Inhibitory Effects of Antiviral Drug Candidates on Canine Parvovirus in F81 cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell and Viruses
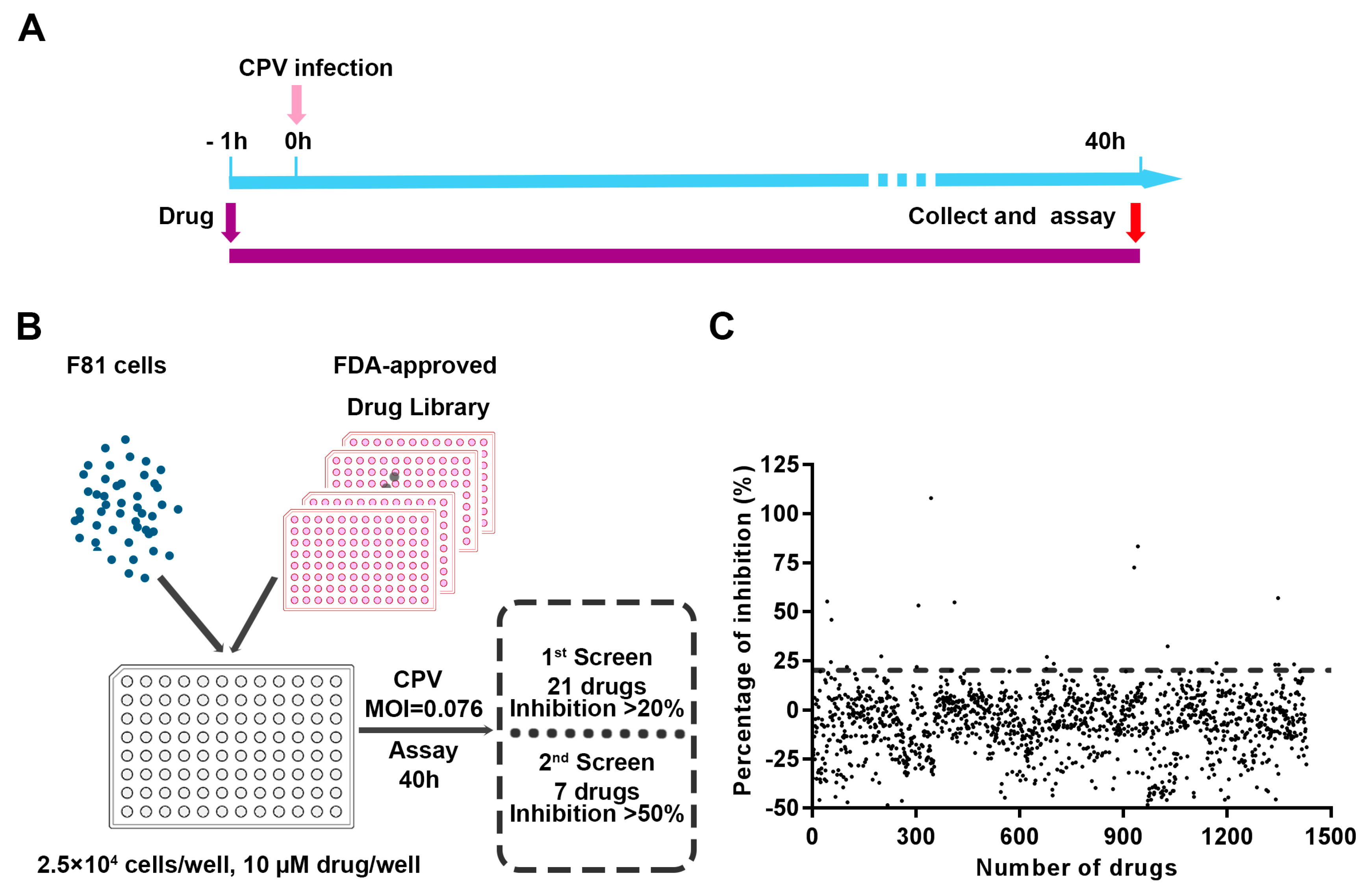
2.2. Cytopathic Effect-Based Antiviral Inhibitors Screening Assay
2.3. 50% Cytotoxicity Concentrations (CC50s) and 50% Antiviral Efficacy Concentrations (EC50s) Assays
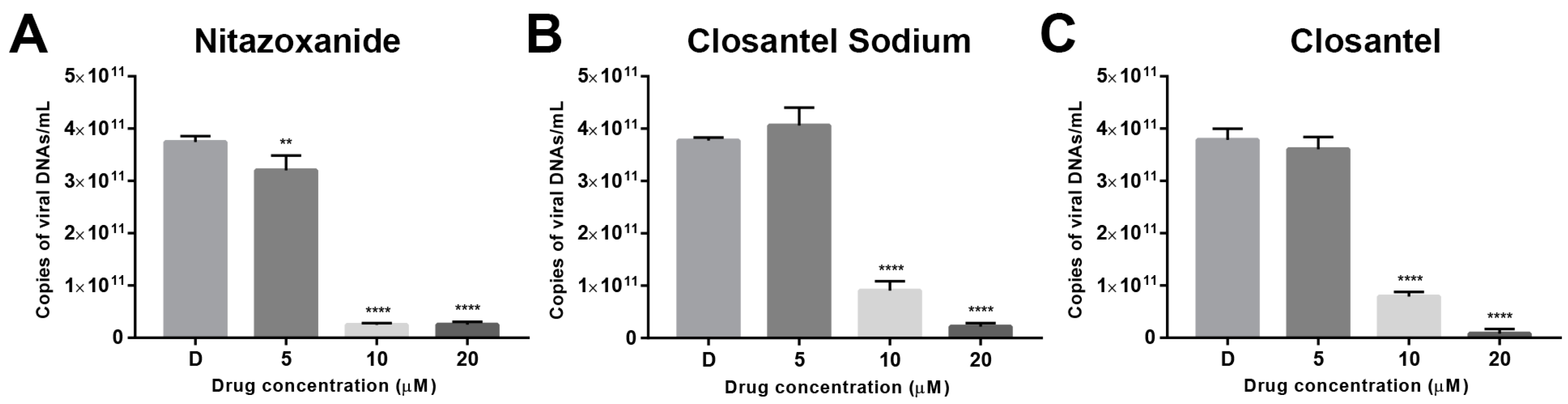
2.4. Absolute Quantitative PCR
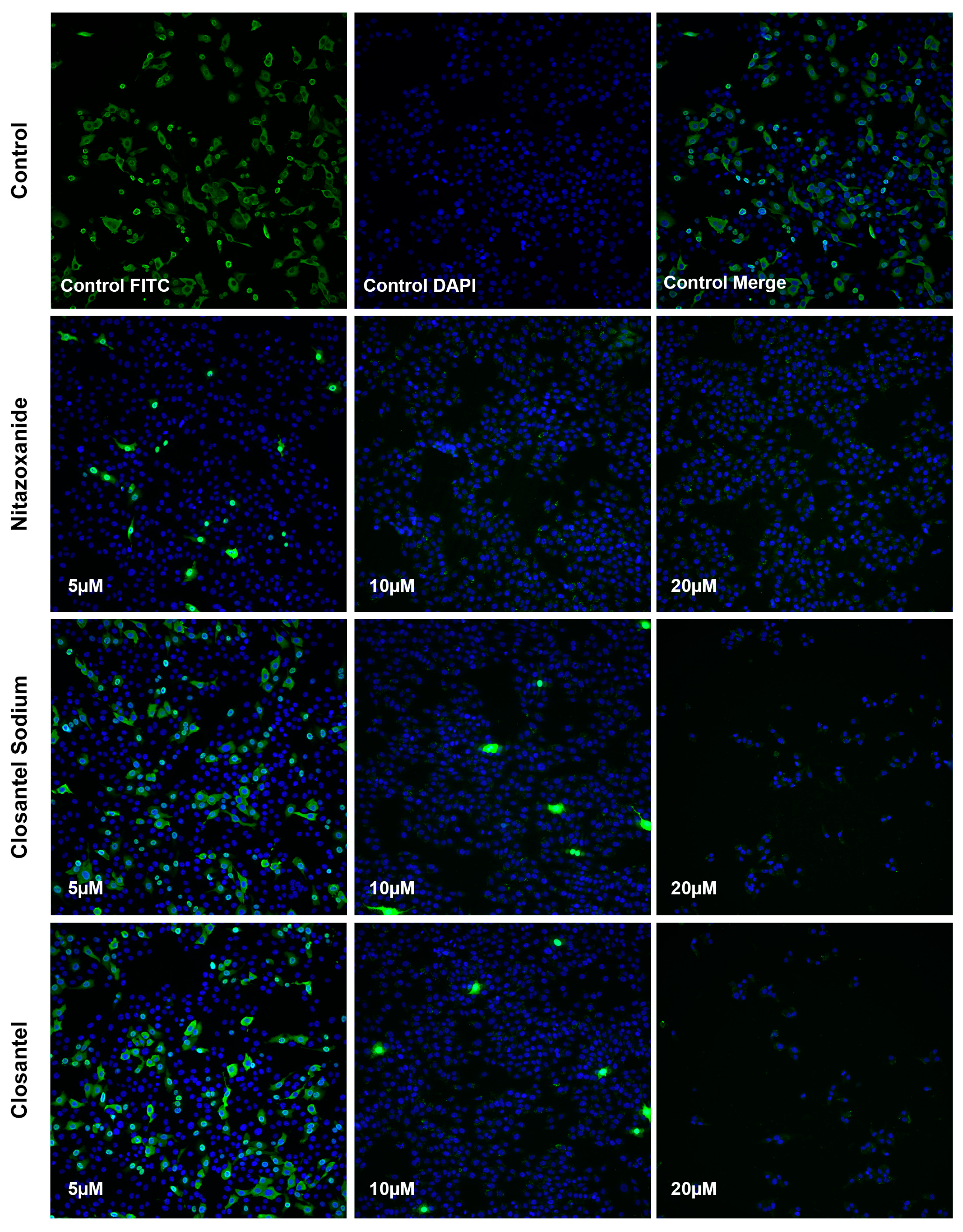
2.5. Immunofluorescence Aassay
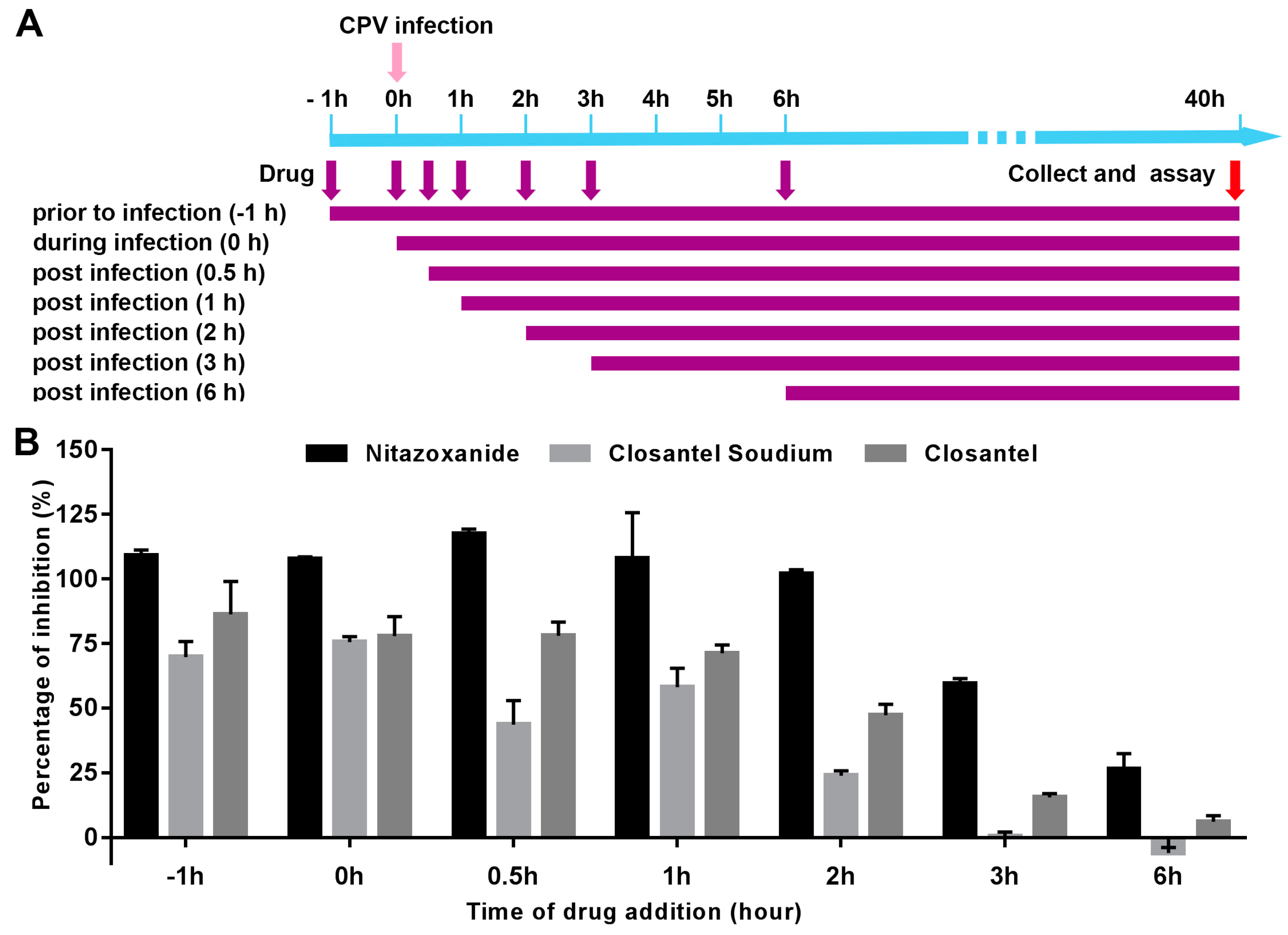
2.6. Time of Addition Study
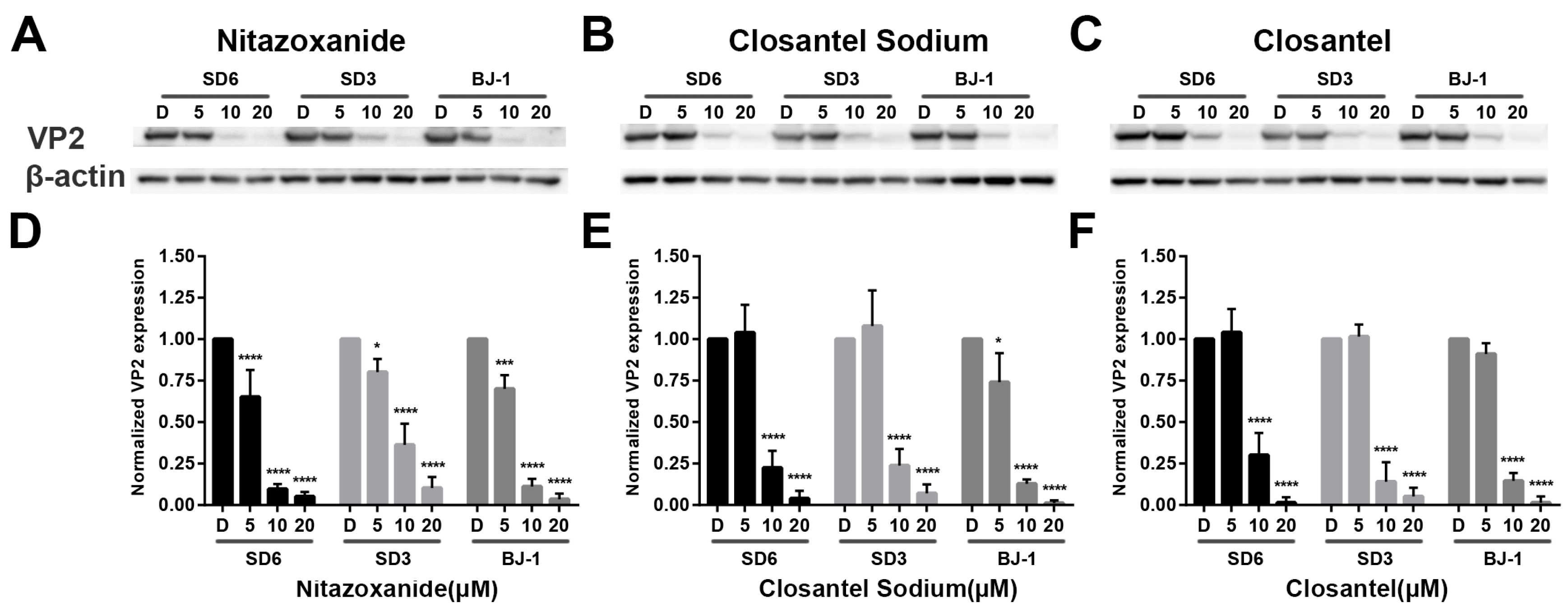
2.7. Immunoblotting
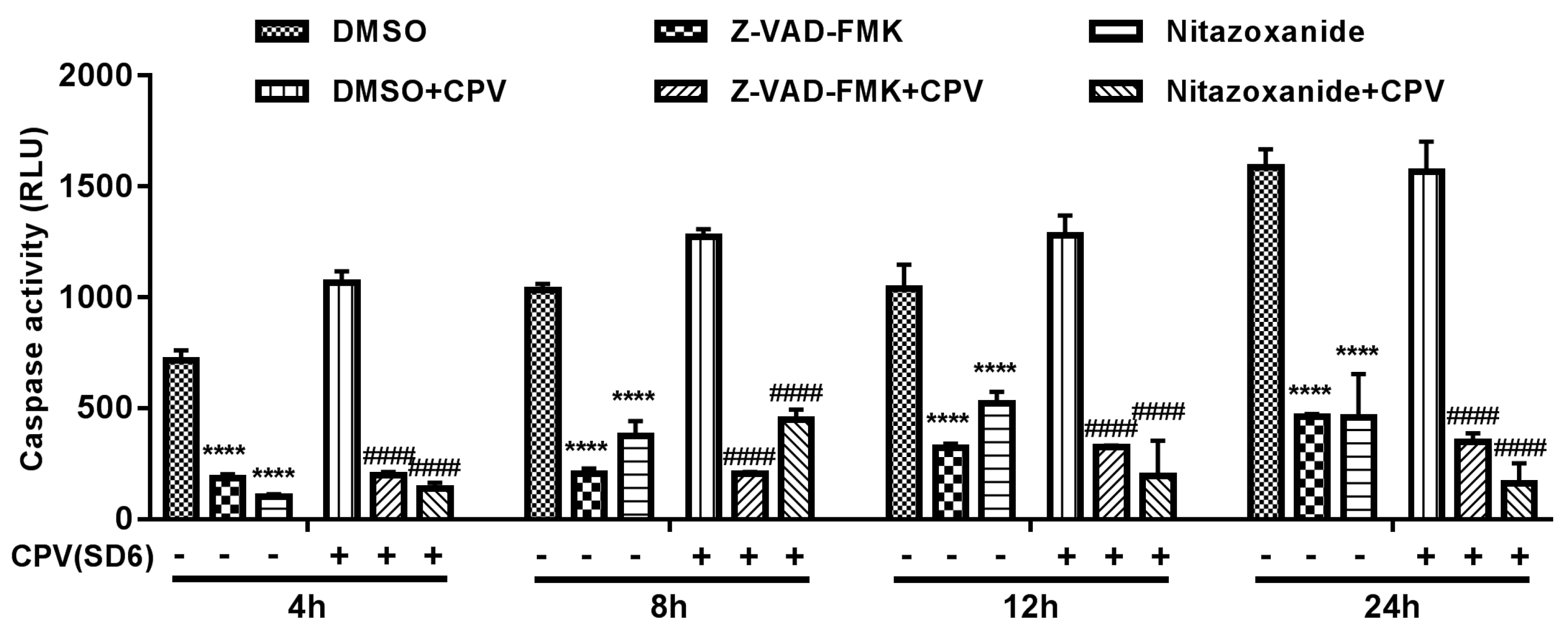
2.8. Caspase-3 Assay
2.9. Statistical Analysis
3. Results
3.1. Screening Drug Inhibitors against CPV Infection in F81 Ccells
3.2. Validation of Anti-CPV Drug Candidates by qPCR and IFA
3.3. Inhibitory Effects of Anti-CPV Drugs at Different Time Points.
3.4. Inhibitory Effects of Anti-CPV Drugs on Different CPV Variants
3.5. Nitazoxanide-Induced Apoptosis Involved in Antiviral Effect.
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Mira, F.; Purpari, G.; Lorusso, E.; Di Bella, S.; Gucciardi, F.; Desario, C.; Macaluso, G.; Decaro, N.; Guercio, A. Introduction of Asian canine parvovirus in Europe through dog importation. Transbound. Emerg. Dis. 2018, 65, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Liu, H.; Ding, K.; Peng, C.; Xue, Q.; Yu, Z.; Xue, Y. Occurrence of canine parvovirus in dogs from Henan province of China in 2009–2014. BMC Veter. Res. 2016, 12, 138. [Google Scholar] [CrossRef] [PubMed]
- Goddard, A.; Leisewitz, A.L. Canine parvovirus. Vet. Clin. N. Am. Small Anim. Pract. 2010, 40, 1041–1053. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Li, X.; Wang, L.; Liu, Y.; Tian, K. Molecular epidemiological survey of canine parvovirus in domestic dogs in four provinces, China. Virus Dis. 2018, 29, 113–117. [Google Scholar] [CrossRef] [PubMed]
- Mira, F.; Dowgier, G.; Purpari, G.; Vicari, D.; Di Bella, S.; Macaluso, G.; Gucciardi, F.; Randazzo, V.; DeCaro, N.; Guercio, A. Molecular typing of a novel canine parvovirus type 2a mutant circulating in Italy. Infect. Genet. Evol. 2018, 61, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Zeng, W.; Zhang, X.; Li, S. The genetic evolution of canine parvovirus—A new perspective. PLoS ONE 2017, 12, e0175035. [Google Scholar] [CrossRef] [PubMed]
- Miranda, C.; Thompson, G. Canine parvovirus: The worldwide occurrence of antigenic variants. J. Gen. Virol. 2016, 97, 2043–2057. [Google Scholar] [CrossRef]
- Duque-García, Y.; Echeverri-Zuluaga, M.; Trejos-Suarez, J.; Ruiz-Saenz, J. Prevalence and molecular epidemiology of Canine parvovirus 2 in diarrheic dogs in Colombia, South America: A possible new CPV-2a is emerging? Veter. Microbiol. 2017, 201, 56–61. [Google Scholar] [CrossRef]
- Hoelzer, K.; Shackelton, L.A.; Holmes, E.C.; Parrish, C.R. Within-Host Genetic Diversity of Endemic and Emerging Parvoviruses of Dogs and Cats. J. Virol. 2008, 82, 11096–11105. [Google Scholar] [CrossRef]
- Shackelton, L.A.; Parrish, C.R.; Truyen, U.; Holmes, E.C. High rate of viral evolution associated with the emergence of carnivore parvovirus. Proc. Natl. Acad. Sci. USA 2005, 102, 379–384. [Google Scholar] [CrossRef]
- Gaykwad, C.; Garkhal, J.; Chethan, G.E.; Nandi, S.; De, U.K. Amelioration of oxidative stress using N-acetylcysteine in canine parvoviral enteritis. J. Vet. Pharmacol. Ther. 2018, 41, 68–75. [Google Scholar] [CrossRef]
- Lamm, C.G.; Rezabek, G.B. Parvovirus infection in domestic companion animals. Vet. Clin. N. Am. Small Anim. Pract. 2008, 38, 837–850. [Google Scholar] [CrossRef] [PubMed]
- Bragg, R.F.; Duffy, A.L.; DeCecco, F.A.; Chung, D.K.; Green, M.T.; Veir, J.K.; Dow, S.W. Clinical evaluation of a single dose of immune plasma for treatment of canine parvovirus infection. J. Am. Veter. Med. Assoc. 2012, 240, 700–704. [Google Scholar] [CrossRef]
- Xu, M.; Lee, E.M.; Wen, Z.; Cheng, Y.; Huang, W.-K.; Qian, X.; Tcw, J.; Kouznetsova, J.; Ogden, S.C.; Hammack, C.; et al. Identification of small-molecule inhibitors of Zika virus infection and induced neural cell death via a drug repurposing screen. Nat. Med. 2016, 22, 1101–1107. [Google Scholar] [CrossRef]
- Liu, G.; Nash, P.J.; Johnson, B.; Pietzsch, C.; Ilagan, M.X.G.; Bukreyev, A.; Basler, C.F.; Bowlin, T.L.; Moir, D.T.; Leung, D.W.; et al. A Sensitive in vitro High-Throughput Screen to Identify Pan-Filoviral Replication Inhibitors Targeting the VP35-NP Interface. ACS Infect. Dis. 2017, 3, 190–198. [Google Scholar] [CrossRef]
- Cheng, H.; Lear-Rooney, C.M.; Johansen, L.; Varhegyi, E.; Chen, Z.W.; Olinger, G.G.; Rong, L. Inhibition of Ebola and Marburg Virus Entry by G Protein-Coupled Receptor Antagonists. J. Virol. 2015, 89, 9932–9938. [Google Scholar] [CrossRef] [PubMed]
- Barrows, N.J.; Campos, R.K.; Powell, S.T.; Prasanth, K.R.; Schott-Lerner, G.; Soto-Acosta, R.; Galarza-Muñoz, G.; McGrath, E.L.; Urrabaz-Garza, R.; Gao, J.; et al. A screen of FDA-approved drugs for inhibitors of Zika virus infection. Cell Host Microbe 2016, 20, 259–270. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F.; Murray, J.L.; Rubin, D.H. Drug Repurposing: New Treatments for Zika Virus Infection? Trends Mol. Med. 2016, 22, 919–921. [Google Scholar] [CrossRef] [PubMed]
- Van de Klundert, M.A.; Zaaijer, H.L.; Kootstra, N.A. Identification of FDA-approved drugs that target hepatitis B virus transcription. J. Viral Hepat. 2016, 23, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-M.; Lu, J.-W.; Lin, C.-C.; Chin, Y.-F.; Wu, T.-Y.; Lin, L.-I.; Lai, Z.-Z.; Kuo, S.-C.; Ho, Y.-J. Antiviral activities of niclosamide and nitazoxanide against chikungunya virus entry and transmission. Antivir. Res. 2016, 135, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Sun, L.; Peng, G.; Xu, J.; Zhou, R.; Cao, S.; Chen, H.; Song, Y. Identification of Three Antiviral Inhibitors against Japanese Encephalitis Virus from Library of Pharmacologically Active Compounds 1280. PLoS ONE 2013, 8, e78425. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.Y.; Cruz, D.J.M.; Ko, Y.; Min, J.-Y. Identification of pyrrolo[3,2-c]pyridin-4-amine compounds as a new class of entry inhibitors against influenza viruses in vitro. Biochem. Biophys. Res. Commun. 2016, 478, 1594–1601. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, H.-H.; Palese, P.; Shaw, M.L. Modulation of influenza virus replication by alteration of sodium ion transport and protein kinase C activity. Antivir. Res. 2008, 80, 124–134. [Google Scholar] [CrossRef] [PubMed]
- Doley, J.; Singh, L.V.; Kumar, G.R.; Sahoo, A.P.; Saxena, L.; Chaturvedi, U.; Saxena, S.; Kumar, R.; Singh, P.K.; Rajmani, R.S.; et al. Canine parvovirus type 2a (CPV-2a)-induced apoptosis in MDCK involves both extrinsic and intrinsic pathways. Appl. Biochem. Biotechnol. 2014, 172, 497–508. [Google Scholar] [CrossRef] [PubMed]
- Savigny, M.R.; Macintire, D.K. Use of oseltamivir in the treatment of canine parvoviral enteritis. J. Veter. Emerg. Crit. Care 2010, 20, 132–142. [Google Scholar] [CrossRef] [PubMed]
- Liekens, S.; Noppen, S.; Gijsbers, S.; Sienaert, R.; Ronca, R.; Tobia, C.; Presta, M. The broad-spectrum anti-DNA virus agent cidofovir inhibits lung metastasis of virus-independent, FGF2-driven tumors. Oncotarget 2015, 6, 4633–4648. [Google Scholar] [CrossRef] [PubMed]
- Hickson, S.E.; Margineantu, D.; Hockenbery, D.M.; Simon, J.A.; Geballe, A.P. Inhibition of vaccinia virus replication by nitazoxanide. Virology 2018, 518, 398–405. [Google Scholar] [CrossRef] [PubMed]
- Korba, B.E.; Montero, A.B.; Farrar, K.; Gaye, K.; Mukerjee, S.; Ayers, M.S.; Rossignol, J.-F. Nitazoxanide, tizoxanide and other thiazolides are potent inhibitors of hepatitis B virus and hepatitis C virus replication. Antivir. Res. 2008, 77, 56–63. [Google Scholar] [CrossRef]
- Stachulski, A.V.; Pidathala, C.; Row, E.C.; Sharma, R.; Berry, N.G.; Iqbal, M.; Bentley, J.; Allman, S.A.; Edwards, G.; Helm, A.; et al. Thiazolides as Novel Antiviral Agents. 1. Inhibition of Hepatitis B Virus Replication. J. Med. Chem. 2011, 54, 4119–4132. [Google Scholar] [CrossRef]
- Mercorelli, B.; Luganini, A.; Nannetti, G.; Tabarrini, O.; Palú, G.; Gribaudo, G.; Loregian, A. Drug Repurposing Approach Identifies Inhibitors of the Prototypic Viral Transcription Factor IE2 that Block Human Cytomegalovirus Replication. Cell Chem. Boil. 2016, 23, 340–351. [Google Scholar] [CrossRef]
- Rossignol, J.F.; La Frazia, S.; Chiappa, L.; Ciucci, A.; Santoro, M.G. Thiazolides, a New Class of Anti-influenza Molecules Targeting Viral Hemagglutinin at the Post-translational Level. J. Boil. Chem. 2009, 284, 29798–29808. [Google Scholar] [CrossRef]
- Keeffe, E.B.; Rossignol, J.-F.; Keeffe, J.-F.E.B.; Rossignol, O. Treatment of chronic viral hepatitis with nitazoxanide and second generation thiazolides. World J. Gastroenterol. 2009, 15, 1805–1808. [Google Scholar] [CrossRef] [PubMed]
- Siddiq, D.M.; Koo, H.L.; Adachi, J.A.; Viola, G.M. Norovirus Gastroenteritis Successfully Treated with Nitazoxanide. J. Infect. 2011, 63, 394–397. [Google Scholar] [CrossRef]
- La Frazia, S.; Ciucci, A.; Arnoldi, F.; Coira, M.; Gianferretti, P.; Angelini, M.; Belardo, G.; Burrone, O.R.; Rossignol, J.-F.; Santoro, M.G. Thiazolides, a New Class of Antiviral Agents Effective against Rotavirus Infection, Target Viral Morphogenesis, Inhibiting Viroplasm Formation. J. Virol. 2013, 87, 11096–11106. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Wei, J.; Deng, X.; Li, S.; Qiu, Y.; Shao, D.; Li, B.; Zhang, K.; Xue, F.; Wang, X.; et al. Nitazoxanide inhibits the replication of Japanese encephalitis virus in cultured cells and in a mouse model. Virol. J. 2014, 11, 10. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Forrest, J.C.; Zhang, X. A screen of the NIH Clinical Collection small molecule library identifies potential anti-coronavirus drugs. Antivir. Res. 2015, 114, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Trabattoni, D.; Gnudi, F.; Ibba, S.V.; Saulle, I.; Agostini, S.; Masetti, M.; Biasin, M.; Rossignol, J.-F.; Clerici, M. Thiazolides Elicit Anti-Viral Innate Immunity and Reduce HIV Replication. Sci. Rep. 2016, 6, 27148. [Google Scholar] [CrossRef] [PubMed]
- Cao, R.-Y.; Xu, Y.-F.; Zhang, T.-H.; Yang, J.-J.; Yuan, Y.; Hao, P.; Shi, Y.; Zhong, J.; Zhong, W. Pediatric Drug Nitazoxanide: A Potential Choice for Control of Zika. Open Forum Infect. Dis. 2017, 4, ofx009. [Google Scholar] [CrossRef] [PubMed]
- Perelygina, L.; Hautala, T.; Seppänen, M.; Adebayo, A.; Sullivan, K.E.; Icenogle, J. Inhibition of rubella virus replication by the broad-spectrum drug nitazoxanide in cell culture and in a patient with a primary immune deficiency. Antivir. Res. 2017, 147, 58–66. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Li, Z.; Brecher, M.; Deng, Y.-Q.; Zhang, J.; Sakamuru, S.; Liu, B.; Huang, R.; A Koetzner, C.; A Allen, C.; A Jones, S.; et al. Existing drugs as broad-spectrum and potent inhibitors for Zika virus by targeting NS2B-NS3 interaction. Cell Res. 2017, 27, 1046–1064. [Google Scholar] [CrossRef] [PubMed]
- Ashiru, O.; Howe, J.D.; Butters, T.D. Nitazoxanide, an antiviral thiazolide, depletes ATP-sensitive intracellular Ca(2+) stores. Virology 2014, 462–463, 135–148. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, E. Discovery of novel SPAK inhibitors that block WNK kinase signaling to cation chloride transporters. J. Am. Soc. Nephrol. 2015, 57, 1525–1536. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.-Y.; Xia, B.; Liu, H.-C.; Xu, Y.-Q.; Huang, C.-J.; Gao, J.-M.; Dong, Q.-X.; Li, C.-Q. Closantel Suppresses Angiogenesis and Cancer Growth in Zebrafish Models. ASSAY Drug Dev. Technol. 2016, 14, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Guo, B.; Xu, Z.; Li, B.; Cai, T.; Zhang, X.; Yu, Y.; Wang, H.; Shi, J.; Zhu, W. Repositioning organohalogen drugs: A case study for identification of potent B-Raf V600E inhibitors via docking and bioassay. Sci. Rep. 2016, 6, 31074. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Tian, D.; Matsuyama, H.; Hamazaki, T.; Shiratsuchi, T.; Terada, N.; Hook, D.J.; Walters, M.A.; Georg, G.I.; Hawkinson, J.E. Human Adenine Nucleotide Translocase (ANT) Modulators Identified by High-Throughput Screening of Transgenic Yeast. J. Biomol. Screen 2016, 21, 381–390. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Alamri, M.A.; Kadri, H.; Alderwick, L.J.; Simpkins, N.S.; Mehellou, Y. Rafoxanide and Closantel Inhibit SPAK and OSR1 Kinases by Binding to a Highly Conserved Allosteric Site on Their C-terminal Domains. ChemMedChem 2017, 12, 639–645. [Google Scholar] [CrossRef] [PubMed]
- Senkowski, W.; Zhang, X.; Olofsson, M.H.; Isacson, R.; Höglund, U.; Gustafsson, M.; Nygren, P.; Linder, S.; Larsson, R.; Fryknäs, M. Three-Dimensional Cell Culture-Based Screening Identifies the Anthelmintic Drug Nitazoxanide as a Candidate for Treatment of Colorectal Cancer. Mol. Cancer Ther. 2015, 14, 1504–1516. [Google Scholar] [CrossRef] [PubMed]
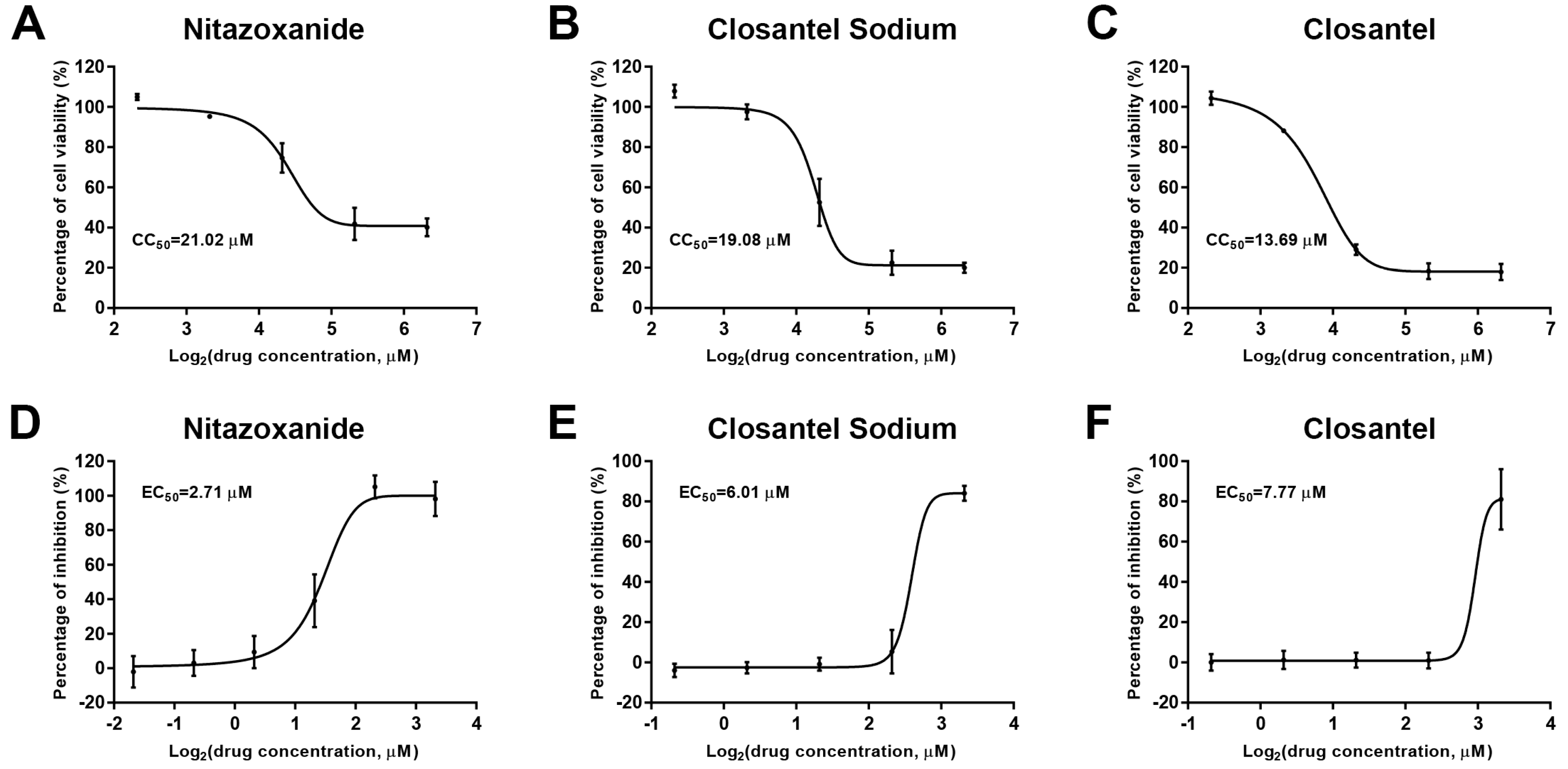
| Hit drugs | CC50 (µM) | EC50 (µM) | SI |
|---|---|---|---|
| Nitazoxanide | 21.02 | 2.71 | 7.76 |
| Closantel Sodium | 19.08 | 6.01 | 3.17 |
| Closantel | 13.69 | 7.77 | 1.76 |
| Gemcitabine HCl | 141.6 | 0.68 | 208.24 |
| Cladribine | 40.21 | 0.32 | 125.66 |
| Gemcitabine | 40.03 | 0.62 | 64.56 |
| Trifluridine | >160 | 9.35 | >17.11 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, H.; Su, X.; Lin, L.; Zhang, J.; Qi, Q.; Guo, F.; Xu, F.; Yang, B. Inhibitory Effects of Antiviral Drug Candidates on Canine Parvovirus in F81 cells. Viruses 2019, 11, 742. https://doi.org/10.3390/v11080742
Zhou H, Su X, Lin L, Zhang J, Qi Q, Guo F, Xu F, Yang B. Inhibitory Effects of Antiviral Drug Candidates on Canine Parvovirus in F81 cells. Viruses. 2019; 11(8):742. https://doi.org/10.3390/v11080742
Chicago/Turabian StyleZhou, Hongzhuan, Xia Su, Lulu Lin, Jin Zhang, Qi Qi, Fangfang Guo, Fuzhou Xu, and Bing Yang. 2019. "Inhibitory Effects of Antiviral Drug Candidates on Canine Parvovirus in F81 cells" Viruses 11, no. 8: 742. https://doi.org/10.3390/v11080742
APA StyleZhou, H., Su, X., Lin, L., Zhang, J., Qi, Q., Guo, F., Xu, F., & Yang, B. (2019). Inhibitory Effects of Antiviral Drug Candidates on Canine Parvovirus in F81 cells. Viruses, 11(8), 742. https://doi.org/10.3390/v11080742




