Transmission of Porcine Circovirus 3 (PCV3) by Xenotransplantation of Pig Hearts into Baboons
Abstract
1. Introduction
2. Materials and Methods
2.1. Orthotopic Pig Heart Transplantation
2.2. Real-Time PCR
2.3. Sequencing
2.4. Infection Experiments with Human Cells
3. Results
3.1. Detection and Quantification of PCV3 in Pigs Generated for Xenotransplantation
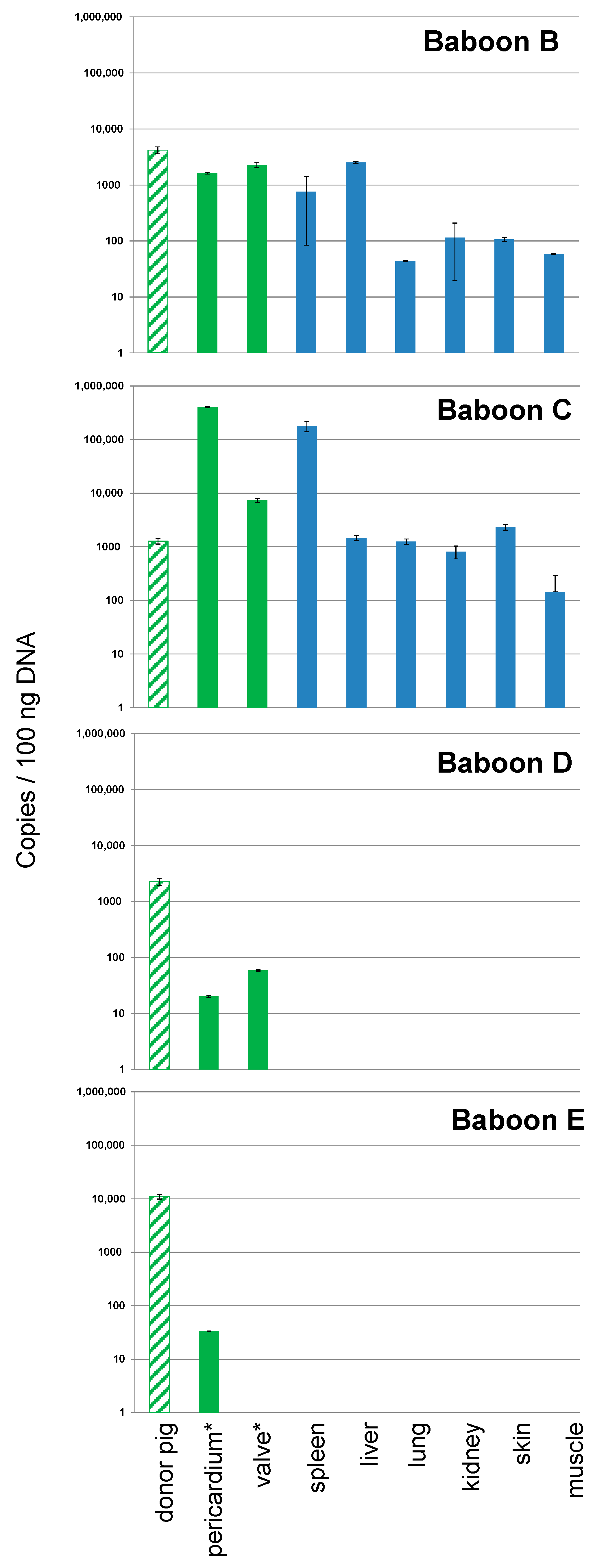
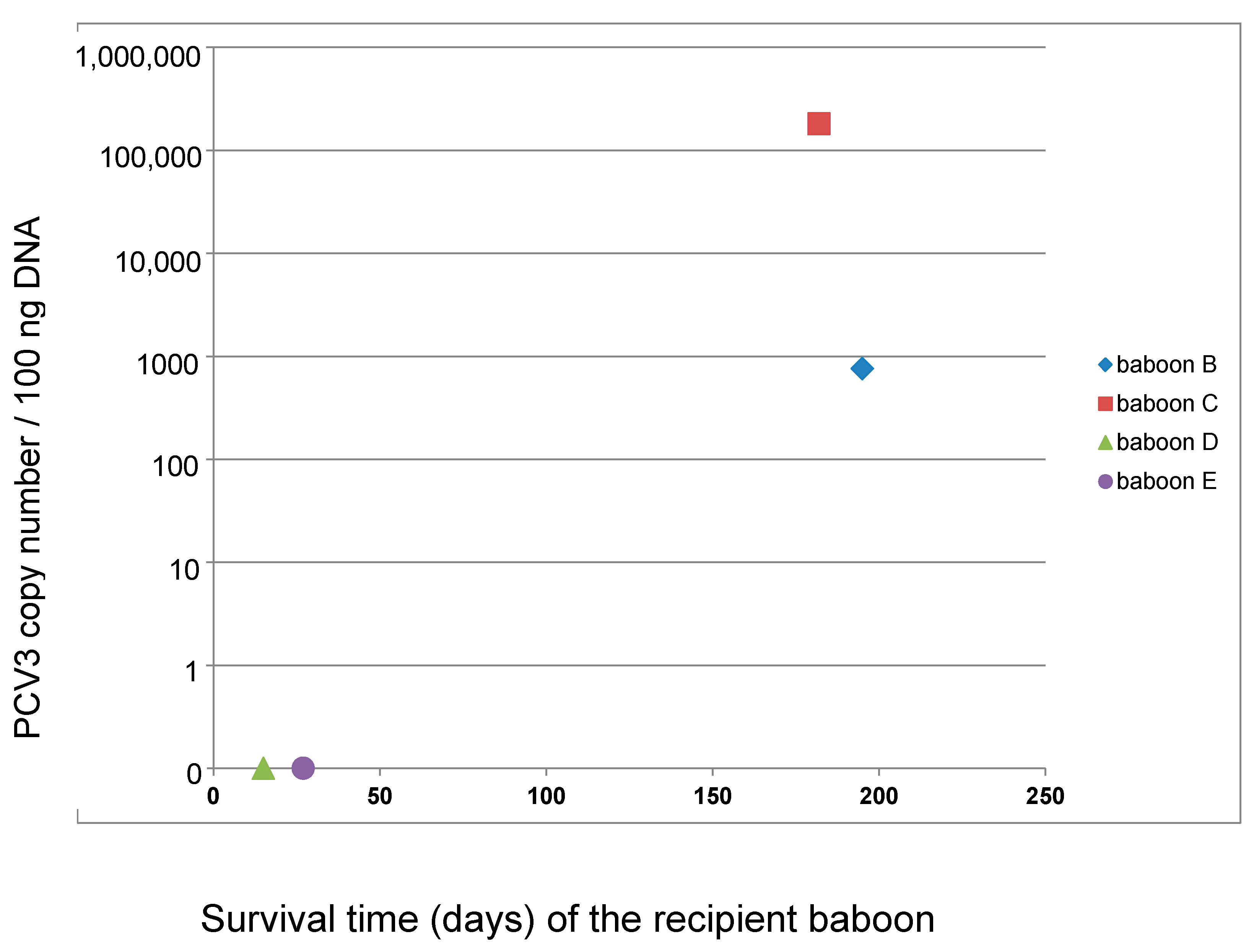
3.2. Detection and Quantification of PCV3 in Recipient Baboons After Transplantation of Pig Hearts
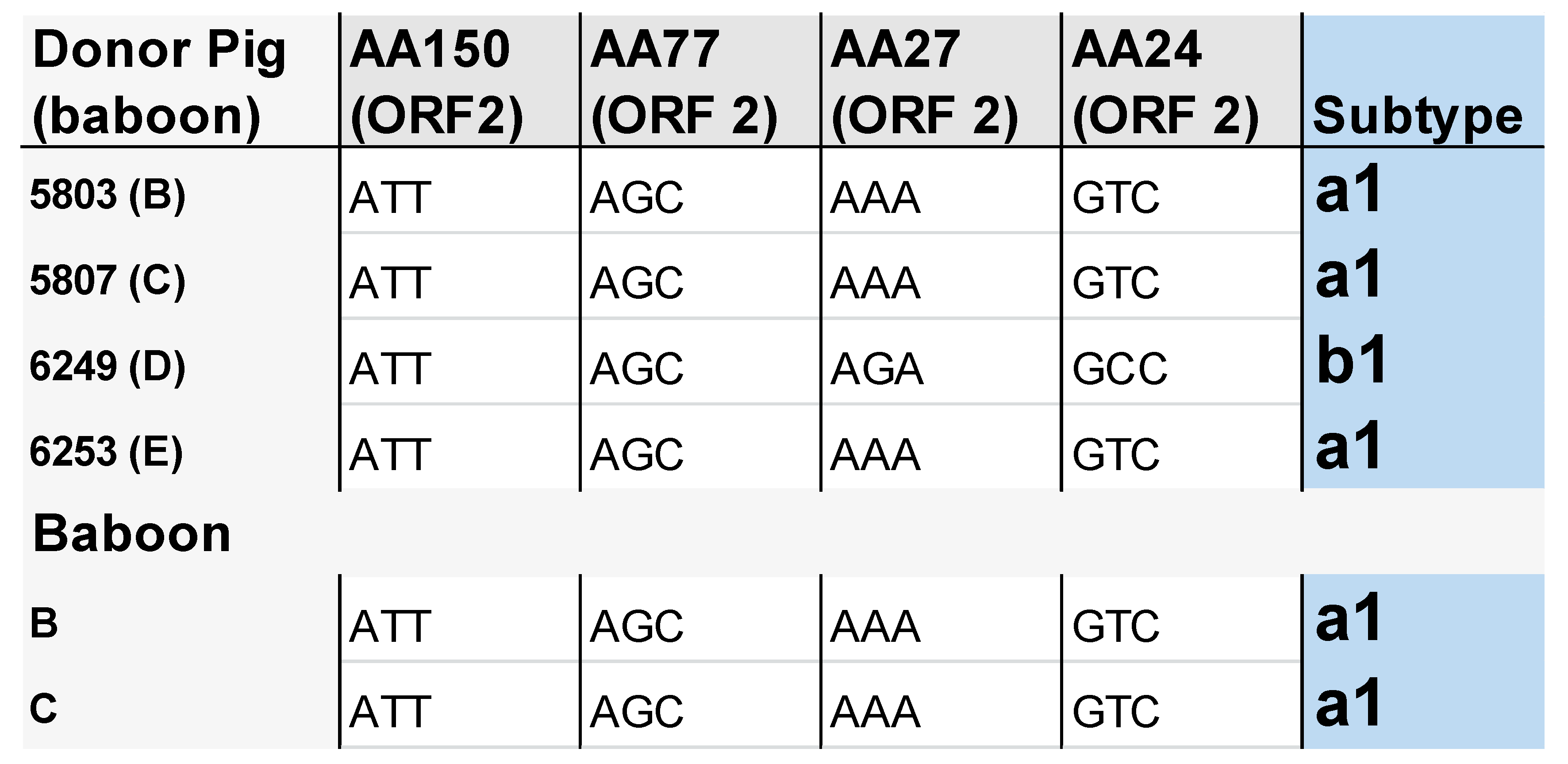
3.3. Subtype Characterisation of PCV3 in the Pigs and Baboons
3.4. Absence of Infection of Human Cells with PCV3
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Finsterbusch, T.; Mankertz, A. Porcine circoviruses—small but powerful. Virus Res. 2009, 143, 177–183. [Google Scholar] [CrossRef]
- Allan, G.M.; Ellis, J.A. Porcine circoviruses: a review. J. Vet. Diagn. Investig. 2000, 12, 2–14. [Google Scholar] [CrossRef]
- Hattermann, K.; Roedner, C.; Schmitt, C.; Finsterbusch, T.; Steinfeldt, T.; Mankertz, A. Infection studies on human cell lines with porcine circovirus type 1 and porcine circovirus type 2. Xenotransplantation 2004, 11, 284–294. [Google Scholar] [CrossRef]
- Liu, X.; Ouyang, T.; Ouyang, H.; Liu, X.; Niu, G.; Huo, W.; Yin, W.; Pang, D.; Ren, L. Human cells are permissive for the productive infection of porcine circovirus type 2 in vitro. Sci. Rep. 2019, 9, 5638. [Google Scholar] [CrossRef]
- Denner, J.; Mankertz, A. Porcine Circoviruses and Xenotransplantation. Viruses 2017, 9, 83. [Google Scholar] [CrossRef]
- Ekser, B.; Li, P.; Cooper, D.K.C. Xenotransplantation: Past, present, future. Curr. Opin. Organ. Transplant. 2017, 22, 513–521. [Google Scholar] [CrossRef]
- Denner, J. Recent Progress in Xenotransplantation, with Emphasis on Virological Safety. Ann. Transplant. 2016, 21, 717–727. [Google Scholar] [CrossRef]
- Heinze, J.; Plotzki, E.; Denner, J. Virus Safety of Xenotransplantation: Prevalence of Porcine Circovirus 2 (PCV2) in Pigs. Ann. Virol. Res. 2016, 2, 1023. [Google Scholar]
- Morozov, V.A.; Ludwig, S.; Ludwig, B.; Rotem, A.; Barkai, U.; Bornstein, S.R.; Denner, J. Islet cell transplantation from Göttingen minipigs to cynomolgus monkeys: analysis of virus safety. Xenotransplantation 2016, 23, 320–327. [Google Scholar] [CrossRef]
- Plotzki, E.; Wolf-van Buerck, L.; Knauf, Y.; Becker, T.; Maetz-Rensing, K.; Schuster, M.; Baehr, A.; Klymiuk, N.; Wolf, E.; Seissler, J.; et al. Virus safety of islet cell transplantation from transgenic pigs to marmosets. Virus Res. 2015, 204, 95–102. [Google Scholar] [CrossRef]
- Längin, M.; Mayr, T.; Reichart, B.; Michel, S.; Buchholz, S.; Guethoff, S.; Dashkevich, A.; Baehr, A.; Egerer, S.; Bauer, A.; et al. Consistent success in life-supporting porcine cardiac xenotransplantation. Nature 2018, 564, 430–433. [Google Scholar]
- Denner, J.; Krüger, L.; Prinz, C.; Robert Koch Institute, Berlin, Germany. All screened igs were PCV2 negative. Unpublished work. 2018. [Google Scholar]
- Klaumann, F.; Correa-Fiz, F.; Franzo, G.; Sibila, M.; Núñez, J.I.; Segalés, J. Current Knowledge on Porcine circovirus 3 (PCV-3): A Novel Virus with a Yet Unknown Impact on the Swine Industry. Front. Vet. Sci. 2018, 5, 315. [Google Scholar] [CrossRef]
- Franzo, G.; Tucciarone, C.M.; Drigo, M.; Cecchinato, M.; Martini, M.; Mondin, A.; Menandro, M.L. First report of wild boar susceptibility to porcine circovirus type 3: high prevalence in the Colli Euganei Regional Park (Italy) in the absence of clinical signs. Transbound. Emerg. Dis. 2018, 65, 957–962. [Google Scholar] [CrossRef]
- Klaumann, F.; Dias-Alves, A.; Cabezón, O.; Mentaberre, G.; Castillo-Contreras, R.; López-Béjar, M.; Casas-Díaz, E.; Sibila, M.; Correa-Fiz, F.; Segalés, J. Porcine circovirus 3 is highly prevalent in serum and tissues and may persistently infect wild boar (Sus scrofa scrofa). Transbound Emerg. Dis. 2019, 66, 91–101. [Google Scholar] [CrossRef]
- Prinz, C.; Stillfried, M.; Neubert, L.K.; Denner, J. Detection of PCV3 in German wild boars. Virol. J. 2019, 16, 25. [Google Scholar] [CrossRef]
- Palinski, R.; Piñeyro, P.; Shang, P.; Yuan, F.; Guo, R.; Fang, Y.; Byers, E.; Hause, B.M. A novel porcine circovirus distantly related to known circoviruses is associated with porcine dermatitis and nephropathy syndrome and reproductive failure. J. Virol. 2017, 91, e01879-16. [Google Scholar] [CrossRef]
- Fux, R.; Söckler, C.; Link, E.K.; Renken, C.; Krejci, R.; Sutter, G.; Eddicks, M. Full genome characterization of porcine circovirus type 3 isolates reveals the existence of two distinct groups of virus strains. Virol. J. 2018, 15, 25. [Google Scholar] [CrossRef]
- Duvigneau, J.C.; Hartl, R.T.; Groiss, S.; Gemeiner, M. Quantitative simultaneous multiplex real-time PCR for the detection of porcine cytokines. J. immunol. methods 2005, 306, 16–27. [Google Scholar] [CrossRef]
- Behrendt, R.; Fiebig, U.; Norley, S.; Gürtler, L.; Kurth, R.; Denner, J. A neutralization assay for HIV-2 based on measurement of provirus integration by duplex real-time PCR. J. virological methods 2009, 159, 40–46. [Google Scholar] [CrossRef]
- Morozov, V.A.; Morozov, A.V.; Denner, J. New PCR diagnostic systems for the detection and quantification of porcine cytomegalovirus (PCMV). Arch. Virol. 2016, 161, 1159–1168. [Google Scholar] [CrossRef]
- Morozov, V.A.; Plotzki, E.; Rotem, A.; Barkai, U.; Denner, J. Extended microbiological characterization of Göttingen minipigs: Porcine cytomegalovirus and other viruses. Xenotransplantation 2016, 23, 490–496. [Google Scholar] [CrossRef]
- Plotzki, E.; Heinrichs, G.; Kubícková, B.; Ulrich, R.G.; Denner, J. Microbiological characterization of a newly established pig breed, Aachen Minipigs. Xenotransplantation 2016, 23, 159–167. [Google Scholar] [CrossRef]
- Morozov, V.A.; Morozov, A.V.; Rotem, A.; Barkai, U.; Bornstein, S.; Denner, J. Extended Microbiological Characterization of Göttingen Minipigs in the Context of Xenotransplantation: Detection and Vertical Transmission of Hepatitis E Virus. PLoS One 2015, 10, e0139893. [Google Scholar] [CrossRef]
- Lefebvre, D.J.; Meerts, P.; Costers, S.; Misinzo, G.; Barbé, F.; Van Reeth, K.; Nauwynck, H.J. Increased porcine circovirus type 2 replication in porcine leukocytes in vitro and in vivo by concanavalin A stimulation. Vet. Microbiol. 2008, 132, 74–86. [Google Scholar] [CrossRef]
- Yang, X.; Chen, F.; Cao, Y.; Pang, D.; Ouyang, H.; Ren, L. Comparative analysis of different methods to enhance porcine circovirus 2 replication. J. Virol. Methods 2013, 187, 368–371. [Google Scholar] [CrossRef]
- Yu, S.; Halbur, P.G.; Thacker, E. Effect of porcine circovirus type 2 infection and replication on activated porcine peripheral blood mononuclear cells in vitro. Vet. Immunol. Immunopathol. 2009, 127, 350–356. [Google Scholar] [CrossRef]
- Fiebig, U.; Abicht, J.M.; Mayr, T.; Längin, M.; Bähr, A.; Guethoff, S.; Falkenau, A.; Wolf, E.; Reichart, B.; Shibahara, T.; et al. Distribution of Porcine Cytomegalovirus in Infected Donor Pigs and in Baboon Recipients of Pig Heart Transplantation. Viruses 2018, 10, 66. [Google Scholar] [CrossRef]
- Tacke, S.J.; Specke, V.; Denner, J. Differences in release and determination of subtype of porcine endogenous retroviruses produced by stimulated normal pig blood cells. Intervirology 2003, 46, 17–24. [Google Scholar] [CrossRef]
- Piroozmand, A.; Yamamoto, Y.; Khamsri, B.; Fujita, M.; Uchiyama, T.; Adachi, A. Generation and characterization of APOBEC3G-positive 293T cells for HIV-1 Vif study. J. Med. Investig. 2007, 54, 154–158. [Google Scholar] [CrossRef][Green Version]
- Denner, J. Why was PERV not transmitted during preclinical and clinical xenotransplantation trials and after inoculation of animals? Retrovirology 2018, 15, 28. [Google Scholar] [CrossRef]
- Arruda, B.; Piñeyro, P.; Derscheid, R.; Hause, B.; Byers, E.; Dion, K.; Long, D.; Sievers, C.; Tangen, J.; Williams, T.; et al. PCV3-associated disease in the United States swine herd. Emerg. Microbes Infect. 2019, 8, 684–698. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Wang, D.; Wang, J.; Zhu, S.; She, R.; Ren, X.; Tian, J.; Quan, R.; Hou, L.; Li, Z.; et al. Induction of Porcine Dermatitis and Nephropathy Syndrome in Piglets by Infection with Porcine Circovirus Type 3. J. Virol. 2019, 93, e02045-18. [Google Scholar] [CrossRef] [PubMed]
- Zhai, S.L.; Zhou, X.; Zhang, H.; Hause, B.M.; Lin, T.; Liu, R.; Chen, Q.L.; Wei, W.K.; Lv, D.H.; Wen, X.H.; et al. Comparative epidemiology of porcine circovirus type 3 in pigs with different clinical presentations. Virol. J. 2017, 14, 222. [Google Scholar] [CrossRef] [PubMed]
- Opriessnig, T.; Meng, X.J.; Halbur, P.G. Porcine circovirus type 2 associated disease: update on current terminology, clinical manifestations, pathogenesis, diagnosis, and intervention strategies. J. Vet. Diagn. Investig. 2007, 19, 591–615. [Google Scholar] [CrossRef] [PubMed]
- Opriessnig, T.; Halbur, P.G. Concurrent infections are important for expression of porcine circovirus associated disease. Virus Res. 2012, 164, 20–32. [Google Scholar] [CrossRef] [PubMed]
- Fenaux, M.; Halbur, P.G.; Haqshenas, G.; Royer, R.; Thomas, P.; Nawagitgul, P.; Gill, M.; Toth, T.E.; Meng, X.J. Cloned genomic DNA of type 2 porcine circovirus is infectious when injected directly into the liver and lymph nodes of pigs: characterization of clinical disease, virus distribution, and pathologic lesions. J. Virol. 2002, 76, 541–551. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.J. Porcine circovirus type 2 (PCV2): pathogenesis and interaction with the immune system. Annu. Rev. Anim. Biosci. 2013, 1, 43–64. [Google Scholar] [CrossRef]
- Darwich, L.; Mateu, E. Immunology of porcine circovirus type 2 (PCV2). Virus Res. 2012, 164, 61–67. [Google Scholar] [CrossRef]
- Denner, J.; Längin, M.; Reichart, B.; Krüger, L.; Wolf, E.; Rieben, R.; Abicht, J.-M. Impact of porcine cytomegalovirus on long-term xenotransplant survival. in preparation.
- Denner, J. Reduction of the survival time of pig xenotransplants by porcine cytomegalovirus. Virol. J. 2018, 15, 171. [Google Scholar] [CrossRef]
| Primer Sets | Primer, Probe | Sequences | Accession Number | Position (nt–nt) | Length of the Amplicon (bp) | Reference |
|---|---|---|---|---|---|---|
| PCV3 screening | PCV3 Palinski_For | AGTGCTCCCCATTGAACG | KT869077 | 1427–1444 | 135 | Palinski et al., 2016 [17] |
| PCV3 Palinski_Rev | ACACAGCCGTTACTTCAC | 1561–1544 | ||||
| PCV3 Palinski_probe | [FAM]-ACCCCATGGCTCAACACATATGACC-[BHQ1] | 1473–1449 | ||||
| Sequencing 2 | PCV3 Palinski_For | AGTGCTCCCCATTGAACG | 1427–1444 | 1007 | Fux et al., 2018 [18] | |
| PCV3 Pal Seq2_Rev | CGACCAAATCCGGGTAAGC | 433–415 | ||||
| Sequencing 5 | PCV3 Pal Seq1_For | CACCGTGTGAGTGGATATAC | 74–93 | 1072 | ||
| PCV3 Fux 1144_Rev | CACCCCAACGCAATAATTGTA | 1144–1124 | ||||
| Sequencing 3 | PCV3 Fux 1137_For | TTGGGGTGGGGGTATTTATT | 1137–1156 | 425 | ||
| PCV3 Palinski_Rev | ACACAGCCGTTACTTCAC | 1561–1544 | ||||
| pGAPDH Set | pGAPDH_For | ACATGGCCTCCAAGGAGTAAGA | n/s | n/s | 106 | Duvigneau et al., 2005 [19] |
| pGAPDH_Rev | GATCGAGTTGGGGCTGTGACT | |||||
| pGAPDH_probe | [HEX]CCACCAACCCCAGCAAGAGCACGC[BHQ1] | |||||
| huGAPDH Set | huGAPDH_For | GGCGATGCTGGCGCTGAGTAC | AF261085 | 365–385 | 148 | Behrendt et al., 2009 [20] |
| huGAPDH_Rev | TGGTCCACACCCATGACGA | 513–495 | ||||
| huGAPDH_probe | [HEX]TTCACCACCATGGAGAAGGCTGGG[BHQ1] | 407–430 |
| Date of Transplantation | Nr. Donor Pig | Recipient Baboon | Transplant Survival Time, Days | Presence of PCV3 1 |
|---|---|---|---|---|
| 04.10.2017 | 5528 | A | 90 | no |
| 07.03.2018 | 5803 | B | 195 | yes |
| 21.03.2018 | 5807 | C | 182 | yes |
| 10.10.2018 | 6249 | D | 15 | yes |
| 24.10.2018 | 6253 | E | 27 | yes |
| 12.12.2018 | 6329 | F | 90 | no |
| Pig Number | PCV3 1 |
|---|---|
| 6493 | No |
| 5295 | No |
| 4776 | No |
| 5806 | No |
| 5870 | Yes |
| 6086 | No |
| 6087 | Yes |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krüger, L.; Längin, M.; Reichart, B.; Fiebig, U.; Kristiansen, Y.; Prinz, C.; Kessler, B.; Egerer, S.; Wolf, E.; Abicht, J.-M.; et al. Transmission of Porcine Circovirus 3 (PCV3) by Xenotransplantation of Pig Hearts into Baboons. Viruses 2019, 11, 650. https://doi.org/10.3390/v11070650
Krüger L, Längin M, Reichart B, Fiebig U, Kristiansen Y, Prinz C, Kessler B, Egerer S, Wolf E, Abicht J-M, et al. Transmission of Porcine Circovirus 3 (PCV3) by Xenotransplantation of Pig Hearts into Baboons. Viruses. 2019; 11(7):650. https://doi.org/10.3390/v11070650
Chicago/Turabian StyleKrüger, Luise, Matthias Längin, Bruno Reichart, Uwe Fiebig, Yannick Kristiansen, Carolin Prinz, Barbara Kessler, Stefanie Egerer, Eckhard Wolf, Jan-Michael Abicht, and et al. 2019. "Transmission of Porcine Circovirus 3 (PCV3) by Xenotransplantation of Pig Hearts into Baboons" Viruses 11, no. 7: 650. https://doi.org/10.3390/v11070650
APA StyleKrüger, L., Längin, M., Reichart, B., Fiebig, U., Kristiansen, Y., Prinz, C., Kessler, B., Egerer, S., Wolf, E., Abicht, J.-M., & Denner, J. (2019). Transmission of Porcine Circovirus 3 (PCV3) by Xenotransplantation of Pig Hearts into Baboons. Viruses, 11(7), 650. https://doi.org/10.3390/v11070650





