Environmental Surveillance of Polioviruses in Armenia, Colombia before Trivalent Oral Polio Vaccine Cessation
Abstract
1. Introduction
2. Material and Methods
2.1. Type of Study and Locations
2.2. Wastewater Sampling
2.3. Wastewater Preparation
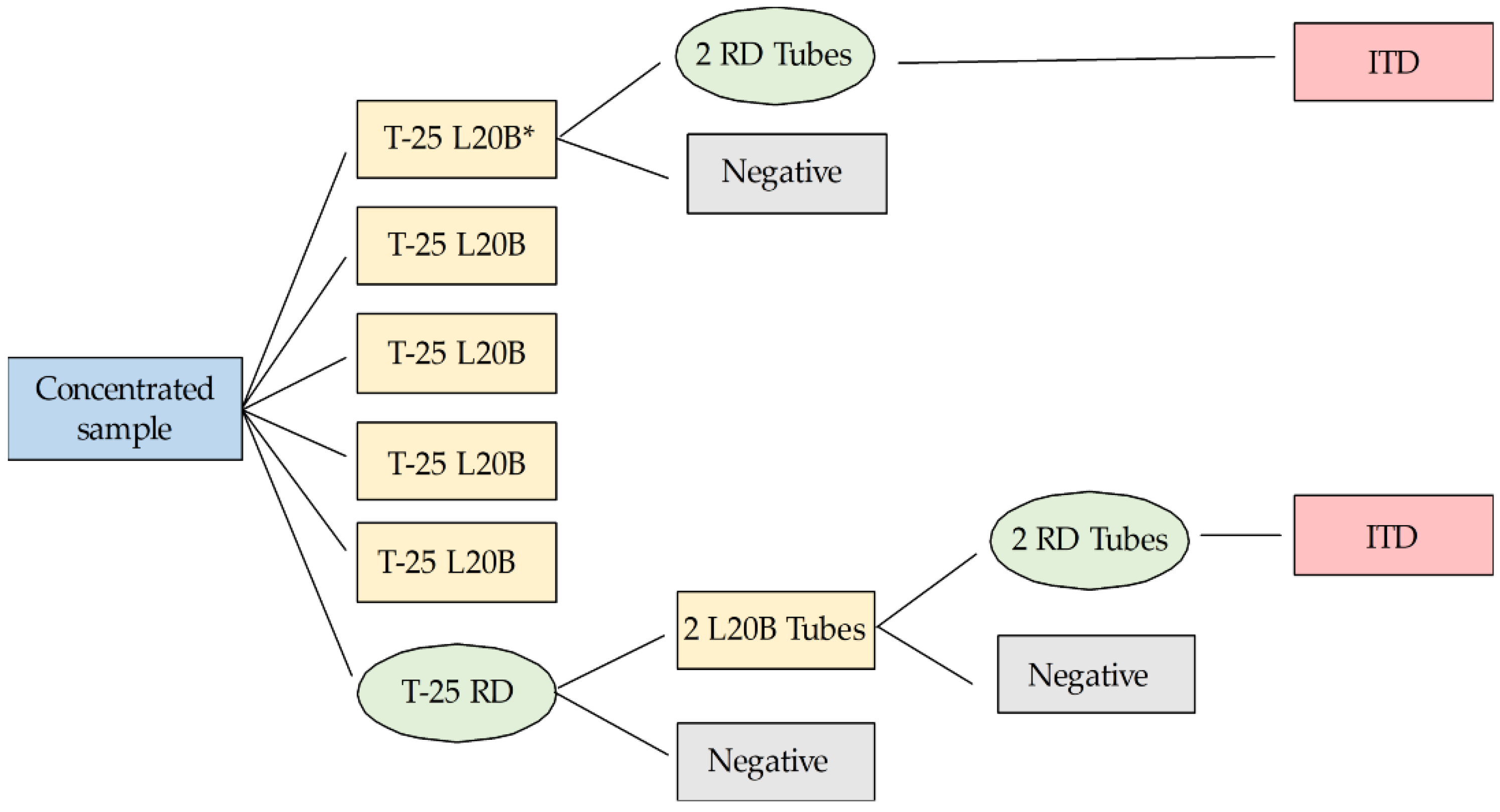
2.4. Viral Isolation
2.5. Identification and Characterization of the Isolate
2.6. Ethics Statement
3. Results
3.1. Environmental Virus Isolations
3.2. Identification and Characterization of Isolated Polioviruses
4. Discussion
Author Contributions
Acknowledgments
Conflicts of Interest
References
- World Health Assembly. Poliomyelitis: Intensification of the Global Eradication Initiative; Report by the Secretariat; World Health Organization: Geneva, Switzerland, 2012. [Google Scholar]
- Global Polio Eradication Initiative. Polio Eradication and Endgame Strategic Plan, 2013–2018; World Health Organization: Geneva, Switzerland, 2013. [Google Scholar]
- Levitt, A.; Diop, O.M.; Tangermann, R.H.; Paladin, F.; Kamgang, J.B.; Burns, C.C.; Chenoweth, P.J.; Goel, A.; Wassilak, S.G.; Centers for Disease Control and Prevention (CDC). Surveillance systems to track progress toward global polio eradication—Worldwide, 2012–2013. MMWR. Morb. Mortal. Wkly. Rep. 2014, 63, 356–361. [Google Scholar] [PubMed]
- Mayor Mora, A.; Menjura, F.; Arias, A. Cruzada Interminable por la Niñez Colombiana: Historia del Programa Ampliado de Inmunizaciones (PAI) en Colombia 1979–2009; Ministerio de la Protección Social, Organización Panamericana de la Salud: Bogotá, Colombia, 2010; Volume 1, pp. 18–148. [Google Scholar]
- Alexander, J.P.; Gary, H.E.; Pallansch, M.A. Duration of Poliovirus Excretion and Its Implications for Acute Flaccid Paralysis Surveillance: A Review of the Literature. J. Infect. Dis. 1997, 175, S176–S182. [Google Scholar] [CrossRef] [PubMed]
- Lago, P.M.; Gary, H.E.; Pérez, L.S.; Cáceres, V.; Olivera, J.B.; Puentes, R.P.; Corredor, M.B.; Jímenez, P.; Pallansch, M.A.; Cruz, R.G. Poliovirus detection in wastewater and stools following an immunization campaign in Havana, Cuba. Int. J. Epidemiol. 2003, 32, 772–777. [Google Scholar] [CrossRef] [PubMed]
- Asghar, H.; Diop, O.M.; Weldegebriel, G.; Malik, F.; Shetty, S.; El Bassioni, L.; Akande, A.O.; Al Maamoun, E.; Zaidi, S.; Adeniji, A.J.; et al. Environmental surveillance for polioviruses in the Global Polio Eradication Initiative. J. Infect. Dis. 2014, 210, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Tebbens, R.J.D.; Zimmermann, M.; Pallansch, M.A.; Thompson, K.M. Insights from a Systematic Search for Information on Designs, Costs, and Effectiveness of Poliovirus Environmental Surveillance Systems. Food Environ. Virol. 2017, 9, 361–382. [Google Scholar] [CrossRef] [PubMed]
- González, M.M.; Castaño, J.C.; Giraldo, A.M.; Salazar, A.; Muñoz, N.J.; Sarmiento, L. Detección de Poliovirus en Aguas Residuales de Armenia, Colombia. Revista de Salud Pública 2006, 8, 813–823. [Google Scholar] [CrossRef]
- Ministerio de Salud y Proteccion Social Colombia. Available online: https://minsalud.gov.co/salud/publica/Vacunacion/Paginas/pai.aspx (accessed on 16 May 2018).
- World Health Organization; Department of Immunization, Vaccines and Biologicals. Guidelines for Environmental Surveillance of Poliovirus Circulation; World Health Organization: Geneva, Switzerland, 2003. [Google Scholar]
- Sobsey, M. Methods for Recovering Viruses from Shellfish, Seawater and Sediments. In Methods for Recovering Viruses in the Environments; Berg, G., Ed.; CRC Press: Boca Raton, FL, USA, 1987; pp. 77–108. [Google Scholar]
- World Health Organization; Department of Immunization, Vaccines and Biologicals. Polio Laboratory Manual, 4th ed.; World Health Organization, Ed.; World Health Organization: Geneva, Switzerland, 2004. [Google Scholar]
- World Health Organization; Department of Immunization, Vaccines and Biologicals. Supplement 1 to the WHO Polio Laboratory Manual. An Alternative Test Algorithm for Poliovirus Isolation and Characteri-zation. Available online: http://www.who.int/immunization_monitoring/Supplement_ polio_lab_manual.pdf (accessed on 20 November 2018).
- Kilpatrick, D.R.; Nottay, B.; Yang, C.F.; Yang, S.J.; Mulders, M.N.; Holloway, B.P.; Pallansch, M.A.; Kew, O.M. Group-specific identification of polioviruses by PCR using primers containing mixed-base or deoxyinosine residue at positions of codon degeneracy. J. Clin. Microbiol. 1996, 34, 2990–2996. [Google Scholar] [PubMed]
- Kilpatrick, D.R.; Nottay, B.; Yang, C.F.; Yang, S.J.; Da Silva, E.; Peñaranda, S.; Pallansch, M.; Kew, O. Serotype-Specific Identification of Polioviruses by PCR Using Primers Containing Mixed-Base or Deoxyinosine Residues at Positions of Codon Degeneracy. J. Clin. Microbiol. 1998, 36, 352–357. [Google Scholar] [PubMed]
- Yang, C.F.; Lina, D.E.; Holloway, B.P.; Pallansch, M.A.; Kew, O.M. Detection and identification of vaccine related polio virus by the Polymerase Chain Reaction. Virus Res. 1991, 20, 159–179. [Google Scholar] [PubMed]
- Kilpatrick, D.R.; Yang, C.F.; Ching, K.; Vincent, A.; Iber, J.; Campagnoli, R.; Mandelbaum, M.; De, L.; Yang, S.J.; Nix, A.; et al. Rapid Group-, Serotype-, and Vaccine Strain-Specific Identification of Poliovirus Isolates by Real-Time Reverse Transcription-PCR Using Degenerate Primers and Probes Containing Deoxyinosine Residues. J. Clin. Microbiol. 2009, 47, 1939–1941. [Google Scholar] [CrossRef] [PubMed]
- Kilpatrick, D.R.; Ching, K.; Iber, J.; Chen, Q.; Yang, S.J.; De, L.; Williams, A.; Mandelbaum, M.; Sun, H.; Oberste, M.S.; et al. Identification of vaccine-derived polioviruses using dual-stage real-time RT-PCR. J. Virol. Methods 2014, 197, 25–28. [Google Scholar] [CrossRef] [PubMed]
- González, M.M.; Sarmiento, L.; Rey-Benito, G.J.; Padilla, L.; Giraldo, A.M.; Castaño, J.C. Absence of poliovirus circulation in Colombian departments with vaccination coverage below 80%. Revista Panamericana de Salud Pública 2012, 32, 156–160. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cáceres, V.M.; Galindo, M.A.; Gary, H.E.; Valcarcel, M.; Barrios, J.; Avalos, I.; Bravo, J.A.; Palomera, R.; Bello, M.; Sarmiento, L.; et al. Persistence of vaccine-derived poliovirus following a mass vaccination campaign in Cuba: Implications for stopping polio vaccination after global eradication. Int. J. Epidemiol. 2001, 30, 1029–1034. [Google Scholar]
- De Oliveira Pereira, J.S.; da Silva, L.R.; de Meireles Nunes, A.; de Souza Oliveira, S.; da Costa, E.V.; da Silva, E.E. Environmental surveillance of polioviruses in Rio de Janeiro, Brazil, in support to the activities of Global Polio Eradication Initiative. Food Environ. Virol. 2016, 8, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Diop, O.M.; Asghar, H.; Gavrilin, E.; Moeletsi, N.G.; Benito, G.R.; Paladin, F.; Pattamadilok, S.; Zhang, Y.; Goel, A.; Quddus, A. Virologic Monitoring of Poliovirus Type 2 after Oral Poliovirus Vaccine Type 2 Withdrawal in April 2016—Worldwide, 2016–2017. Morb. Mortal. Wkly. Rep. 2017, 66, 538–542. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Figas, A.; Wieczorek, M.; Żuk-Wasek, A.; Litwińska, B. Isolation of Sabin-like Polioviruses from Sewage in Poland. Pol. J. Microbiol. 2018, 67, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Esteves-Jaramillo, A.; Estivariz, C.F.; Peñaranda, S.; Richardson, V.L.; Reyna, J.; Coronel, D.L.; Carrion, V.; Landaverde, J.M.; Wassilak, S.G.F.; Perez-Sanchez, E.E.; et al. Detection of Vaccine-Derived Polioviruses in Mexico Using Environmental Surveillance. J. Infect. Dis. 2014, 210, 315–323. [Google Scholar] [CrossRef]
- González, M.M.; Sarmiento, L.; Giraldo, A.; Padilla, L.; Rey-Benito, G.; Castaño, J.C. Sero-prevalence of antibodies to measles, rubella, mumps, hepatitis B viruses and all three poliovirus serotypes among children in Quindío, Colombia. Revista de Salud Pública 2016, 18, 95–103. [Google Scholar]
| Communes | Collector SewerLocation | No. Inhabitants in Catchment Area | No. of Samples/Month (March to September) | Total | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| M | A | M | J | J | A | S | ||||
| Quimbaya | Aldana | 46,614 | 1 | 1 | 1 | 1 | 4 | |||
| El Cafetero | C. Diablo | 37,808 | 1 | 1 | 1 | 3 | ||||
| Rufino José Cuervo Sur | Sta Rita | 49,224 | 1 | 1 | 1 | 1 | 4 | |||
| Centenario | Pinares | 33,111 | 1 | 1 | 1 | 3 | ||||
| Rufino José Cuervo Sur | Cristales | 49,224 | 1 | 1 | 2 | |||||
| Francisco de Paula Santander | Miraflores | 14,429 | 1 | 1 | 1 | 3 | ||||
| Alfonso López | M Beltran | 33,796 | 1 | 1 | 1 | 3 | ||||
| Rufino José Cuervo Sur | Los Quindos | 49,224 | 1 | 1 | 2 | |||||
| All | 24 | |||||||||
| Primers | Sequences |
|---|---|
| Generic enterovirus | EVS 5′-CTCCGGCCCCTGAATGCGGCT A-3′ EVA 5′-ATTGTCACCATAAGCAGCC-3′ |
| Group of polioviruses | Pan PV S 5′-TTIAIIGC(A/G)TGICC(A/G)TT(A/G)TT-3′ Pan PVA 5′-CITAITCI(A/C)GITT(C/T)GA(C/T)ATG-3′ |
| Poliovirus type 1 | PV1 2S 5′-TGCGIGA(C/T)ACIACICA(C/T)AT-3′ PV1 A 5′-ATCATICT(C/T)TCIA(A/G)CAT(C/T)TG-3′ |
| Poliovirus type 2 | PV 2S 5′-TGCGIGA(C/T)ACIACICA(C/T)AT-3′ PV2 A 5′-A(C/T)ICC(C/T)TCIACI(A/G)CICC(C/T)TC-3′ |
| Poliovirus type 3 | PV3 S 5′-AA(C/T)CCITCI(A/G)TITT(C/T)TA(C/T)AC-3′ PV3 A, 5′-CCIAI(C/T)TGITC(A/G)TTIG(C/T)(A/G)TC-3′ |
| Sabin type 1 | Sabin 1R 5′-TCCACTGGCITCAGTGTT-3′ Sabin 1S 5′-AGGTCAGATGCTTGAAAGC-3′ |
| Sabin type 2 | Sabin 2A 5′-CGGCTTGTGTCCAGGC-3′ Sabin 2S 5′-CCGTTGAAGGGATTACTAAA-3′ |
| Sabin type 3 | Sabin 3A 5′-TAAGCTATCCTGTTGCC-3′ Sabin 3S 5′-AGGGCGCCCTAACIYTG-3′ |
| Date | Location | Isolation | RT-PCR | Intratypic Differentiation (ITD) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RD | L20B | EV | PV | P1 | P2 | P3 | S1 | S2 | S3 | |||
| May | Aldana | + | + | + | + | + | - | - | + | - | - | Sabin poliovirus 1 |
| Sept | Aldana | + | - | + | N/A | N/A | N/A | N/A | N/A | N/A | N/A | Non-polio enterovirus |
| June | C. Diablo | + | + | + | + | + | - | - | + | - | - | Sabin poliovirus 1 |
| April | Sta Rita | + | + | + | + | + | - | - | + | - | - | Sabin poliovirus 1 |
| Sept | Sta Rita | + | + | + | + | + | - | - | + | - | - | Sabin poliovirus 1 |
| June | Cristales | + | + | + | + | + | - | - | + | - | - | Sabin poliovirus 1 |
| March | Miraflores | + | + | + | + | + | - | - | + | - | - | Sabin poliovirus 1 |
| June | Miraflores | + | + | + | + | + | - | - | + | - | - | Sabin poliovirus 1 |
| Sept | Miraflores | + | + | + | + | + | - | - | + | - | - | Sabin poliovirus 1 |
| April | Y.M. Beltran | + | + | + | + | - | - | + | - | - | + | Sabin poliovirus 3 |
| May | Y.M. Beltran | + | + | + | + | - | - | + | - | - | + | Sabin poliovirus 3 |
| July | Y.M. Beltran | + | + | + | + | + | - | - | + | - | - | Sabin poliovirus 1 |
| Aug | Los Quindios | + | - | + | N/A | N/A | N/A | N/A | N/A | N/A | N/A | Non-polio enterovirus |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
González, M.M.; Fonseca, M.C.; Rodríguez, C.A.; Giraldo, A.M.; Vila, J.J.; Castaño, J.C.; Padilla, L.; Sarmiento, L. Environmental Surveillance of Polioviruses in Armenia, Colombia before Trivalent Oral Polio Vaccine Cessation. Viruses 2019, 11, 775. https://doi.org/10.3390/v11090775
González MM, Fonseca MC, Rodríguez CA, Giraldo AM, Vila JJ, Castaño JC, Padilla L, Sarmiento L. Environmental Surveillance of Polioviruses in Armenia, Colombia before Trivalent Oral Polio Vaccine Cessation. Viruses. 2019; 11(9):775. https://doi.org/10.3390/v11090775
Chicago/Turabian StyleGonzález, María Mercedes, Magile C. Fonseca, Carlos Andrés Rodríguez, Alejandra María Giraldo, José Joaquín Vila, Jhon Carlos Castaño, Leonardo Padilla, and Luis Sarmiento. 2019. "Environmental Surveillance of Polioviruses in Armenia, Colombia before Trivalent Oral Polio Vaccine Cessation" Viruses 11, no. 9: 775. https://doi.org/10.3390/v11090775
APA StyleGonzález, M. M., Fonseca, M. C., Rodríguez, C. A., Giraldo, A. M., Vila, J. J., Castaño, J. C., Padilla, L., & Sarmiento, L. (2019). Environmental Surveillance of Polioviruses in Armenia, Colombia before Trivalent Oral Polio Vaccine Cessation. Viruses, 11(9), 775. https://doi.org/10.3390/v11090775





