGTA1) knock-out transgenic miniature pigs expressing human CD39. Transgenic Res. 2017, 26, 209–224. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Chee, H.K.; Yang, J.; Hwang, S.; Han, K.H.; Kang, J.; Park, J.H.; Kim, J.S.; Lee, S.J.; Ock, S.A.; et al. Outcomes of Alpha 1,3-GT-knockout Porcine Heart Transplants Into a Preclinical Nonhuman Primate Model (vol 45, pg 3085, 2013). Transplant Proc. 2014, 46, 301. [Google Scholar] [CrossRef]
- Lee, D.; Lee, J.; Kim, H.; Park, H.; Kim, Y. Detection and Classification of Porcine Endogenous Retroviruses by Polymerase Chain Reaction. J. Anim. Sci. Technol. 2007, 49, 405–414. [Google Scholar]
- Soncini, M.; Signoroni, P.B.; Bailo, M.; Zatti, D.; Gregori, A.; Lombardi, G.; Albertini, A.; Wengler, G.S.; Parolini, O. Use of highly sensitive mitochondrial probes to detect microchimerism in xenotransplantation models. Xenotransplantation 2006, 13, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Morozov, V.A.; Wynyard, S.; Matsumoto, S.; Abalovich, A.; Denner, J.; Elliott, R. No PERV transmission during a clinical trial of pig islet cell transplantation. Virus. Res. 2017, 227, 34–40. [Google Scholar] [CrossRef]
- Yamada, K.; Sykes, M.; Sachs, D.H. Tolerance in xenotransplantation. Curr. Opin. Organ Transpl. 2017, 22, 522–528. [Google Scholar] [CrossRef] [PubMed]
- Moscoso, I.; Hermida-Prieto, M.; Manez, R.; Lopez-Pelaez, E.; Centeno, A.; Diaz, T.M.; Domenech, N. Lack of cross-species transmission of porcine endogenous retrovirus in pig-to-baboon xenotransplantation with sustained depletion of anti-alpha Gal antibodies. Transplantation 2005, 79, 777–782. [Google Scholar] [CrossRef]
- Issa, N.C.; Wilkinson, R.A.; Griesemer, A.; Cooper, D.K.C.; Yamada, K.; Sachs, D.H.; Fishman, J.A. Absence of Replication of Porcine Endogenous Retrovirus and Porcine Lymphotropic Herpesvirus Type 1 with Prolonged Pig Cell Microchimerism after Pig-to-Baboon Xenotransplantation. J. Virol. 2008, 82, 12441–12448. [Google Scholar] [CrossRef][Green Version]
- Denner, J. Why was PERV not transmitted during preclinical and clinical xenotransplantation trials and after inoculation of animals? Retrovirology 2018, 15, 28. [Google Scholar] [CrossRef] [PubMed]
- Gola, J.; Mazurek, U. Detection of porcine endogenous retrovirus in xenotransplantation. Reprod. Biol. 2014, 14, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Specke, V.; Langford, G.; Schuurman, H.J.; Coulibaly, C.; Plesker, R.; Kurth, R.; Denner, J. Porcine endogenous retroviruses (PERVs): Studies on transmission in vitro and inoculation of small animals and non-human primates in vivo. Xenotransplantation 2001, 8, 34–35. [Google Scholar]
- Dinsmore, J.H.; Manhart, C.; Raineri, R.; Jacoby, D.B.; Moore, A. No evidence for infection of human cells with porcine endogenous retrovirus (PERV) after exposure to porcine fetal neuronal cells. Transplantation 2000, 70, 1382–1389. [Google Scholar] [CrossRef] [PubMed]
- Denner, J.; Specke, V.; Tacke, S.; Schwendemann, J.; Coulibaly, C.; Plesker, R.; Kurth, R.; Langford, G.; Schuuman, H.J. PERVs: Diagnostics, adaptation to human cells, but no transmission to small animals, non-human primates and man. Xenotransplantation 2001, 8, 126–127. [Google Scholar]
| Type | Transgenic Pig | Rhesus Macaques | Survival Date (After Transplantation, Day) |
|---|---|---|---|
| Heart | GT-MCP/-MCP 1 | NHP23-16 | 60 |
| Heart | GT-CD39/-CD39 2 | NHP20-01 | 18 |
| Kidney | GT-CD39/-CD39 | NHP20-06 | 32 |
| Kidney | GT-CD39/-CD39 | NHP23-30 | 25 |
| Name | Sequence (5′ to 3′) |
|---|---|
| PERV-pol-F | GATGAGCGTAAGGGAGTAGC |
| PERV-pol-R | TGCTTCCGTCAGTGAACCAG |
| PERV-gag-F | CCCGATCAGGAGCCCTATATCCTTACGTG |
| PERV-gag-R | CGCAGCGGTAATGTCGCGATCTCGT |
| PERV LTR 1-F | ATGCCCCCGAATTCCAGA |
| PERV LTR 1-R | GGTTAGGTTGCATTTTCATCCTT |
| PERV LTR 2-F | CCCCGAATTCCAGACCCT |
| PERV LTR 2-R | AGGTTGCATTTTCATCCTTTCATT |
| Porcine cytochrome B-F | CATTGGAGTAGTCCTACTATTTCCG |
| Porcine cytochrome B-R | CATTGGAGTAGTCCTACTATTTCCG |
| 18sRNA-F | GTTCCGACCATAAACGATGCC |
| 18sRNA-R | TGGTGGTGCCCTTCCGTCAAT |
| Junction Sequence 1 | Position | Species |
|---|---|---|
| ATGCCCCCGAATTCCAGACCCTGTTCCCTATAGGTAAAAGATCATGGTACTTAGACAGCA | LOC110259374 | Sus scrofa |
| ATGCCCCCGAATTCCAGACCCTGCTCCCTGCCAATAAATAGGTAGAAGGTCACACTTCTT | CH242-417C1 on chromosome 4 | Pig |
| ATGCCCCCGAATTCCAGACCCTGCTCCCTGCCAGTAAATCGGTAGAAGGTCACACTTCT | LOC110261659 | Sus scrofa |
| ATGCCCCGAATTCCAGACCCTGTTCCCTATAGGTAAAAGATCATGGTACTTAGACAGCAG | LOC110256025 | Sus scrofa |
| ATGCCCCGAATTCCAGATCCTTTCATTCCCCACTTCTTCTCTTGTTAATAGTTCTAA | LOC110261658 | Sus scrofa |
| ATGCCCCGAATTCCAGATACCAAGGCCTTCCGAGCTAAGGAGAAACTGACCTTTAGCCT | CH242-160D12 on chromosome X | Sus scrofa |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heo, Y.; Cho, Y.; Oh, K.B.; Park, K.H.; Cho, H.; Choi, H.; Kim, M.; Yun, I.J.; Lee, H.J.; Kim, Y.B. Detection of Pig Cells Harboring Porcine Endogenous Retroviruses in Non-Human Primate Bladder After Renal Xenotransplantation. Viruses 2019, 11, 801. https://doi.org/10.3390/v11090801
Heo Y, Cho Y, Oh KB, Park KH, Cho H, Choi H, Kim M, Yun IJ, Lee HJ, Kim YB. Detection of Pig Cells Harboring Porcine Endogenous Retroviruses in Non-Human Primate Bladder After Renal Xenotransplantation. Viruses. 2019; 11(9):801. https://doi.org/10.3390/v11090801
Chicago/Turabian StyleHeo, Yoonki, Yeondong Cho, Keon Bong Oh, Ki Hoon Park, Hansam Cho, Hanul Choi, Minjee Kim, Ik Jin Yun, Hee Jung Lee, and Young Bong Kim. 2019. "Detection of Pig Cells Harboring Porcine Endogenous Retroviruses in Non-Human Primate Bladder After Renal Xenotransplantation" Viruses 11, no. 9: 801. https://doi.org/10.3390/v11090801
APA StyleHeo, Y., Cho, Y., Oh, K. B., Park, K. H., Cho, H., Choi, H., Kim, M., Yun, I. J., Lee, H. J., & Kim, Y. B. (2019). Detection of Pig Cells Harboring Porcine Endogenous Retroviruses in Non-Human Primate Bladder After Renal Xenotransplantation. Viruses, 11(9), 801. https://doi.org/10.3390/v11090801





