The Adenosine Analogue NITD008 has Potent Antiviral Activity against Human and Animal Caliciviruses
Abstract
1. Introduction
2. Materials and Methods
2.1. Drugs, Cell Lines, Viruses
2.2. Toxicity Analysis
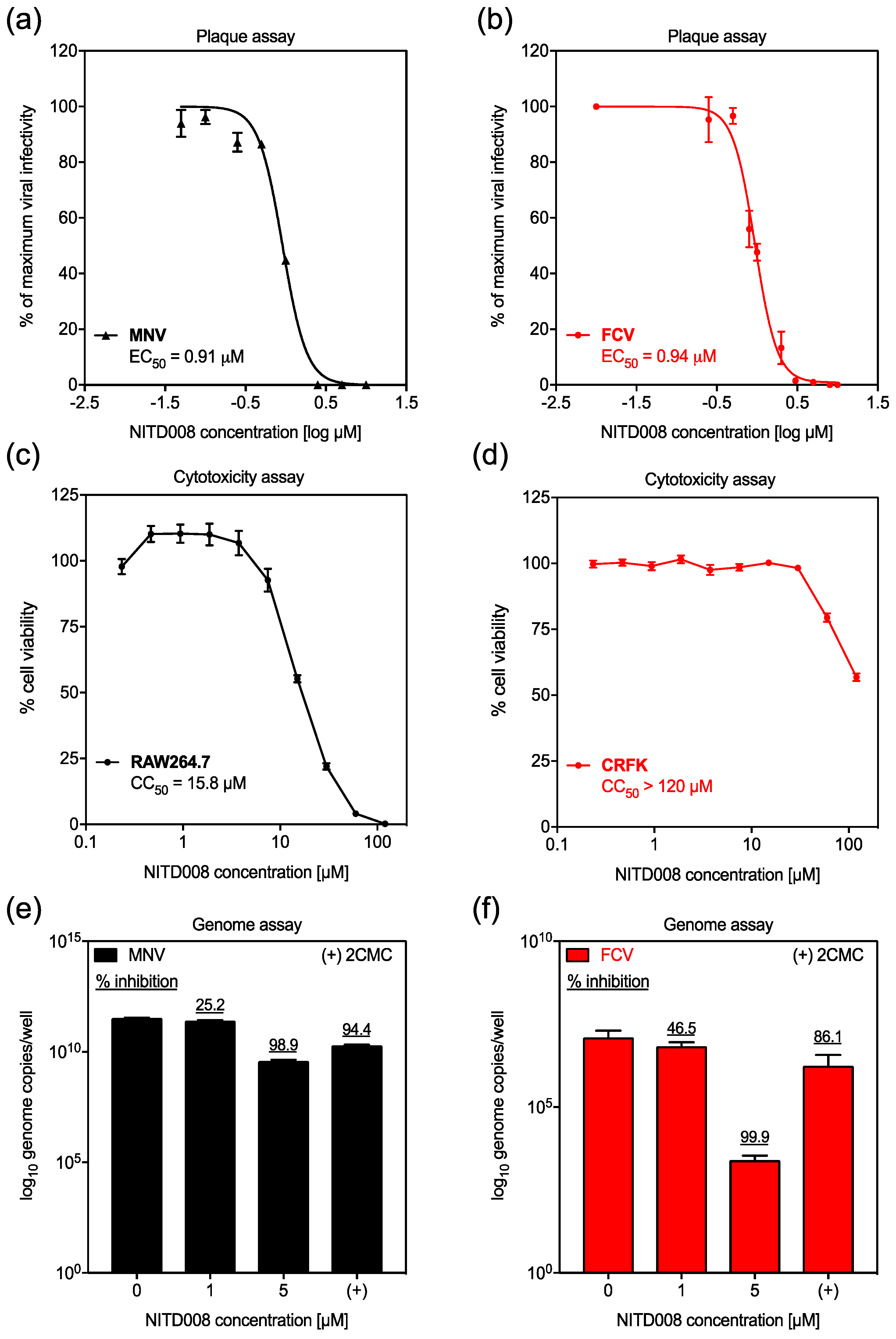
2.3. Inhibition of Viral Infectivity
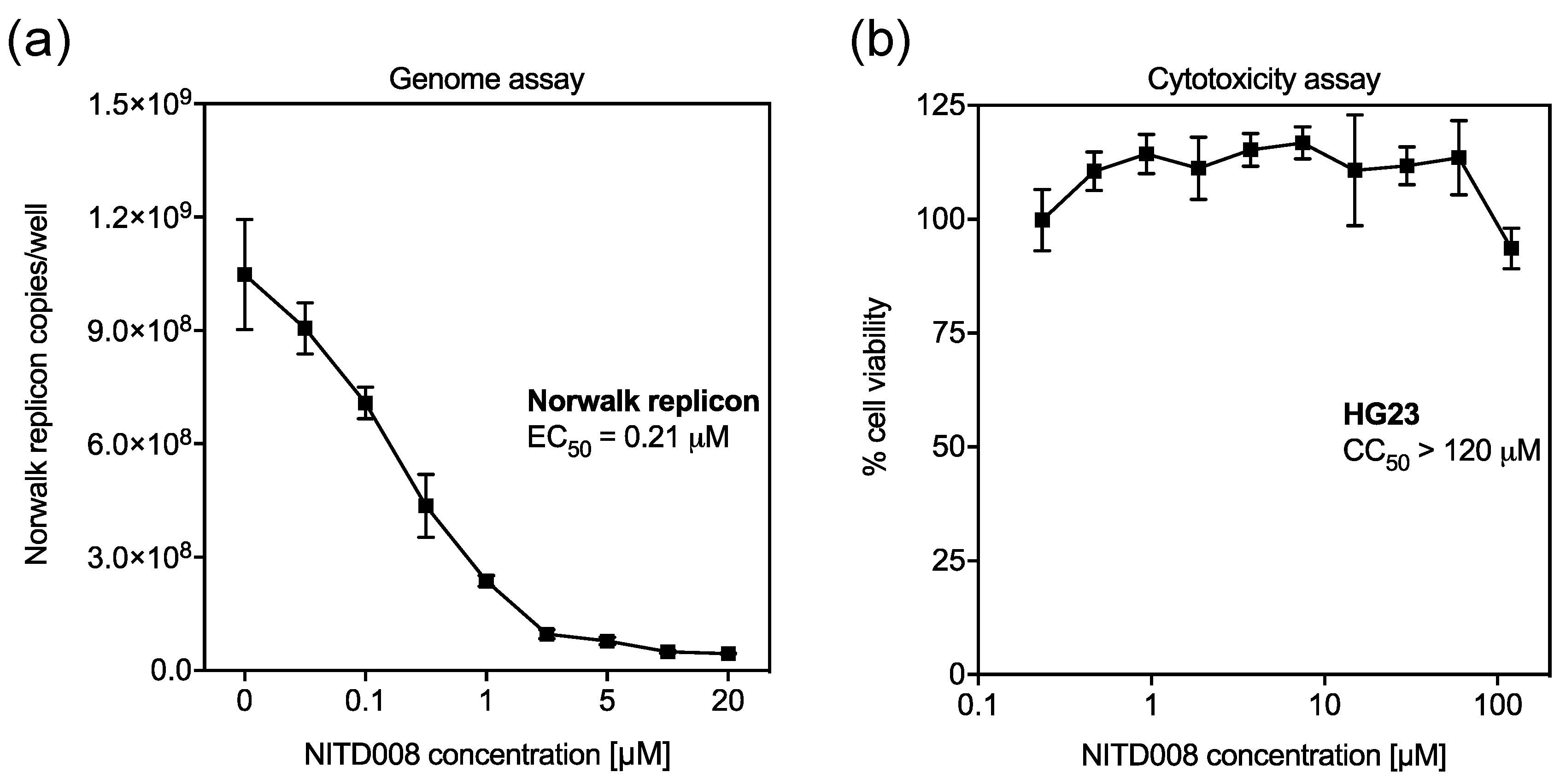
2.4. Inhibition of Viral Replication
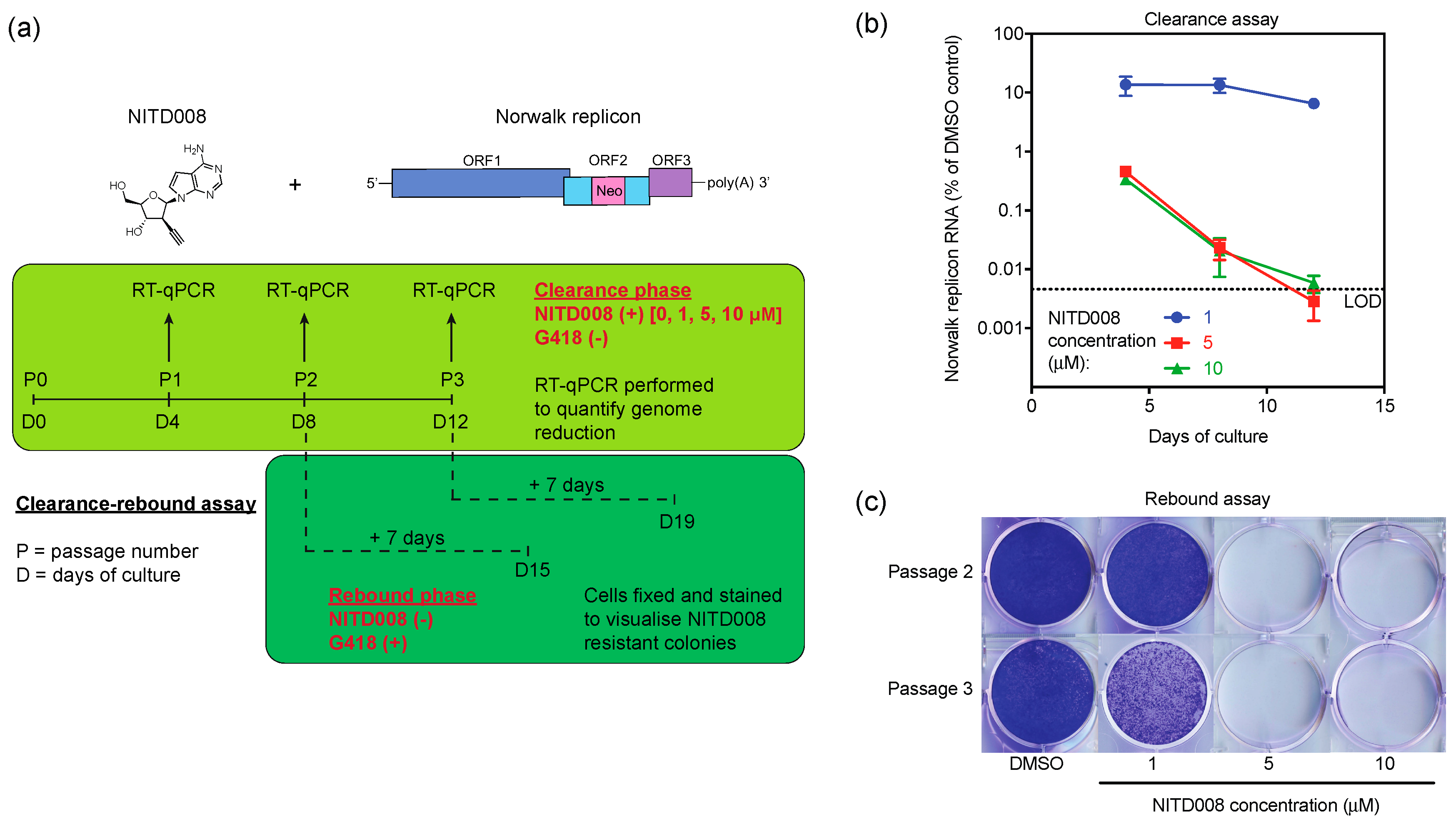
2.5. Clearance-Rebound Studies
3. Results
3.1. NITD008 is Inhibitory against Animal Calicivirus Infection In vitro
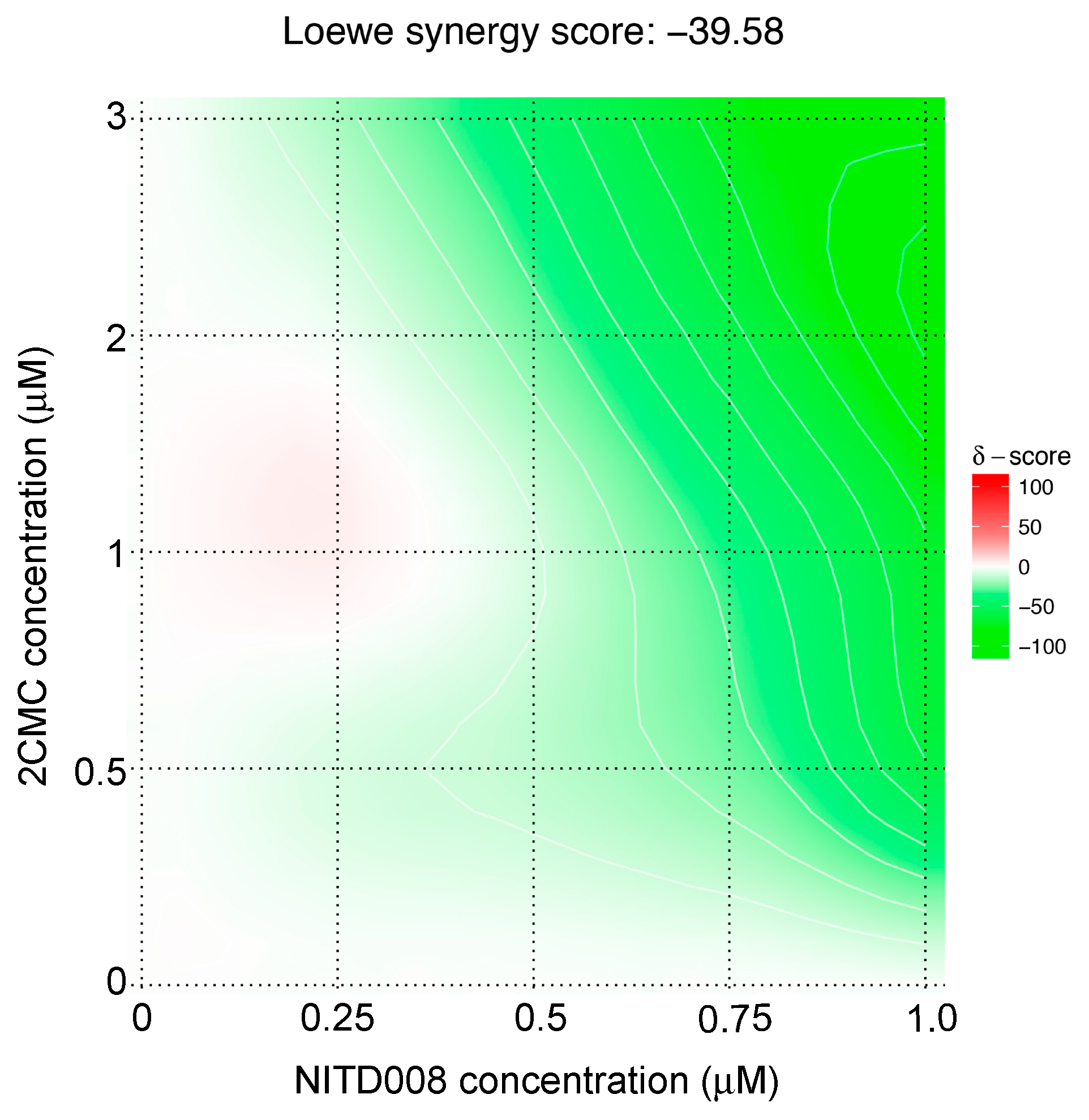
3.2. NITD008 Combined with 2CMC has an Antagonistic Antiviral Effect
3.3. NITD008 is a Potent Inhibitor of Human Norovirus Replication
3.4. NITD008 Effectively Clears the Human Norovirus Replicon from Cells
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Clarke, I.N.; Estes, M.K.; Green, K.Y.; Hansman, G.S.; Knowles, N.J.; Koopmans, M.K.; Matson, D.O.; Meyers, G.; Neill, J.D.; Radford, A.; et al. Caliciviridae; 9th Report; Elsevier: San Diego, CA, USA, 2012; pp. 977–986. [Google Scholar]
- Rohayem, J.; Bergmann, M.; Gebhardt, J.; Gould, E.; Tucker, P.; Mattevi, A.; Unge, T.; Hilgenfeld, R.; Neyts, J. Antiviral strategies to control calicivirus infections. Antivir. Res. 2010, 87, 162–178. [Google Scholar] [CrossRef] [PubMed]
- Green, K.; Chanock, R.; Kapikian, A. Fields virology. In Human Caliciviruses; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2001; pp. 841–874. [Google Scholar]
- Ahmed, S.M.; Hall, A.J.; Robinson, A.E.; Verhoef, L.; Premkumar, P.; Parashar, U.D.; Koopmans, M.; Lopman, B.A. Global prevalence of norovirus in cases of gastroenteritis: A systematic review and meta-analysis. Lancet Infect. Dis. 2014, 14, 725–730. [Google Scholar] [CrossRef]
- Pires, S.M.; Fischer-Walker, C.L.; Lanata, C.F.; Devleesschauwer, B.; Hall, A.J.; Kirk, M.D.; Duarte, A.S.; Black, R.E.; Angulo, F.J. Aetiology-specific estimates of the global and regional incidence and mortality of diarrhoeal diseases commonly transmitted through food. PLoS ONE 2015, 10, e0142927. [Google Scholar] [CrossRef]
- Bartsch, S.M.; Lopman, B.A.; Ozawa, S.; Hall, A.J.; Lee, B.Y. Global economic burden of norovirus gastroenteritis. PLoS ONE 2016, 11, e0151219. [Google Scholar] [CrossRef] [PubMed]
- Radford, A.D.; Coyne, K.P.; Dawson, S.; Porter, C.J.; Gaskell, R.M. Feline calicivirus. Vet. Res. 2007, 38, 319–335. [Google Scholar] [CrossRef]
- Rocchi, M.; Dagleish, M. Diagnosis and prevention of RHDV2 infection. Vet. Rec. 2018, 182, 604. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, D.I.; Atmar, R.L.; Lyon, G.M.; Treanor, J.J.; Chen, W.H.; Jiang, X.; Vinjé, J.; Gregoricus, N.; Frenck Jr, R.W.; Moe, C.L. Norovirus vaccine against experimental human GII.4 virus illness: A challenge study in healthy adults. J. Infect. Dis. 2014, 211, 870–878. [Google Scholar] [CrossRef]
- Atmar, R.L.; Bernstein, D.I.; Lyon, G.M.; Treanor, J.J.; Al-Ibrahim, M.S.; Graham, D.Y.; Vinjé, J.; Jiang, X.; Gregoricus, N.; Frenck, R.W. Serological correlates of protection against a GII.4 norovirus. Clin. Vaccine Immunol. 2015, 22, 923–929. [Google Scholar] [CrossRef]
- Atmar, R.L.; Baehner, F.; Cramer, J.P.; Song, E.; Borkowski, A.; Mendelman, P.M.; Group, N.-S.; Al-Ibrahim, M.S.; Bernstein, D.L.; Brandon, D.M. Rapid responses to 2 virus-like particle norovirus vaccine candidate formulations in healthy adults: A randomized controlled trial. J. Infect. Dis. 2016, 214, 845–853. [Google Scholar] [CrossRef]
- Leroux-Roels, G.; Cramer, J.P.; Mendelman, P.M.; Sherwood, J.; Clemens, R.; Aerssens, A.; de Coster, I.; Borkowski, A.; Baehner, F.; van Damme, P. Safety and immunogenicity of different formulations of norovirus vaccine candidate in healthy adults: A randomized, controlled, double-blind clinical trial. J. Infect. Dis. 2017, 217, 597–607. [Google Scholar] [CrossRef]
- Bárcena, J.; Guerra, B.; Angulo, I.; González, J.; Valcárcel, F.; Mata, C.P.; Castón, J.R.; Blanco, E.; Alejo, A. Comparative analysis of rabbit hemorrhagic disease virus (RHDV) and new RHDV2 virus antigenicity, using specific virus-like particles. Vet. Res. 2015, 46, 106. [Google Scholar] [CrossRef] [PubMed]
- Abrantes, J.; Van Der Loo, W.; Le Pendu, J.; Esteves, P.J. Rabbit haemorrhagic disease (RHD) and rabbit haemorrhagic disease virus (RHDV): A review. Vet. Res. 2012, 43, 12. [Google Scholar] [CrossRef] [PubMed]
- Peacock, D.; Kovaliski, J.; Sinclair, R.; Mutze, G.; Iannella, A.; Capucci, L. RHDV2 overcoming RHDV immunity in wild rabbits (Oryctolagus cuniculus) in Australia. Vet. Rec. 2017, 180, 280. [Google Scholar] [CrossRef]
- Coyne, K.; Jones, B.; Kipar, A.; Chantrey, J.; Porter, C.; Barber, P.; Dawson, S.; Gaskell, R.; Radford, A. Lethal outbreak of disease associated with feline calicivirus infection in cats. Vet. Rec. 2006, 158, 544. [Google Scholar] [CrossRef]
- Radford, A.D.; Dawson, S.; Coyne, K.P.; Porter, C.J.; Gaskell, R.M. The challenge for the next generation of feline calicivirus vaccines. Vet. Microbiol. 2006, 117, 14–18. [Google Scholar] [CrossRef]
- Chang, K.-O.; Sosnovtsev, S.V.; Belliot, G.; King, A.D.; Green, K.Y. Stable expression of a Norwalk virus RNA replicon in a human hepatoma cell line. Virology 2006, 353, 463–473. [Google Scholar] [CrossRef]
- Fumian, T.; Tuipulotu, D.; Netzler, N.; Lun, J.; Russo, A.; Yan, G.; White, P. Potential therapeutic agents for feline calicivirus infection. Viruses 2018, 10, 433. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, S.S.; Green, K.Y.; Korba, B.E. Treatment of norovirus infections: Moving antivirals from the bench to the bedside. Antivir. Res. 2014, 105, 80–91. [Google Scholar] [CrossRef] [PubMed]
- Urakova, N.; Netzler, N.; Kelly, A.G.; Frese, M.; White, P.A.; Strive, T. Purification and biochemical characterisation of rabbit calicivirus RNA-dependent RNA polymerases and identification of non-nucleoside inhibitors. Viruses 2016, 8, 100. [Google Scholar] [CrossRef] [PubMed]
- Van Dycke, J.; Arnoldi, F.; Papa, G.; Vandepoele, J.; Burrone, O.R.; Mastrangelo, E.; Tarantino, D.; Heylen, E.; Neyts, J.; Rocha-Pereira, J. A single nucleoside viral polymerase inhibitor against norovirus, rotavirus, and sapovirus-induced diarrhea. J. Infect. Dis. 2018, 218, 1753–1758. [Google Scholar] [CrossRef]
- Vashist, S.; Bailey, D.; Putics, A.; Goodfellow, I. Model systems for the study of human norovirus biology. Future Virol. 2009, 4, 353–367. [Google Scholar] [CrossRef]
- Weerasekara, S.; Prior, A.M.; Hua, D.H. Current tools for norovirus drug discovery. Expert Opin. Drug Discov. 2016, 11, 529–541. [Google Scholar] [CrossRef]
- Najera, I. Resistance to HCV nucleoside analogue inhibitors of hepatitis C virus RNA-dependent RNA polymerase. Curr. Opin. Virol. 2013, 3, 508–513. [Google Scholar] [CrossRef]
- Benzaria, S.; Bardiot, D.; Bouisset, T.; Counor, C.; Rabeson, C.; Pierra, C.; Storer, R.; Loi, A.G.; Cadeddu, A.; Mura, M. 2′-C-methyl branched pyrimidine ribonucleoside analogues: Potent inhibitors of RNA virus replication. Antivir. Chem. Chemother. 2007, 18, 225–242. [Google Scholar] [CrossRef][Green Version]
- Jin, Z.; Tucker, K.; Lin, X.; Kao, C.C.; Shaw, K.; Tan, H.; Symons, J.; Behera, I.; Rajwanshi, V.K.; Dyatkina, N. Biochemical evaluation of the inhibition properties of favipiravir (T-705) and 2′-C-methyl-cytidine triphosphates against human and mouse norovirus RNA polymerases. Antimicrob. Agents Chemother. 2015, 59, 7504–7516. [Google Scholar] [CrossRef]
- Kolawole, A.O.; Rocha-Pereira, J.; Elftman, M.D.; Neyts, J.; Wobus, C.E. Inhibition of human norovirus by a viral polymerase inhibitor in the B cell culture system and in the mouse model. Antivir. Res. 2016, 132, 46–49. [Google Scholar] [CrossRef]
- Rocha-Pereira, J.; Jochmans, D.; Neyts, J. Prophylactic treatment with the nucleoside analogue 2′-C-methylcytidine completely prevents transmission of norovirus. J. Antimicrob. Chemother. 2014, 70, 190–197. [Google Scholar] [CrossRef]
- Costantini, V.P.; Whitaker, T.; Barclay, L.; Lee, D.; McBrayer, T.R.; Schinazi, R.F.; Vinjé, J. Antiviral activity of nucleoside analogues against norovirus. Antivir. Ther. 2012, 17, 981. [Google Scholar] [CrossRef]
- Rocha-Pereira, J.; Jochmans, D.; Debing, Y.; Verbeken, E.; Nascimento, M.S.; Neyts, J. The viral polymerase inhibitor 2′-C-methylcytidine inhibits Norwalk virus replication and protects against norovirus-induced diarrhea and mortality in a mouse model. J. Virol. 2013, 87, 11798–11805. [Google Scholar] [CrossRef]
- Rocha-Pereira, J.; Jochmans, D.; Dallmeier, K.; Leyssen, P.; Cunha, R.; Costa, I.; Nascimento, M.; Neyts, J. Inhibition of norovirus replication by the nucleoside analogue 2′-C-methylcytidine. Biochem. Biophys. Res. Commun. 2012, 427, 796–800. [Google Scholar] [CrossRef]
- Netzler, N.E.; Enosi Tuipulotu, D.; White, P.A. Norovirus antivirals: Where are we now? Med. Res. Rev. 2018, 39, 860–886. [Google Scholar] [CrossRef]
- Ruis, C.; Brown, L.-A.K.; Roy, S.; Atkinson, C.; Williams, R.; Burns, S.O.; Yara-Romero, E.; Jacobs, M.; Goldstein, R.; Breuer, J. Mutagenesis in norovirus in response to favipiravir treatment. N. Engl. J. Med. 2018, 379, 2173–2176. [Google Scholar] [CrossRef]
- Yin, Z.; Chen, Y.-L.; Schul, W.; Wang, Q.-Y.; Gu, F.; Duraiswamy, J.; Kondreddi, R.R.; Niyomrattanakit, P.; Lakshminarayana, S.B.; Goh, A. An adenosine nucleoside inhibitor of dengue virus. Proc. Natl. Acad. Sci. USA 2009, 106, 20435–20439. [Google Scholar] [CrossRef]
- Deng, Y.-Q.; Zhang, N.-N.; Li, C.-F.; Tian, M.; Hao, J.-N.; Xie, X.-P.; Shi, P.-Y.; Qin, C.-F. Adenosine analog NITD008 is a potent inhibitor of Zika virus. Open Forum Infect. Dis. 2016, 3, ofw175. [Google Scholar] [CrossRef]
- Qing, J.; Luo, R.; Wang, Y.; Nong, J.; Wu, M.; Shao, Y.; Tang, R.; Yu, X.; Yin, Z.; Sun, Y. Resistance analysis and characterization of NITD008 as an adenosine analog inhibitor against hepatitis C virus. Antivir. Res. 2016, 126, 43–54. [Google Scholar] [CrossRef]
- Nelson, J.; Roe, K.; Orillo, B.; Shi, P.-Y.; Verma, S. Combined treatment of adenosine nucleoside inhibitor NITD008 and histone deacetylase inhibitor vorinostat represents an immunotherapy strategy to ameliorate West Nile virus infection. Antivir. Res. 2015, 122, 39–45. [Google Scholar] [CrossRef]
- Shang, L.; Wang, Y.; Qing, J.; Shu, B.; Cao, L.; Lou, Z.; Gong, P.; Sun, Y.; Yin, Z. An adenosine nucleoside analogue NITD008 inhibits EV71 proliferation. Antivir. Res. 2014, 112, 47–58. [Google Scholar] [CrossRef]
- Deng, C.-L.; Yeo, H.; Ye, H.-Q.; Liu, S.-Q.; Shang, B.-D.; Gong, P.; Alonso, S.; Shi, P.-Y.; Zhang, B. Inhibition of enterovirus 71 by adenosine analog NITD008. J. Virol. 2014, 88, 11915–11923. [Google Scholar] [CrossRef]
- Netzler, N.E.; Tuipulotu, D.E.; Vasudevan, S.G.; Mackenzie, J.M.; White, P.A. Antiviral candidates for treating hepatitis E virus infection. Antimicrob. Agents Chemother. 2019, 63, e00003–19. [Google Scholar] [CrossRef]
- Eltahla, A.A.; Lim, K.L.; Eden, J.-S.; Kelly, A.G.; Mackenzie, J.M.; White, P.A. Non-nucleoside inhibitors of the norovirus RNA polymerase; scaffolds for rational drug design. Antimicrob. Agents Chemother. 2014, 58, 3115–3123. [Google Scholar] [CrossRef]
- Ferla, S.; Netzler, N.E.; Ferla, S.; Veronese, S.; Tuipulotu, D.E.; Guccione, S.; Brancale, A.; White, P.A.; Bassetto, M. In silico screening for human norovirus antivirals reveals a novel non-nucleoside inhibitor of the viral polymerase. Sci. Rep. 2018, 8, 4129. [Google Scholar] [CrossRef]
- Netzler, N.E.; Tuipulotu, D.E.; Eltahla, A.A.; Lun, J.H.; Ferla, S.; Brancale, A.; Urakova, N.; Frese, M.; Strive, T.; Mackenzie, J.M. Broad-spectrum non-nucleoside inhibitors for caliciviruses. Antivir. Res. 2017, 146, 65–75. [Google Scholar] [CrossRef]
- Tuipulotu, D.E.; Netzler, N.E.; Lun, J.H.; Mackenzie, J.M.; White, P.A. TLR7 agonists display potent antiviral effects against norovirus infection via innate stimulation. Antimicrob. Agents Chemother. 2018, 62, e02417-17. [Google Scholar] [CrossRef] [PubMed]
- Ianevski, A.; He, L.; Aittokallio, T.; Tang, J. Synergyfinder: A web application for analyzing drug combination dose–response matrix data. Bioinformatics 2017, 33, 2413–2415. [Google Scholar] [CrossRef]
- Loewe, S. Die quantitativen probleme der pharmakologie. Ergeb. Physiol. 1928, 27, 47–187. [Google Scholar] [CrossRef]
- Kitano, M.; Hosmillo, M.; Emmott, E.; Lu, J.; Goodfellow, I. Selection and characterization of rupintrivir-resistant Norwalk virus replicon cells in vitro. Antimicrob. Agents Chemother. 2018, 62, e00201-18. [Google Scholar] [CrossRef]
- Pawlotsky, J.M. Treatment failure and resistance with direct-acting antiviral drugs against hepatitis C virus. Hepatology 2011, 53, 1742–1751. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Enosi Tuipulotu, D.; Fumian, T.M.; Netzler, N.E.; Mackenzie, J.M.; White, P.A. The Adenosine Analogue NITD008 has Potent Antiviral Activity against Human and Animal Caliciviruses. Viruses 2019, 11, 496. https://doi.org/10.3390/v11060496
Enosi Tuipulotu D, Fumian TM, Netzler NE, Mackenzie JM, White PA. The Adenosine Analogue NITD008 has Potent Antiviral Activity against Human and Animal Caliciviruses. Viruses. 2019; 11(6):496. https://doi.org/10.3390/v11060496
Chicago/Turabian StyleEnosi Tuipulotu, Daniel, Tulio M. Fumian, Natalie E. Netzler, Jason M. Mackenzie, and Peter A. White. 2019. "The Adenosine Analogue NITD008 has Potent Antiviral Activity against Human and Animal Caliciviruses" Viruses 11, no. 6: 496. https://doi.org/10.3390/v11060496
APA StyleEnosi Tuipulotu, D., Fumian, T. M., Netzler, N. E., Mackenzie, J. M., & White, P. A. (2019). The Adenosine Analogue NITD008 has Potent Antiviral Activity against Human and Animal Caliciviruses. Viruses, 11(6), 496. https://doi.org/10.3390/v11060496





