Co-Isolation and Characterization of Two Pandoraviruses and a Mimivirus from a Riverbank in Japan
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection and Virus Isolation
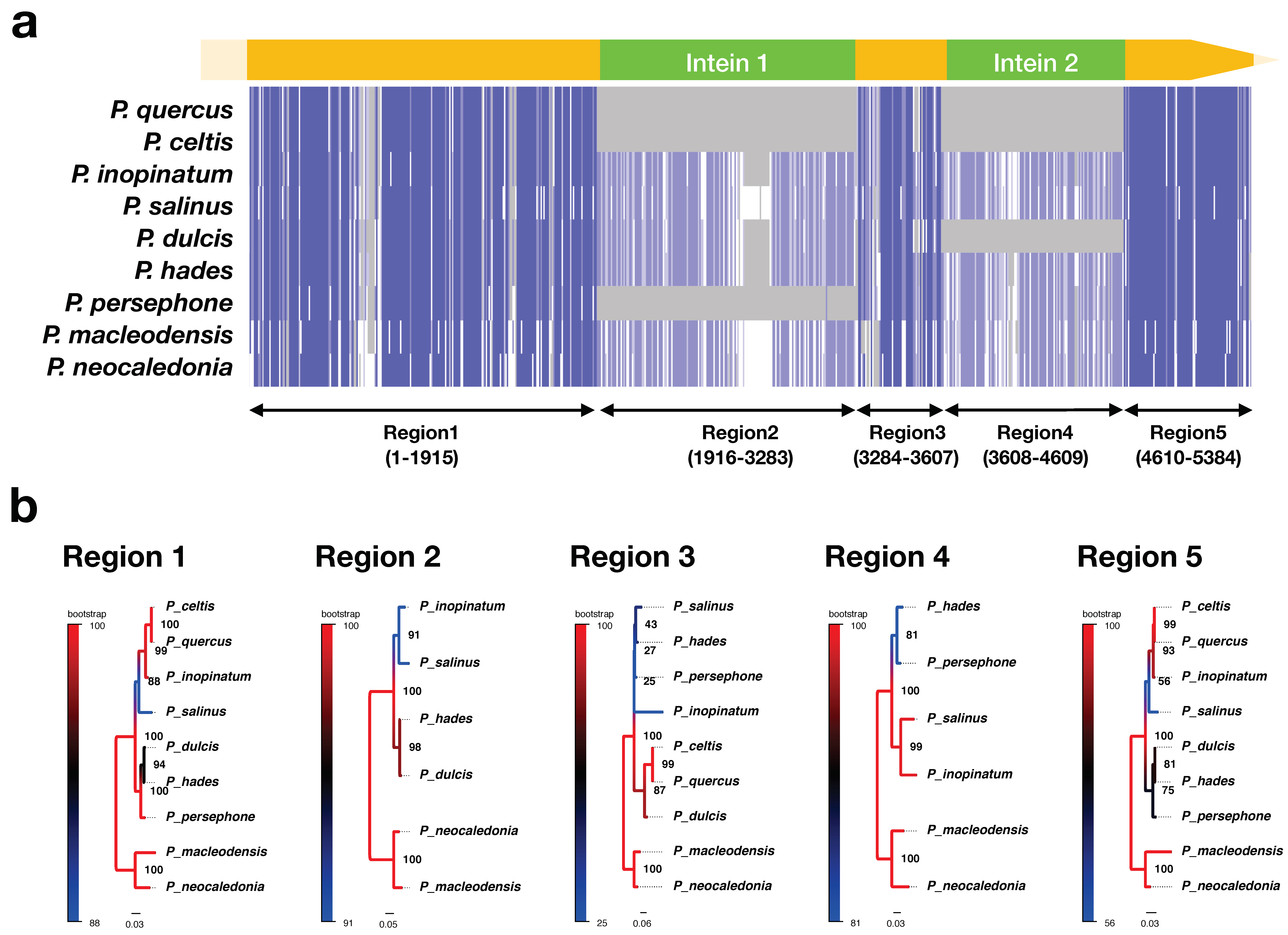
2.2. Sequencing Analysis of the Family B DNA Polymerase (polB)
2.3. Molecular Phylogenetic Analysis
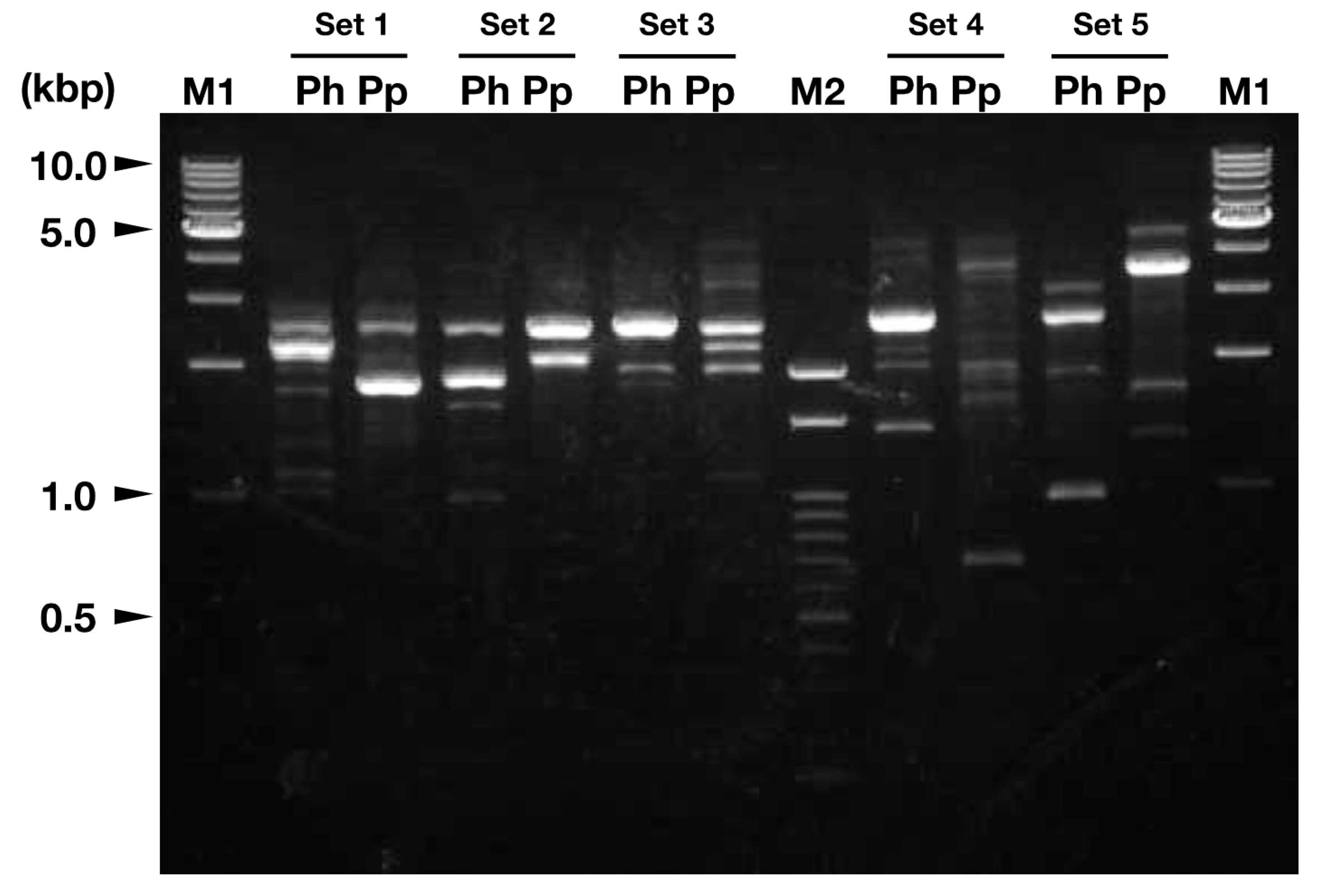
2.4. Random Amplified Polymorphic DNA (RAPD) Analysis
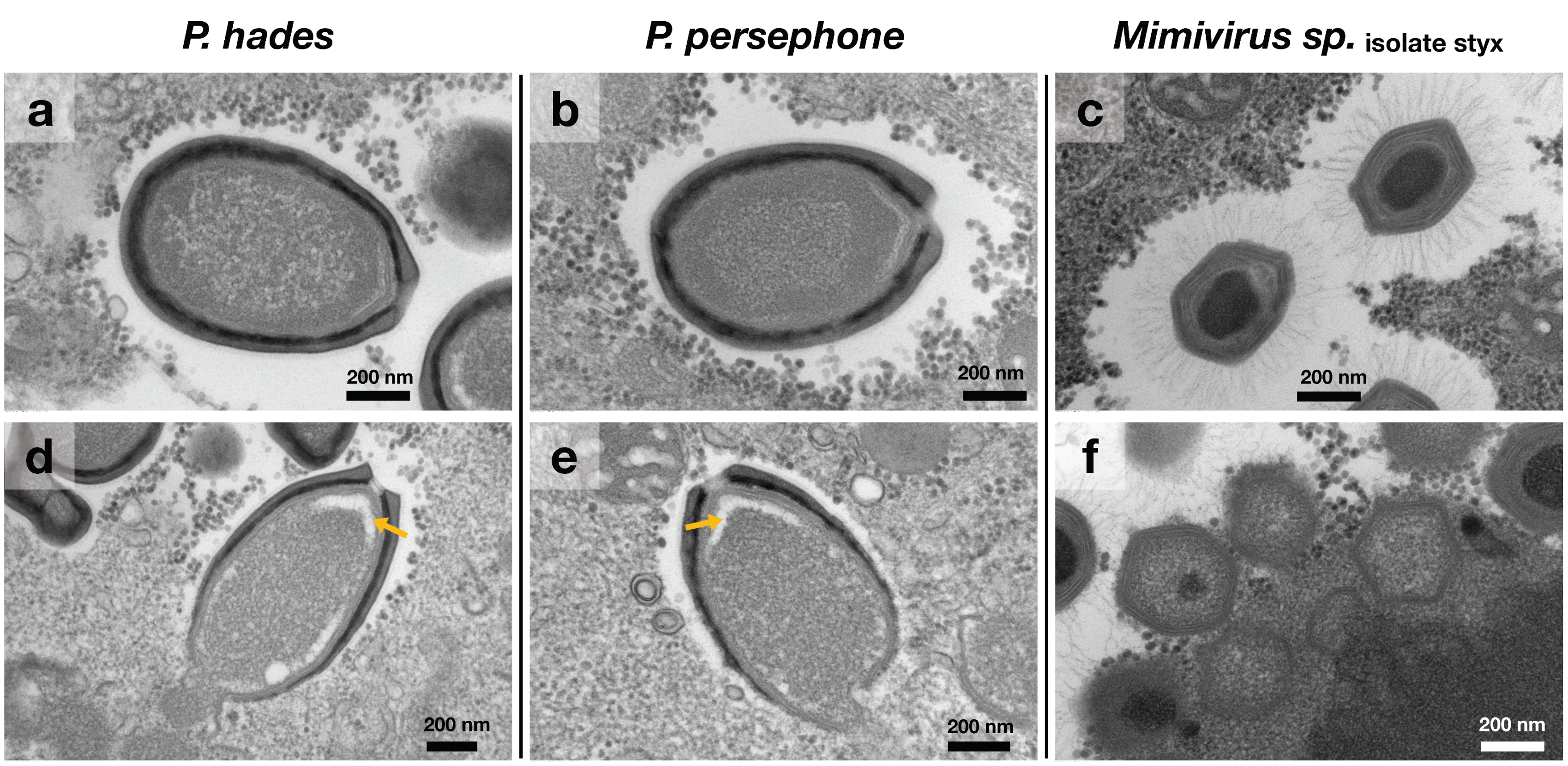
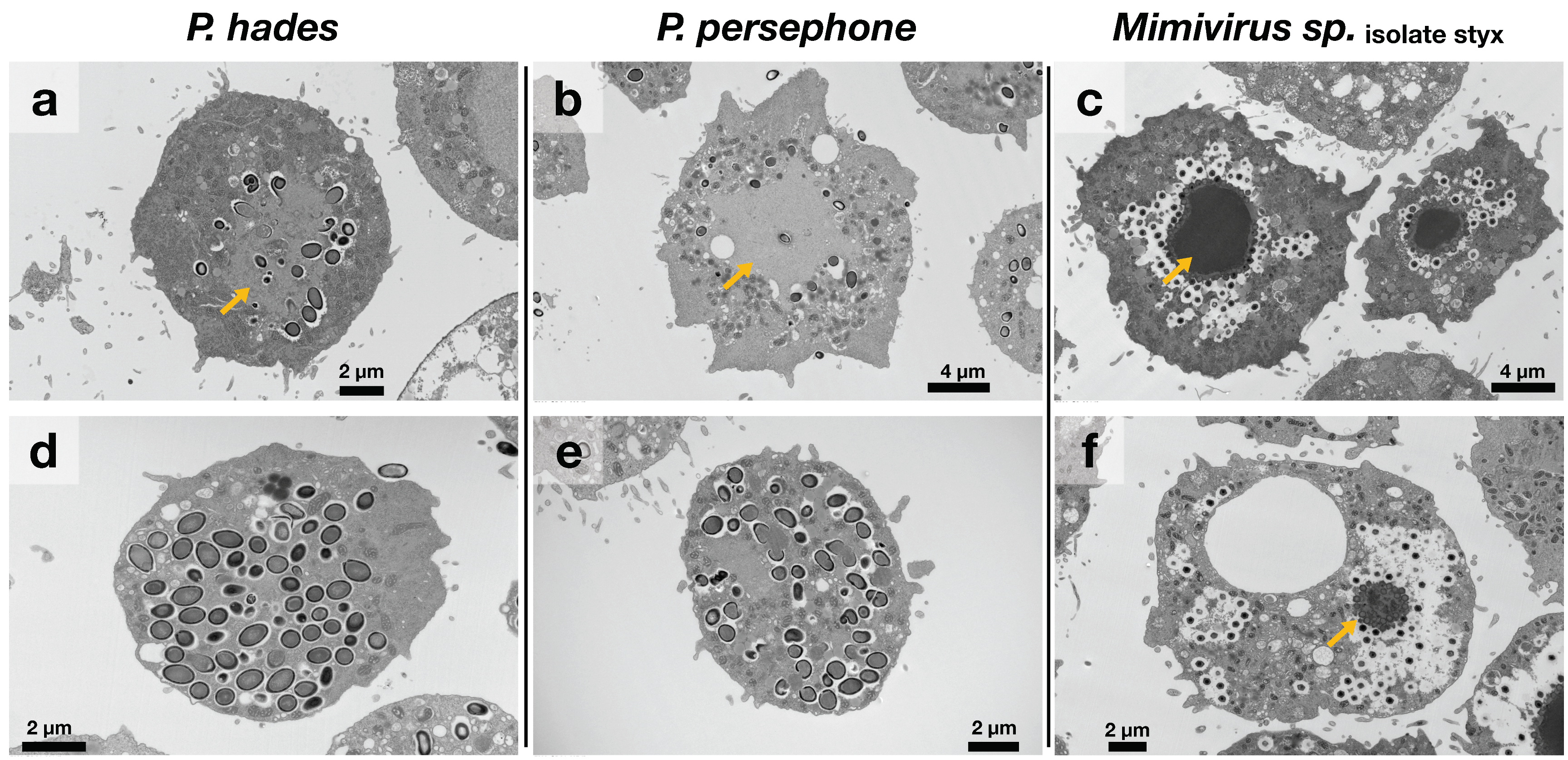
2.5. Transmission Electron Microscopic Observation
2.6. DNA Staining
2.7. Time-Lapse Analysis of Mimivirus-Infected Amoeba Cell
3. Results
3.1. Two Pandoraviruses and One Mimivirus Co-Isolated from a Riverbank Soil Sample
3.2. Sub-Classification of Isolated Viruses
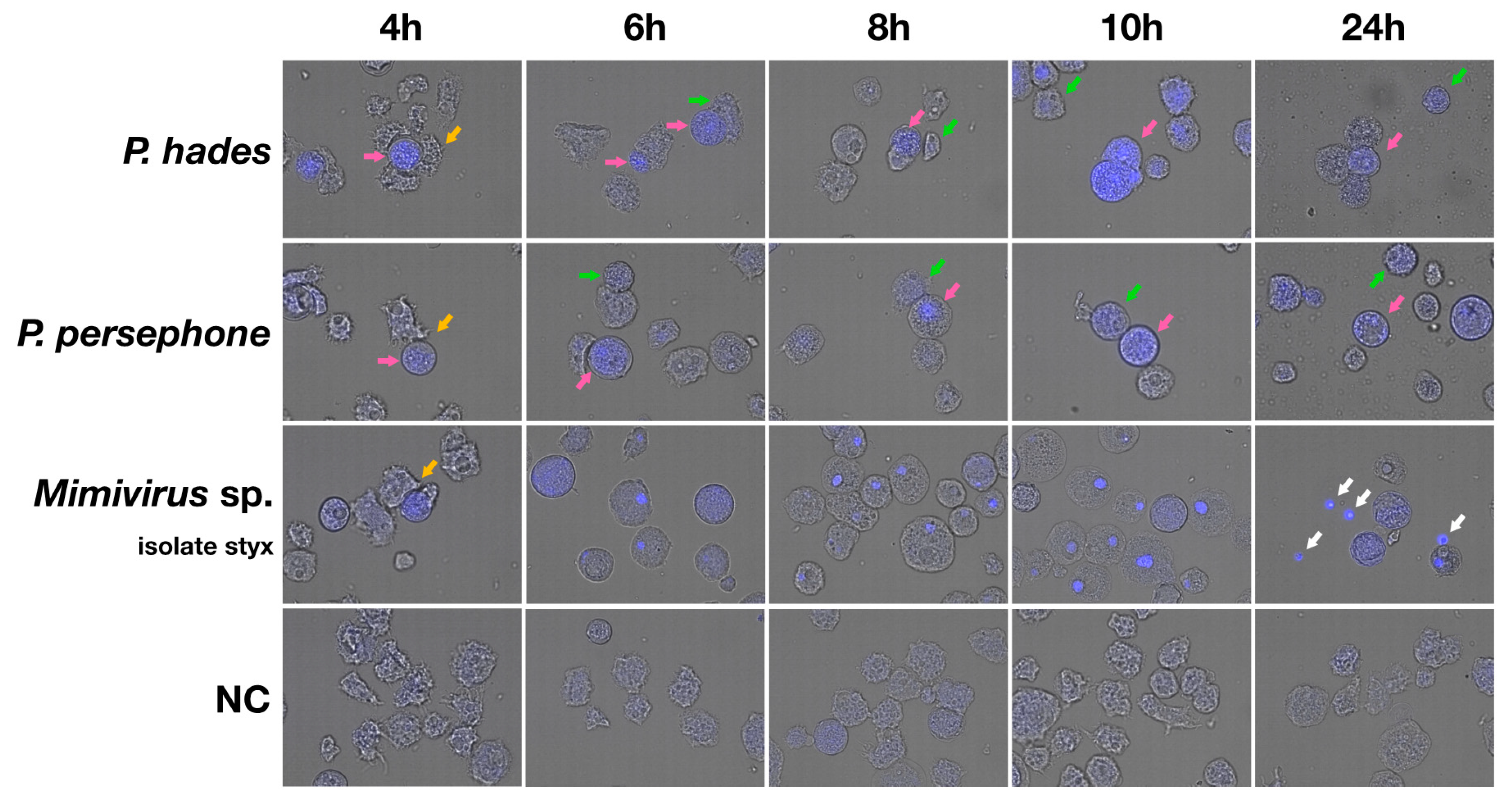
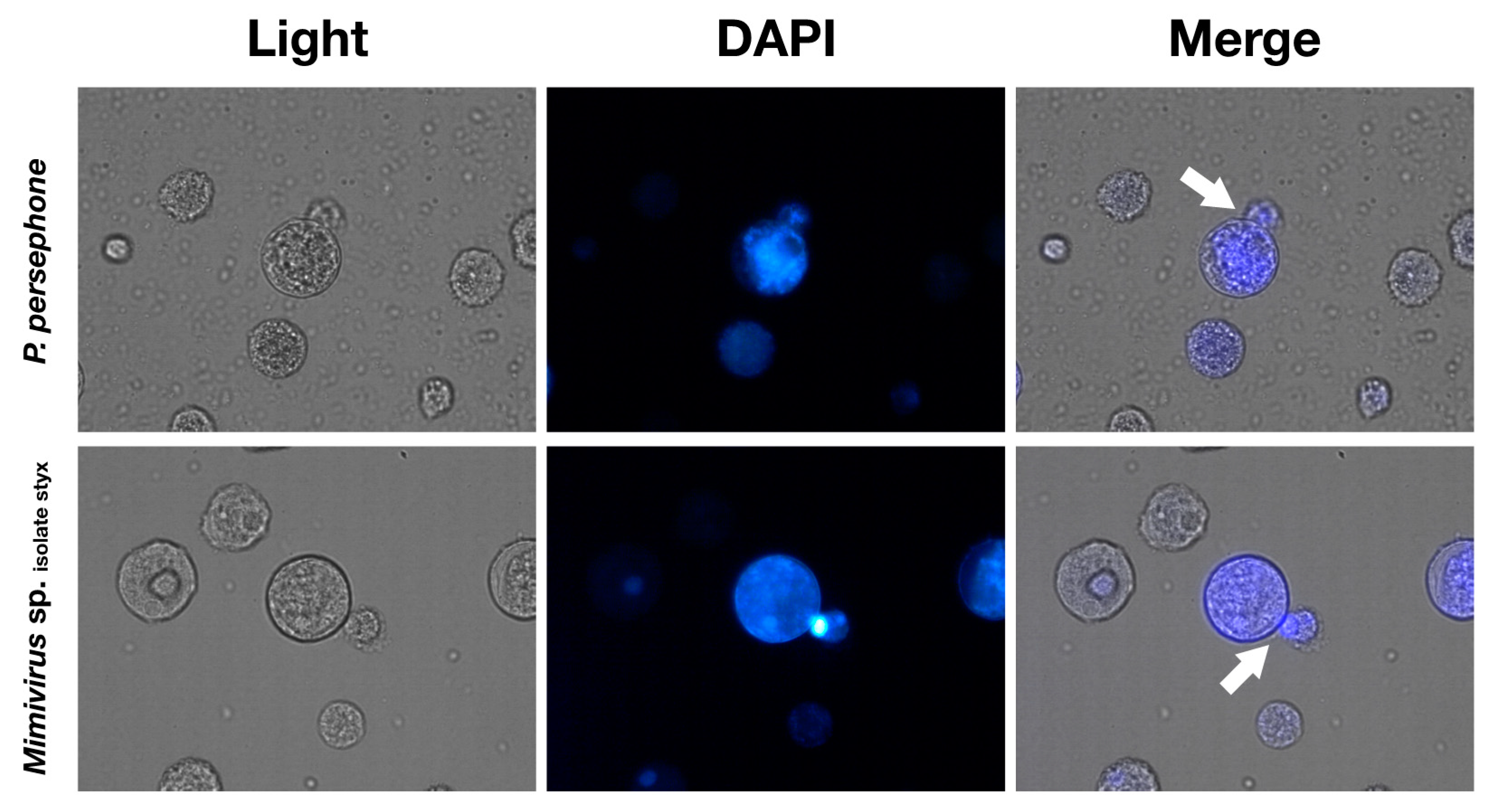
3.3. Isolated Pandoraviruses Proliferate Asynchronously in Amoeba Cells
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- La Scola, B.; Audic, S.; Robert, C.; Jungang, L.; De Lamballerie, X.; Drancourt, M.; Birtles, R.; Claverie, J.M.; Raoult, D. A giant virus in amoebae. Science 2003, 299, 2033. [Google Scholar] [CrossRef] [PubMed]
- Arslan, D.; Legendre, M.; Seltzer, V.; Abergel, C.; Claverie, J.M. Distant Mimivirus relative with a larger genome highlights the fundamental features of Megaviridae. Proc. Natl. Acad. Sci. USA 2011, 108, 17486–17491. [Google Scholar] [CrossRef] [PubMed]
- Boyer, M.; Yutin, N.; Pagnier, I.; Barrassi, L.; Fournous, G.; Espinosa, L.; Robert, C.; Azza, S.; Sun, S.; Rossmann, M.G.; et al. Giant Marseillevirus highlights the role of amoebae as a melting pot in emergence of chimeric microorganisms. Proc. Natl. Acad. Sci. USA 2009, 106, 21848–21853. [Google Scholar] [CrossRef] [PubMed]
- Takemura, M. Morphological and Taxonomic Properties of Tokyovirus, the First Marseilleviridae Member Isolated from Japan. Microbes Environ. 2016, 31, 442–448. [Google Scholar] [CrossRef] [PubMed]
- Legendre, M.; Bartoli, J.; Shmakova, L.; Jeudy, S.; Labadie, K.; Adrait, A.; Lescot, M.; Poirot, O.; Bertaux, L.; Bruley, C.; et al. Thirty-thousand-year-old distant relative of giant icosahedral DNA viruses with a pandoravirus morphology. Proc. Natl. Acad. Sci. USA 2014, 111, 4274–4279. [Google Scholar] [CrossRef] [PubMed]
- Abrahão, J.; Silva, L.; Silva, L.S.; Khalil, J.Y.B.; Rodrigues, R.; Arantes, T.; Assis, F.; Boratto, P.; Andrade, M.; Kroon, E.G.; et al. Tailed giant Tupanvirus possesses the most complete translational apparatus of the known virosphere. Nat. Commun. 2018, 9, 749. [Google Scholar] [CrossRef]
- Yoshikawa, G.; Blanc-Mathieu, R.; Song, C.; Kayama, Y.; Mochizuki, T.; Murata, K.; Ogata, H.; Takemura, M. Medusavirus, a novel large DNA virus discovered from hot spring water. J. Virol. 2019, 93, e02130-18. [Google Scholar] [CrossRef]
- Scheid, P.; Zöller, L.; Pressmar, S.; Richard, G.; Michel, R. An extraordinary endocytobiont in Acanthamoeba sp. isolated from a patient with keratitis. Parasitol. Res. 2008, 102, 945–950. [Google Scholar] [CrossRef]
- Scheid, P.; Hauröder, B.; Michel, R. Investigations of an extraordinary endocytobiont in Acanthamoeba sp.: Development and replication. Parasitol. Res. 2010, 106, 1371–1377. [Google Scholar] [CrossRef]
- Philippe, N.; Legendre, M.; Doutre, G.; Couté, Y.; Poirot, O.; Lescot, M.; Arslan, D.; Seltzer, V.; Bertaux, L.; Bruley, C.; et al. Pandoraviruses: Amoeba viruses with genomes up to 2.5 Mb reaching that of parasitic eukaryotes. Science 2013, 341, 281–286. [Google Scholar] [CrossRef]
- Scheid, P.; Balczun, C.; Schaub, G.A. Some secrets are revealed: Parasitic keratitis amoebae as vectors of the scarcely described pandoraviruses to humans. Parasitol. Res. 2014, 113, 3759–3764. [Google Scholar] [CrossRef] [PubMed]
- Antwerpen, M.H.; Georgi, E.; Zoeller, L.; Woelfel, R.; Stoecker, K.; Scheid, P. Whole-genome sequencing of a pandoravirus isolated from keratitis-inducing Acanthamoeba. Genome Announc. 2015, 3, e00136-15. [Google Scholar] [CrossRef] [PubMed]
- Scheid, P. A strange endocytobiont revealed as largest virus. Curr. Opin. Microbiol. 2016, 31, 58–62. [Google Scholar] [CrossRef]
- dos S. P. Andrade, A.C.; Arantes, T.S.; Rodrigues, R.A.L.; Machado, T.B.; Dornas, F.P.; Landell, M.F.; Furst, C.; Borges, L.G.A.; Dutra, L.A.L.; Almeida, G.; et al. Ubiquitous giants: A plethora of giant viruses found in Brazil and Antarctica. Virol. J. 2018, 15, 1–10. [Google Scholar]
- Andrade, A.C.D.S.P.; Victor de Miranda Boratto, P.; Rodrigues, R.A.L.; Bastos, T.M.; Azevedo, B.L.; Dornas, F.P.; Oliveira, D.B.; Drumond, B.P.; Kroon, E.G.; Abrahão, J.S. New Isolates of Pandoraviruses: Contribution to the Study of Replication Cycle Steps. J. Virol. 2019, 93, e01942-18. [Google Scholar] [CrossRef] [PubMed]
- Aherfi, S.; Andreani, J.; Baptiste, E.; Oumessoum, A.; Dornas, F.P.; Andrade, A.C.D.S.P.; Chabriere, E.; Abrahao, J.; Levasseur, A.; Raoult, D.; et al. A Large Open Pangenome and a Small Core Genome for Giant Pandoraviruses. Front. Microbiol. 2018, 9, 1486. [Google Scholar] [CrossRef] [PubMed]
- Legendre, M.; Fabre, E.; Poirot, O.; Jeudy, S.; Lartigue, A.; Alempic, J.M.; Beucher, L.; Philippe, N.; Bertaux, L.; Christo-Foroux, E.; et al. Diversity and evolution of the emerging Pandoraviridae family. Nat. Commun. 2018, 9, 2285. [Google Scholar] [CrossRef]
- Legendre, M.; Alempic, J.-M.; Philippe, N.; Lartigue, A.; Jeudy, S.; Poirot, O.; Ta, N.T.; Nin, S.; Couté, Y.; Abergel, C.; et al. Pandoravirus Celtis Illustrates the Microevolution Processes at Work in the Giant Pandoraviridae Genomes. Front. Microbiol. 2019, 10, 430. [Google Scholar] [CrossRef]
- Aoki, K.; Hagiwara, R.; Akashi, M.; Sasaki, K.; Murata, K.; Ogata, H.; Takemura, M. Fifteen Marseilleviruses Newly Isolated From Three Water Samples in Japan Reveal Local Diversity of Marseilleviridae. Front. Microbiol. 2019, 10, 1152. [Google Scholar] [CrossRef]
- Binder, M. TCID50 Calculator (v2.1-20-01-2017_MB). Available online: https://www.klinikum.uni-heidelberg.de/fileadmin/inst_hygiene/molekulare_virologie/Downloads/TCID50_calculator_v2_17-01-20_MB.xlsx (accessed on 21 May 2018).
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evolut. 2013, 30, 772–780. [Google Scholar] [CrossRef]
- Rice, P.; Longden, I.; Bleasby, A. EMBOSS: The European Molecular Biology Open Software Suite. Trends Genet. 2000, 16, 276–277. [Google Scholar] [CrossRef]
- Waterhouse, A.M.; Procter, J.B.; Martin, D.M.A.; Clamp, M.; Barton, G.J. Jalview Version 2—a multiple sequence alignment editor and analysis workbench. Bioinformatics 2009, 25, 1189–1191. [Google Scholar] [CrossRef] [PubMed]
- Tanabe, A.S. Phylogears (v2-2.0.2016.09.06). Available online: https://www.fifthdimension.jp/ (accessed on 22 May 2018).
- Stamatakis, A. RAxML version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 2014, 30, 1312–1313. [Google Scholar] [CrossRef] [PubMed]
- Ronquist, F.; Teslenko, M.; Van Der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef]
- Tanabe, A.S. Kakusan4 and Aminosan: Two programs for comparing nonpartitioned, proportional and separate models for combined molecular phylogenetic analyses of multilocus sequence data. Mol. Ecol. Resour. 2011, 11, 914–921. [Google Scholar] [CrossRef]
- Rambaut, A. Figtree Software (v.1.4.4). Available online: http://tree.bio.ed.ac.uk/software/figtree/ (accessed on 28 February 2019).
- Sievers, F.; Wilm, A.; Dineen, D.; Gibson, T.J.; Karplus, K.; Li, W.; Lopez, R.; McWilliam, H.; Remmert, M.; Söding, J.; et al. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol. Syst. Biol. 2011, 7, 539. [Google Scholar] [CrossRef]
- R Core Team. The R Project for Statistical Computing. Available online: https://www.r-project.org (accessed on 22 October 2018).
- Govarthanan, M.; Arunapriya, S.; Guruchandar, A.; Selvankumar, T.; Gnanasekaran, N.; Koildhasan, M. Genetic variability among Coleus sp. studied by RAPD banding pattern analysis. Nat. Preced. 2011, 2, 202–208. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Takemura, M.; Mikami, T.; Murono, S. Nearly Complete Genome Sequences of Two Mimivirus Strains Isolated from a Japanese Freshwater Pond and River Mouth. Genome Announc. 2016, 4, e01378-16. [Google Scholar] [CrossRef]
- Mutsafi, Y.; Fridmann-Sirkis, Y.; Milrot, E.; Hevroni, L.; Minsky, A. Infection cycles of large DNA viruses: Emerging themes and underlying questions. Virology 2014, 466–467, 3–14. [Google Scholar] [CrossRef]
- Sá, O.; Pereira, J.A.; Baptista, P. Optimization of DNA Extraction for RAPD and ISSR Analysis of Arbutus unedo L. Leaves. Int. J. Mol. Sci. 2011, 12, 4156–4164. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akashi, M.; Takemura, M. Co-Isolation and Characterization of Two Pandoraviruses and a Mimivirus from a Riverbank in Japan. Viruses 2019, 11, 1123. https://doi.org/10.3390/v11121123
Akashi M, Takemura M. Co-Isolation and Characterization of Two Pandoraviruses and a Mimivirus from a Riverbank in Japan. Viruses. 2019; 11(12):1123. https://doi.org/10.3390/v11121123
Chicago/Turabian StyleAkashi, Motohiro, and Masaharu Takemura. 2019. "Co-Isolation and Characterization of Two Pandoraviruses and a Mimivirus from a Riverbank in Japan" Viruses 11, no. 12: 1123. https://doi.org/10.3390/v11121123
APA StyleAkashi, M., & Takemura, M. (2019). Co-Isolation and Characterization of Two Pandoraviruses and a Mimivirus from a Riverbank in Japan. Viruses, 11(12), 1123. https://doi.org/10.3390/v11121123




