Novel Antiviral Activities of Obatoclax, Emetine, Niclosamide, Brequinar, and Homoharringtonine
Abstract
1. Introduction
2. Materials and Methods
2.1. Compounds
2.2. Cells
2.3. Viruses
2.4. Microscopy
2.5. Cell Viability and Toxicity Assays
2.6. Drug Combination Experiment
2.7. Virus Titration
3. Results
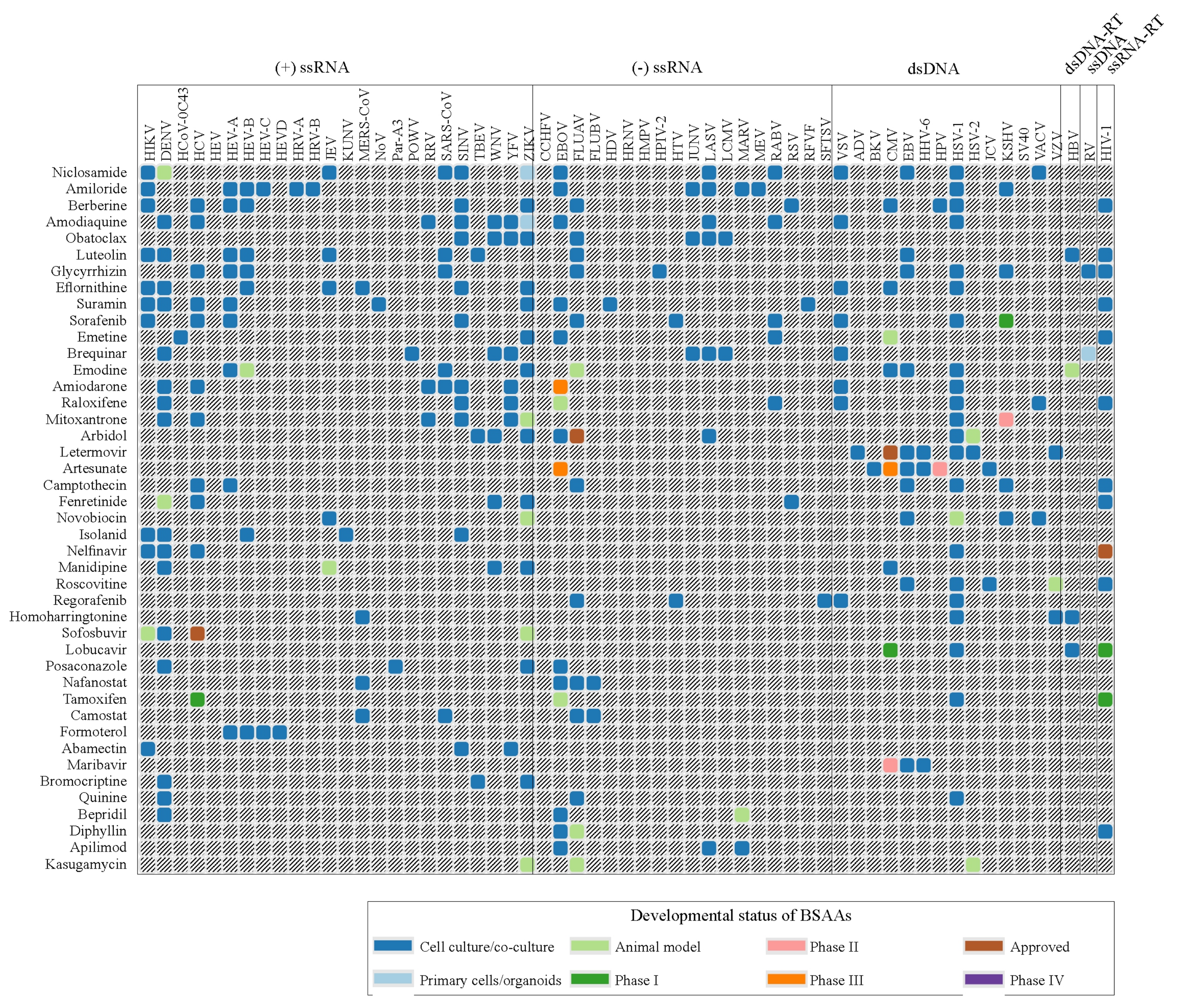
3.1. Forty-Three BSAAs Target 52 Viruses
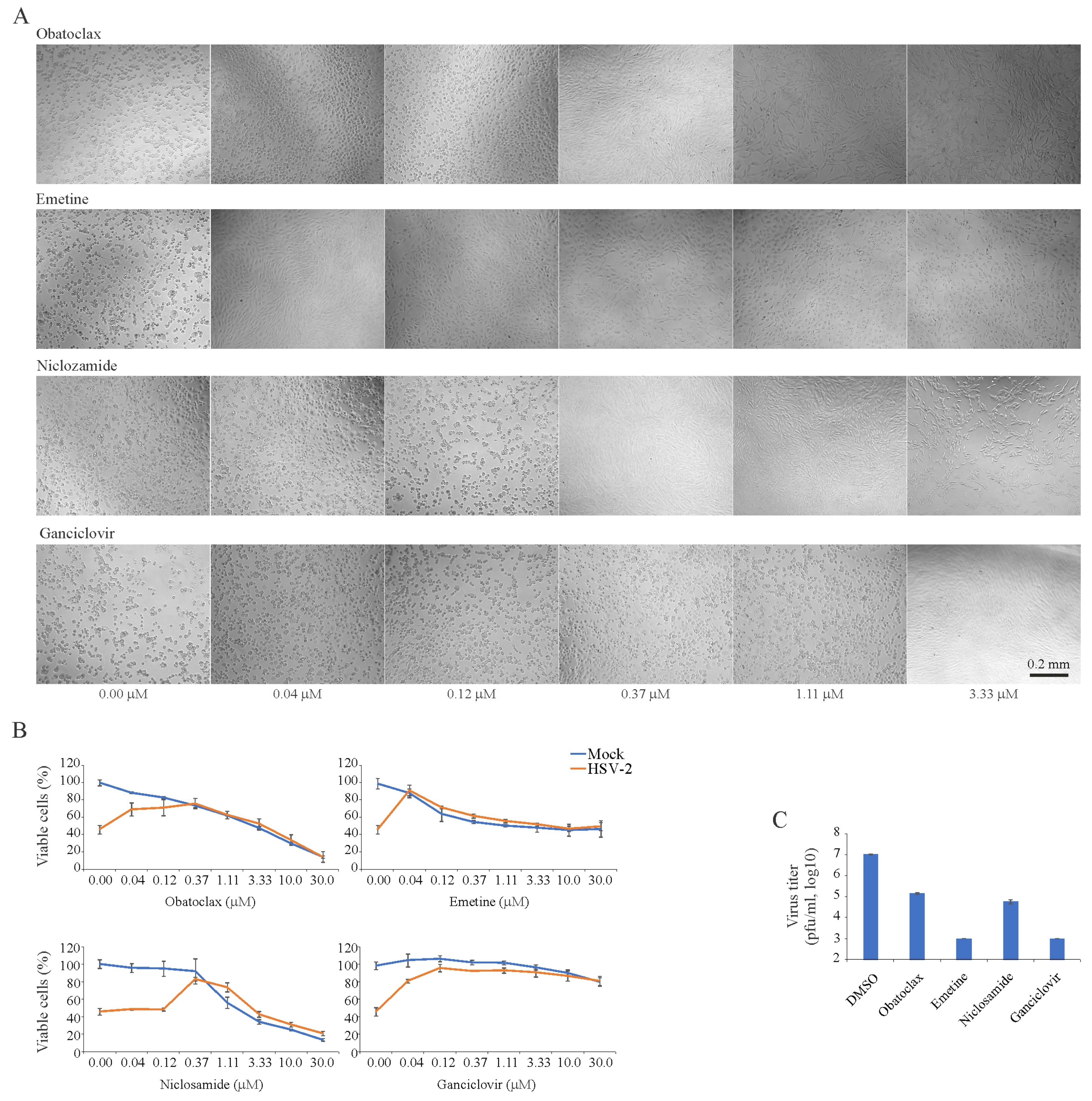
3.2. Obatoclax, Emetine, Niclosamide and Ganciclovir Inhibit HSV-2 Replication in RPE Cells
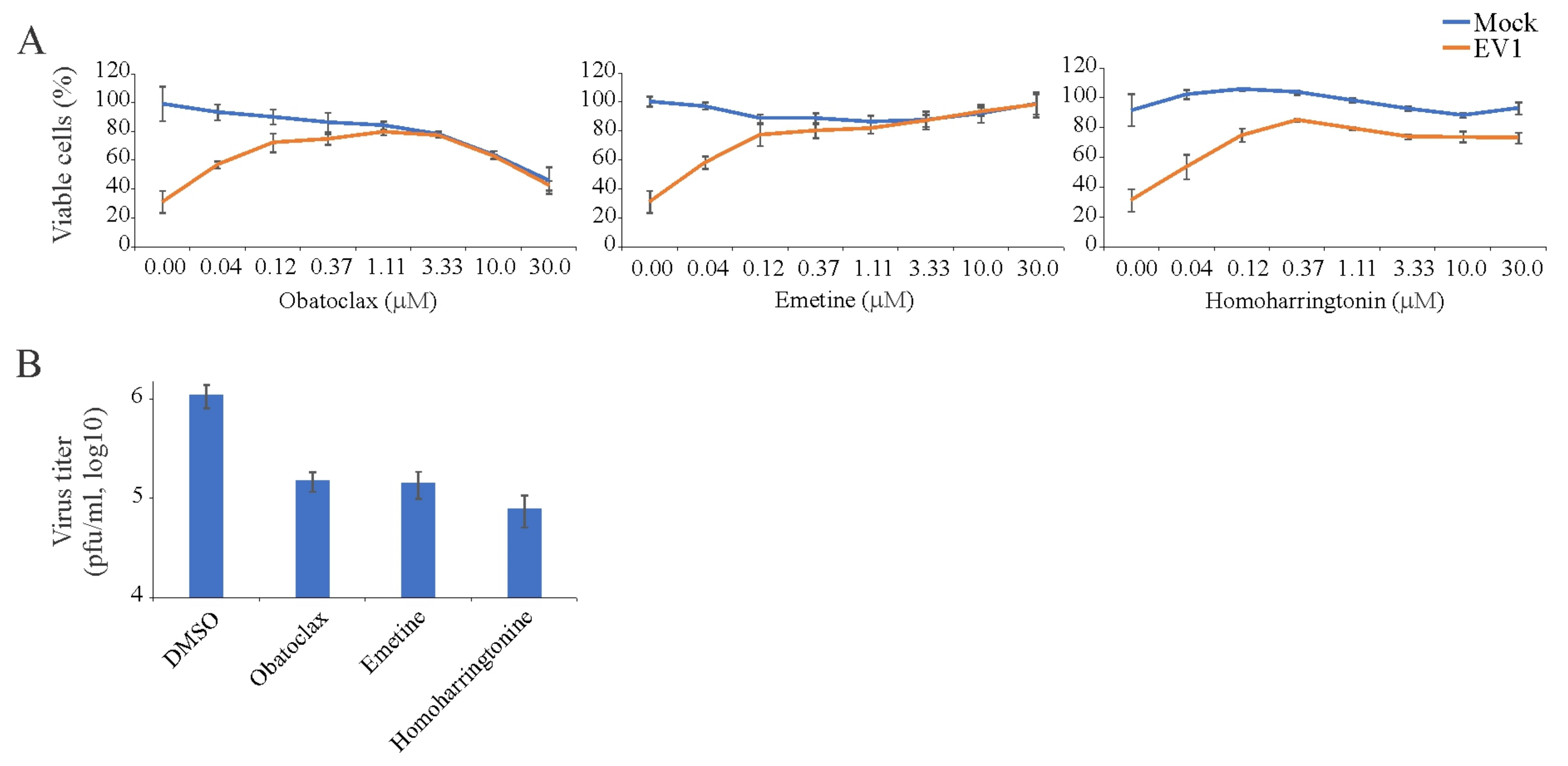
3.3. Obatoclax, Emetine, and Homoharringtonin Inhibit EV1 Replication in RPE Cells
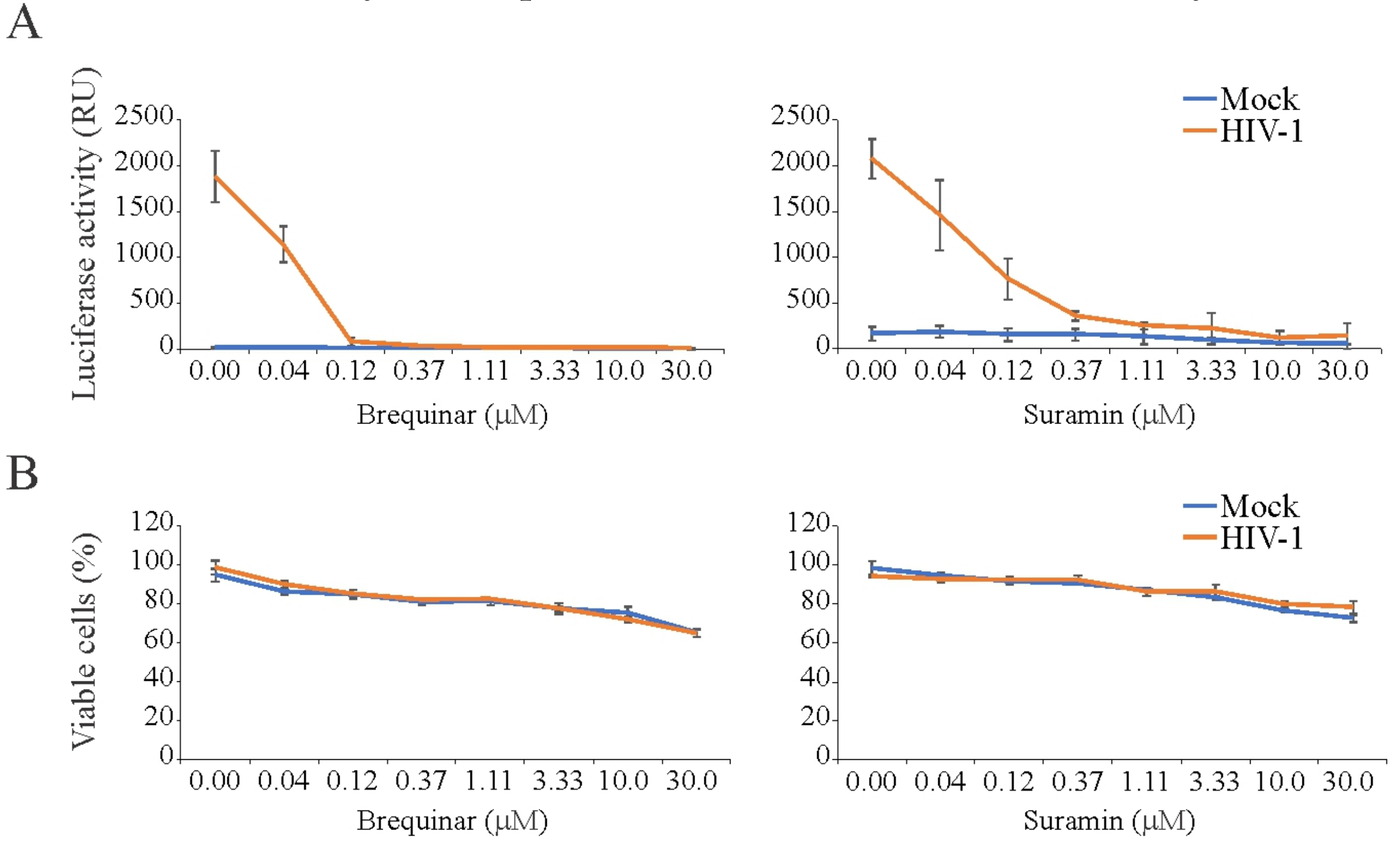
3.4. Brequinar and Suramin Inhibit HIV-1-Mediated Luciferase Expression in TZM-bl Cells
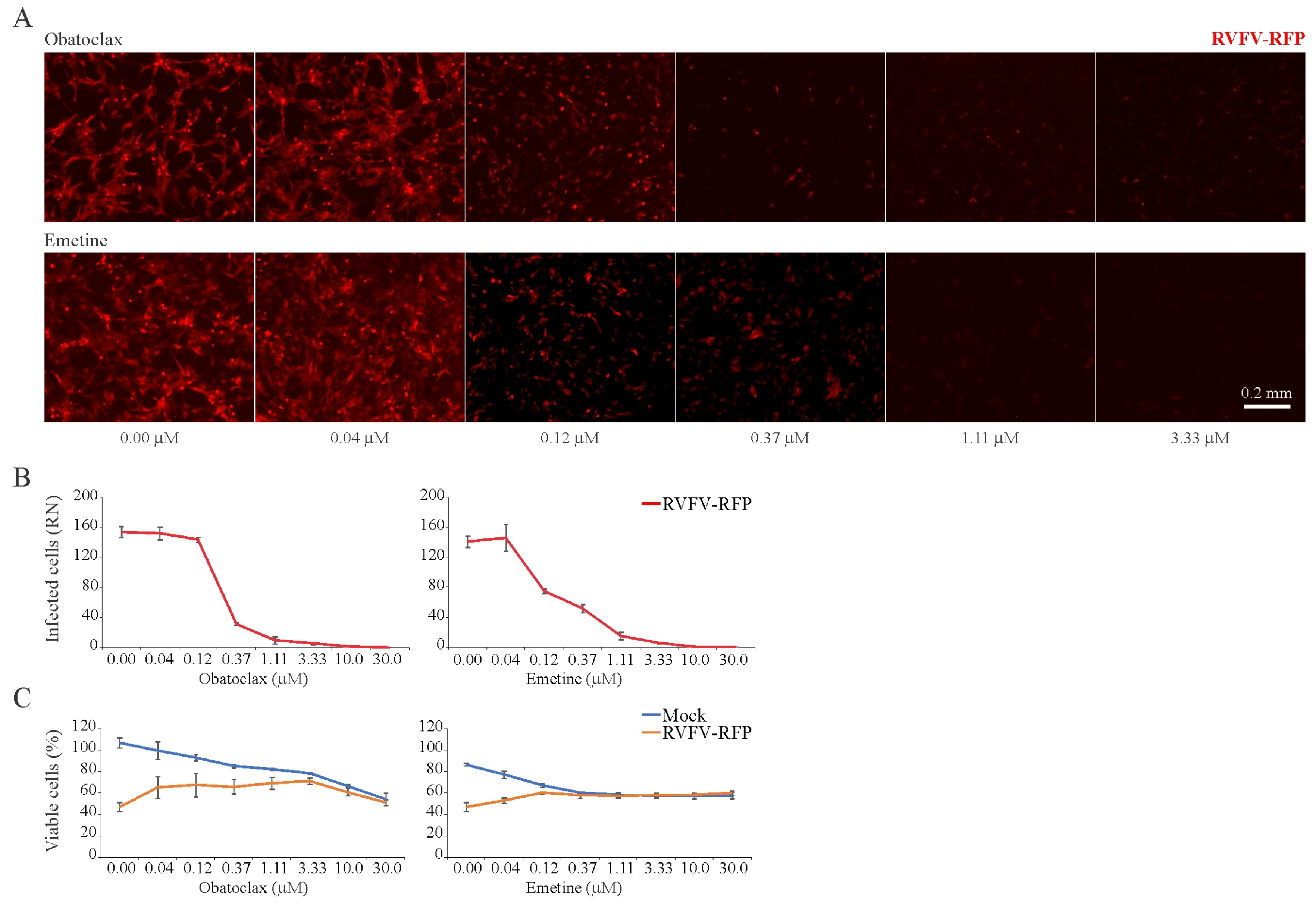
3.5. Obatoclax and Emetine Inhibit RVFV-Mediated RFP Expression in RPE Cells
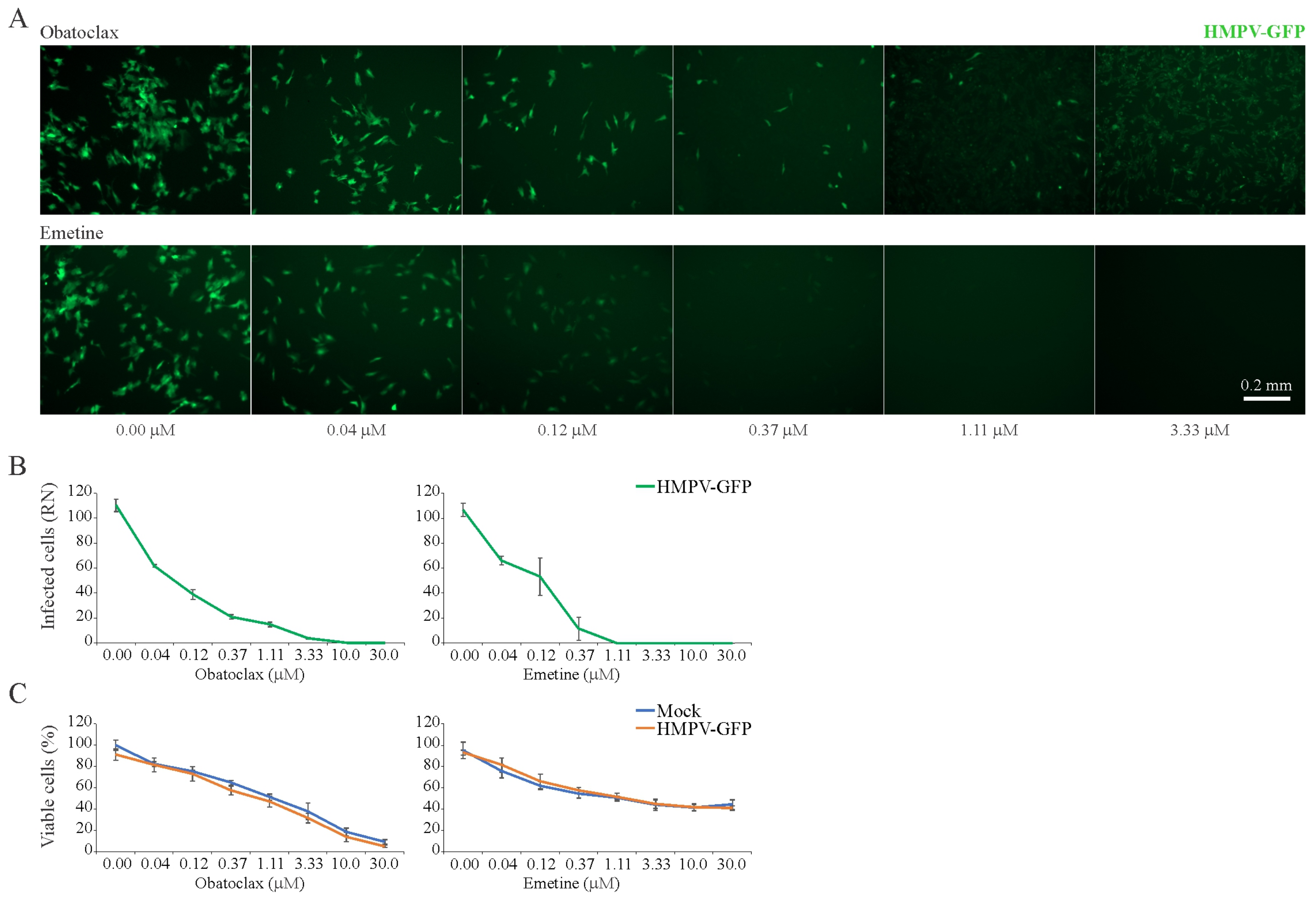
3.6. Obatoclax and Emetine Inhibit HMPV-Mediated GFP Expression in RPE Cells
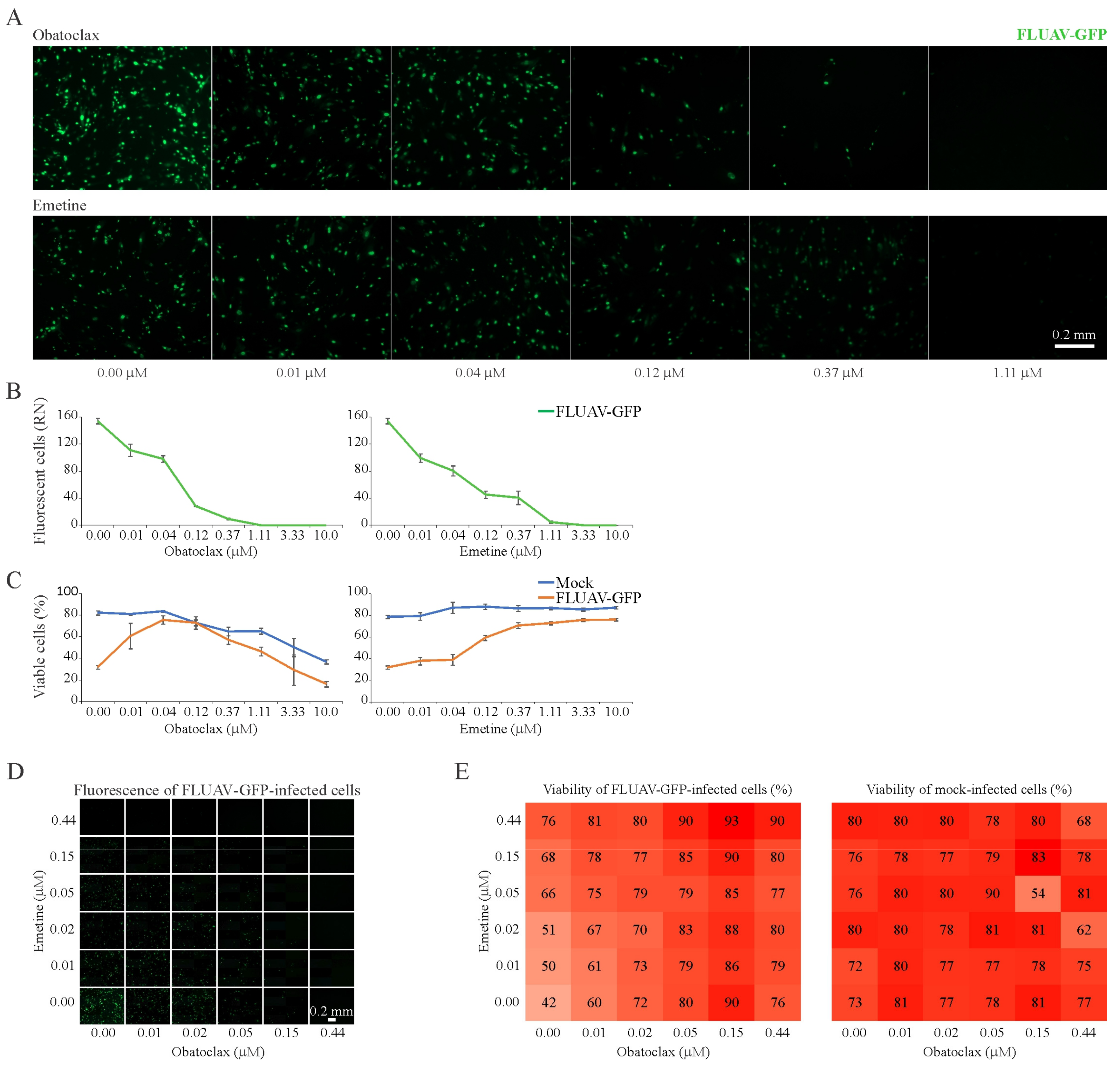
3.7. Obatoclax and Emetine Inhibit FLUAV-Mediated GFP Expression in RPE Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- WHO. WHO Publishes List of Top Emerging Diseases Likely to Cause Major Epidemics. 2015. Available online: wwwwhoint/medicines/ebola-treatment/WHO-list-of-top-emerging-diseases/en/ (accessed on 10 October 2019).
- Howard, C.R.; Fletcher, N.F. Emerging virus diseases: Can we ever expect the unexpected? Emerg. Microbes Infect. 2012, 1, e46–e49. [Google Scholar] [CrossRef] [PubMed]
- GBD 2017 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1789–1858. [Google Scholar] [CrossRef]
- GBD 2017 DALYs and HALE Collaborators. Global, regional, and national disability-adjusted life-years (DALYs) for 359 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1859–1922. [Google Scholar] [CrossRef]
- De Clercq, E.; Li, G. Approved Antiviral Drugs over the Past 50 Years. Clin. Microbiol. Rev. 2016, 29, 695–747. [Google Scholar] [CrossRef] [PubMed]
- Bekerman, E.; Einav, S. Infectious disease. Combating emerging viral threats. Science 2015, 348, 282–283. [Google Scholar] [CrossRef]
- Yuan, S.; Chu, H.; Chan, J.F.; Ye, Z.W.; Wen, L.; Yan, B.; Lai, P.M.; Tee, K.M.; Huang, J.; Chen, D.; et al. SREBP-dependent lipidomic reprogramming as a broad-spectrum antiviral target. Nat. Commun. 2019, 10, 120. [Google Scholar] [CrossRef]
- Hayden, E.C. Experimental drugs poised for use in Ebola outbreak. Nature 2018, 557, 475–476. [Google Scholar] [CrossRef]
- Jaishankar, D.; Yakoub, A.M.; Yadavalli, T.; Agelidis, A.; Thakkar, N.; Hadigal, S.; Ames, J.; Shukla, D. An off-target effect of BX795 blocks herpes simplex virus type 1 infection of the eye. Sci. Transl. Med. 2018, 10, eaan5861. [Google Scholar] [CrossRef]
- Schor, S.; Einav, S. Repurposing of Kinase Inhibitors as Broad-Spectrum Antiviral Drugs. DNA Cell Boil. 2018, 37, 63–69. [Google Scholar] [CrossRef]
- Debing, Y.; Neyts, J.; Delang, L. The future of antivirals: Broad-spectrum inhibitors. Curr. Opin. Infect. Dis. 2015, 28, 596–602. [Google Scholar] [CrossRef]
- Yu, F.; Lu, L.; Du, L.; Zhu, X.; Debnath, A.K.; Jiang, S. Approaches for Identification of HIV-1 Entry Inhibitors Targeting gp41 Pocket. Viruses 2013, 5, 127–149. [Google Scholar] [CrossRef] [PubMed]
- Pushpakom, S.; Iorio, F.; Eyers, P.A.; Escott, K.J.; Hopper, S.; Wells, A.; Doig, A.; Guilliams, T.; Latimer, J.; McNamee, C.; et al. Drug repurposing: Progress, challenges and recommendations. Nat. Rev. Drug Discov. 2019, 18, 41–58. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, Y.; Hara, H. Editorial: Drug Repositioning: Current Advances and Future Perspectives. Front. Pharmacol. 2018, 9, 1068. [Google Scholar] [CrossRef] [PubMed]
- Xue, H.; Li, J.; Xie, H.; Wang, Y. Review of Drug Repositioning Approaches and Resources. Int. J. Boil. Sci. 2018, 14, 1232–1244. [Google Scholar] [CrossRef] [PubMed]
- Ianevski, A.; Andersen, P.I.; Merits, A.; Bjørås, M.; Kainov, D. Expanding the activity spectrum of antiviral agents. Drug Discov. Today 2019, 24, 1224–1228. [Google Scholar] [CrossRef]
- Bosl, K.; Ianevski, A.; Than, T.T.; Andersen, P.I.; Kuivanen, S.; Teppor, M.; Zusinaite, E.; Dumpis, U.; Vitkauskiene, A.; Cox, R.J.; et al. Common Nodes of Virus—Host Interaction Revealed through an Integrated Network Analysis. Front. Immunol. 2019. accepted. [Google Scholar] [CrossRef]
- Ianevski, A.; Žusinaite, E.; Kuivanen, S.; Strand, M.; Lysvand, H.; Teppor, M.; Kakkola, L.; Paavilainen, H.; Laajala, M.; Kallio-Kokko, H.; et al. Novel activities of safe-in-human broad-spectrum antiviral agents. Antivir. Res. 2018, 154, 174–182. [Google Scholar] [CrossRef]
- Marjomäki, V.; Pietiäinen, V.; Matilainen, H.; Upla, P.; Ivaska, J.; Nissinen, L.; Reunanen, H.; Huttunen, P.; Hyypiä, T.; Heino, J. Internalization of Echovirus 1 in Caveolae. J. Virol. 2002, 76, 1856–1865. [Google Scholar] [CrossRef]
- Habjan, M.; Penski, N.; Spiegel, M.; Weber, F. T7 RNA polymerase-dependent and -independent systems for cDNA-based rescue of Rift Valley fever virus. J. Gen. Virol. 2008, 89, 2157–2166. [Google Scholar] [CrossRef]
- De Graaf, M.; Herfst, S.; Schrauwen, E.J.; Hoogen, B.G.V.D.; Osterhaus, A.D.; Fouchier, R.A. An improved plaque reduction virus neutralization assay for human metapneumovirus. J. Virol. Methods 2007, 143, 169–174. [Google Scholar] [CrossRef]
- Kittel, C.; Sereinig, S.; Ferko, B.; Stasakova, J.; Romanova, J.; Wolkerstorfer, A.; Katinger, H.; Egorov, A. Rescue of influenza virus expressing GFP from the NS1 reading frame. Virology 2004, 324, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Bulanova, D.; Ianevski, A.; Bugai, A.; Akimov, Y.; Kuivanen, S.; Paavilainen, H.; Kakkola, L.; Nandania, J.; Turunen, L.; Ohman, T.; et al. Antiviral Properties of Chemical Inhibitors of Cellular Anti-Apoptotic Bcl-2 Proteins. Viruses 2017, 9, 271. [Google Scholar] [CrossRef] [PubMed]
- Denisova, O.V.; Kakkola, L.; Feng, L.; Stenman, J.; Nagaraj, A.; Lampe, J.; Yadav, B.; Aittokallio, T.; Kaukinen, P.; Ahola, T.; et al. Obatoclax, Saliphenylhalamide, and Gemcitabine Inhibit Influenza A Virus Infection. J. Boil. Chem. 2012, 287, 35324–35332. [Google Scholar] [CrossRef] [PubMed]
- Kakkola, L.; Denisova, O.V.; Tynell, J.; Viiliäinen, J.; Ysenbaert, T.; Matos, R.C.; Nagaraj, A.; Öhman, T.; Kuivanen, S.; Paavilainen, H.; et al. Anticancer compound ABT-263 accelerates apoptosis in virus-infected cells and imbalances cytokine production and lowers survival rates of infected mice. Cell Death Dis. 2013, 4, e742. [Google Scholar] [CrossRef]
- Kuivanen, S.; Bespalov, M.M.; Nandania, J.; Ianevski, A.; Velagapudi, V.; De Brabander, J.K.; Kainov, D.E.; Vapalahti, O. Obatoclax, saliphenylhalamide and gemcitabine inhibit Zika virus infection in vitro and differentially affect cellular signaling, transcription and metabolism. Antivir. Res. 2017, 139, 117–128. [Google Scholar] [CrossRef]
- Müller, K.H.; Kainov, D.E.; El Bakkouri, K.; Saelens, X.; De Brabander, J.K.; Kittel, C.; Samm, E.; Muller, C.P. The proton translocation domain of cellular vacuolar ATPase provides a target for the treatment of influenza A virus infections. Br. J. Pharmacol. 2011, 164, 344–357. [Google Scholar] [CrossRef]
- Müller, K.H.; Spoden, G.A.; Scheffer, K.D.; Brunnhöfer, R.; De Brabander, J.K.; Maier, M.E.; Florin, L.; Muller, C.P. Inhibition by Cellular Vacuolar ATPase Impairs Human Papillomavirus Uncoating and Infection. Antimicrob. Agents Chemother. 2014, 58, 2905–2911. [Google Scholar] [CrossRef]
- Ianevski, A.; He, L.; Aittokallio, T.; Tang, J. SynergyFinder: A web application for analyzing drug combination dose-response matrix data. Bioinformatics 2017, 33, 2413–2415. [Google Scholar] [CrossRef]
- Tan, S.; Li, J.-Q.; Cheng, H.; Li, Z.; Lan, Y.; Zhang, T.-T.; Yang, Z.-C.; Li, W.; Qi, T.; Qiu, Y.-R.; et al. The anti-parasitic drug suramin potently inhibits formation of seminal amyloid fibrils and their interaction with HIV-1. J. Boil. Chem. 2019, 294, 13740–13754. [Google Scholar] [CrossRef]
- Field, A.K.; Davies, M.E.; DeWitt, C.; Perry, H.C.; Liou, R.; Germershausen, J.; Karkas, J.D.; Ashton, W.T.; Johnston, D.B.; Tolman, R.L. 9-([2-hydroxy-1-(hydroxymethyl)ethoxy]methyl)guanine: A selective inhibitor of herpes group virus replication. Proc. Natl. Acad. Sci. USA 1983, 80, 4139–4143. [Google Scholar] [CrossRef]
- Kim, Y.-J.; Cubitt, B.; Chen, E.; Hull, M.V.; Chatterjee, A.K.; Cai, Y.; Kuhn, J.H.; De La Torre, J.C. The ReFRAME library as a comprehensive drug repurposing library to identify mammarenavirus inhibitors. Antivir. Res. 2019, 169, 104558. [Google Scholar] [CrossRef] [PubMed]
- Varghese, F.S.; Rausalu, K.; Hakanen, M.; Saul, S.; Kümmerer, B.M.; Susi, P.; Merits, A.; Ahola, T. Obatoclax Inhibits Alphavirus Membrane Fusion by Neutralizing the Acidic Environment of Endocytic Compartments. Antimicrob. Agents Chemother. 2017, 61, e02227-16. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Niu, J.; Wang, C.; Huang, B.; Wang, W.; Zhu, N.; Deng, Y.; Wang, H.; Ye, F.; Cen, S.; et al. High-Throughput Screening and Identification of Potent Broad-Spectrum Inhibitors of Coronaviruses. J. Virol. 2019, 93. [Google Scholar] [CrossRef] [PubMed]
- MacGibeny, M.A.; Koyuncu, O.O.; Wirblich, C.; Schnell, M.J.; Enquist, L.W. Retrograde axonal transport of rabies virus is unaffected by interferon treatment but blocked by emetine locally in axons. PLoS Pathog. 2018, 14, e1007188. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Xu, M.; Lee, E.M.; Gorshkov, K.; Shiryaev, S.A.; He, S.; Sun, W.; Cheng, Y.-S.; Hu, X.; Tharappel, A.M.; et al. Emetine inhibits Zika and Ebola virus infections through two molecular mechanisms: Inhibiting viral replication and decreasing viral entry. Cell Discov. 2018, 4, 31. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, R.; Roy, S.; Venkatadri, R.; Su, Y.P.; Ye, W.; Barnaeva, E.; Mathews Griner, L.; Southall, N.; Hu, X.; Wang, A.Q.; et al. Efficacy and Mechanism of Action of Low Dose Emetine against Human Cytomegalovirus. PLoS Pathog. 2016, 12, e1005717. [Google Scholar] [CrossRef] [PubMed]
- Chaves Valadao, A.L.; Abreu, C.M.; Dias, J.Z.; Arantes, P.; Verli, H.; Tanuri, A.; de Aguiar, R.S. Natural Plant Alkaloid (Emetine) Inhibits HIV-1 Replication by Interfering with Reverse Transcriptase Activity. Molecules 2015, 20, 11474–11489. [Google Scholar] [CrossRef]
- Khandelwal, N.; Chander, Y.; Rawat, K.D.; Riyesh, T.; Nishanth, C.; Sharma, S.; Jindal, N.; Tripathi, B.N.; Barua, S.; Kumar, N. Emetine inhibits replication of RNA and DNA viruses without generating drug-resistant virus variants. Antivir. Res. 2017, 144, 196–204. [Google Scholar] [CrossRef]
- Mazzon, M.; Ortega-Prieto, A.M.; Imrie, D.; Luft, C.; Hess, L.; Czieso, S.; Grove, J.; Skelton, J.K.; Farleigh, L.; Bugert, J.J.; et al. Identification of Broad-Spectrum Antiviral Compounds by Targeting Viral Entry. Viruses 2019, 11, 176. [Google Scholar] [CrossRef]
- Hulseberg, C.E.; Feneant, L.; Szymanska-de Wijs, K.M.; Kessler, N.P.; Nelson, E.A.; Shoemaker, C.J.; Schmaljohn, C.S.; Polyak, S.J.; White, J.M. Arbidol and Other Low-Molecular-Weight Drugs That Inhibit Lassa and Ebola Viruses. J. Virol. 2019, 93. [Google Scholar] [CrossRef]
- Kao, J.-C.; Huangfu, W.-C.; Tsai, T.-T.; Ho, M.-R.; Jhan, M.-K.; Shen, T.-J.; Tseng, P.-C.; Wang, Y.-T.; Lin, C.-F. The antiparasitic drug niclosamide inhibits dengue virus infection by interfering with endosomal acidification independent of mTOR. PLoS Negl. Trop. Dis. 2018, 12, e0006715. [Google Scholar] [CrossRef] [PubMed]
- Cairns, D.M.; Boorgu, D.S.S.K.; Levin, M.; Kaplan, D.L. Niclosamide rescues microcephaly in a humanized in vivo model of Zika infection using human induced neural stem cells. Boil. Open 2018, 7, bio031807. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Yang, M.; Yuan, Y.; Li, X.; Kuang, E. Niclosamide inhibits lytic replication of Epstein-Barr virus by disrupting mTOR activation. Antivir. Res. 2017, 138, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-M.; Lu, J.-W.; Lin, C.-C.; Chin, Y.-F.; Wu, T.-Y.; Lin, L.-I.; Lai, Z.-Z.; Kuo, S.-C.; Ho, Y.-J. Antiviral activities of niclosamide and nitazoxanide against chikungunya virus entry and transmission. Antivir. Res. 2016, 135, 81–90. [Google Scholar] [CrossRef]
- Fang, J.; Sun, L.; Peng, G.; Xu, J.; Zhou, R.; Cao, S.; Chen, H.; Song, Y. Identification of Three Antiviral Inhibitors against Japanese Encephalitis Virus from Library of Pharmacologically Active Compounds 1280. PLoS ONE 2013, 8, e78425. [Google Scholar] [CrossRef]
- Jurgeit, A.; McDowell, R.; Moese, S.; Meldrum, E.; Schwendener, R.; Greber, U.F. Niclosamide Is a Proton Carrier and Targets Acidic Endosomes with Broad Antiviral Effects. PLoS Pathog. 2012, 8, e1002976. [Google Scholar] [CrossRef]
- Stachulski, A.V.; Pidathala, C.; Row, E.C.; Sharma, R.; Berry, N.G.; Lawrenson, A.S.; Moores, S.L.; Iqbal, M.; Bentley, J.; Allman, S.A.; et al. Thiazolides as novel antiviral agents. 2. Inhibition of hepatitis C virus replication. J. Med. Chem. 2011, 54, 8670–8680. [Google Scholar] [CrossRef]
- Wu, C.-J.; Jan, J.-T.; Chen, C.-M.; Hsieh, H.-P.; Hwang, D.-R.; Liu, H.-W.; Liu, C.-Y.; Huang, H.-W.; Chen, S.-C.; Hong, C.-F.; et al. Inhibition of Severe Acute Respiratory Syndrome Coronavirus Replication by Niclosamide. Antimicrob. Agents Chemother. 2004, 48, 2693–2696. [Google Scholar] [CrossRef]
- Kim, J.E.; Song, Y.J. Anti-varicella-zoster virus activity of cephalotaxine esters in vitro. J. Microbiol. 2019, 57, 74–79. [Google Scholar] [CrossRef]
- Dong, H.J.; Wang, Z.H.; Meng, W.; Li, C.C.; Hu, Y.X.; Zhou, L.; Wang, X.J. The Natural Compound Homoharringtonine Presents Broad Antiviral Activity in vitro and in vivo. Viruses 2018, 10, 601. [Google Scholar] [CrossRef]
- Cao, J.; Forrest, J.C.; Zhang, X. A screen of the NIH Clinical Collection small molecule library identifies potential anti-coronavirus drugs. Antivir. Res. 2015, 114, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Romero, M.R.; Serrano, M.A.; Efferth, T.; Álvarez, M.; Marin, J.J.G. Effect of Cantharidin, Cephalotaxine and Homoharringtonine on “in vitro” Models of Hepatitis B Virus (HBV) and Bovine Viral Diarrhoea Virus (BVDV) Replication. Planta Med. 2007, 73, 552–558. [Google Scholar] [CrossRef] [PubMed]
- Qing, M.; Zou, G.; Wang, Q.-Y.; Xu, H.Y.; Dong, H.; Yuan, Z.; Shi, P.-Y. Characterization of Dengue Virus Resistance to Brequinar in Cell Culture. Antimicrob. Agents Chemother. 2010, 54, 3686–3695. [Google Scholar] [CrossRef] [PubMed]
| Compound | Virus | Cell Line | Time (h) | CC50 (mM) | EC50-1 (mM) | EC50-2 (mM) | SI |
|---|---|---|---|---|---|---|---|
| Obatoclax | HSV-2 | RPE | 72 | 1.23 ± 0.02 | 0.10 ± 0.02 | 12 | |
| Emetine | HSV-2 | RPE | 72 | 1.12 ± 0.07 | 0.03 ± 0.01 | 37 | |
| Niclosamide | HSV-2 | RPE | 72 | 1.31 ± 0.02 | 0.43 ± 0.04 | 3 | |
| Ganciclovir | HSV-2 | RPE | 72 | >30 | 0.04±0.01 | >750 | |
| Obatoclax | EV1 | RPE | 48 | 3.21 ± 0.04 | 0.12 ± 0.01 | 25 | |
| Emetine | EV1 | RPE | 48 | >30 | 0.12 ± 0.04 | >300 | |
| Homoharringtonine | EV1 | RPE | 48 | >30 | 0.14 ± 0.03 | >300 | |
| Brequinar | HIV-1 | TZM-bl | 24 | >30 | 0.04 ± 0.01 | >750 | |
| Suramin | HIV-1 | TZM-bl | 24 | >30 | 0.08 ± 0.03 | >375 | |
| Obatoclax | RVFV | RPE | 24 | >30 | 0.04 ± 0.01 | 0.32 ± 0.09 | >100 |
| Emetine | RVFV | RPE | 24 | >30 | 0.10 ± 0.02 | 0.43 ± 0.10 | >75 |
| Obatoclax | HMPV | RPE | 96 | 0.60 ± 0.04 | 0.12 ± 0.02 | 6 | |
| Emetine | HMPV | RPE | 96 | 1 | 0.14 ± 0.05 | 10 | |
| Obatoclax | FLUAV | RPE | 24 | 3.11 ± 0.09 | 0.04 ± 0.01 | 0.10 ± 0.01 | 31 |
| Emetine | FLUAV | RPE | 24 | >30 | 0.13 ± 0.05 | 0.12 ± 0.03 | >300 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andersen, P.I.; Krpina, K.; Ianevski, A.; Shtaida, N.; Jo, E.; Yang, J.; Koit, S.; Tenson, T.; Hukkanen, V.; Anthonsen, M.W.; et al. Novel Antiviral Activities of Obatoclax, Emetine, Niclosamide, Brequinar, and Homoharringtonine. Viruses 2019, 11, 964. https://doi.org/10.3390/v11100964
Andersen PI, Krpina K, Ianevski A, Shtaida N, Jo E, Yang J, Koit S, Tenson T, Hukkanen V, Anthonsen MW, et al. Novel Antiviral Activities of Obatoclax, Emetine, Niclosamide, Brequinar, and Homoharringtonine. Viruses. 2019; 11(10):964. https://doi.org/10.3390/v11100964
Chicago/Turabian StyleAndersen, Petter I., Klara Krpina, Aleksandr Ianevski, Nastassia Shtaida, Eunji Jo, Jaewon Yang, Sandra Koit, Tanel Tenson, Veijo Hukkanen, Marit W. Anthonsen, and et al. 2019. "Novel Antiviral Activities of Obatoclax, Emetine, Niclosamide, Brequinar, and Homoharringtonine" Viruses 11, no. 10: 964. https://doi.org/10.3390/v11100964
APA StyleAndersen, P. I., Krpina, K., Ianevski, A., Shtaida, N., Jo, E., Yang, J., Koit, S., Tenson, T., Hukkanen, V., Anthonsen, M. W., Bjoras, M., Evander, M., Windisch, M. P., Zusinaite, E., & Kainov, D. E. (2019). Novel Antiviral Activities of Obatoclax, Emetine, Niclosamide, Brequinar, and Homoharringtonine. Viruses, 11(10), 964. https://doi.org/10.3390/v11100964







