Mechanistic Insights into Chemoresistance Mediated by Oncogenic Viruses in Lymphomas
Abstract
1. Introduction
2. Current Status of Chemotherapy in Virus-Associated Lymphomas
2.1. KSHV-Associated Lymphoma
2.2. EBV-Associated Lymphomas
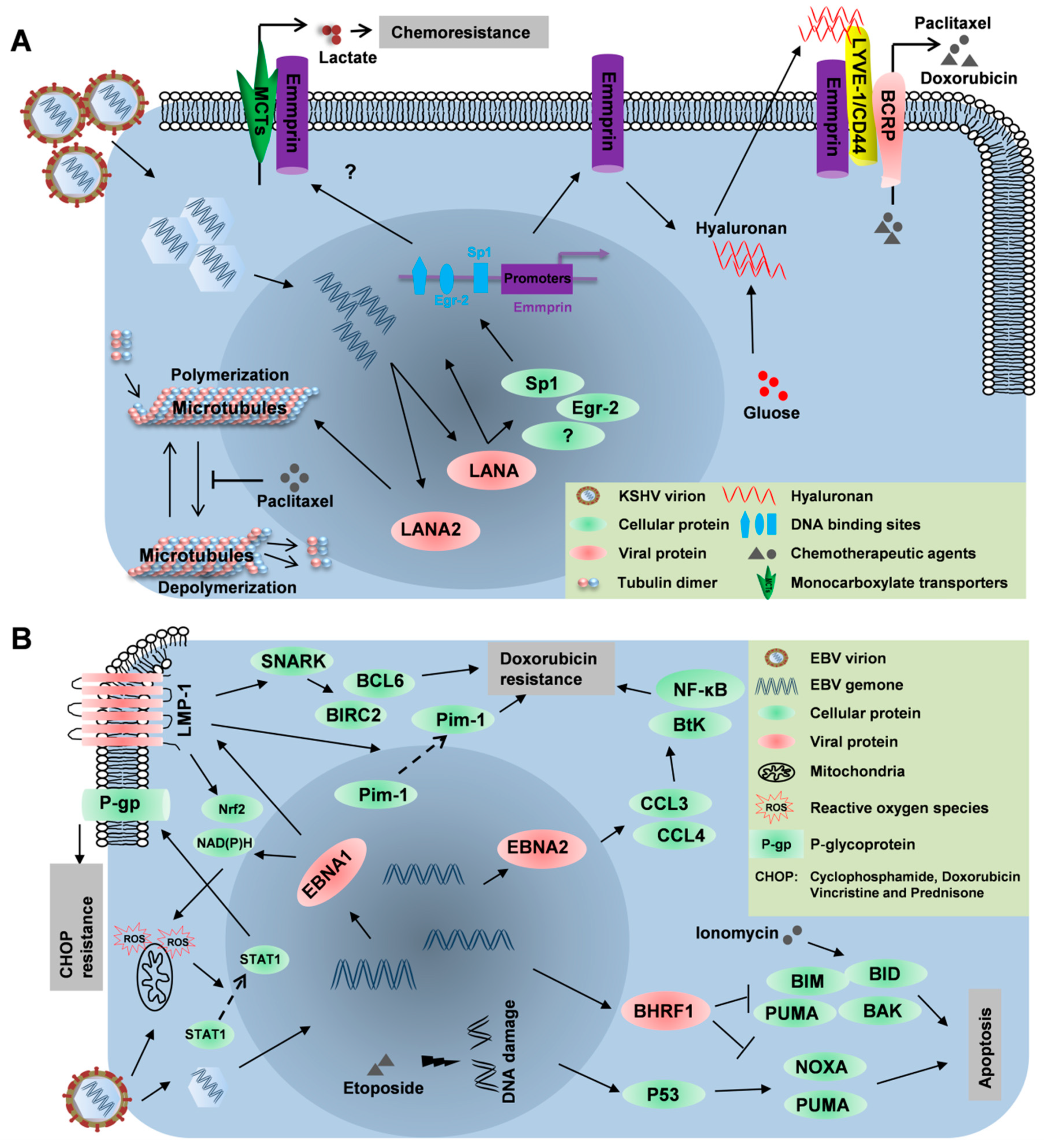
3. Involvement of KSHV-Encoded Proteins into Drug Resistance of Lymphomas
3.1. LANA
3.2. LANA2
3.3. Other Viral Proteins
4. Involvement of EBV Components into Drug Resistance of Lymphomas
4.1. LMP1
4.2. EBNA2
4.3. BHRF1
5. HTLV-1
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Castillo, J.J.; Reagan, J.L.; Bishop, K.D.; Apor, E. Viral lymphomagenesis: From pathophysiology to the rationale for novel therapies. Br. J. Haematol. 2014, 165, 300–315. [Google Scholar] [CrossRef]
- Alexander, D.D.; Mink, P.J.; Adami, H.O.; Chang, E.T.; Cole, P.; Mandel, J.S.; Trichopoulos, D. The non-Hodgkin lymphomas: A review of the epidemiologic literature. Int. J. Cancer 2007, 120, 1–39. [Google Scholar] [CrossRef]
- Grulich, A.E.; Vajdic, C.M. The epidemiology of non-Hodgkin lymphoma. Pathology 2005, 37, 409–419. [Google Scholar] [CrossRef]
- Cesarman, E.; Chang, Y.; Moore, P.S.; Said, J.W.; Knowles, D.M. Kaposi’s sarcoma–Associated herpesvirus-like DNA sequences in AIDS-related body-cavity–based lymphomas. N. Engl. J. Med. 1995, 332, 1186–1191. [Google Scholar] [CrossRef]
- Thompson, M.P.; Kurzrock, R. Epstein-barr virus and cancer. Clin. Cancer Res. 2004, 10, 803–821. [Google Scholar] [CrossRef]
- De Vita, S.; Sacco, C.; Sansonno, D.; Gloghini, A.; Dammacco, F.; Crovatto, M.; Santini, G.; Dolcetti, R.; Boiocchi, M.; Carbone, A.; et al. Characterization of overt B-cell lymphomas in patients with hepatitis C virus infection. Blood 1997, 90, 776–782. [Google Scholar] [CrossRef]
- Shimoyama, M. Diagnostic-criteria and classification of clinical subtypes of adult T-cell leukemia-lymphoma—A report from the Lymphoma-Study-Group (1984–1987). Br. J. Haematol. 1991, 79, 428–437. [Google Scholar] [CrossRef]
- Tarantul, V.Z. Virus-associated lymphomagenesis. Int. J. Biomed. Sci. 2006, 2, 101–113. [Google Scholar]
- Ziegler, J.L.; Beckstead, J.A.; Volberding, P.A.; Abrams, D.I.; Levine, A.M.; Lukes, R.J.; Gill, P.S.; Burkes, R.L.; Meyer, P.R.; Metroka, C.E.; et al. Non-Hodgkin’s lymphoma in 90 homosexual men. Relation to generalized lymphadenopathy and the acquired immunodeficiency syndrome. N. Engl. J. Med. 1984, 311, 565–570. [Google Scholar] [CrossRef]
- Dolcetti, R.; Giagulli, C.; He, W.; Selleri, M.; Caccuri, F.; Eyzaguirre, L.M.; Mazzuca, P.; Corbellini, S.; Campilongo, F.; Marsico, S.; et al. Role of HIV-1 matrix protein p17 variants in lymphoma pathogenesis. Proc. Natl. Acad. Sci. USA 2015, 112, 14331–14336. [Google Scholar] [CrossRef]
- Dolcetti, R.; Gloghini, A.; Caruso, A.; Carbone, A. A lymphomagenic role for HIV beyond immune suppression? Blood 2016, 127, 1403–1409. [Google Scholar] [CrossRef] [PubMed]
- Manners, O.; Murphy, J.C.; Coleman, A.; Hughes, D.J.; Whitehouse, A. Contribution of the KSHV and EBV lytic cycles to tumourigenesis. Curr. Opin. Virol. 2018, 32, 60–70. [Google Scholar] [CrossRef]
- Hardie, D.R. Human gamma-herpesviruses: A review of 2 divergent paths to oncogenesis. Transfus. Apher. Sci. 2010, 42, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Jha, H.C.; Banerjee, S.; Robertson, E.S. The role of gammaherpesviruses in cancer pathogenesis. Pathogens 2016, 5, 18. [Google Scholar] [CrossRef]
- Li, X.; Burton, E.M.; Koganti, S.; Zhi, J.; Doyle, F.; Tenenbaum, S.A.; Horn, B.; Bhaduri-McIntosh, S. KRAB-ZFP Repressors Enforce Quiescence of Oncogenic Human Herpesviruses. J. Virol. 2018, 92, e00298-18. [Google Scholar] [CrossRef]
- Gantt, S.; Casper, C. Human herpesvirus 8-associated neoplasms: The roles of viral replication and antiviral treatment. Curr. Opin. Infect. Dis. 2011, 24, 295–301. [Google Scholar] [CrossRef]
- Coen, N.; Duraffour, S.; Snoeck, R.; Andrei, G. KSHV targeted therapy: An update on inhibitors of viral lytic replication. Viruses 2014, 6, 4731–4759. [Google Scholar] [CrossRef]
- Pagano, J.S.; Whitehurst, C.B.; Andrei, G. Antiviral drugs for EBV. Cancers 2018, 10, 197. [Google Scholar] [CrossRef]
- Dittmer, D.; Lagunoff, M.; Renne, R.; Staskus, K.; Haase, A.; Ganem, D. A cluster of latently expressed genes in Kaposi’s sarcoma-associated herpesvirus. J. Virol. 1998, 72, 8309–8315. [Google Scholar]
- Greene, W.; Kuhne, K.; Ye, F.; Chen, J.; Zhou, F.; Lei, X.; Gao, S.J. Molecular biology of KSHV in relation to AIDS-associated oncogenesis. Cancer Treat. Res. 2007, 133, 69–127. [Google Scholar]
- Rosario, S.A.; Santiago, G.E.; Mesri, E.A.; Verdun, R.E. Kaposi’s sarcoma-associated herpesvirus-encoded viral IL-6 (vIL-6) enhances immunoglobulin class-switch recombination. Front. Microbiol. 2018, 9, 3119. [Google Scholar] [CrossRef] [PubMed]
- Okada, S.; Goto, H.; Yotsumoto, M. Current status of treatment for primary effusion lymphoma. Intractable Rare Dis. Res. 2014, 3, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Ansari, M.Q.; Dawson, D.B.; Nador, R.; Rutherford, C.; Schneider, N.R.; Latimer, M.J.; Picker, L.; Knowles, D.M.; McKenna, R.W. Primary body cavity-based AIDS-related lymphomas. Am. J. Clin. Pathol. 1996, 105, 221–229. [Google Scholar] [CrossRef]
- Simonelli, C.; Spina, M.; Cinelli, R.; Talamini, R.; Tedeschi, R.; Gloghini, A.; Vaccher, E.; Carbone, A.; Tirelli, U. Clinical features and outcome of primary effusion lymphoma in HIV-infected patients: A single-institution study. J. Clin. Oncol. 2003, 21, 3948–3954. [Google Scholar] [CrossRef]
- Dunleavy, K.; Wilson, W.H. How I treat HIV-associated lymphoma. Blood 2012, 119, 3245–3255. [Google Scholar] [CrossRef]
- Carbone, A.; Gloghini, A.; Dotti, G. EBV-associated lymphoproliferative disorders: Classification and treatment. Oncologist 2008, 13, 577–585. [Google Scholar] [CrossRef]
- Calderwood, M.A.; Venkatesan, K.; Xing, L.; Chase, M.R.; Vazquez, A.; Holthaus, A.M.; Ewence, A.E.; Li, N.; Hirozane-Kishikawa, T.; Hill, D.E.; et al. Epstein-Barr virus and virus human protein interaction maps. Proc. Natl. Acad. Sci. USA 2007, 104, 7606–7611. [Google Scholar] [CrossRef]
- Hutzinger, R.; Feederle, R.; Mrazek, J.; Schiefermeier, N.; Balwierz, P.J.; Zavolan, M.; Polacek, N.; Delecluse, H.J.; Huttenhofer, A. Expression and processing of a small nucleolar RNA from the Epstein-Barr virus genome. PLoS Pathog. 2009, 5, e1000547. [Google Scholar] [CrossRef]
- Niedobitek, G.; Young, L.S. Epstein-Barr virus persistence and virus-associated tumours. Lancet 1994, 343, 333–335. [Google Scholar] [CrossRef]
- Rezk, S.A.; Weiss, L.M. Epstein-Barr virus-associated lymphoproliferative disorders. Hum. Pathol. 2007, 38, 1293–1304. [Google Scholar] [CrossRef]
- Onnis, A.; Navari, M.; Antonicelli, G.; Morettini, F.; Mannucci, S.; De Falco, G.; Vigorito, E.; Leoncini, L. Epstein-Barr nuclear antigen 1 induces expression of the cellular microRNA hsa-miR-127 and impairing B-cell differentiation in EBV-infected memory B cells. New insights into the pathogenesis of Burkitt lymphoma. Blood Cancer J. 2012, 2, e84. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y.; Kawamura, Y.; Iwata, S.; Kawada, J.; Yoshikawa, T.; Kimura, H. Demonstration of type II latency in T lymphocytes of Epstein-Barr Virus-associated hemophagocytic lymphohistiocytosis. Pediatr. Blood Cancer 2013, 60, 326–328. [Google Scholar] [CrossRef] [PubMed]
- Kis, L.L.; Gerasimcik, N.; Salamon, D.; Persson, E.K.; Nagy, N.; Klein, G.; Severinson, E.; Klein, E. STAT6 signaling pathway activated by the cytokines IL-4 and IL-13 induces expression of the Epstein-Barr virus-encoded protein LMP-1 in absence of EBNA-2: Implications for the type II EBV latent gene expression in Hodgkin lymphoma. Blood 2011, 117, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Grywalska, E.; Rolinski, J. Epstein-Barr virus-associated lymphomas. Semin. Oncol. 2015, 42, 291–303. [Google Scholar] [CrossRef] [PubMed]
- Niedobitek, G.; Young, L.S.; Herbst, H. Epstein-Barr virus infection and the pathogenesis of malignant lymphomas. Cancer Surv. 1997, 30, 143–162. [Google Scholar] [PubMed]
- Ramos, J.C.; Lossos, I.S. Newly emerging therapies targeting viral-related lymphomas. Curr. Oncol. Rep. 2011, 13, 416–426. [Google Scholar] [CrossRef]
- Ghosh, S.K.; Perrine, S.P.; Faller, D.V. Advances in virus-directed therapeutics against Epstein-Barr virus-associated malignancies. Adv. Virol. 2012, 2012, 509296. [Google Scholar] [CrossRef]
- Messick, T.E.; Smith, G.R.; Soldan, S.S.; McDonnell, M.E.; Deakyne, J.S.; Malecka, K.A.; Tolvinski, L.; van den Heuvel, A.P.J.; Gu, B.W.; Cassel, J.A.; et al. Structure-based design of small-molecule inhibitors of EBNA1 DNA binding blocks Epstein-Barr virus latent infection and tumor growth. Sci. Transl. Med. 2019, 11, eaau5612. [Google Scholar] [CrossRef]
- Qin, Z.; Dai, L.; Bratoeva, M.; Slomiany, M.G.; Toole, B.P.; Parsons, C. Cooperative roles for emmprin and LYVE-1 in the regulation of chemoresistance for primary effusion lymphoma. Leukemia 2011, 25, 1598–1609. [Google Scholar] [CrossRef]
- Qin, Z.; Dai, L.; Slomiany, M.G.; Toole, B.P.; Parsons, C. Direct activation of emmprin and associated pathogenesis by an oncogenic herpesvirus. Cancer Res. 2010, 70, 3884–3889. [Google Scholar] [CrossRef]
- Munoz-Fontela, C.; Marcos-Villar, L.; Hernandez, F.; Gallego, P.; Rodriguez, E.; Arroyo, J.; Gao, S.J.; Avila, J.; Rivas, C. Induction of paclitaxel resistance by the Kaposi’s sarcoma-associated herpesvirus latent protein LANA2. J. Virol. 2008, 82, 1518–1525. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Kim, W.S.; Hong, J.Y.; Ryu, K.J.; Kim, S.J.; Park, C. Epstein-Barr virus EBNA2 directs doxorubicin resistance of B cell lymphoma through CCL3 and CCL4-mediated activation of NF-κB and Btk. Oncotarget 2017, 8, 5361–5370. [Google Scholar] [PubMed]
- Kim, J.H.; Kim, W.S.; Park, C. SNARK, a novel downstream molecule of EBV latent membrane protein 1, is associated with resistance to cancer cell death. Leuk. Lymphoma 2008, 49, 1392–1398. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Kim, W.S.; Yun, Y.; Park, C. Epstein-Barr virus latent membrane protein 1 increases chemo-resistance of cancer cells via cytoplasmic sequestration of Pim-1. Cell Signal 2010, 22, 1858–1863. [Google Scholar] [CrossRef] [PubMed]
- Pujals, A.; Favre, L.; Pioche-Durieu, C.; Robert, A.; Meurice, G.; Le Gentil, M.; Chelouah, S.; Martin-Garcia, N.; Le Cam, E.; Guettier, C.; et al. Constitutive autophagy contributes to resistance to TP53-mediated apoptosis in Epstein-Barr virus-positive latency III B-cell lymphoproliferations. Autophagy 2015, 11, 2275–2287. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Howell, M.E.A.; McPeak, B.; Riggs, K.; Kohne, C.; Yohanon, J.U.; Foxler, D.E.; Sharp, T.V.; Moorman, J.P.; Yao, Z.Q.; et al. LIMD1 is induced by and required for LMP1 signaling, and protects EBV-transformed cells from DNA damage-induced cell death. Oncotarget 2017, 9, 6282–6297. [Google Scholar] [CrossRef] [PubMed]
- Fitzsimmons, L.; Cartlidge, R.; Chang, C.; Sejic, N.; Galbraith, L.C.A.; Suraweera, C.D.; Croom-Carter, D.; Dewson, G.; Tierney, R.J.; Bell, A.I.; et al. EBV BCL-2 homologue BHRF1 drives chemoresistance and lymphomagenesis by inhibiting multiple cellular pro-apoptotic proteins. Cell Death Differ. 2019. [Google Scholar] [CrossRef]
- Hall, A.G.; Tilby, M.J. Mechanisms of action of, and modes of resistance to, alkylating agents used in the treatment of haematological malignancies. Blood Rev. 1992, 6, 163–173. [Google Scholar] [CrossRef]
- Tacar, O.; Sriamornsak, P.; Dass, C.R. Doxorubicin: An update on anticancer molecular action, toxicity and novel drug delivery systems. J. Pharm. Pharmacol. 2013, 65, 157–170. [Google Scholar] [CrossRef]
- Pommier, Y.; Leo, E.; Zhang, H.L.; Marchand, C. DNA topoisomerases and their poisoning by anticancer and antibacterial drugs. Chem. Biol. 2010, 17, 421–433. [Google Scholar] [CrossRef]
- Pufall, M.A. Glucocorticoids and cancer. Adv. Exp. Med. Biol 2015, 872, 315–333. [Google Scholar] [PubMed]
- Nitiss, J.L. Targeting DNA topoisomerase II in cancer chemotherapy. Nat. Rev. Cancer 2009, 9, 338–350. [Google Scholar] [CrossRef] [PubMed]
- Noll, D.M.; Mason, T.M.; Miller, P.S. Formation and repair of interstrand cross-links in DNA. Chem. Rev. 2006, 106, 277–301. [Google Scholar] [CrossRef] [PubMed]
- Stanton, R.A.; Gernert, K.M.; Nettles, J.H.; Aneja, R. Drugs That target dynamic microtubules: A new molecular perspective. Med. Res. Rev. 2011, 31, 443–481. [Google Scholar] [CrossRef]
- Wilson, W.H.; Grossbard, M.L.; Pittaluga, S.; Cole, D.; Pearson, D.; Drbohlav, N.; Steinberg, S.M.; Little, R.F.; Janik, J.; Gutierrez, M.; et al. Dose-adjusted EPOCH chemotherapy for untreated large B-cell lymphomas: A pharmacodynamic approach with high efficacy. Blood 2002, 99, 2685–2693. [Google Scholar] [CrossRef] [PubMed]
- Jessamy, K.; Ojevwe, F.O.; Doobay, R.; Naous, R.; Yu, J.; Lemke, S.M. Primary effusion lymphoma: Is dose-adjusted-EPOCH worthwhile therapy? Case Rep. Oncol. 2016, 9, 273–279. [Google Scholar] [CrossRef]
- Boulanger, E.; Gerard, L.; Gabarre, J.; Molina, J.M.; Rapp, C.; Abino, J.F.; Cadranel, J.; Chevret, S.; Oksenhendler, E. Prognostic factors and outcome of human herpesvirus 8-associated primary effusion lymphoma in patients with AIDS. J. Clin. Oncol. 2005, 23, 4372–4380. [Google Scholar] [CrossRef]
- Guillet, S.; Gerard, L.; Meignin, V.; Agbalika, F.; Cuccini, W.; Denis, B.; Katlama, C.; Galicier, L.; Oksenhendler, E. Classic and extracavitary primary effusion lymphoma in 51 HIV-infected patients from a single institution. Am. J. Hematol. 2016, 91, 233–237. [Google Scholar] [CrossRef]
- Ramos, J.C.; Sparano, J.A.; Rudek, M.A.; Moore, P.C.; Cesarman, E.; Reid, E.G.; Henry, D.; Ratner, L.; Aboulafia, D.; Lee, J.Y.; et al. Safety and preliminary efficacy of vorinostat with R-EPOCH in high-risk HIV-associated Non-Hodgkin’s lymphoma (AMC-075). Clin. Lymphoma Myeloma Leuk. 2018, 18, 180–190e2. [Google Scholar] [CrossRef]
- Siddiqi, T.; Joyce, R.M. A case of HIV-negative primary effusion lymphoma treated with bortezomib, pegylated liposomal doxorubicin, and rituximab. Clin. Lymphoma Myeloma 2008, 8, 300–304. [Google Scholar] [CrossRef]
- Yiakoumis, X.; Pangalis, G.A.; Kyrtsonis, M.C.; Vassilakopoulos, T.P.; Kontopidou, F.N.; Kalpadakis, C.; Korkolopoulou, P.; Levidou, G.; Androulaki, A.; Siakantaris, M.P.; et al. Primary effusion lymphoma in two HIV-negative patients successfully treated with pleurodesis as first-line therapy. Anticancer Res. 2010, 30, 271–276. [Google Scholar] [PubMed]
- Antar, A.; El Hajj, H.; Jabbour, M.; Khalifeh, I.; El-Merhi, F.; Mahfouz, R.; Bazarbachi, A. Primary effusion lymphoma in an elderly patient effectively treated by lenalidomide: Case report and review of literature. Blood Cancer J. 2014, 4, e190. [Google Scholar] [CrossRef] [PubMed]
- Sin, S.H.; Roy, D.; Wang, L.; Staudt, M.R.; Fakhari, F.D.; Patel, D.D.; Henry, D.; Harrington, W.J.; Damania, B.A.; Dittmer, D.P. Rapamycin is efficacious against primary effusion lymphoma (PEL) cell lines in vivo by inhibiting autocrine signaling. Blood 2007, 109, 2165–2173. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Ko, Y.H.; Kim, J.E.; Lee, S.S.; Lee, H.; Park, G.; Paik, J.H.; Cha, H.J.; Choi, Y.D.; Han, J.H.; et al. Epstein-Barr virus-associated lymphoproliferative disorders: Review and update on 2016 WHO classification. J. Pathol. Transl. Med. 2017, 51, 352–358. [Google Scholar] [CrossRef] [PubMed]
- Carbone, A.; Gloghini, A. Epstein Barr virus-associated Hodgkin lymphoma. Cancers 2018, 10, 163. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, Y.; Choi, J.W.; Kim, Y.S. Prevalence and prognostic significance of Epstein-Barr virus infection in classical Hodgkin’s lymphoma: A meta-analysis. Arch. Med. Res. 2014, 45, 417–431. [Google Scholar] [CrossRef]
- Devita, V.T., Jr.; Simon, R.M.; Hubbard, S.M.; Young, R.C.; Berard, C.W.; Moxley, J.H., III; Frei, E., III; Carbone, P.P.; Canellos, G.P. Curability of advanced Hodgkin’s disease with chemotherapy: Long-term follow-up of MOPP-treated patients at the National Cancer Institute. Ann. Intern. Med. 1980, 92, 587–595. [Google Scholar] [CrossRef]
- Canellos, G.P.; Anderson, J.R.; Propert, K.J.; Nissen, N.; Cooper, M.R.; Henderson, E.S.; Green, M.R.; Gottlieb, A.; Peterson, B.A. Chemotherapy of advanced Hodgkin’s disease with MOPP, ABVD, or MOPP alternating with ABVD. N. Engl. J. Med. 1992, 327, 1478–1484. [Google Scholar] [CrossRef]
- Engert, A. ABVD or BEACOPP for advanced Hodgkin lymphoma. J. Clin. Oncol. 2016, 34, 1167–1169. [Google Scholar] [CrossRef]
- Von Tresckow, B.; Kreissl, S.; Goergen, H.; Brockelmann, P.J.; Pabst, T.; Fridrik, M.; Rummel, M.; Jung, W.; Thiemer, J.; Sasse, S.; et al. Intensive treatment strategies in advanced-stage Hodgkin’s lymphoma (HD9 and HD12): Analysis of long-term survival in two randomised trials. Lancet Haematol. 2018, 5, e462–e473. [Google Scholar] [CrossRef]
- Yustein, J.T.; Dang, C.V. Biology and treatment of Burkitt’s lymphoma. Curr. Opin. Hematol. 2007, 14, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Brady, G.; MacArthur, G.J.; Farrell, P.J. Epstein-Barr virus and Burkitt lymphoma. J. Clin. Pathol. 2007, 60, 1397–1402. [Google Scholar] [CrossRef]
- Magrath, I.; Adde, M.; Shad, A.; Venzon, D.; Seibel, N.; Gootenberg, J.; Neely, J.; Arndt, C.; Nieder, M.; Jaffe, E.; et al. Adults and children with small non-cleaved-cell lymphoma have a similar excellent outcome when treated with the same chemotherapy regimen. J. Clin. Oncol. 1996, 14, 925–934. [Google Scholar] [CrossRef] [PubMed]
- Boue, F.; Gabarre, J.; Gisselbrecht, C.; Reynes, J.; Cheret, A.; Bonnet, F.; Billaud, E.; Raphael, M.; Lancar, R.; Costagliola, D. Phase II trial of CHOP plus rituximab in patients with HIV-associated non-Hodgkin’s lymphoma. J. Clin. Oncol. 2006, 24, 4123–4128. [Google Scholar] [CrossRef] [PubMed]
- Ribrag, V.; Koscielny, S.; Bosq, J.; Leguay, T.; Casasnovas, O.; Fornecker, L.M.; Recher, C.; Ghesquieres, H.; Morschhauser, F.; Girault, S.; et al. Rituximab and dose-dense chemotherapy for adults with Burkitt’s lymphoma: A randomised, controlled, open-label, phase 3 trial. Lancet 2016, 387, 2402–2411. [Google Scholar] [CrossRef]
- Davis, D.A.; Shrestha, P.; Aisabor, A.I.; Stream, A.; Galli, V.; Pise-Masison, C.A.; Tagawa, T.; Ziegelbauer, J.M.; Franchini, G.; Yarchoan, R. Pomalidomide increases immune surface marker expression and immune recognition of oncovirus-infected cells. Oncoimmunology 2019, 8, e1546544. [Google Scholar] [CrossRef]
- Park, S.; Lee, J.; Ko, Y.H.; Han, A.; Jun, H.J.; Lee, S.C.; Hwang, I.G.; Park, Y.H.; Ahn, J.S.; Jung, C.W.; et al. The impact of Epstein-Barr virus status on clinical outcome in diffuse large B-cell lymphoma. Blood 2007, 110, 972–978. [Google Scholar] [CrossRef]
- Lu, T.X.; Liang, J.H.; Miao, Y.; Fan, L.; Wang, L.; Qu, X.Y.; Cao, L.; Gong, Q.X.; Wang, Z.; Zhang, Z.H.; et al. Epstein-Barr virus positive diffuse large B-cell lymphoma predict poor outcome, regardless of the age. Sci. Rep. 2015, 5, 12168. [Google Scholar] [CrossRef]
- Feugier, P.; Hoof, A.V.; Sebban, C.; Solal-Celigny, P.; Bouabdallah, R.; Fermé, C.; Christian, B.; Lepage, E.; Tilly, H.; Morschhauser, F.; et al. Long-term results of the R-CHOP study in the treatment of elderly patients with diffuse large B-cell lymphoma: A study by the Groupe d’etude des Lymphomes de l’adulte. J. Clin. Oncol. 2005, 23, 4117–4126. [Google Scholar] [CrossRef]
- Habermann, T.M.; Weller, E.A.; Morrison, V.A.; Gascoyne, R.D.; Cassileth, P.A.; Cohn, J.B.; Dakhil, S.R.; Woda, B.; Fisher, R.I.; Peterson, B.A.; et al. Rituximab-CHOP versus CHOP alone or with maintenance rituximab in older patients with diffuse large B-cell lymphoma. J. Clin. Oncol. 2006, 24, 3121–3127. [Google Scholar] [CrossRef]
- Pfreundschuh, M.; Kuhnt, E.; Trümper, L.; Österborg, A.; Trneny, M.; Shepherd, L.; Gill, D.S.; Walewski, J.; Pettengell, R.; Jaeger, U.; et al. CHOP-like chemotherapy with or without rituximab in young patients with good-prognosis diffuse large-B-cell lymphoma: 6-year results of an open-label randomised study of the MabThera International Trial (MInT) Group. Lancet Oncol. 2011, 12, 1013–1022. [Google Scholar] [CrossRef]
- Castillo, J.J.; Beltran, B.E.; Miranda, R.N.; Young, K.H.; Chavez, J.C.; Sotomayor, E.M. EBV-positive diffuse large B-cell lymphoma of the elderly: 2016 update on diagnosis, risk-stratification, and management. Am. J. Hematol. 2016, 91, 529–537. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, M.; Miyazaki, K. Current treatment approaches for NK/T-cell lymphoma. J. Clin. Exp. Hematophol. 2017, 57, 98–108. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Tobinai, K.; Oguchi, M.; Ishizuka, N.; Kobayashi, Y.; Isobe, Y.; Ishizawa, K.; Maseki, N.; Itoh, K.; Usui, N.; et al. Phase I/II study of concurrent chemoradiotherapy for localized nasal natural killer/T-cell lymphoma: Japan Clinical Oncology Group Study JCOG0211. J. Clin. Oncol. 2009, 27, 5594–5600. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, M.; Tobinai, K.; Oguchi, M.; Ishizuka, N.; Kobayashi, Y.; Isobe, Y.; Ishizawa, K.; Maseki, N.; Itoh, K.; Usui, N.; et al. Concurrent chemoradiotherapy for localized nasal natural killer/T-cell lymphoma: An updated analysis of the Japan clinical oncology group study JCOG0211. J. Clin. Oncol. 2012, 30, 4044–4046. [Google Scholar] [CrossRef]
- Kim, S.J.; Kim, K.; Kim, B.S.; Kim, C.Y.; Suh, C.; Huh, J.; Lee, S.W.; Kim, J.S.; Cho, J.; Lee, G.W.; et al. Phase II trial of concurrent radiation and weekly cisplatin followed by VIPD chemotherapy in newly diagnosed, stage IE to IIE, nasal, extranodal NK/T-Cell Lymphoma: Consortium for Improving Survival of Lymphoma study. J. Clin. Oncol. 2009, 27, 6027–6032. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Kwong, Y.L.; Kim, W.S.; Maeda, Y.; Hashimoto, C.; Suh, C.; Izutsu, K.; Ishida, F.; Isobe, Y.; Sueoka, E.; et al. Phase II study of SMILE chemotherapy for newly diagnosed stage IV, relapsed, or refractory extranodal natural killer (NK)/T-cell lymphoma, nasal type: The NK-cell tumor study group study. J. Clin. Oncol. 2011, 29, 4410–4416. [Google Scholar] [CrossRef]
- Kwong, Y.L.; Kim, W.S.; Lim, S.T.; Kim, S.J.; Tang, T.; Tse, E.; Leung, A.Y.; Chim, C.S. SMILE for natural killer/T-cell lymphoma: Analysis of safety and efficacy from the Asia lymphoma study group. Blood 2012, 120, 2973–2980. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Z.H.; Chen, X.Q.; Wang, K.F.; Huang, H.Q.; Xia, Z.J. First-line combination of GELOX followed by radiation therapy for patients with stage IE/IIE ENKTL: An updated analysis with long-term follow-up. Oncol. Lett. 2015, 10, 1036–1040. [Google Scholar] [CrossRef]
- Zheng, W.; Gao, Y.H.; Ke, X.Y.; Zhang, W.J.; Su, L.P.; Ren, H.Y.; Lin, N.J.; Xie, Y.; Tu, M.F.; Liu, W.P.; et al. PEG-L-CHOP treatment is safe and effective in adult extranodal NK/T-cell lymphoma with a low rate of clinical hypersensitivity. BMC Cancer 2018, 18, 910. [Google Scholar] [CrossRef]
- Ai, W.B.; Jing, Q.C. Clinical observation of LOP chemotherapy combined with radiotherapy in the treatment of early nasal NK/T cell lymphoma. Lin chung er bi yan hou tou jing wai ke za zhi (J. Clin. Otorhinolaryngol. Head Neck Surg.) 2019, 33, 990–992. [Google Scholar]
- Kanakry, J.A.; Ambinder, R.F. EBV-related lymphomas: New approaches to treatment. Curr. Treat. Options Oncol. 2013, 14, 224–236. [Google Scholar] [CrossRef] [PubMed]
- Otvos, R.; Skribek, H.; Kis, L.L.; Gloghini, A.; Markasz, L.; Flaberg, E.; Eksborg, S.; Konya, J.; Gergely, L.; Carbone, A.; et al. Drug sensitivity patterns of HHV8 carrying body cavity lymphoma cell lines. BMC Cancer 2011, 11, 441. [Google Scholar] [CrossRef] [PubMed]
- Wei, F.; Gan, J.; Wang, C.; Zhu, C.; Cai, Q. Cell cycle regulatory functions of the KSHV oncoprotein LANA. Front. Microbiol. 2016, 7, 334. [Google Scholar] [CrossRef]
- Lee, H.R.; Lee, S.; Chaudhary, P.M.; Gill, P.; Jung, J.U. Immune evasion by Kaposi’s sarcoma-associated herpesvirus. Future Microbiol. 2010, 5, 1349–1365. [Google Scholar] [CrossRef]
- Dai, L.; Bai, L.; Lu, Y.; Xu, Z.; Reiss, K.; Del Valle, L.; Kaleeba, J.; Toole, B.P.; Parsons, C.; Qin, Z. Emmprin and KSHV: New partners in viral cancer pathogenesis. Cancer Lett. 2013, 337, 161–166. [Google Scholar] [CrossRef]
- Dai, L.; Bratoeva, M.; Toole, B.P.; Qin, Z.Q.; Parsons, C. KSHV activation of VEGF secretion and invasion for endothelial cells is mediated through viral upregulation of emmprin-induced signal transduction. Int. J. Cancer 2012, 131, 834–843. [Google Scholar] [CrossRef]
- Pelicano, H.; Martin, D.S.; Xu, R.H.; Huang, P. Glycolysis inhibition for anticancer treatment. Oncogene 2006, 25, 4633–4646. [Google Scholar] [CrossRef]
- Gatenby, R.A.; Gillies, R.J. Why do cancers have high aerobic glycolysis? Nat. Rev. Cancer 2004, 4, 891–899. [Google Scholar] [CrossRef]
- Bhatt, A.P.; Jacobs, S.R.; Freemerman, A.J.; Makowski, L.; Rathmell, J.C.; Dittmer, D.P.; Damania, B. Dysregulation of fatty acid synthesis and glycolysis in non-Hodgkin lymphoma. Proc. Natl. Acad. Sci. USA 2012, 109, 11818–11823. [Google Scholar] [CrossRef]
- Delgado, T.; Carroll, P.A.; Punjabi, A.S.; Margineantu, D.; Hockenbery, D.M.; Lagunoff, M. Induction of the Warburg effect by Kaposi’s sarcoma herpesvirus is required for the maintenance of latently infected endothelial cells. Proc. Natl. Acad Sci. USA 2010, 107, 10696–10701. [Google Scholar] [CrossRef] [PubMed]
- Rivas, C.; Thlick, A.E.; Parravicini, C.; Moore, P.S.; Chang, Y. Kaposi’s sarcoma-associated herpesvirus LANA2 is a B-cell-specific latent viral protein that inhibits p53. J. Virol. 2001, 75, 429–438. [Google Scholar] [CrossRef] [PubMed]
- Esteban, M.; García, M.A.; Domingo-Gil, E.; Arroyo, J.; Nombela, C.; Rivas, C. The latency protein LANA2 from Kaposi’s sarcoma-associated herpesvirus inhibits apoptosis induced by dsRNA-activated protein kinase but not RNase L activation. J. Gen. Virol. 2003, 84, 1463–1470. [Google Scholar] [CrossRef] [PubMed]
- Lubyova, B.; Pitha, P.M. Characterization of a novel human herpesvirus 8-encoded protein, vIRF-3, that shows homology to viral and cellular interferon regulatory factors. J. Virol. 2000, 74, 8194–8201. [Google Scholar] [CrossRef]
- Marcos-Villar, L.; Gallego, P.; Munoz-Fontela, C.; de la Cruz-Herrera, C.F.; Campagna, M.; Gonzalez, D.; Lopitz-Otsoa, F.; Rodriguez, M.S.; Rivas, C. Kaposi’s sarcoma-associated herpesvirus lana2 protein interacts with the pocket proteins and inhibits their sumoylation. Oncogene 2014, 33, 495–503. [Google Scholar] [CrossRef]
- Marcos-Villar, L.; Lopitz-Otsoa, F.; Gallego, P.; Munoz-Fontela, C.; Gonzalez-Santamaria, J.; Campagna, M.; Shou-Jiang, G.; Rodriguez, M.S.; Rivas, C. Kaposi’s sarcoma-associated herpesvirus protein LANA2 disrupts PML oncogenic domains and inhibits PML-mediated transcriptional repression of the survivin gene. J. Virol. 2009, 83, 8849–8858. [Google Scholar] [CrossRef]
- Gonnella, R.; Santarelli, R.; Farina, A.; Granato, M.; D’Orazi, G.; Faggioni, A.; Cirone, M. Kaposi sarcoma associated herpesvirus (KSHV) induces AKT hyperphosphorylation, bortezomib-resistance and GLUT-1 plasma membrane exposure in THP-1 monocytic cell line. J. Exp. Clin. Cancer Res. 2013, 32, 79. [Google Scholar] [CrossRef][Green Version]
- Lee, S.; Jang, J.; Jeon, H.; Lee, J.; Yoo, S.M.; Park, J.; Lee, M.S. Latent Kaposi’s sarcoma-associated herpesvirus infection in bladder cancer cells promotes drug resistance by reducing reactive oxygen species. J. Microbiol. 2016, 54, 782–788. [Google Scholar] [CrossRef]
- Masuda, Y.; Noguchi, K.; Segawa, H.; Tanaka, N.; Katayama, K.; Mitsuhashi, J.; Sugimoto, Y. Novel regulatory role for Kaposi’s sarcoma-associated herpesvirus-encoded vFLIP in chemosensitization to bleomycin. Biochem. Biophys. Res. Commun. 2011, 415, 305–312. [Google Scholar] [CrossRef]
- Leao, M.; Anderton, E.; Wade, M.; Meekings, K.; Aday, M.J. Epstein-Barr virus-induced resistance to drugs that activate the mitotic spindle assembly checkpoint in Burkitt’s lymphoma cells. J. Virol. 2007, 81, 248–260. [Google Scholar] [CrossRef]
- Seo, J.S.; Kim, T.G.; Hong, Y.S.; Chen, J.Y.; Lee, S.K. Contribution of Epstein-Barr virus infection to chemoresistance of gastric carcinoma cells to 5-fluorouracil. Arch. Pharm. Res. 2011, 34, 635–643. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.J.; Kim, D.N.; Lee, S.K. Association between Epstein-Barr virus infection and chemoresistance to docetaxel in gastric carcinoma. Mol. Cells 2011, 32, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, A.S.; Pal, A.D.; Banerjee, S. Epstein-Barr virus-encoded small non-coding RNAs induce cancer cell chemoresistance and migration. Virology 2013, 443, 294–305. [Google Scholar] [CrossRef] [PubMed]
- Dirmeier, U.; Neuhierl, B.; Kilger, E.; Reisbach, G.; Sandberg, M.L.; Hammerschmidt, W. Latent membrane protein 1 is critical for efficient growth transformation of human B cells by Epstein-Barr virus. Cancer Res. 2003, 63, 2982–2989. [Google Scholar] [PubMed]
- Saha, A.; Robertson, E.S. Mechanisms of B-Cell oncogenesis induced by Epstein-Barr virus. J. Virol. 2019, 93, e00238-19. [Google Scholar] [CrossRef]
- Zhang, B.; Kracker, S.; Yasuda, T.; Casola, S.; Vanneman, M.; Homig-Holzel, C.; Wang, Z.; Derudder, E.; Li, S.; Chakraborty, T.; et al. Immune surveillance and therapy of lymphomas driven by Epstein-Barr virus protein LMP1 in a mouse model. Cell 2012, 148, 739–751. [Google Scholar] [CrossRef]
- Lee, D.Y.; Sugden, B. The latent membrane protein 1 oncogene modifies B-cell physiology by regulating autophagy. Oncogene 2008, 27, 2833–2842. [Google Scholar] [CrossRef]
- Pratt, Z.L.; Zhang, J.Z.; Sugden, B. The latent membrane protein 1 (LMP1) oncogene of Epstein-Barr virus can simultaneously induce and inhibit apoptosis in B cells. J. Virol. 2012, 86, 4380–4393. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Kita, K.; Miwa, H.; Nishii, K.; Oka, K.; Ohno, T.; Shirakawa, S.; Fukumoto, M. Frequent expression of P-glycoprotein/MDR1 by nasal T-cell lymphoma cells. Cancer 1995, 76, 2351–2356. [Google Scholar] [CrossRef]
- Yoshimori, M.; Takada, H.; Imadome, K.I.; Kurata, M.; Yamamoto, K.; Koyama, T.; Shimizu, N.; Fujiwara, S.; Miura, O.; Arai, A. P-glycoprotein is expressed and causes resistance to chemotherapy in EBV-positive T-cell lymphoproliferative diseases. Cancer Med. 2015, 4, 1494–1504. [Google Scholar] [CrossRef]
- Wartenberg, M.; Hoffmann, E.; Schwindt, H.; Grunheck, F.; Petros, J.; Arnold, J.R.; Hescheler, J.; Sauer, H. Reactive oxygen species-linked regulation of the multidrug resistance transporter P-glycoprotein in Nox-1 overexpressing prostate tumor spheroids. FEBS Lett. 2005, 579, 4541–4549. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Nagy, N.; Masucci, M.G. The Epstein–Barr virus nuclear antigen-1 upregulates the cellular antioxidant defense to enable B-cell growth transformation and immortalization. Oncogene 2019, 1–14. [Google Scholar] [CrossRef]
- Nam, Y.S.; Kim, N.; Song, Y.; Lee, J.S.; Jeon, Y.W.; Cho, S.G.; Im, K.-I. Down-regulation of intracellular reactive oxygen species attenuates P-glycoprotein-associated chemoresistance in Epstein-Barr virus-positive NK/T-cell lymphoma. Am. J. Transl. Res. 2019, 11, 1359. [Google Scholar] [PubMed]
- Sun, J.; Hu, C.Y.; Zhu, Y.H.; Sun, R.; Fang, Y.J.; Fan, Y.H.; Xu, F. LMP1 increases expression of NADPH oxidase (NOX) and its regulatory subunit p22 in NP69 nasopharyngeal cells and makes them sensitive to a treatment by a NOX inhibitor. PLoS ONE 2015, 10, e0134896. [Google Scholar] [CrossRef] [PubMed]
- Yun, S.-M.; Kim, Y.S.; Hur, D.Y. LMP1 and 2A induce the expression of Nrf2 through Akt signaling pathway in Epstein-Barr virus-transformed B cells. Transl. Oncol. 2019, 12, 775–783. [Google Scholar] [CrossRef] [PubMed]
- Gahn, T.A.; Sugden, B. An EBNA-1-dependent enhancer acts from a distance of 10 kilobase pairs to increase expression of the Epstein-Barr virus LMP gene. J. Virol. 1995, 69, 2633–2636. [Google Scholar]
- Desbien, A.L.; Kappler, J.W.; Marrack, P. The Epstein-Barr virus Bcl-2 homolog, BHRF1, blocks apoptosis by binding to a limited amount of Bim. Proc. Natl. Acad. Sci. USA 2009, 106, 5663–5668. [Google Scholar] [CrossRef]
- Bouillet, P.; Metcalf, D.; Huang, D.C.; Tarlinton, D.M.; Kay, T.W.; Kontgen, F.; Adams, J.M.; Strasser, A. Proapoptotic Bcl-2 relative Bim required for certain apoptotic responses, leukocyte homeostasis, and to preclude autoimmunity. Science 1999, 286, 1735–1738. [Google Scholar] [CrossRef]
- Villunger, A.; Michalak, E.M.; Coultas, L.; Mullauer, F.; Bock, G.; Ausserlechner, M.J.; Adams, J.M.; Strasser, A. p53- and drug-induced apoptotic responses mediated by BH3-only proteins puma and noxa. Science 2003, 302, 1036–1038. [Google Scholar] [CrossRef]
- Mahieux, R.; Gessain, A. Adult T-cell leukemia/lymphoma and HTLV-1. Curr. Hematol. Malig. Rep. 2007, 2, 257–264. [Google Scholar] [CrossRef]
- Hermine, O.; Ramos, J.C.; Tobinai, K. A review of new findings in adult T-cell Leukemia-lymphoma: A focus on current and emerging treatment strategies. Adv. Ther. 2018, 35, 135–152. [Google Scholar] [CrossRef] [PubMed]
| Viruses | Transcripts | Drugs/Agents | Lymphomas | Mechanisms | Ref. |
|---|---|---|---|---|---|
| KSHV | LANA | Doxorubicin | PEL | Emmprin, LYVE-1, BCRP and hyaluronan signaling | [39,40] |
| Paclitaxel | PEL | Emmprin, LYVE-1, BCRP and hyaluronan signaling | [39] | ||
| LANA2 | Paclitaxel | PEL | Modulating microtubule dynamics to prevent microtubule stabilization induced by paclitaxel | [41] | |
| EBV | EBNA2 | Doxorubicin | DLBCL | Regulating CCL3 and CCL4-mediated activation of NF-κB and Btk signaling | [42] |
| LMP1 | Doxorubicin | T-cell lymphoma | Upregulating SNARK to increase expression of anti-apoptotic genes, such as BCL6 and BIRC2; Enhancing expression of Pim-1 and translocating Pim-1 to the cytoplasm | [43,44] | |
| Nutlin-3 | DLBCL/PTLD | Upregulating pro-autophagic proteins such as Beclin 1 and Sestrin 1 through LMP1-activated NF-κB signaling | [45] | ||
| Ionomycin | BL | Upregulating LIMD1 through LMP1-activated IRF4 and NF-κB signaling as well as autophagy | [46] | ||
| BHFR1 | Etoposide | BL | Interacting with the cellular pro-apoptotic BCL-2 proteins, BIM, BID, PUMA and BAK, to regulate apoptosis | [47] | |
| Ionomycin |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.; Kendrick, S.; Qin, Z. Mechanistic Insights into Chemoresistance Mediated by Oncogenic Viruses in Lymphomas. Viruses 2019, 11, 1161. https://doi.org/10.3390/v11121161
Chen J, Kendrick S, Qin Z. Mechanistic Insights into Chemoresistance Mediated by Oncogenic Viruses in Lymphomas. Viruses. 2019; 11(12):1161. https://doi.org/10.3390/v11121161
Chicago/Turabian StyleChen, Jungang, Samantha Kendrick, and Zhiqiang Qin. 2019. "Mechanistic Insights into Chemoresistance Mediated by Oncogenic Viruses in Lymphomas" Viruses 11, no. 12: 1161. https://doi.org/10.3390/v11121161
APA StyleChen, J., Kendrick, S., & Qin, Z. (2019). Mechanistic Insights into Chemoresistance Mediated by Oncogenic Viruses in Lymphomas. Viruses, 11(12), 1161. https://doi.org/10.3390/v11121161





