Specific Interactions between Human Norovirus and Environmental Matrices: Effects on the Virus Ecology
Abstract
1. Introduction
2. Specific Adsorbents for Human Noroviruses
3. Specific Interactions between HBGA-Like Substances and Human Norovirus
4. Effects of the Interactions with HBGA-Like Substances on Norovirus Ecology
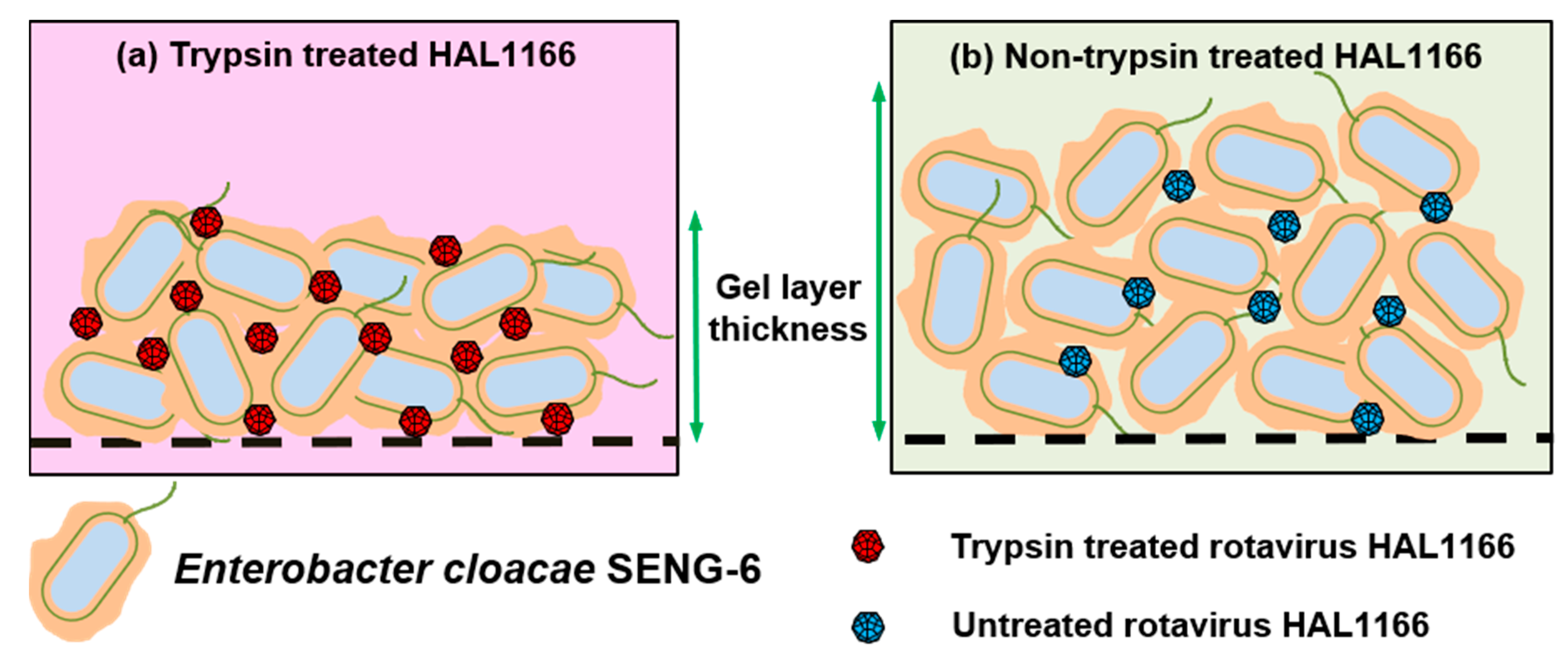
4.1. Norovirus Removal
4.2. Norovirus Cell Attachment and Infectivity
4.3. Norovirus Persistence, Survival, and Circulation in the Environment
5. Further Studies
Author Contributions
Funding
Conflicts of Interest
References
- Glass, R.; Parashar, U.; Estes, M. Norovirus gastroenteritis. N. Engl. J. Med. 2009, 361, 1776–1785. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.M.; Hall, A.J.; Robinson, A.E.; Verhoef, L.; Premkumar, P.; Parashar, U.D.; Koopmans, M.; Lopman, B.A. Global prevalence of norovirus in cases of gastroenteritis: a systematic review and meta-analysis. Lancet Infect. Dis. 2014, 14, 725–730. [Google Scholar] [CrossRef]
- Bartsch, S.M.; Lopman, B.A.; Ozawa, S.; Hall, A.J.; Lee, B.Y. Global economic burden of norovirus gastroenteritis. PLoS ONE 2016, 11, e0151219. [Google Scholar] [CrossRef] [PubMed]
- Karst, S.M.; Wobus, C.E.; Goodfellow, I.G.; Green, K.Y.; Virgin, H.W. Advances in Norovirus Biology. Cell Host Microbe 2014, 15, 668–680. [Google Scholar] [CrossRef] [PubMed]
- Vinjé, J. Advances in laboratory methods for detection and typing of norovirus. J. Clin. Microbiol. 2015, 53, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Parra, G.I.; Squires, R.B.; Karangwa, C.K.; Johnson, J.A.; Lepore, C.J.; Sosnovtsev, S.V.; Green, K.Y. Static and evolving norovirus genotypes: Implications for epidemiology and immunity. PLOS Pathog. 2017, 13, e1006136. [Google Scholar] [CrossRef] [PubMed]
- Atmar, R.L.; Opekun, A.R.; Gilger, M.A.; Estes, M.K.; Crawford, S.E.; Neill, F.H.; Graham, D.Y. Norwalk virus shedding after experimental human infection. Emerg. Infect. Dis. 2008, 14, 1553–1557. [Google Scholar] [CrossRef] [PubMed]
- Sima, L.C.; Schaeffer, J.; Le Saux, J.C.; Parnaudeau, S.; Elimelech, M.; Le Guyader, F.S. Calicivirus removal in a membrane bioreactor wastewater treatment plant. Appl. Environ. Microbiol. 2011, 77, 5170–5177. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, A.K.; Le Saux, J.-C.; Parnaudeau, S.; Pommepuy, M.; Elimelech, M.; Le Guyader, F.S. Evaluation of removal of noroviruses during wastewater treatment, using real-time reverse transcription-PCR: different behaviors of genogroups I and II. Appl. Environ. Microbiol. 2007, 73, 7891–7897. [Google Scholar] [CrossRef] [PubMed]
- Myrmel, M.; Berg, E.M.M.; Grinde, B.; Rimstad, E. Enteric viruses in inlet and outlet samples from sewage treatment plants. J. Water Health 2006, 4, 197–209. [Google Scholar] [CrossRef] [PubMed]
- Blanco, A.; Guix, S.; Fuster, N.; Fuentes, C.; Bartolomé, R.; Cornejo, T.; Pintó, R.M.; Bosch, A. Norovirus in bottled water associated with gastroenteritis outbreak, Spain, 2016. Emerg. Infect. Dis. 2017, 23, 1531–1534. [Google Scholar] [CrossRef] [PubMed]
- El-Senousy, W.M.; Costafreda, M.I.; Pintó, R.M.; Bosch, A. Method validation for norovirus detection in naturally contaminated irrigation water and fresh produce. Int. J. Food Microbiol. 2013, 167, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Beaudequin, D.; Harden, F.; Roiko, A.; Mengersen, K. Utility of Bayesian networks in QMRA-based evaluation of risk reduction options for recycled water. Sci. Total Environ. 2016, 541, 1393–1409. [Google Scholar] [CrossRef] [PubMed]
- Mara, D.; Sleigh, A. Estimation of norovirus and Ascaris infection risks to urban farmers in developing countries using wastewater for crop irrigation. J. Water Health 2010, 8, 572–576. [Google Scholar] [CrossRef] [PubMed]
- Mara, D.; Sleigh, A. Estimation of norovirus infection risks to consumers of wastewater-irrigated food crops eaten raw. J. Water Health 2010, 8, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Bernard, H.; Faber, M.; Wilking, H.; Haller, S.; Höhle, M.; Schielke, A.; Ducomble, T.; Siffczyk, C.; Merbecks, S.S. Large multistate outbreak of norovirus gastroenteritis associated with frozen strawberries, Germany, 2012. Euro Surveill. 2014, 19, 20719. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mäde, D.; Trübner, K.; Neubert, E.; Höhne, M.; Johne, R. Detection and typing of norovirus from frozen strawberries involved in a large-scale gastroenteritis outbreak in Germany. Food Environ. Virol. 2013, 5, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Bellou, M.; Kokkinos, P.; Vantarakis, A. Shellfish-Borne Viral Outbreaks: A Systematic Review. Food Environ. Virol. 2013, 5, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Kauppinen, A.; Al-Hello, H.; Zacheus, O.; Kilponen, J.; Maunula, L.; Huusko, S.; Lappalainen, M.; Miettinen, I.; Blomqvist, S.; Rimhanen-Finne, R. Increase in outbreaks of gastroenteritis linked to bathing water in Finland in summer 2014. Eurosurveillance 2017, 22, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Schets, F.; van den Berg, H.; Vennema, H.; Pelgrim, M.; Collé, C.; Rutjes, S.; Lodder, W. Norovirus outbreak associated with swimming in a recreational lake not influenced by external human fecal sources in The Netherlands, August 2012. Int. J. Environ. Res. Public Health 2018, 15, 2550. [Google Scholar] [CrossRef] [PubMed]
- Gerba, C.P. Applied and theoretical aspects of virus adsorption to surfaces. Adv. Appl. Microbiol. 1984, 30, 133–168. [Google Scholar] [PubMed]
- Da Silva, A.K.; Le Guyader, F.S.; Le Saux, J.C.; Pommepuy, M.; Montgomery, M.A.; Elimelech, M. Norovirus removal and particle association in a waste stabilization pond. Environ. Sci. Technol. 2008, 42, 9151–9157. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, A.K.; Kavanagh, O.V.; Estes, M.K.; Elimelech, M. Adsorption and aggregation properties of norovirus GI and GII virus-like particles demonstrate differing responses to solution chemistry. Environ. Sci. Technol. 2011, 45, 520–526. [Google Scholar] [CrossRef] [PubMed]
- Miura, T.; Schaeffer, J.; Le Saux, J.-C.; Le Mehaute, P.; Le Guyader, F.S. Virus type-specific removal in a full-scale membrane bioreactor treatment process. Food Environ. Virol. 2018, 10, 176–186. [Google Scholar] [CrossRef] [PubMed]
- Miura, T.; Okabe, S.; Nakahara, Y.; Sano, D. Removal properties of human enteric viruses in a pilot-scale membrane bioreactor (MBR) process. Water Res. 2015, 75, 282–291. [Google Scholar] [CrossRef] [PubMed]
- Chaudhry, R.M.; Nelson, K.L.; Drewes, J.E. Mechanisms of pathogenic virus removal in a full-scale membrane bioreactor. Environ. Sci. Technol. 2015, 49, 2815–2822. [Google Scholar] [CrossRef] [PubMed]
- Deng, W.; Gibson, K.E. Interaction of microorganisms within leafy green phyllospheres: Where do human noroviruses fit in? Int. J. Food Microbiol. 2017, 258, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Busscher, H.J.; Weerkamp, A.H. Specific and non-specific interactions in bacterial adhesion to solid substrata. FEMS Microbiol. Lett. 1987, 46, 165–173. [Google Scholar] [CrossRef]
- Chaudhry, R.M.; Holloway, R.W.; Cath, T.Y.; Nelson, K.L. Impact of virus surface characteristics on removal mechanisms within membrane bioreactors. Water Res. 2015, 84, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Marionneau, S.; Ruvoën, N.; Le Moullac–Vaidye, B.; Clement, M.; Cailleau–Thomas, A.; Ruiz–Palacois, G.; Huang, P.; Jiang, X.; Le Pendu, J. Norwalk virus binds to histo-blood group antigens present on gastroduodenal epithelial cells of secretor individuals. Gastroenterology 2002, 122, 1967–1977. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.; Jiang, X. Norovirus and its histo-blood group antigen receptors: an answer to a historical puzzle. Trends Microbiol. 2005, 13, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.; Jiang, X. Norovirus-host interaction: implications for disease control and prevention. Expert Rev. Mol. Med. 2007, 9, 1–22. [Google Scholar] [CrossRef] [PubMed]
- de Graaf, M.; van Beek, J.; Koopmans, M.P.G. Human norovirus transmission and evolution in a changing world. Nat. Rev. Microbiol. 2016, 14, 421–433. [Google Scholar] [CrossRef] [PubMed]
- Robilotti, E.; Deresinski, S.; Pinsky, B.A. Norovirus. Clin. Microbiol. Rev. 2015, 28, 134–164. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.; Farkas, T.; Zhong, W.; Ruvoe, N.; Morrow, A.L.; Altaye, M.; Pickering, L.K.; Newburg, D.S.; Lependu, J.; Jiang, X. Noroviruses Bind to Human ABO, Lewis, and Secretor Histo—Blood Group Antigens: Identification of 4 Distinct Strain-Specific Patterns. J. Infect. Dis. 2003, 3039, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.; Farkas, T.; Zhong, W.; Tan, M.; Thornton, S.; Morrow, A.L.; Jiang, X. Norovirus and histo-blood group antigens: Demonstration of a wide spectrum of strain specificities and classification of two major binding groups among multiple binding patterns. J. Virol. 2005, 79, 6714–6722. [Google Scholar] [CrossRef] [PubMed]
- Nasir, W. A Study of Norovirus-HBGA Interactions. Master’s Thesis, Chalmers University of Technology, University of Gothenburg, Gothenburg, Sweden, 2009. [Google Scholar]
- Cao, S.; Lou, Z.; Tan, M.; Chen, Y.; Liu, Y.; Zhang, Z.; Zhang, X.C.; Jiang, X.; Li, X.; Rao, Z. Structural basis for the recognition of blood group trisaccharides by norovirus. J. Virol. 2007, 81, 5949–5957. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.; Xia, M.; Cao, S.; Huang, P.; Farkas, T.; Meller, J.; Hegde, R.S.; Li, X.; Rao, Z.; Jiang, X. Elucidation of strain-specific interaction of a GII-4 norovirus with HBGA receptors by site-directed mutagenesis study. Virology 2008, 379, 324–334. [Google Scholar] [CrossRef] [PubMed]
- Nasir, W.; Frank, M.; Kunze, A.; Bally, M.; Parra, F.; Nyholm, P.-G.; Höök, F.; Larson, G. Histo-blood group antigen presentation is critical for binding of norovirus VLP to glycosphingolipids in model membranes. ACS Chem. Biol. 2017, 12, 1288–1296. [Google Scholar] [CrossRef] [PubMed]
- de Rougemont, A.; Ruvoen-Clouet, N.; Simon, B.; Estienney, M.; Elie-Caille, C.; Aho, S.; Pothier, P.; Le Pendu, J.; Boireau, W.; Belliot, G. Qualitative and quantitative analysis of the binding of GII.4 norovirus variants onto human blood group antigens. J. Virol. 2011, 85, 4057–4070. [Google Scholar] [CrossRef] [PubMed]
- Ruvoën-clouet, N.; Belliot, G.; Le Pendu, J. Noroviruses and histo-blood groups: the impact of common host genetic polymorphisms on virus transmission and evolution. Rev. Med. Virol. 2013, 23, 355–366. [Google Scholar] [CrossRef] [PubMed]
- Tamura, M.; Natori, K.; Kobayashi, M.; Miyamura, T.; Takeda, N. Genogroup II noroviruses efficiently bind to Heparan Sulfate proteoglycan associated with the cellular membrane. J. Virol. 2004, 78, 3817–3826. [Google Scholar] [CrossRef] [PubMed]
- Wegener, H.; Mallagaray, Á.; Schöne, T.; Peters, T.; Lockhauserbäumer, J.; Yan, H.; Uetrecht, C.; Hansman, G.S.; Taube, S. Human norovirus GII.4(MI001) P dimer binds fucosylated and sialylated carbohydrates. Glycobiology 2017, 27, 1027–1037. [Google Scholar] [CrossRef] [PubMed]
- Sano, D.; Matsuo, T.; Omura, T. Virus-binding proteins recovered from bacterial culture derived from activated sludge by affinity chromatography assay using a viral capsid peptide. Appl. Environ. Microbiol. 2004, 70, 3434–3442. [Google Scholar] [CrossRef] [PubMed]
- Imai, T.; Sano, D.; Miura, T.; Okabe, S.; Wada, K.; Masago, Y.; Omura, T. Adsorption characteristics of an enteric virus-binding protein to norovirus, rotavirus and poliovirus. BMC Biotechnol. 2011, 11, 123. [Google Scholar] [CrossRef] [PubMed]
- Tamura, M.; Natori, K.; Kobayashi, M.; Miyamura, T.; Takeda, N. Interaction of recombinant Norwalk virus particles with the 105-kiloDalton cellular binding protein, a candidate receptor molecule for virus attachment. J. Virol. 2000, 74, 11589–11597. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, K.M.; Mandrell, R.E.; Tian, P. Binding of virus-like particles of Norwalk virus to romaine lettuce veins. Appl. Environ. Microbiol. 2010, 76, 7997–8003. [Google Scholar] [CrossRef] [PubMed]
- Almand, E.A.; Moore, M.D.; Outlaw, J.; Jaykus, L.-A. Human norovirus binding to select bacteria representative of the human gut microbiota. PLoS ONE 2017, 12, e0173124. [Google Scholar] [CrossRef] [PubMed]
- Shang, J.; Piskarev, V.E.; Xia, M.; Huang, P.; Jiang, X.; Likhosherstov, L.M.; Novikova, O.S.; Newburg, D.S.; Ratner, D.M. Identifying human milk glycans that inhibit norovirus binding using surface plasmon resonance. Glycobiology 2013, 23, 1491–1498. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Chen, Y.; Jiang, X.; Xia, M.; Yang, Y.; Tan, M.; Li, X.; Rao, Z. A unique human norovirus lineage with a distinct HBGA binding interface. PLoS Pathog. 2015, 11, e1005025. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-F.; Dai, Y.-C.; Zhong, W.; Tan, M.; Lv, Z.-P.; Zhou, Y.-C.; Jiang, X. Tannic acid inhibited norovirus binding to HBGA receptors, a study of 50 Chinese medicinal herbs. Bioorg. Med. Chem. 2012, 20, 1616–1623. [Google Scholar] [CrossRef] [PubMed]
- Almand, E.A.; Moore, M.D.; Jaykus, L.A. Norovirus binding to ligands beyond histo-blood group antigens. Front. Microbiol. 2017, 8, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Taube, S.; Mallagaray, A.; Peters, T. Norovirus, glycans and attachment. Curr. Opin. Virol. 2018, 31, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Taube, S.; Perry, J.W.; Yetming, K.; Patel, S.P.; Auble, H.; Shu, L.; Nawar, H.F.; Lee, C.H.; Connell, T.D.; Shayman, J.A.; et al. Ganglioside-linked terminal sialic acid moieties on murine macrophages function as attachment receptors for murine noroviruses. J. Virol. 2009, 83, 4092–5101. [Google Scholar] [CrossRef] [PubMed]
- Taube, S.; Perry, J.W.; McGreevy, E.; Yetming, K.; Perkins, C.; Henderson, K.; Wobus, C.E. Murine noroviruses bind glycolipid and glycoprotein attachment receptors in a strain-dependent manner. J. Virol. 2012, 86, 5584–5593. [Google Scholar] [CrossRef] [PubMed]
- Orchard, R.C.; Wilen, C.B.; Doench, J.G.; Baldridge, M.T.; McCune, B.T.; Lee, Y.C.J.; Lee, S.; Pruett-Miller, S.M.; Nelson, C.A.; Fremont, D.H.; et al. Discovery of a proteinaceous cellular receptor for a norovirus. Science 2016, 353, 933–936. [Google Scholar] [CrossRef] [PubMed]
- Haga, K.; Fujimoto, A.; Takai-Todaka, R.; Miki, M.; Doan, Y.H.; Murakami, K.; Yokoyama, M.; Murata, K.; Nakanishi, A.; Katayama, K. Functional receptor molecules CD300lf and CD300ld within the CD300 family enable murine noroviruses to infect cells. Proc. Natl. Acad. Sci. USA 2016, 113, E6248–E6255. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Huang, P.; Zou, L.; Lowary, T.L.; Tan, M.; Jiang, X. Tulane Virus recognizes the A type 3 and B histo-blood group antigens. J. Virol. 2015, 89, 1419–1427. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.; Wei, C.; Huang, P.; Fan, Q.; Quigley, C.; Xia, M.; Fang, H.; Zhang, X.; Zhong, W.; Klassen, J.S.; et al. Tulane virus recognizes sialic acids as cellular receptors. Sci. Rep. 2015, 5, 11784. [Google Scholar] [CrossRef] [PubMed]
- Miura, T.; Sano, D.; Suenaga, A.; Yoshimura, T.; Fuzawa, M.; Nakagomi, T.; Nakagomi, O.; Okabe, S. Histo-blood group antigen-like substances of human enteric bacteria as specific adsorbents for human noroviruses. J. Virol. 2013, 87, 9441–9451. [Google Scholar] [CrossRef] [PubMed]
- Tian, P.; Bates, A.H.; Jensen, H.M.; Mandrell, R.E. Norovirus binds to blood group A-like antigens in oyster gastrointestinal cells. Lett. Appl. Microbiol. 2006, 43, 645–651. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Esseili, M.A.; Lu, Z.; Saif, L.J.; Wang, Q. Recognition of histo-blood group antigen-like carbohydrates in lettuce by human GII.4 norovirus. Appl. Environ. Microbiol. 2016, 82, 2966–2974. [Google Scholar] [CrossRef] [PubMed]
- Le Guyader, F.S.; Atmar, R.L.; Le Pendu, J. Transmission of viruses through shellfish: when specific ligands come into play. Curr. Opin. Virol. 2012, 2, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Tian, P.; Engelbrektson, A.L.; Jiang, X.; Zhong, W.; Mandrell, R.E. Norovirus recognizes histo-blood group antigens on gastrointestinal cells of clams, mussels, and oysters: a possible mechanism of bioaccumulation. J. Food Prot. 2007, 70, 2140–2147. [Google Scholar] [CrossRef] [PubMed]
- Morozov, V.; Hanisch, F.-G.; Wegner, K.M.; Schroten, H. Pandemic GII.4 Sydney and epidemic GII.17 Kawasaki308 noroviruses display distinct specificities for histo-blood group antigens leading to different transmission vector dynamics in Pacific oysters. Front. Microbiol. 2018, 9, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Le Guyader, F.S.; Loisy, F.; Atmar, R.L.; Hutson, A.M.; Estes, M.K.; Ruvoën-Clouet, N.; Pommepuy, M.; Le Pendu, J. Norwalk virus-specific binding to oyster digestive tissues. Emerg. Infect. Dis. 2006, 12, 931–936. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Breiman, A.; Le Pendu, J.; Uyttendaele, M. Binding to histo-blood group antigen-expressing bacteria protects human norovirus from acute heat stress. Front. Microbiol. 2015, 6, 659. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Wang, D.; Yang, D.; Shan, L.; Tian, P. Binding of Escherichia coli does not protect Tulane virus from heat-inactivation regardless the expression of HBGA-like molecules. Front. Microbiol. 2017, 8, 1746. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Rong, S.; Tian, P.; Zhou, Y.; Guan, S.; Li, Q.; Wang, D. Bacterial surface-displayed GII.4 human norovirus capsid proteins bound to HBGA-like molecules in Romaine lettuce. Front. Microbiol. 2017, 8, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Amarasiri, M.; Hashiba, S.; Miura, T.; Nakagomi, T.; Nakagomi, O.; Ishii, S.; Okabe, S.; Sano, D. Bacterial histo-blood group antigens contributing to genotype-dependent removal of human noroviruses with a microfiltration membrane. Water Res. 2016, 95, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Liu, H.; Su, L.; Zhao, F.; Zhou, D.; Duan, D. Histo-blood group antigens in Crassostrea gigas and binding profiles with GII.4 Norovirus. J. Oceanol. Limnol. 2018, 36, 1383–1391. [Google Scholar] [CrossRef]
- Hu, L.; Crawford, S.E.; Czako, R.; Cortes-Penfield, N.W.; Smith, D.F.; Le Pendu, J.; Estes, M.K.; Prasad, B.V.V. Cell attachment protein VP8* of a human rotavirus specifically interacts with A-type histo-blood group antigen. Nature 2012, 485, 256–259. [Google Scholar] [CrossRef] [PubMed]
- Coulson, B.S. Expanding diversity of glycan receptor usage by rotaviruses. Curr. Opin. Virol. 2015, 15, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Amarasiri, M.; Kawai, H.; Kitajima, M.; Okabe, S.; Sano, D. Specific interactions of rotavirus HAL1166 with Enterobacter cloacae SENG-6 and their contribution on rotavirus HAL1166 removal. Water Sci. Technol. 2019. [Google Scholar] [CrossRef]
- Jones, M.K.; Watanabe, M.; Zhu, S.; Graves, C.L.; Keyes, L.R.; Grau, K.R.; Gonzalez-Hernandez, M.B.; Iovine, N.M.; Wobus, C.E.; Vinje, J.; et al. Enteric bacteria promote human and mouse norovirus infection of B cells. Science 2014, 346, 755–759. [Google Scholar] [CrossRef] [PubMed]
- Baldridge, M.T.; Nice, T.J.; McCune, B.T.; Yokoyama, C.C.; Kambal, A.; Wheadon, M.; Diamond, M.S.; Ivanova, Y.; Artyomov, M.; Virgin, H.W. Commensal microbes and interferon-λ determine persistence of enteric murine norovirus infection. Science 2015, 347, 266–269. [Google Scholar] [CrossRef] [PubMed]
- Nice, T.J.; Baldridge, M.T.; McCune, B.T.; Norman, J.M.; Lazear, H.M.; Artyomov, M.; Diamond, M.S.; Virgin, H.W. Interferon-λ cures persistent murine norovirus infection in the absence of adaptive immunity. Science 2015, 347, 269–273. [Google Scholar] [CrossRef] [PubMed]
- Pfeiffer, J.K.; Virgin, H.W. Viral immunity: Transkingdom control of viral infection and immunity in the mammalian intestine. Science 2016, 351, aad5872. [Google Scholar] [CrossRef] [PubMed]
- Karst, S.M. The influence of commensal bacteria on infection with enteric viruses. Nat. Rev. Microbiol. 2016, 14, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Lei, S.; Samuel, H.; Twitchell, E.; Bui, T.; Ramesh, A.; Wen, K.; Weiss, M.; Li, G.; Yang, X.; Jiang, X.; et al. Enterobacter cloacae inhibits human norovirus infectivity in gnotobiotic pigs. Sci. Rep. 2016, 6, 25017. [Google Scholar] [CrossRef] [PubMed]
- Uchida, H.; Kinoshita, H.; Kawai, Y.; Kitazawa, H.; Miura, K.; Shiiba, K.; Horii, A.; Kimura, K.; Taketomo, N.; Oda, M.; et al. Lactobacilli binding human A-antigen expressed in intestinal mucosa. Res. Microbiol. 2006, 157, 659–665. [Google Scholar] [CrossRef] [PubMed]
- Uchida, H.; Kawai, Y.; Kinoshita, H.; Kitazawa, H.; Miura, K.; Shiiba, K.; Horii, A.; Kimura, K.; Taketomo, N.; Oda, M.; et al. Lactic acid bacteria (LAB) bind to human B- or H-antigens expressed on intestinal mucosa. Biosci. Biotechnol. Biochem. 2006, 70, 3073–3076. [Google Scholar] [CrossRef] [PubMed]
- Rubio-del-Campo, A.; Coll-Marqués, J.M.; Yebra, M.J.; Buesa, J.; Pérez-Martínez, G.; Monedero, V.; Rodríguez-Díaz, J. Noroviral P-particles as an in vitro model to assess the interactions of noroviruses with probiotics. PLoS ONE 2014, 9, 21–25. [Google Scholar] [CrossRef]
- Randazzo, W.; López-Gálvez, F.; Allende, A.; Aznar, R.; Sánchez, G. Evaluation of viability PCR performance for assessing norovirus infectivity in fresh-cut vegetables and irrigation water. Int. J. Food Microbiol. 2016, 229, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Seitz, S.R.; Leon, J.S.; Schwab, K.J.; Lyon, G.M.; Dowd, M.; McDaniels, M.; Abdulhafid, G.; Fernandez, M.L.; Lindesmith, L.C.; Baric, R.S.; et al. Norovirus infectivity in humans and persistence in water. Appl. Environ. Microbiol. 2011, 77, 6884–6888. [Google Scholar] [CrossRef] [PubMed]
- Esseili, M.A.; Gao, X.; Tegtmeier, S.; Saif, L.J.; Wang, Q. Abiotic stress and phyllosphere bacteria influence the survival of human norovirus and its surrogates on preharvest leafy greens. Appl. Environ. Microbiol. 2016, 82, 352–363. [Google Scholar] [CrossRef] [PubMed]
- Bae, J.; Schwab, K.J. Evaluation of Murine Norovirus, Feline Calicivirus, Poliovirus, and MS2 as Surrogates for Human Norovirus in a Model of Viral Persistence in Surface Water and Groundwater. Appl. Environ. Microbiol. 2008, 74, 477–484. [Google Scholar] [CrossRef] [PubMed]
- Kotwal, G.; Cannon, J.L. Environmental persistence and transfer of enteric viruses. Curr. Opin. Virol. 2014, 4, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Colas de la Noue, A.; Estienney, M.; Aho, S.; Perrier-Cornet, J.M.; de Rougemont, A.; Pothier, P.; Gervais, P.; Belliot, G. Absolute humidity influences the seasonal persistence and infectivity of human norovirus. Appl. Environ. Microbiol. 2014, 80, 7196–7205. [Google Scholar] [CrossRef] [PubMed]
- López-Gálvez, F.; Truchado, P.; Sánchez, G.; Aznar, R.; Gil, M.I.; Allende, A. Occurrence of enteric viruses in reclaimed and surface irrigation water: relationship with microbiological and physicochemical indicators. J. Appl. Microbiol. 2016, 121, 1180–1188. [Google Scholar] [CrossRef] [PubMed]
- Iritani, N.; Kaida, A.; Abe, N.; Kubo, H.; Sekiguchi, J.-I.; Yamamoto, S.P.; Goto, K.; Tanaka, T.; Noda, M. Detection and genetic characterization of human enteric viruses in oyster-associated gastroenteritis outbreaks between 2001 and 2012 in Osaka City, Japan. J. Med. Virol. 2014, 86, 2019–2025. [Google Scholar] [CrossRef] [PubMed]
- Maalouf, H.; Zakhour, M.; Le Pendu, J.; Le Saux, J.-C.; Atmar, R.L.; Le Guyader, F.S. Distribution in tissue and seasonal variation of norovirus genogroup I and II ligands in oysters. Appl. Environ. Microbiol. 2010, 76, 5621–5630. [Google Scholar] [CrossRef] [PubMed]
- Hewitt, J.; Greening, G.E. Survival and persistence of norovirus, hepatitis A virus, and feline calicivirus in marinated mussels. J. Food Prot. 2004, 67, 1743–1750. [Google Scholar] [CrossRef] [PubMed]
- Hewitt, J.; Greening, G.E. Effect of heat treatment on hepatitis A virus and norovirus in New Zealand greenshell mussels (Perna canaliculus) by quantitative real-time reverse transcription PCR and cell culture. J. Food Prot. 2006, 69, 2217–2223. [Google Scholar] [CrossRef] [PubMed]
- Choi, C.; Kingsley, D.H. Temperature-dependent persistence of human norovirus within oysters (Crassostrea virginica). Food Environ. Virol. 2016, 8, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Schaeffer, J.; Treguier, C.; Piquet, J.-C.; Gachelin, S.; Cochennec-Laureau, N.; Le Saux, J.-C.; Garry, P.; Le Guyader, F.S. Improving the efficacy of sewage treatment decreases norovirus contamination in oysters. Int. J. Food Microbiol. 2018, 286, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Maalouf, H.; Pommepuy, M.; Le Guyader, F.S. Environmental cconditions leading to shellfish contamination and related outbreaks. Food Environ. Virol. 2010, 2, 136–145. [Google Scholar] [CrossRef]
- Araud, E.; DiCaprio, E.; Ma, Y.; Lou, F.; Gao, Y.; Kingsley, D.; Hughes, J.H.; Li, J. Thermal inactivation of enteric viruses and bioaccumulation of enteric foodborne viruses in live oysters (Crassostrea virginica). Appl. Environ. Microbiol. 2016, 82, 2086–2099. [Google Scholar] [CrossRef] [PubMed]
- Esseili, M.A.; Wang, Q.; Saif, L.J. Binding of human GII.4 norovirus virus-like particles to carbohydrates of romaine lettuce leaf cell wall materials. Appl. Environ. Microbiol. 2012, 78, 786–794. [Google Scholar] [CrossRef] [PubMed]
- Prevost, B.; Lucas, F.S.; Goncalves, A.; Richard, F.; Moulin, L.; Wurtzer, S. Large scale survey of enteric viruses in river and waste water underlines the health status of the local population. Environ. Int. 2015, 79, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Farkas, K.; Cooper, D.M.; McDonald, J.E.; Malham, S.K.; de Rougemont, A.; Jones, D.L. Seasonal and spatial dynamics of enteric viruses in wastewater and in riverine and estuarine receiving waters. Sci. Total Environ. 2018, 634, 1174–1183. [Google Scholar] [CrossRef] [PubMed]
- Kazama, S.; Masago, Y.; Tohma, K.; Souma, N.; Imagawa, T.; Suzuki, A.; Liu, X.; Saito, M.; Oshitani, H.; Omura, T. Temporal dynamics of norovirus determined through monitoring of municipal wastewater by pyrosequencing and virological surveillance of gastroenteritis cases. Water Res. 2016, 92, 244–253. [Google Scholar] [CrossRef] [PubMed]
- Kazama, S.; Miura, T.; Masago, Y.; Konta, Y.; Tohma, K.; Manaka, T.; Liu, X.; Nakayama, D.; Tanno, T.; Saito, M.; et al. Environmental Surveillance of norovirus genogroups I and II for sensitive detection of epidemic variants. Appl. Environ. Microbiol. 2017, 83, e03406-16. [Google Scholar] [CrossRef] [PubMed]
- Haramoto, E.; Kitajima, M.; Hata, A.; Torrey, J.R.; Masago, Y.; Sano, D.; Katayama, H. A review on recent progress in the detection methods and prevalence of human enteric viruses in water. Water Res. 2018, 135, 168–186. [Google Scholar] [CrossRef] [PubMed]
- Amarasiri, M.; Kitajima, M.; Miyamura, A.; Santos, R.; Monteiro, S.; Miura, T.; Kazama, S.; Okabe, S.; Sano, D. Reverse transcription-quantitative PCR assays for genotype-specific detection of human noroviruses in clinical and environmental samples. Int. J. Hyg. Environ. Health 2018, 221, 578–585. [Google Scholar] [CrossRef] [PubMed]
- Thongprachum, A.; Okitsu, S.; Khamrin, P.; Maneekarn, N.; Hayakawa, S.; Ushijima, H. Emergence of norovirus GII.2 and its novel recombination during the gastroenteritis outbreak in Japanese children in mid-2016. Infect. Genet. Evol. 2017, 51, 86–88. [Google Scholar] [CrossRef] [PubMed]
- Iritani, N.; Kaida, A.; Abe, N.; Sekiguchi, J.; Kubo, H.; Takakura, K.; Goto, K.; Ogura, H.; Seto, Y. Increase of GII.2 norovirus infections during the 2009–2010 season in Osaka city, Japan. J. Med. Virol. 2012, 84, 517–525. [Google Scholar] [CrossRef] [PubMed]
- Templeton, M.R.; Andrews, R.C.; Hofmann, R. Particle-associated viruses in water: Impacts on disinfection processes. Crit. Rev. Environ. Sci. Technol. 2008, 38, 137–164. [Google Scholar] [CrossRef]
- Waldman, P.; Meseguer, A.; Lucas, F.; Moulin, L.; Wurtzer, S. Interaction of human enteric viruses with microbial compounds: Implication for virus persistence and disinfection treatments. Environ. Sci. Technol. 2017, 51, 13633–13640. [Google Scholar] [CrossRef] [PubMed]
- Keswick, B.H.; Satterwhite, T.K.; Johnson, P.C.; DuPont, H.L.; Secor, S.L.; Bitsura, J.A.; Gary, G.W.; Hoff, J.C. Inactivation of Norwalk virus in drinking water by chlorine. Appl. Environ. Microbiol. 1985, 50, 261–264. [Google Scholar] [CrossRef] [PubMed]
- Shin, G.-A.; Sobsey, M.D. Inactivation of norovirus by chlorine disinfection of water. Water Res. 2008, 42, 4562–4568. [Google Scholar] [CrossRef] [PubMed]
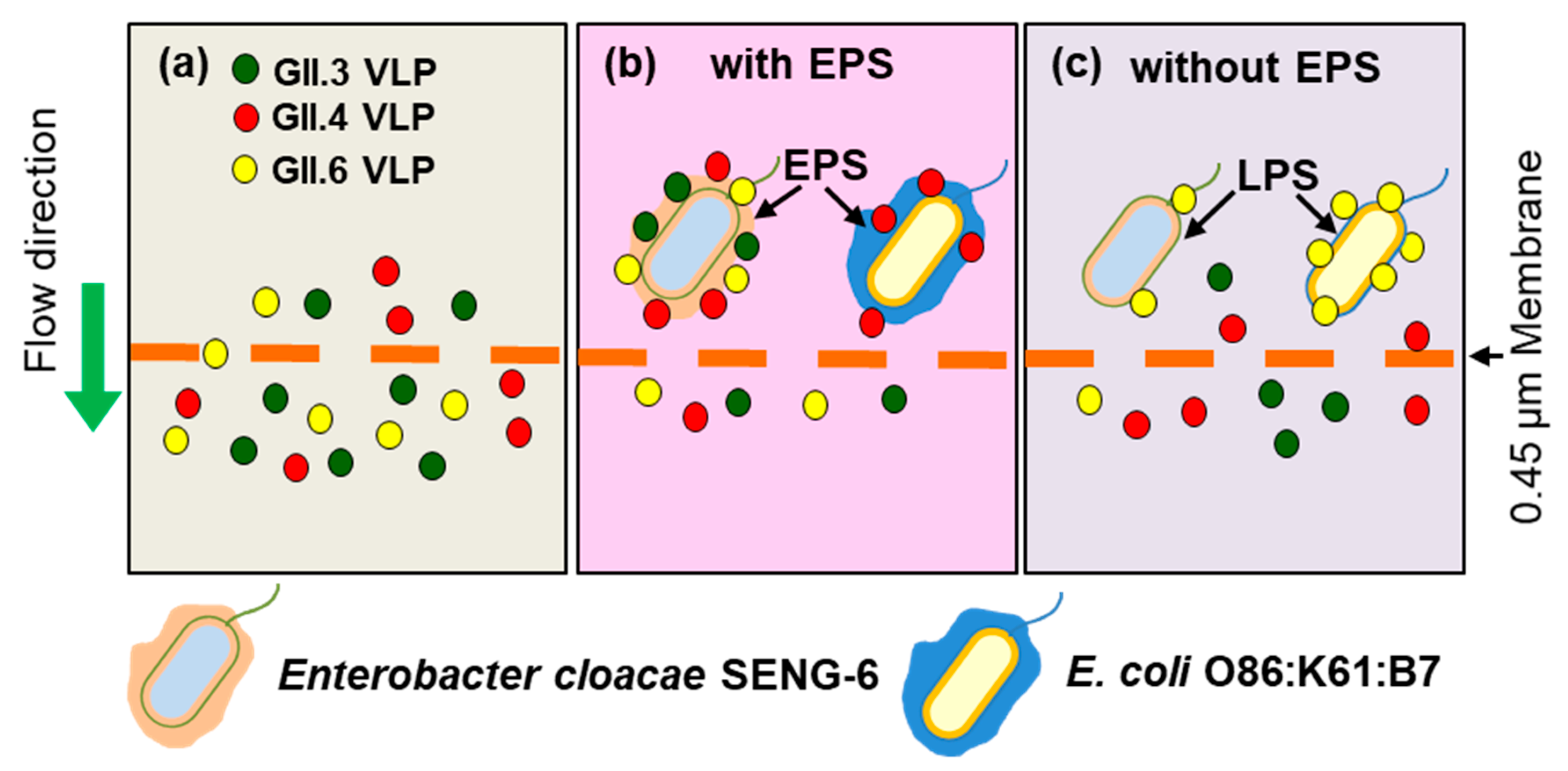
| Cell/Tissue | HBGA Activity | Location of HBGA-Like Substances | Interacting Norovirus Strains | Ref |
|---|---|---|---|---|
| Enterobacter cloacae SENG-6 | A, B, H | EPS | GI.7, GII.3, GII.6 GII.4 (DenHaag 2006b) | [61] |
| Escherichia coli O86:K61:B7 | B | LPS | GII.6 | [71] |
| E. coli LMG8223 | A, B, H, Lea, Leb, Lex, Ley | GI.1, GII.4 (Dijon 1996) | [68] | |
| E. coli LFMFP861 | B, Lea | |||
| E. coli LFMFP289 | B, Leb | |||
| Enterobacter aerogenes | Lea, Leb, Ley | GI.1 | ||
| Clostridium difficile | Lea | GI.1 | ||
| E. coli O86:H2 | B | Tulane virus | [69] | |
| Romaine lettuce | A, B, H | Leaf | GII.4 (Sydney 2012) | [70] |
| A, B, H | Vein | GII.4 (Sydney 2012) | ||
| Lettuce | H | Cell wall | GII.4 (DenHaag 2006b) | [63] |
| Crassostrea virginica oysters | A | Gastrointestinal cells | GI.1 (8FIIa) | [62] |
| Crassostrea virginica oysters | A, H | Gastrointestinal cells | GI.1 (8FIIa) US 95/96 (VA387) GII.9 (VA207) | [65] |
| Crassostrea sikamea oysters | A, H | |||
| Crassostrea gigas oysters | A, H | |||
| Venerupis japonica clams | A | Gastrointestinal cells | GI.1 (8FIIa) GII.9 (VA207) | |
| Mytilis edulis mussels | A | Gastrointestinal cells | GI.1 (8FIIa) GII.9 (VA207) | |
| Crassostrea gigas oysters | A | Digestive tissues and palps | GI.1 (West Chester) GII.4 (Sydney 2012) | [66] |
| H | Digestive tissues, gills and palps | |||
| Crassostrea gigas oysters | A (100%) | Gut | GII.4 (DenHaag 2006b) GII.4 (US 95/96) | [72] |
| A (61%) Leb (91%) | Gills |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amarasiri, M.; Sano, D. Specific Interactions between Human Norovirus and Environmental Matrices: Effects on the Virus Ecology. Viruses 2019, 11, 224. https://doi.org/10.3390/v11030224
Amarasiri M, Sano D. Specific Interactions between Human Norovirus and Environmental Matrices: Effects on the Virus Ecology. Viruses. 2019; 11(3):224. https://doi.org/10.3390/v11030224
Chicago/Turabian StyleAmarasiri, Mohan, and Daisuke Sano. 2019. "Specific Interactions between Human Norovirus and Environmental Matrices: Effects on the Virus Ecology" Viruses 11, no. 3: 224. https://doi.org/10.3390/v11030224
APA StyleAmarasiri, M., & Sano, D. (2019). Specific Interactions between Human Norovirus and Environmental Matrices: Effects on the Virus Ecology. Viruses, 11(3), 224. https://doi.org/10.3390/v11030224






