Identification and Analysis of Potential Genes Regulated by an Alphasatellite (TYLCCNA) that Contribute to Host Resistance against Tomato Yellow Leaf Curl China Virus and Its Betasatellite (TYLCCNV/TYLCCNB) Infection in Nicotiana benthamiana
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials, Growth Conditions and Agroinoculations
2.2. Genomic DNA Extraction and Southern Blot Analysis
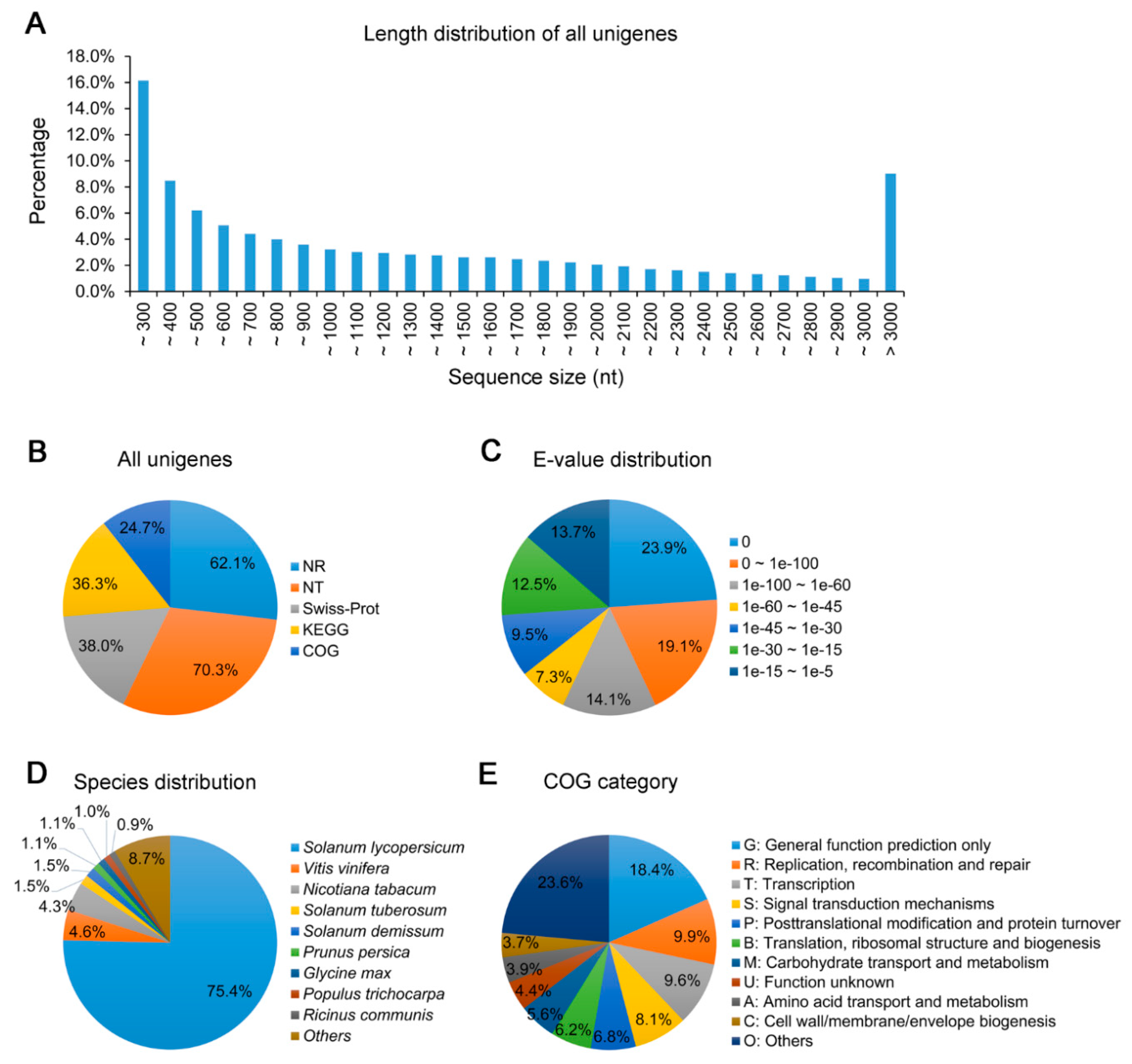
2.3. Transcriptome Sequencing and Unigene Annotation
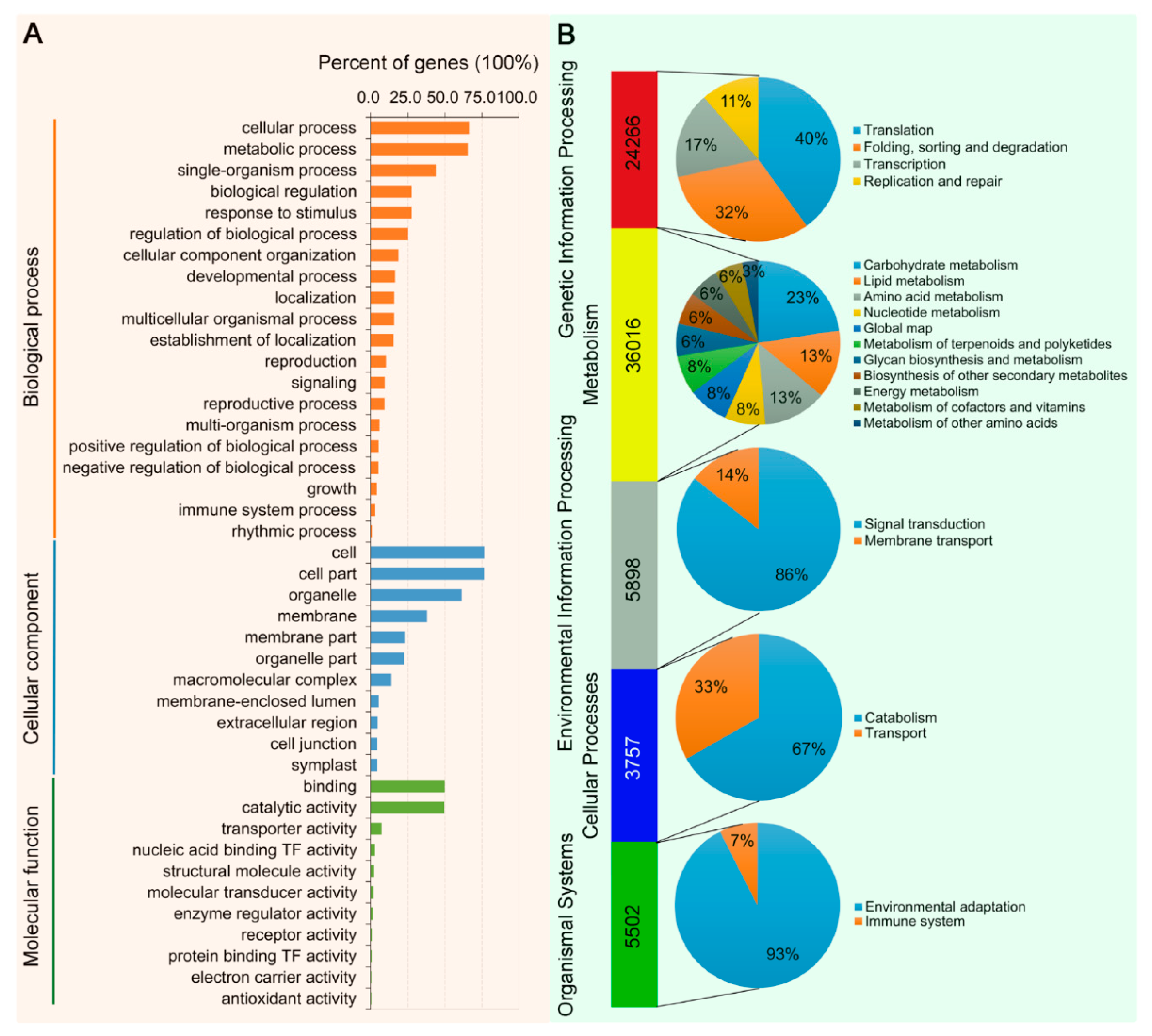
2.4. Differential Gene Expression, GO and KEGG Enrichment Analyses
2.5. Reverse Transcription Quantitative Real-Time PCR (RT-qPCR) Analysis
2.6. Virus-Induced Gene Silencing (VIGS) Assay
2.7. Statistical Analysis
3. Results
3.1. Effects of TYLCCNA on Virus-Infected Symptoms and Accumulation of Viral Genomic DNAs
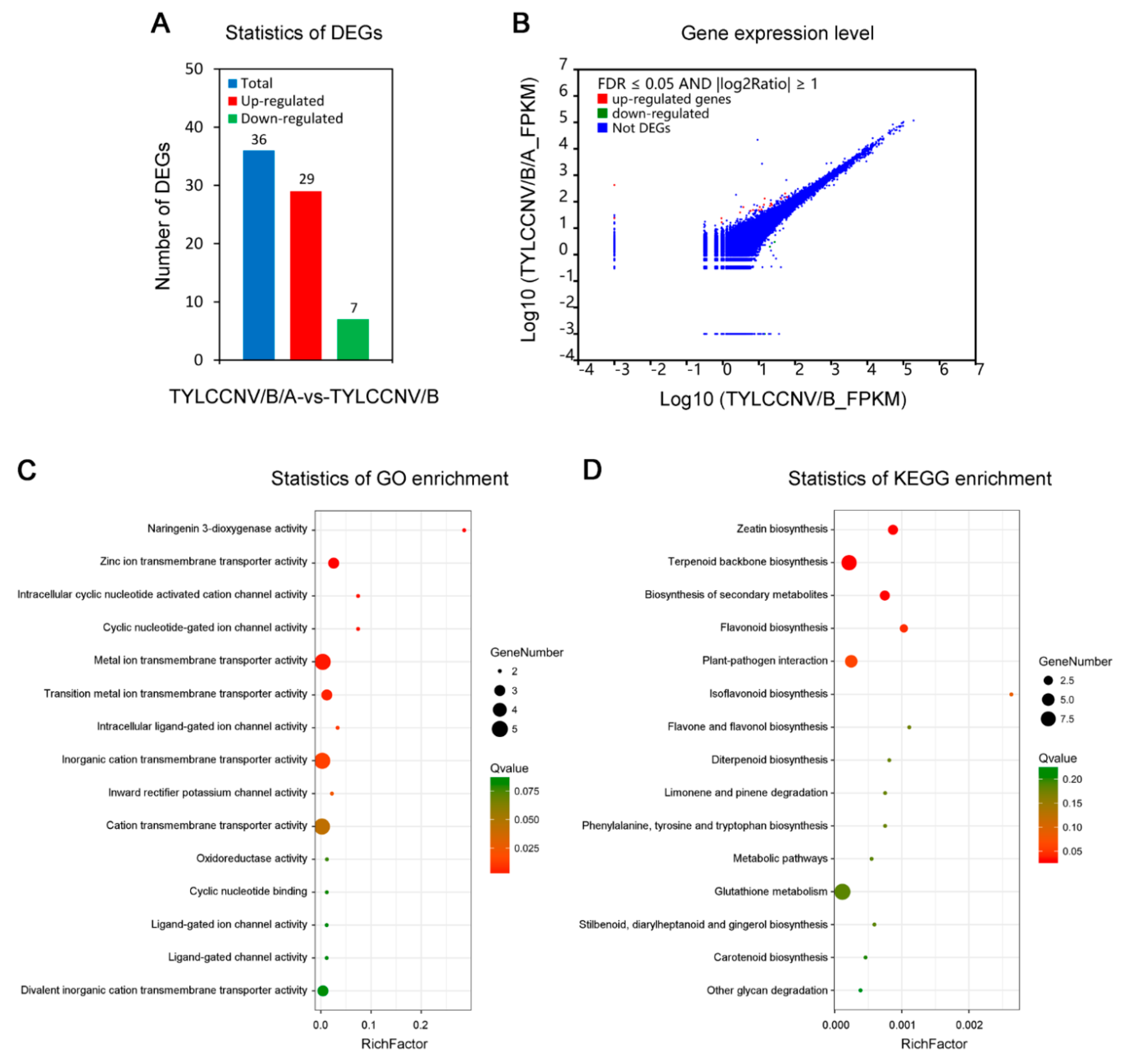
3.2. Transcriptome Sequencing of Leaves of N. benthamiana Infected by TYLCCNV/TYLCCNB with or without TYLCCNA
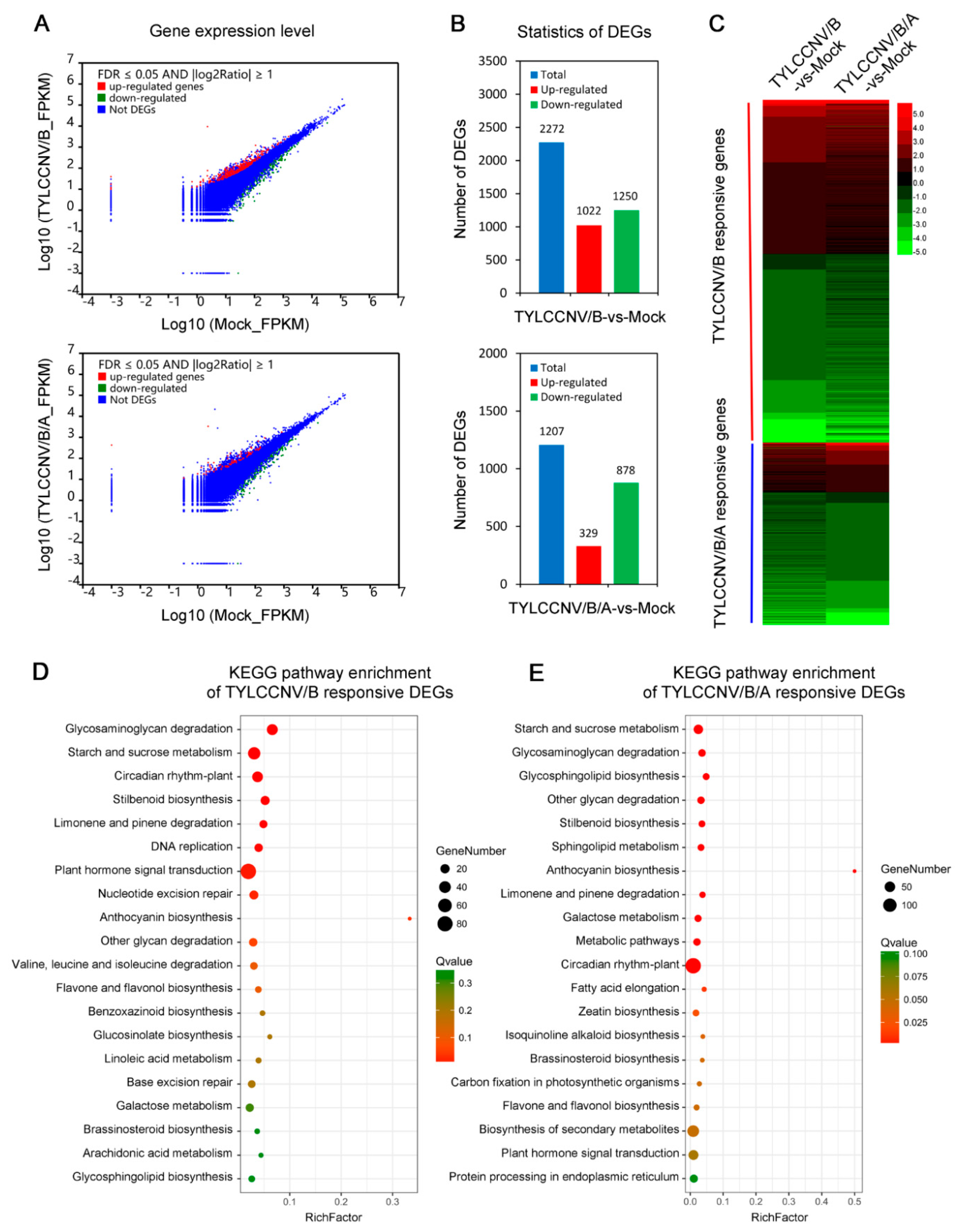
3.3. Screening of DEGs Responding to TYLCCNV/TYLCCNB and TYLCCNV/TYLCCNB/TYLCCNA
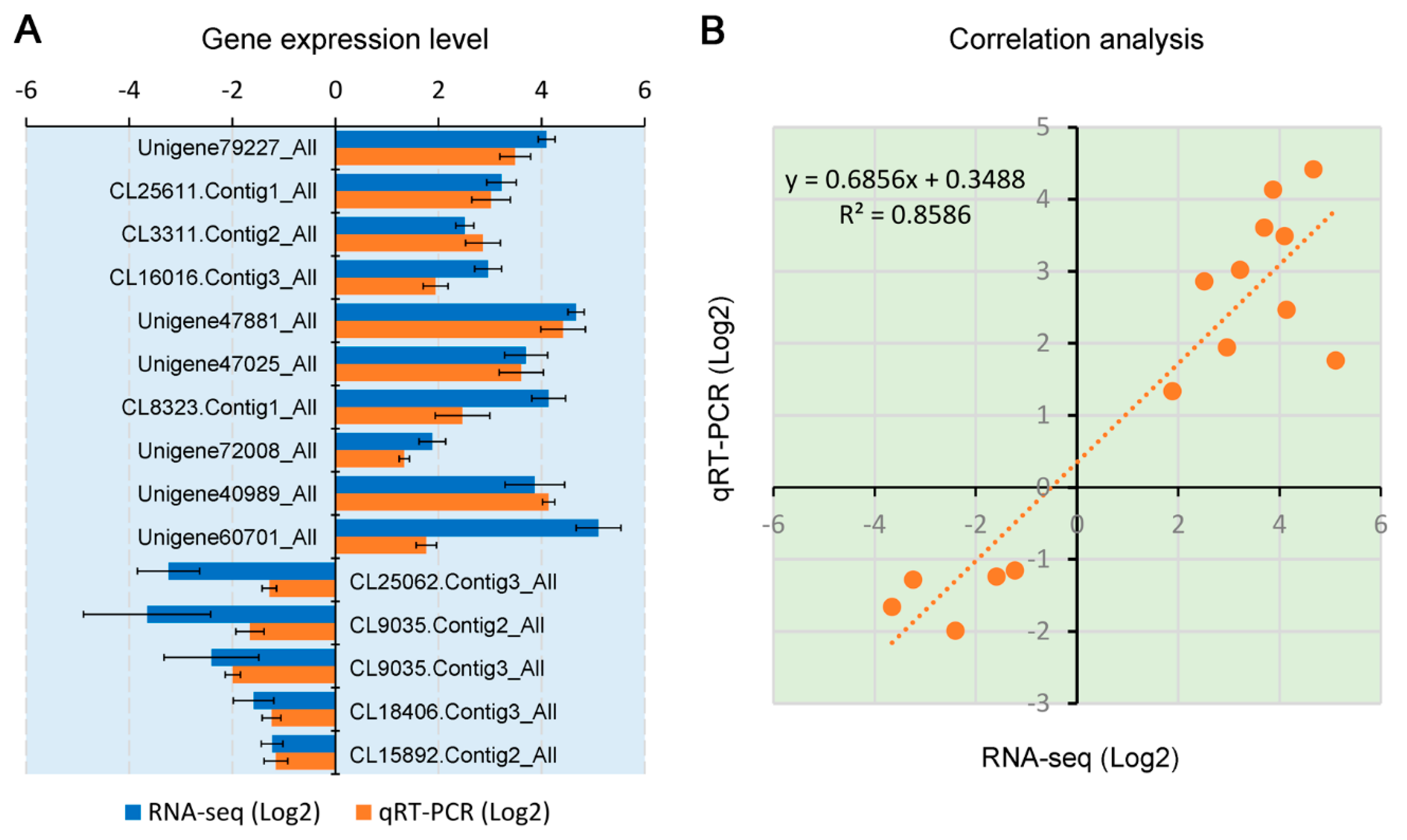
3.4. Validation of Gene Expression by RT-qPCR Analysis
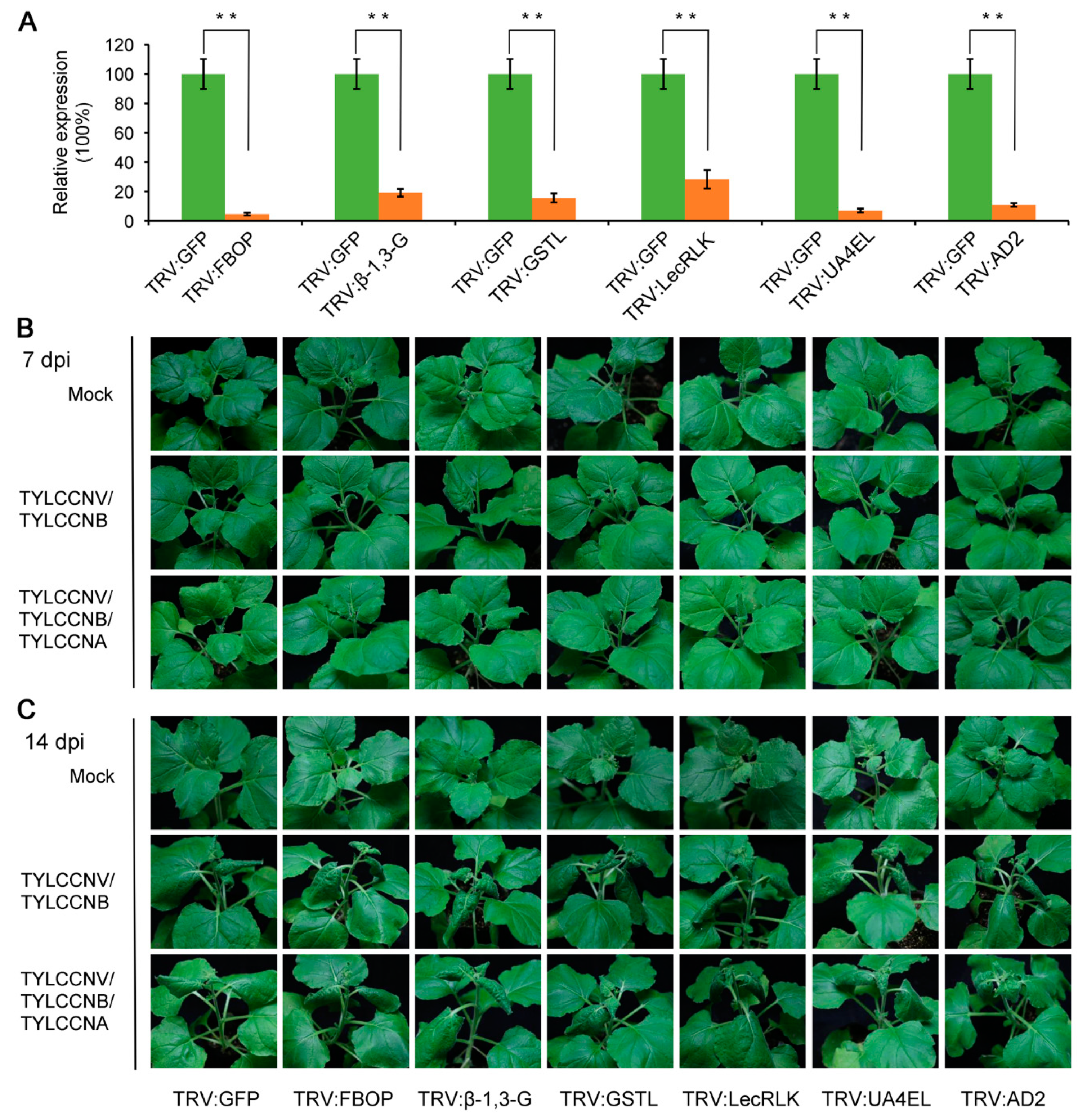
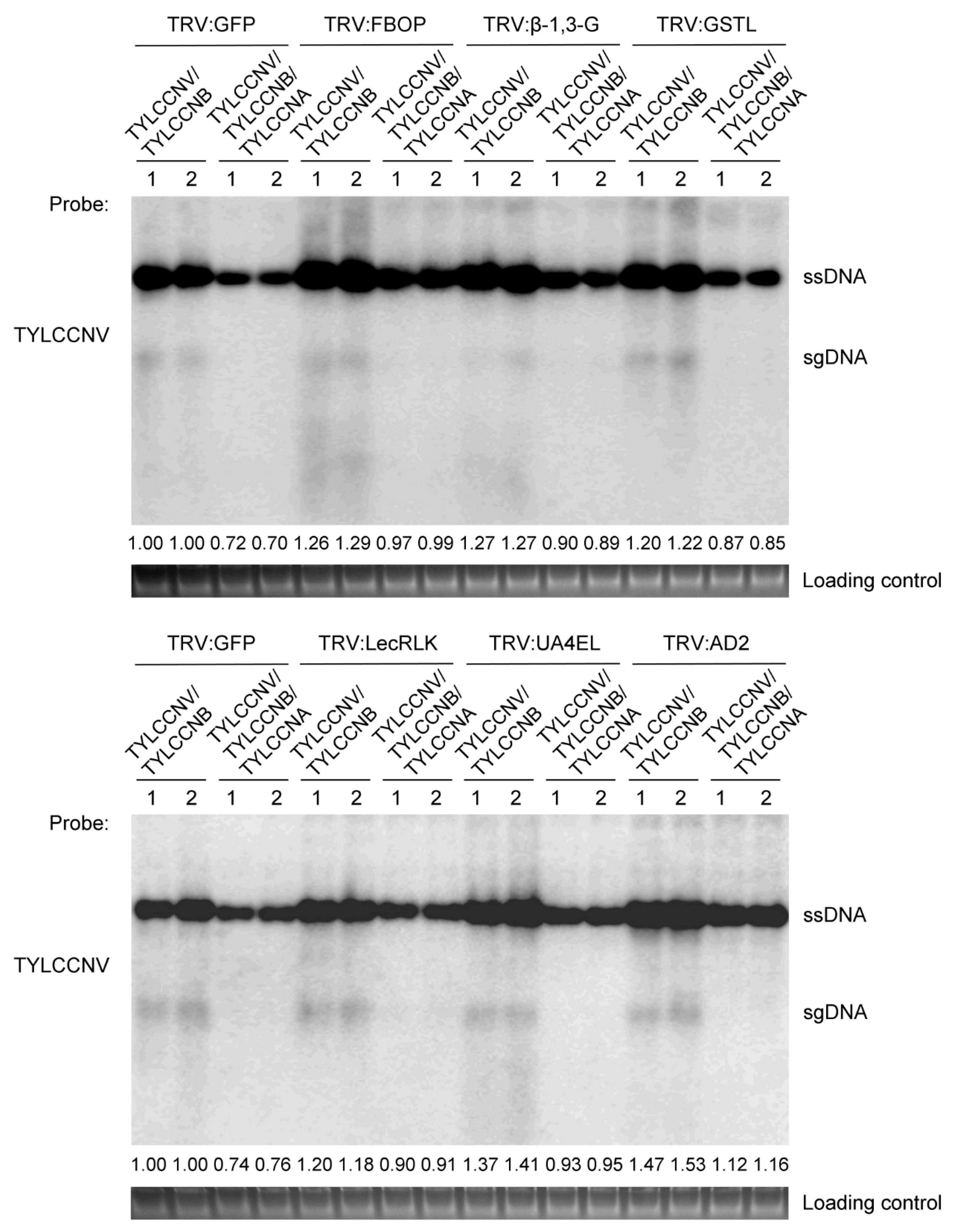
3.5. Disruption of TYLCCNA Responsive Genes Increases Susceptibility to TYLCCNV/TYLCCNB Infection
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Rojas, M.R.; Hagen, C.; Lucas, W.J.; Gilbertson, R.L. Exploiting chinks in the plant’s armor: Evolution and emergence of geminiviruses. Annu. Rev. Phytopathol. 2005, 43, 361–394. [Google Scholar] [CrossRef] [PubMed]
- Jeske, H. Geminiviruses. Curr. Top. Microbiol. Immunol. 2009, 331, 185–226. [Google Scholar] [PubMed]
- Hanley-Bowdoin, L.; Bejarano, E.R.; Robertson, D.; Mansoor, S. Geminiviruses: Masters at redirecting and reprogramming plant processes. Nat. Rev. Microbiol. 2013, 11, 777–788. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.; Wang, Z.Q.; Xiao, R.; Wang, Y.; Xie, Y.; Zhou, X. iTRAQ analysis of the tobacco leaf proteome reveals that RNA-directed DNA methylation (RdDM) has important roles in defense against geminivirus-betasatellite infection. J. Proteomics 2017, 152, 88–101. [Google Scholar] [CrossRef] [PubMed]
- Varsani, A.; Roumagnac, P.; Fuchs, M.; Navas-Castillo, J.; Moriones, E.; Idris, A.; Briddon, R.W.; Rivera-Bustamante, R.; Murilo Zerbini, F.; Martin, D.P. Capulavirus and grablovirus: Two new genera in the family geminiviridae. Arch. Virol. 2017, 162, 1819–1831. [Google Scholar] [CrossRef] [PubMed]
- Harrison, B.; Robinson, D. Natural genomic and antigenic variation in whitefly- transmitted geminiviruses (begomoviruses). Annu. Rev. Phytopathol. 1999, 37, 369–398. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.K.; Zerbini, F.M.; Navascastillo, J.; Moriones, E.; Ramossobrinho, R.; Silva, J.C.; Fiallo-Olivé, E.; Briddon, R.W.; Hernández-Zepeda, C.; Idris, A.; et al. Revision of begomovirus taxonomy based on pairwise sequence comparisons. Arch. Virol. 2015, 160, 1593–1619. [Google Scholar] [CrossRef]
- Mansoor, S.; Zafar, Y.; Briddon, R.W. Geminivirus disease complexes: The threat is spreading. Trends Plant Sci. 2006, 11, 209–212. [Google Scholar] [CrossRef]
- Navascastillo, J.; Fialloolivé, E.; Sánchezcampos, S. Emerging virus diseases transmitted by whiteflies. Annu. Rev. Phytopathol. 2011, 49, 219–248. [Google Scholar] [CrossRef]
- Rojas, M.R.; Macedo, M.A.; Maliano, M.R.; Soto-Aguilar, M.; Souza, J.O.; Briddon, R.W.; Kenyon, L.; Rivera Bustamante, R.F.; Zerbini, F.M.; Adkins, S.; et al. World Management of Geminiviruses. Annu. Rev. Phytopathol. 2018, 56, 637–677. [Google Scholar] [CrossRef]
- Sattar, M.N.; Kvarnheden, A.; Saeed, M.; Briddon, R.W. Cotton leaf curl disease-an emerging threat to cotton production worldwide. J. Gen. Virol. 2013, 94, 695–710. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, A.L.; Duffy, S.; Sseruwagi, P. Whitefly-transmitted viruses threatening cassava production in Africa. Curr. Opin. Virol. 2018, 33, 167–176. [Google Scholar] [CrossRef]
- Zhou, X. Advances in understanding begomovirus satellites. Annu. Rev. Phytopathol. 2013, 51, 357–381. [Google Scholar] [CrossRef] [PubMed]
- Briddon, R.W.; Brown, J.K.; Moriones, E.; Stanley, J.; Zerbini, M.; Zhou, X.; Fauquet, C.M. Recommendations for the classification and nomenclature of the DNA-beta satellites of begomoviruses. Arch. Virol. 2008, 153, 763–781. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Li, G.; Wang, D.; Hu, D.; Zhou, X. A Begomovirus DNAbeta-encoded protein binds DNA, functions as a suppressor of RNA silencing, and targets the cell nucleus. J. Virol. 2005, 79, 10764–10775. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Huang, C.; Li, Z.; Zhou, X. Suppression of RNA silencing by a plant DNA virus satellite requires a host calmodulin-like protein to repress RDR6 expression. PLoS Pathog. 2014, 10, e1003921. [Google Scholar] [CrossRef]
- Zhong, X.; Wang, Z.Q.; Xiao, R.; Cao, L.; Wang, Y.; Xie, Y.; Zhou, X. Mimic phosphorylation of a βC1 protein encoded by TYLCCNB impairs its functions as a viral suppressor of RNA silencing and a symptom determinant. J. Virol. 2017, 91, e00300-17. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.P.; Usha, R.; Zrachya, A.; Levy, Y.; Spanov, H.; Gafni, Y. Protein-protein interactions and nuclear trafficking of coat protein and βC1 protein associated with Bhendi yellow vein mosaic disease. Virus Res. 2006, 122, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.Y.; Iwasaki, M.; Machida, C.; Machida, Y.; Zhou, X.; Chua, N.H. βC1, the pathogenicity factor of TYLCCNV, interacts with AS1 to alter leaf development and suppress selective jasmonic acid responses. Genes Dev. 2008, 22, 2564–2577. [Google Scholar] [CrossRef]
- Zhang, T.; Luan, J.B.; Qi, J.F.; Huang, C.J.; Li, M.; Zhou, X.P.; Liu, S.S. Begomovirus-whitefly mutualism is achieved through repression of plant defences by a virus pathogenicity factor. Mol. Ecol. 2012, 21, 1294–1304. [Google Scholar] [CrossRef]
- Briddon, R.W.; Bull, S.E.; Amin, I.; Mansoor, S.; Bedford, I.D.; Rishi, N.; Siwatch, S.S.; Zafar, Y.; Abdel-Salam, A.M.; Markham, P.G. Diversity of DNA 1: A satellite-like molecule associated with monopartite begomovirus-DNA beta complexes. Virology 2004, 324, 462–474. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Wu, P.; Liu, P.; Gong, H.; Zhou, X. Characterization of alphasatellites associated with monopartite begomovirus/betasatellite complexes in Yunnan, China. Virol. J. 2010, 7, 178. [Google Scholar] [CrossRef] [PubMed]
- Leke, W.N.; Sattar, M.N.; Ngane, E.B.; Ngeve, J.M.; Kvarnheden, A.; Brown, J.K. Molecular characterization of begomoviruses and DNA satellites associated with okra leaf curl disease in Cameroon. Virus Res. 2013, 174, 116–125. [Google Scholar] [CrossRef]
- Briddon, R.W.; Martin, D.P.; Roumagnac, P.; Navas-Castillo, J.; Fiallo-Olivé, E.; Moriones, E.; Lett, J.M.; Zerbini, F.M.; Varsani, A. Alphasatellitidae: A new family with two subfamilies for the classification of geminivirus- and nanovirus-associated alphasatellites. Arch. Virol. 2018, 163, 2587–2600. [Google Scholar] [CrossRef]
- Kumar, S.; Srivastava, A.; Jaidi, M.; Chauhan, P.S.; Raj, S.K. Molecular Characterization of a begomovirus, α-satellite, and β-satellite associated with leaf curl disease of Parthenium hysterophorus in India. Plant Dis. 2016, 100, 2299–2305. [Google Scholar] [CrossRef] [PubMed]
- Kon, T.; Rojas, M.R.; Abdourhamane, I.K.; Gilbertson, R.L. Roles and interactions of begomoviruses and satellite DNAs associated with okra leaf curl disease in Mali, West Africa. J. Gen. Virol. 2009, 90(Pt4), 1001–1013. [Google Scholar] [CrossRef]
- Nawaz-Ul-Rehman, M.S.; Nahid, N.; Mansoor, S.; Briddon, R.W.; Fauquet, C.M. Post-transcriptional gene silencing suppressor activity of two non-pathogenic alphasatellites associated with a begomovirus. Virology 2010, 405, 300–308. [Google Scholar] [CrossRef]
- Idris, A.M.; Shahid, M.S.; Briddon, R.W.; Khan, A.J.; Zhu, J.K.; Brown, J.K. An unusual alphasatellite associated with monopartite begomoviruses attenuates symptoms and reduces betasatellite accumulation. J. Gen. Virol. 2011, 92(Pt3), 706–717. [Google Scholar] [CrossRef]
- Mar, T.B.; Mendes, I.R.; Lau, D.; Fiallo-Olivé, E.; Navas-Castillo, J.; Alves, M.S.; Murilo Zerbini, F. Interaction between the New World begomovirus Euphorbia yellow mosaic virus and its associated alphasatellite: Effects on infection and transmission by the whitefly Bemisia tabaci. J. Gen. Virol. 2017, 98, 1552–1562. [Google Scholar] [CrossRef]
- Zulfiqar, A.; Zhang, J.; Cui, X.; Qian, Y.; Zhou, X.; Xie, Y. A new begomovirus associated with alpha- and beta-satellite molecules isolated from Vernonia cinerea in China. Arch. Virol. 2012, 157, 189–191. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Xie, Y.; Zhao, L.; Ren, H.; Li, Z. A naturally occurring defective DNA satellite associated with a monopartite begomovirus: Evidence for recombination between alphasatellite and betasatellite. Viruses 2013, 5, 2116–2128. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Xie, Y.; Raja, P.; Li, S.; Wolf, J.N.; Shen, Q.; Bisaro, D.M.; Zhou, X. Suppression of methylation-mediated transcriptional gene silencing by βC1-sahh protein interaction during geminivirus-betasatellite infection. PLoS Pathog. 2011, 7, e1002329. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Xie, Y.; Tao, X.; Zhang, Z.; Li, Z.; Fauquet, C.M. Characterization of DNAbeta associated with begomoviruses in China and evidence for co-evolution with their cognate viral DNA-A. J. Gen. Virol. 2003, 84, 237–247. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.Q.; Li, G.Z.; Gong, Q.Q.; Li, G.X.; Zheng, S.J. OsTCTP, encoding a translationally controlled tumor protein, plays an important role in mercury tolerance in rice. BMC Plant Biol. 2015, 15, 123. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, D.K.; Chen, W.; Zheng, Y.; Kaur, N.; Wintermantel, W.M.; Simmons, A.M.; Fei, Z.; Ling, K.S. Comparative transcriptome analysis reveals networks of genes activated in the whitefly, Bemisia tabaci when fed on tomato plants infected with Tomato yellow leaf curl virus. Virology 2018, 513, 52–64. [Google Scholar] [CrossRef] [PubMed]
- Grabherr, M.G.; Haas, B.J.; Yassour, M.; Levin, J.Z.; Thompson, D.A.; Amit, I.; Adiconis, X.; Fan, L.; Raychowdhury, R.; Zeng, Q.; et al. Trinity: Reconstructing a full-length transcriptome without a genome from RNA-Seq data. Nat. Biotechnol. 2011, 29, 644–652. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhu, W.; Azam, S.; Li, H.; Zhu, F.; Li, H.; Hong, Y.; Liu, H.; Zhang, E.; Wu, H.; et al. Deep sequencing analysis of the transcriptomes of peanut aerial and subterranean young pods identifies candidate genes related to early embryo abortion. Plant Biotechnol. J. 2013, 11, 115–127. [Google Scholar] [CrossRef]
- Du, J.; Wang, S.; He, C.; Zhou, B.; Ruan, Y.L.; Shou, H. Identification of regulatory networks and hub genes controlling soybean seed set and size using RNA sequencing analysis. J. Exp. Bot. 2017, 68, 1955–1972. [Google Scholar] [CrossRef]
- Liu, K.; Li, H.; Li, W.; Zhong, J.; Chen, Y.; Shen, C.; Yuan, C. Comparative transcriptomic analyses of normal and malformed flowers in sugar apple (Annona squamosa L.) to identify the differential expressed genes between normal and malformed flowers. BMC Plant Biol. 2017, 17, 170. [Google Scholar] [CrossRef]
- Shen, C.; Guo, H.; Chen, H.; Shi, Y.; Meng, Y.; Lu, J.; Feng, S.; Wang, H. Identification and analysis of genes associated with the synthesis of bioactive constituents in Dendrobium officinale using RNA-Seq. Sci Rep. 2017, 7, 187. [Google Scholar] [CrossRef] [PubMed]
- Trapnell, C.; Williams, B.A.; Pertea, G.; Mortazavi, A.; Kwan, G.; van Baren, M.J.; Salzberg, S.L.; Wold, B.J.; Pachter, L. Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat. Biotechnol. 2010, 28, 511–515. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.C.; Wu, K.M.; Chiang, T.Y.; Huang, C.Y.; Chou, C.H.; Li, S.J.; Chiang, Y.C. Comparative transcriptome analysis of Gastrodia elata (Orchidaceae) in response to fungus symbiosis to identify gastrodin biosynthesis-related genes. BMC Genomics 2016, 17, 212. [Google Scholar] [CrossRef]
- Yu, C.; Guo, H.; Zhang, Y.; Song, Y.; Pi, E.; Yu, C.; Zhang, L.; Dong, M.; Zheng, B.; Wang, H.; Shen, C. Identification of potential genes that contributed to the variation in the taxoid contents between two Taxus species (Taxus media and Taxus mairei). Tree Physiol. 2017, 37, 1659–1671. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.; Yang, Y.; Liu, K.; Zhang, L.; Guo, H.; Sun, T.; Wang, H. Involvement of endogenous salicylic acid in iron-deficiency responses in Arabidopsis. J. Exp. Bot. 2016, 67, 4179–4193. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Schiff, M.; Dinesh-Kumar, S.P. Virus-induced gene silencing in tomato. Plant J. 2002, 31, 777–786. [Google Scholar] [CrossRef]
- Saunders, K.; Stanley, J. A nanovirus-like DNA component associated with yellow vein disease of Ageratum conyzoides: Evidence for interfamilial recombination between plant DNA viruses. Virology 1999, 264, 142–152. [Google Scholar] [CrossRef]
- Paprotka, T.; Metzler, V.; Jeske, H. The first DNA 1-like alpha satellites in association with New World begomoviruses in natural infections. Virology 2010, 404, 148–157. [Google Scholar] [CrossRef]
- Romay, G.; Chirinos, D.; Geraud-Pouey, F.; Desbiez, C. Association of an atypical alphasatellite with a bipartite New World begomovirus. Arch. Virol. 2010, 155, 1843–1847. [Google Scholar] [CrossRef]
- Cui, X.; Tao, X.; Xie, Y.; Fauquet, C.M.; Zhou, X. A DNAbeta associated with Tomato yellow leaf curl China virus is required for symptom induction. J. Virol. 2004, 78, 13966–13974. [Google Scholar] [CrossRef]
- Ascencio-Ibáñez, J.T.; Sozzani, R.; Lee, T.J.; Chu, T.M.; Wolfinger, R.D.; Cella, R.; Hanley-Bowdoin, L. Global analysis of Arabidopsis gene expression uncovers a complex array of changes impacting pathogen response and cell cycle during geminivirus infection. Plant Physiol. 2008, 148, 436–454. [Google Scholar] [CrossRef]
- Góngora-Castillo, E.; Ibarra-Laclette, E.; Trejo-Saavedra, D.L.; Rivera-Bustamante, R.F. Transcriptome analysis of symptomatic and recovered leaves of geminivirus-infected pepper (Capsicum annuum). Virol. J. 2012, 9, 295. [Google Scholar] [CrossRef]
- Seo, J.K.; Kim, M.K.; Kwak, H.R.; Choi, H.S.; Nam, M.; Choe, J.; Choi, B.; Han, S.J.; Kang, J.H.; Jung, C. Molecular dissection of distinct symptoms induced by tomato chlorosis virus and tomato yellow leaf curl virus based on comparative transcriptome analysis. Virology 2018, 516, 1–20. [Google Scholar] [CrossRef]
- Li, K.; Wu, G.; Li, M.; Ma, M.; Du, J.; Sun, M.; Sun, X.; Qing, L. Transcriptome analysis of Nicotiana benthamiana infected by Tobacco curly shoot virus. Virol. J. 2018, 15, 138. [Google Scholar] [CrossRef]
- Pierce, E.J.; Rey, M.E. Assessing global transcriptome changes in response to South African Cassava Mosaic Virus [ZA-99] infection in susceptible Arabidopsis thaliana. PLoS ONE 2013, 8, e67534. [Google Scholar] [CrossRef]
- Bachan, S.; Dinesh-Kumar, S.P. Tobacco rattle virus (TRV)-based virus-induced gene silencing. Methods Mol. Biol. 2012, 894, 83–92. [Google Scholar]
- Lange, M.; Yellina, A.L.; Orashakova, S.; Becker, A. Virus-induced gene silencing (VIGS) in plants: An overview of target species and the virus-derived vector systems. Methods Mol. Biol. 2013, 975, 1–14. [Google Scholar]
- Senthil-Kumar, M.; Mysore, K.S. Tobacco rattle virus-based virus-induced gene silencing in Nicotiana benthamiana. Nat. Protoc. 2014, 9, 1549–1562. [Google Scholar] [CrossRef]
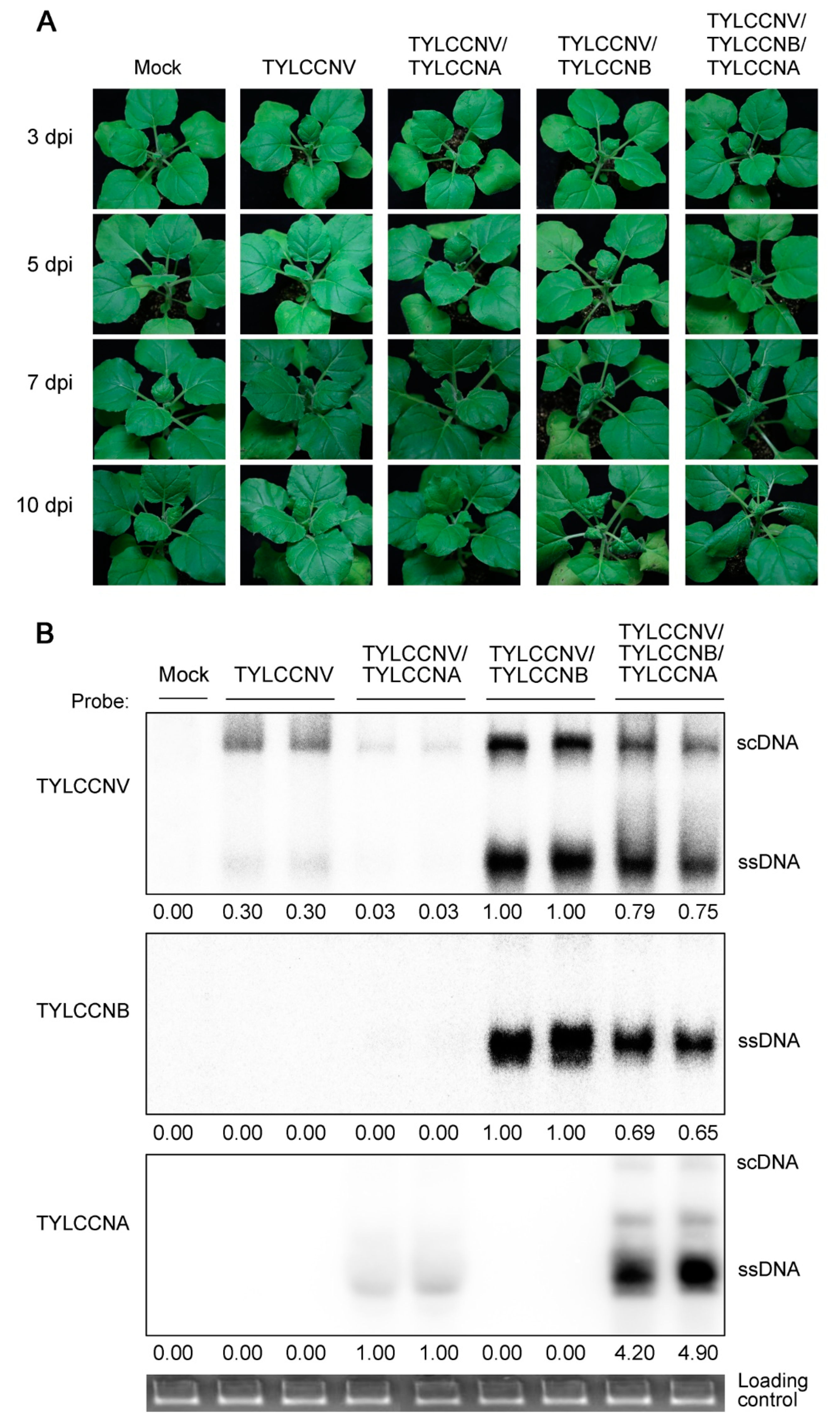
| Plant Species | Inoculum a | Infectivity b | Incidence c |
|---|---|---|---|
| N. benthamiana | Mock | 0/20 | 0/20 |
| TYLCCNV | 20/20 | 0/20 | |
| TYLCCNV/TYLCCNA | 20/20 | 0/20 | |
| TYLCCNV/TYLCCNB | 20/20 | 20/20 | |
| TYLCCNV/TYLCCNB/TYLCCNA | 20/20 | 20/20 |
| Gene-ID | Log2 (Ratio) ± SD a | p-Value | FDR b | Up-Down Regulation | Annotation |
|---|---|---|---|---|---|
| CL16016.Contig3_All | 2.963 ± 0.260 | 6.96 × 10−12 | 1.8 × 10−7 | up | Beta-1,3-glucanase |
| CL22155.Contig3_All | 2.590 ± 0.447 | 6.74 × 10−9 | 9.1 × 10−5 | up | UDP-glycosyltransferase |
| CL22420.Contig2_All | 1.753 ± 0.029 | 8.83 × 10−8 | 8.5 × 10−4 | up | Probable 2-oxoglutarate/Fe(II)-dependent dioxygenase |
| CL25117.Contig26_All | 1.404 ± 0.212 | 6.76 × 10−7 | 5.07 × 10−3 | up | Pleiotropic drug resistance protein 1 |
| CL2512.Contig3_All | 2.291 ± 0.314 | 1.19 × 10−9 | 2.2 × 10−5 | up | DNA-binding protein 3 |
| CL25611.Contig1_All | 3.222 ± 0.286 | 1.39 × 10−18 | 1.4 × 10−13 | up | Cyclic nucleotide-gated ion channel 1-like |
| CL2689.Contig5_All | 1.970 ± 0.324 | 1.06 × 10−8 | 1.3 × 10−4 | up | WRKY transcription factor 6 |
| CL3311.Contig2_All | 2.511 ± 0.175 | 1.03 × 10−14 | 5.2 × 10−10 | up | Flavonoid biosynthesis oxidoreductase protein |
| CL4894.Contig1_All | 2.145 ± 0.249 | 1.49 × 10−9 | 2.5 × 10−5 | up | Zinc finger CCCH domain-containing protein 2-like |
| CL6208.Contig1_All | 1.583 ± 0.221 | 2.12 × 10−6 | 1.43 × 10−2 | up | Receptor-like protein kinase |
| CL6421.Contig3_All | 2.214 ± 0.536 | 3.31 × 10−6 | 2.09 × 10−2 | up | Cucumber peeling cupredoxin-like |
| CL8323.Contig1_All | 4.138 ± 0.328 | 8.51 × 10−8 | 8.5 × 10−4 | up | Lectin-domain receptor-like kinase |
| Unigene13300_All | 1.627 ± 0.290 | 2.64 × 10−8 | 2.8 × 10−4 | up | Putative disease resistance protein |
| Unigene23199_All | 1.979 ± 0.044 | 1.07 × 10−17 | 7.2 × 10−13 | up | Retrovirus-related Pol polyprotein |
| Unigene23569_All | 1.878 ± 0.203 | 1.95 × 10−7 | 1.71 × 10−3 | up | Copia-type pol polyprotein |
| Unigene23982_All | 1.479 ± 0.254 | 7.24 × 10−6 | 4.07 × 10−2 | up | Putative polyprotein |
| Unigene2441_All | 2.759 ± 0.302 | 6.62 × 10−6 | 3.83 × 10−2 | up | Cytochrome P450 |
| Unigene28354_All | 1.823 ± 0.140 | 3.77 × 10−9 | 5.8 × 10−5 | up | Putative gag protein |
| Unigene30685_All | 1.627 ± 0.264 | 3.58 × 10−7 | 3.02 × 10−3 | up | Cyclic nucleotide-gated ion channel 1-like |
| Unigene40989_All | 3.869 ± 0.578 | 1.62 × 10−6 | 1.13 × 10−2 | up | Arogenate dehydrogenase 2 |
| Unigene46047_All | 2.831 ± 0.298 | 1.16 × 10−12 | 3.9 × 10−8 | up | Ethylene-responsive transcription factor 1B-like |
| Unigene47025_All | 3.700 ± 0.417 | 3.38 × 10−10 | 6.9 × 10−6 | up | GDSL esterase/lipase 2-like |
| Unigene47881_All | 4.669 ± 0.157 | 3.19 × 10−11 | 7.2 × 10−7 | up | Glutathione S-transferase-like |
| Unigene60701_All | 5.105 ± 0.434 | 3.07 × 10−6 | 2.01 × 10−2 | up | Glutaredoxin-C9-like |
| Unigene72008_All | 1.880 ± 0.258 | 4.38 × 10−7 | 3.55 × 10−3 | up | UDP-arabinose 4-epimerase 1-like |
| Unigene79227_All | 4.098 ± 0.163 | 3.41 × 10−22 | 6.9 × 10−17 | up | Aspartic proteinase nepenthesin-2-like |
| CL15892.Contig2_All | −1.229 ± 0.210 | 8.02 × 10−6 | 4.39 × 10−2 | down | Zinc transporter 4 |
| CL18406.Contig3_All | −1.589 ± 0.393 | 6.45 × 10−6 | 3.83 × 10−2 | down | Probable carboxylesterase 15-like |
| CL25062.Contig3_All | −3.238 ± 0.604 | 8.07 × 10−9 | 1.0 × 10−4 | down | Prolyl endopeptidase-like |
| CL9035.Contig2_All | −3.655 ± 1.234 | 1.01 × 10−7 | 9.3 × 10−4 | down | Zinc transporter 1-like |
| CL9035.Contig3_All | −2.406 ± 0.918 | 4.75 × 10−7 | 3.7 × 10−3 | down | Zinc transporter 8 |
| Unigene6461_All | 12.250 ± 0.227 | 2.19 × 10−14 | 8.9 × 10−10 | up | Uncharacterized |
| Unigene75053_All | 14.336 ± 1.124 | 4.41 × 10−12 | 1.3 × 10−7 | up | Uncharacterized |
| Unigene78447_All | 1.329 ± 0.207 | 1.16 × 10−8 | 1.3 × 10−4 | up | Uncharacterized |
| CL25997.Contig7_All | −8.840 ± 0.000 | 4.03 × 10−9 | 5.8 × 10−5 | down | Uncharacterized |
| CL4777.Contig2_All | −6.532 ± 4.144 | 3.65 × 10−6 | 2.24 × 10−2 | down | Uncharacterized |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, C.; Wang, Z.Q.; Liu, X.; Zhao, L.; Zhou, X.; Xie, Y. Identification and Analysis of Potential Genes Regulated by an Alphasatellite (TYLCCNA) that Contribute to Host Resistance against Tomato Yellow Leaf Curl China Virus and Its Betasatellite (TYLCCNV/TYLCCNB) Infection in Nicotiana benthamiana. Viruses 2019, 11, 442. https://doi.org/10.3390/v11050442
Luo C, Wang ZQ, Liu X, Zhao L, Zhou X, Xie Y. Identification and Analysis of Potential Genes Regulated by an Alphasatellite (TYLCCNA) that Contribute to Host Resistance against Tomato Yellow Leaf Curl China Virus and Its Betasatellite (TYLCCNV/TYLCCNB) Infection in Nicotiana benthamiana. Viruses. 2019; 11(5):442. https://doi.org/10.3390/v11050442
Chicago/Turabian StyleLuo, Chaohu, Zhan Qi Wang, Xianan Liu, Liling Zhao, Xueping Zhou, and Yan Xie. 2019. "Identification and Analysis of Potential Genes Regulated by an Alphasatellite (TYLCCNA) that Contribute to Host Resistance against Tomato Yellow Leaf Curl China Virus and Its Betasatellite (TYLCCNV/TYLCCNB) Infection in Nicotiana benthamiana" Viruses 11, no. 5: 442. https://doi.org/10.3390/v11050442
APA StyleLuo, C., Wang, Z. Q., Liu, X., Zhao, L., Zhou, X., & Xie, Y. (2019). Identification and Analysis of Potential Genes Regulated by an Alphasatellite (TYLCCNA) that Contribute to Host Resistance against Tomato Yellow Leaf Curl China Virus and Its Betasatellite (TYLCCNV/TYLCCNB) Infection in Nicotiana benthamiana. Viruses, 11(5), 442. https://doi.org/10.3390/v11050442






