Abstract
Bacteriophages, viruses that only kill specific bacteria, are receiving substantial attention as nontraditional antibacterial agents that may help alleviate the growing antibiotic resistance problem in medicine. We describe the design and preclinical development of AB-SA01, a fixed-composition bacteriophage product intended to treat Staphylococcus aureus infections. AB-SA01 contains three naturally occurring, obligately lytic myoviruses related to Staphylococcus phage K. AB-SA01 component phages have been sequenced and contain no identifiable bacterial virulence or antibiotic resistance genes. In vitro, AB-SA01 killed 94.5% of 401 clinical Staphylococcus aureus isolates, including methicillin-resistant and vancomycin-intermediate ones for a total of 95% of the 205 known multidrug-resistant isolates. The spontaneous frequency of resistance to AB-SA01 was ≤3 × 10−9, and resistance emerging to one component phage could be complemented by the activity of another component phage. In both neutropenic and immunocompetent mouse models of acute pneumonia, AB-SA01 reduced lung S. aureus populations equivalently to vancomycin. Overall, the inherent characteristics of AB-SA01 component phages meet regulatory and generally accepted criteria for human use, and the preclinical data presented here have supported production under good manufacturing practices and phase 1 clinical studies with AB-SA01.
1. Introduction
The use of bacteriophages (phages) as antibacterial drugs, frequently referred to as “phage therapy” has been discussed and deployed since these bacterial viruses were discovered in the early 1900s. Interest in phage therapy has waxed and waned in various parts of the world, heavily influenced by the availability, affordability, and efficacy of potent small-molecule antibiotics [1]. The current resurgence of interest is persisting in light of the growing urgency of the antimicrobial resistance (AMR) crisis, which predicts that AMR will be the leading cause of human death by 2050, causing 10 million global deaths per year [2].
Staphylococcus aureus, one of the ESKAPE pathogens (Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter spp.) [3], is a problem in both hospital-associated and community-associated infections [4,5]. It is a significant problem in many clinical settings and the antibiotic-resistant forms are classified as a “High Priority” pathogen by the World Health Organization (WHO) [6] and a “Serious Threat” by the U.S. Centers for Disease Control and Prevention (CDC) [7]. Since 1999, nine antibiotics targeting methicillin-resistant S. aureus (MRSA) have been approved (linezolid, daptomycin, tigecycline, ceftobiprole, telavancin, ceftaroline, dalbavancin, oritavancin, tedizolid) [8]. Of these, only the oxazolidinones, now nearly 20 years old, were a completely new class [9]. Clinical resistance has already been observed for all nine of these drugs, though it can be difficult to predict how quickly or widely some of these resistances will spread [10,11,12,13,14,15,16,17,18,19]. Side effects such as renal toxicity and cross-resistance (e.g. among glyo- and lipoglycopeptides) can limit clinical use [8]. Moreover, many antibiotics have reduced efficacy against Staphylococcus spp. when it grows in biofilms [20], as is often the case with device-associated infections or endocarditis. Thus, there remains an urgent need for anti-staphylococcal drugs, especially ones with fundamentally different mechanisms of action. Here, we describe the design and composition of AB-SA01, a highly characterized anti-Staphylococcus phage product that is being developed to treat acute and chronic S. aureus infections in humans, including those caused by MRSA.
Phage therapy has frequently been cited as a form of technology that could help address the AMR problem, provided that high-quality evidence can be gathered in controlled studies focused on testing product efficacy in well-defined clinical indications and administration parameters [7,21,22,23]. Phages are unable to infect mammalian cells and are usually specific for one or a few bacterial species or strains. Obligately lytic phages would comprise a self-replicating, self-limiting antimicrobial that can be administered by a variety of routes, and that functions via an entirely different mechanism of action compared to small-molecule antibiotics. Humans are continuously exposed to phages present in the environment and as part of the human microbiome, and there is no evidence of any direct toxicity resulting from intentionally administered phages as long as non-phage contaminants such as endotoxins are removed [24,25,26].
While there remains some debate about the optimal features of therapeutic phages, there is widespread agreement that the traits listed below are either required or particularly desirable for phage products and their individual components [27,28,29,30,31]. Individual phages should be:
- Obligately lytic, to avoid specialized transduction of bacterial genes, and maximize chances for bacterial killing;
- Not known, by empirical testing and/or inference from genomics, to be prone to generalized transduction; and,
- Fully sequenced, to avoid phages with known antibiotic resistance or bacterial virulence genes, and to help assess other lifestyle traits.
Collectively, phages used together to treat a patient should:
- Have broad activity against the target pathogen but not other species, to maximize potential utility and minimize off-target effects; and,
- Be capable of complementation, in which resistant mutants arising to one phage are sensitive to another phage.
In addition to characteristics of the phages themselves, material for clinical use should be produced in such a way as to give confidence that the final product retains these characteristics (i.e., are still the same phages) and does not contain potentially harmful (or harmful amounts) of impurities such as endotoxin or host cell proteins. AB-SA01 satisfies these criteria and has entered clinical development.
2. Methods
2.1. Bacteriophages, Source and Propagation
Each of the selected phages was isolated from an environmental source and subsequently paired to a well-characterized S. aureus strain that serves as its manufacturing host. Host-paired phages were purified to ensure that the resulting master stocks produced genetically and phenotypically consistent batches of each phage. Unless otherwise stated, all data is derived from the host-paired, plaque-purified phages. Phages were propagated in liquid culture using vegetable peptone media (VP0101, Oxoid, Hampshire, UK). Lysates were passed through a 0.2-µm filter to remove large cellular debris and, depending on the needs of subsequent testing, optionally subjected to a proprietary process of column-based purification steps to further remove host cell proteins and other bacterial debris and to replace growth medium with phosphate-buffered saline (PBS; Oxoid, Hampshire, UK) containing 10 mM magnesium sulfate (PBS+Mg).
2.2. Bacteria
AB-SA01 manufacturing hosts are S. aureus strains originally isolated from humans. The S. aureus diversity panel and the species-specificity panel were sourced from the American Type Culture Collection (Manassas, VA, USA), the Walter Reed Army Institute of Research Multidrug-resistant Organism Repository and Surveillance Network (“MRSN”, Silver Spring, MD, USA), and clinical sites in Australia and the United Kingdom. Global surveillance panels of S. aureus strains were obtained from JMI Laboratories (North Liberty, IA, USA). Targeted interest panels included chronic rhinosinusitis (CRS) strains from Belgium, and vancomycin intermediate (VISA) strains from the CDC and Food and Drug Administration (FDA) Antimicrobial Resistance Isolate Bank (Atlanta, GA, USA). The definition of multidrug resistant (MDR) strains is according to Magiorakos et al [32].
2.3. Phage Sensitivity Assays
Testing on the S. aureus panels used Heart Infusion Broth (BD, Franklin Lakes, NJ, USA), amended with 1.5% agar (Oxoid, Hampshire, UK) for plates or 0.7% agar for overlays. Phage activity was assessed using a modification of the small drop agar overlay method [33]. Briefly, 100 µL of 16–18 h planktonic bacterial culture was mixed with molten 0.7% top agar and poured evenly over an agar plate. When the top agar layer was set, serial dilutions of standardized phage solutions were spotted onto the overlay and plates incubated overnight at 37 °C. Phage activity was indicated by clearing of the bacterial lawn at the site of phage application, and by the development of individual plaques as the phage sample is diluted. Strains were only considered sensitive if discrete plaques could be observed as the sample was diluted, indicating phage replication. The titer for each phage+bacteria combination tested was calculated from the drop dilutions. Testing on the species-specificity panel was conducted similarly, using media recommended for the specific bacterial species and bacterial culture volumes suitable to produce a uniform lawn.
2.4. Frequency of Resistance and Complementation
Complementation studies conducted during product selection used apparent bacteriophage-insensitive mutant (BIM) colonies that were isolated after infecting a sensitive S. aureus strain with the individual candidate phages. Surviving colonies were streak-purified once on agar plates. The double-drop method was then used to screen for phage sensitivity: 10 µL spots of PBS or phage (~1 × 109 plaque-forming units (PFU)/mL) were spotted onto nutrient agar plates and after 10 min, 5 µL of overnight nutrient broth culture from each BIM or the parental strain was applied to each phage spot. After 24 h incubation at 37 °C, phage+bacteria spots were compared to PBS+bacteria controls and scored as R (resistant, no difference from control spot), I (intermediate, phage activity seen within bacterial spot), or S (sensitive, <10 bacterial colonies in spot).
For the final AB-SA01 composition, the frequency of spontaneous phage resistance in triplicate populations of the same S. aureus strain was assessed using a modification of the method of O’Flynn et al. [34]. In a final volume of 200 µL, 6–8 × 108 colony forming units (CFU) in nutrient broth was mixed with 2–3 × 109 PFU of purified phage (AB-SA01 or individual components), incubated for 10 min at 37 °C, then mixed with 3 mL molten 0.4% nutrient agar and poured over a 90-mm round 1.5% nutrient agar plate. Bacterial colonies were counted after 24 h and 48 h of incubation at 37 °C. The apparent frequency of BIMs was calculated as the number of colonies on each test plate divided by the input number of bacteria in that replicate. Results were compared using a repeated measures ANOVA and a priori planned comparisons between AB-SA01 and the three component phages were conducted using paired t-tests. Up to 10 BIMs from each phage+host combination (all BIMs if <10) were picked and streak-purification was attempted on agar plates.
2.5. Genome Sequencing and Analysis
Phage genomic DNA was purified from filtered lysates or purified preparations and sequenced by Illumina paired-end (ACGT, Wheeling, IL, USA) or PacBio technologies (Expression Analysis, Durham, NC, USA), using PCR-free libraries (Illumina TruSeq PCR-free Library Prep kit, PacBio SMRTbell library). Nucleotide sequences have been deposited in GenBank (Sa83: MK417514, Sa87: MK417515, J-Sa36: MK417516). Annotation was conducted using myRAST v36 (http://blog.theseed.org/servers/). Similarities of (1) annotated proteins to all Staphylococcus integrases in GenBank and (2) annotated genes to a proprietary database of bacterial virulence and antibiotic resistance genes were assessed using BLAST searches requiring at least 30% identity across 50% of the sequence, and E ≤ 0.05; any hits were manually inspected for validity based on factors such as the likely accuracy of the hit’s original annotation and evidence from secondary structure predicted by HHPred (https://toolkit.tuebingen.mpg.de) [35,36]. Genome alignments were constructed using Progressive Mauve (http://darlinglab.org/mauve/mauve.html) with the default parameters [37].
2.6. Animal Studies
Purified phage material was used for all animal studies. (1) Prototype 4-phage product: Six groups of five female CD-1 mice (Harlan Laboratories, Houston, TX, USA) were rendered neutropenic by administering 150 and 100 mg/kg cyclophosphamide on day -4 and day -1 prior to infection, respectively. Mice were anaesthetized by intraperitoneal (IP) injection of 0.15 mL of a mixture of ketamine HCl (100 mg/kg body weight) plus xylazine (10 mg/kg body weight). Once anaesthetized, an inoculum of 9.5 × 106 CFU of MRSA strain UNT144-3 was delivered intranasally (IN) in a 50 µL volume. At 2 and 6 hours post-infection (hpi), untreated controls received 50 µL PBS+Mg IN, antibiotic controls received 100 mg/kg vancomycin as a subcutaneous (SC) injection, and the three phage treatment groups received 1 × 109 PFU per phage, 1 × 108 PFU per phage, or 1 × 107 PFU per phage in a 50-µL IN dose. At 24 hpi, mice were euthanized by CO2 inhalation and lungs processed for bacterial load. Bacterial counts were enumerated on Brain Heart Infusion agar with 0.5% activated charcoal. (2) AB-SA01: Three groups of five female BALB/c mice were anesthetized and an inoculum of 3.0 × 108 CFU of methicillin-sensitive S. aureus strain Xen29 was delivered IN in a volume of 35 µL. At 2 and 6 hpi, untreated controls received 50 µL PBS-Mg IN and the phage treatment group received 5 × 108 PFU per phage in a 50 µL IN dose. At 2, 6, and 12 hpi, the antibiotic controls received 110 mg/kg vancomycin as a SC injection. At 24 hpi, mice were euthanized by CO2 inhalation and lungs processed for bacterial load. Care was taken to ensure tissue samples were kept cold and processed promptly for bacterial presence. Bacterial counts were enumerated on Mueller Hinton agar. After both mouse studies, bacteria recovered from mouse lung tissue were tested for phage sensitivity according to the method of 2.3. Statistical Analysis: Treatments were compared by one-way ANOVA on log10-transformed values with Tukey’s test for all pairwise comparisons. Adjusted p-values are reported. Bacterial strains: S. aureus strains were provided by the vendors conducting the studies. UNT144-3 is MRSA and carries the tetM gene. Xen29 [38] is available from Perkin Elmer, Inc. Media choice for bacterial enumeration was per each vendor’s standard practice. Each vendor had previously established both the dosing for their vancomycin control groups and the bacterial inoculation methods yielding consistent infection outcomes for each specific mouse and S. aureus strain combination.
2.7. Animal Welfare
Mouse studies were conducted by external vendors. Study “AmpliPhi 2014-01”: The University of North Texas Health Science Center Animal Facility is a member in good standing with the Association for Assessment and Accreditation of Laboratory Animal Care International. Study “APP004-2” (study approved21 March, 2016): KWS BioTest conducts all in-life experimental procedures in accordance with United Kingdom Animals (Scientific Procedures) Act 1986. Their local Ethical Review Process occurred under the auspices of the University of Bristol’s Animal Welfare and Ethical Review Body (AWERB).
3. Results
3.1. Physicochemical Characteristics of AB-SA01 Component Phages
All three AB-SA01 component phages produce small, clear plaques when plated on their paired S. aureus hosts. Transmission electron microscopy (TEM) images of the three AB-SA01 component phages show the straight, contractile tail and narrow neck that are characteristic of phages belonging to the order Caudovirales, family Myoviridae (Figure 1).
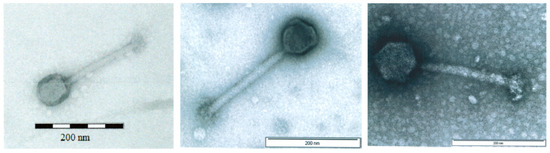
Figure 1.
Transmission electron microscopy images of (left to right) Sa83, Sa87, and J-Sa36. Scale bars: 200 nm. Filtered lysates were PEG8000 precipitated, suspended in salt-magnesium buffer, stained with 2% uranyl acetate, and imaged at 80–100kV [39].
All AB-SA01 component phages were sequenced from amplification-free libraries capable of revealing the relative frequencies of genome regions. Read-mapping data showed regions of approximately doubled coverage identifying the genome termini and associated fixed direct terminal repeats between approximately 8 and 10 kb. These genome structures indicate a sequence-specific packaging mechanism not associated with generalized transduction. The pairwise relatedness of the collinear single-copy component phage genomes ranges from 93 to 97% nucleotide identity (Figure 2) and all are related to well-studied S. aureus myovirus phage K. No identifiable integrases were found in the AB-SA01 component phage genomes and none of the ca. 200 predicted phage genes in each of the three phages were similar to known bacterial virulence or antibiotic resistance genes.

Figure 2.
A Progressive Mauve alignment of (top to bottom) Sa83, Sa87, J-Sa36, and phage K (GenBank K766114), each showing annotated genes (white boxes) and long terminal repeats (small red boxes immediately below white gene blocks). The large red blocks above each annotated genome (connected by the red vertical line at approximately 75 kb) represent local collinear blocks of genomes identity; interruptions in these red blocks indicate differences among the four aligned nucleotide sequences.
3.2. In Vitro Activity of AB-SA01
The target species for AB-SA01 is S. aureus. Overall, 94.5% of 401 clinical S. aureus isolates were sensitive to AB-SA01 (Table 1), including 95% of the 205 total isolates known to be MDR, and with little apparent variation by genetic lineage (Supplementary Table S1), year of isolation, or infection type. When tested on representatives of normal human microflora and related staphylococci, AB-SA01 and its component phages showed some activity against two of five tested S. epidermidis strains, but no cross-genus activity (Table 2). When tested on S. aureus strains, no evidence of interference among the component phages was observed. The titers observed for AB-SA01 were mostly consistent with the component phage activities, except for a few cases of apparent synergy in which AB-SA01 generated plaques on the S. aureus strain even though none of the individual component phages did so. Since testing was conducted in triplicate, this observation of synergy is likely to be real, as opposed to a case of borderline results in which plaques were a bit more obvious with AB-SA01 by simple chance.

Table 1.
In vitro antibacterial activity of AB-SA01 and its component phages on Staphylococcus aureus.

Table 2.
In vitro activity of AB-SA01 and its component phages on bacterial species other than S. aureus.
The AB-SA01 component phages were selected partly based on the 68-member diversity panel, which included representatives of all major community-acquired (CA-) and hospital-acquired (HA-) MRSA lineages [4,5]. Each component phage had a different host range, with most bacterial strains being sensitive to more than one of the AB-SA01 phages. AB-SA01 activity was similarly high across panels of isolates that represent globally prevalent S. aureus from blood, wound, lung, urinary, and other infections in later years. Using targeted interest panels, AB-SA01 was also shown to have activity on strains being relatively rare but concerning the VISA phenotype, a panel of exclusively CRS isolates, and a variety of the clinically significant USA300 lineage.
3.3. Frequency of Resistance and Complementation
The potential for phages to complement each other in the event that bacterial resistance arises was considered as part of AB-SA01 development. During product selection, six candidate phages with broad or differing host ranges were assessed on a sensitive S. aureus strain. Using the double-drop method of 2.4, BIMs that were generated using one phage were first tested to confirm whether they truly exhibited reduced phage sensitivity after streak-purification, then cross-resistance to other phages was tested (Table 3). Sa83, Sa81, and Sa76 were similarly able to complement Sa87-induced resistance and had previously shown very similar host ranges. Of these, only Sa83 was retained because it made a slightly better contribution to the total host range and complementation profile of AB-SA01. J-Sa37 more often exhibited cross-resistance than complementation and was not included in AB-SA01. J-Sa36 exhibited different complementation behavior as compared to Sa87 or Sa83.

Table 3.
Complementation among candidate phages.
After AB-SA01 composition was finalized, the mean apparent frequency of resistance to AB-SA01 was lower than the values observed for the individual phages, both at 24 h and 48 h (Table 4). However, this trend was not statistically significant (p > 0.05), possibly because the values observed in this study were close to the limit of detection. This suggests that the spontaneous frequency of AB-SA01 resistance among sensitive S. aureus populations is no greater than ~3 × 10−9. None of the BIM colonies observed in this study could be recovered by picking and re-streaking on agar to isolate them away from phages on the original test plate, implying that their growth on the test plates was not due to stable, heritable phage resistance, but was instead a temporary phenotype or a spatial phenomenon in which cells escape contact with the phages during incubation of the phage-bacteria mixture before plating.

Table 4.
Apparent frequency of intrinsic phage resistance in populations of S. aureus sensitive to AB-SA01 and its component phages.
3.4. In Vivo Activity of AB-SA01
AB-SA01 showed efficacy equivalent to vancomycin in two murine acute lung infection models, each of which used a different S. aureus challenge strain, murine genetic background, and immune status. In the first murine pneumonia model (Figure 3A), three doses of the AB-SA01 prototype were tested in neutropenic CD-1 mice. This prototype contained the three phage components of AB-SA01 plus the J-Sa37 phage that was later removed from the product because its fractional contribution to in vitro host range and complementation were deemed insufficient to justify manufacturing a fourth component phage. At 24 hpi, lung homogenates from mice treated with 4 × 109 or 4 × 108 total PFU contained significantly fewer bacteria than mice treated with buffer and were statistically equivalent (all p > 0.79) to mice that had been treated with vancomycin at the same time points. The mean reductions in lung bacterial load relative to untreated mice were 3.63 log10CFU (p < 0.0001) for the vancomycin group, 3.09 log10CFU (p < 0.0001) for the highest AB-SA01 dose group, and 3.02 log10CFU (p < 0.0001) for the medium AB-SA01 dose groups. These results suggested that doses higher than 4 × 107 PFU were required for efficacy in this model.
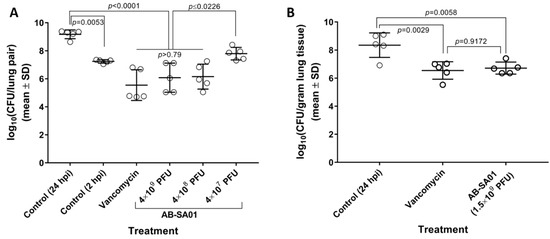
Figure 3.
AB-SA01 reduces lung bacterial burden in (A) neutropenic CD-1 mice and (B) immunocompetent BALB/c mice. Phage doses are given as total plaque-forming units (PFU) per dose.
In a follow-up experiment using the final AB-SA01 composition (Figure 3B), a 1.5 × 109 total PFU dose group was tested in immunocompetent BALB/c mice. At 24 hpi, lung homogenates from mice that had received two doses of AB-SA01 contained statistically fewer bacteria than those from untreated mice (p = 0.0058), and were statistically equivalent to mice that had received three doses of vancomycin (p = 0.9172). The mean reductions in lung bacterial load relative to the untreated control were 1.64 log10CFU for AB-SA01-treated mice and 1.80 log10CFU for vancomycin-treated mice.
In both mouse studies, S. aureus colonies recovered from infected animals showed patterns of sensitivity to AB-SA01 and its component phages that were similar to their respective parental strains, and no phage-resistant colonies were observed.
4. Discussion
The suitability of a medicinal product for human administration depends in part on the intrinsic characteristics of its active components. While no phage product to treat human infections has yet received market approval from the FDA or most of its global equivalents, the characteristics that make individual phages suitable for human use are commonly accepted within the phage research community [27,28,29,31] and generally supported by the FDA in public commentary on the subject [30]. AB-SA01, which is being developed to treat S. aureus infections, consists of three component phages that each meet these criteria in that they are: obligately lytic (not temperate), kill a wide range of clinical S. aureus strains, are incapable of specialized transduction and likely incapable of generalized transduction, and no bacterial virulence factors or drug resistance genes were identified by whole genome sequence analysis. Since a potential advantage of phage therapy is that it can be targeted to a pathogen of interest and therefore cause less disruption of the patient’s commensal flora than a broad-spectrum antibiotic, it is relevant that the AB-SA01 component phages appear to be specific to Staphylococcus spp., exhibiting no in vitro cross-genus activity.
In addition to the characteristics of individual phages, there is a rationale for the specific combination of phages that makes up AB-SA01. Within AB-SA01, phages Sa83, Sa87, and J-Sa36 each contribute different anti-S. aureus activity to AB-SA01; there is evidence of occasional synergy to kill otherwise non-susceptible S. aureus strains, the intrinsic frequency of resistance within populations of sensitive bacteria is low, and complementation is possible when resistance does develop.
The clinical utility of an antibacterial agent depends in large part on its spectrum of activity against target pathogens and non-target bacteria. The in vitro activity of AB-SA01 is high and the percentage of susceptible isolates is nearly identical on MDR and non-MDR S. aureus strains. This is similar to results from an external study that looked at two of the three AB-SA01 component phages and found no significant association between phage susceptibility and antibiotic resistance among 65 clinical S. aureus isolates [44]. The apparently lower activity of AB-SA01 on the VISA strains is difficult to interpret because this panel represents a diversity of vancomycin resistance determinants and not a diversity of S. aureus strain backgrounds. For S. aureus strains with a known multilocus sequence type there was no apparent association between genetic lineage and phage sensitivity, which is not unexpected given that the housekeeping genes on which bacterial strain typing systems are based are not expected to affect phage adsorption, replication, or lysis. It is possible for bacteria to become resistant to phages by a variety of mechanisms such as mutations in cell surface receptors and CRISPR, restriction-modification, or abortive infection systems [45]. The AB-SA01 component phages were partially chosen based on empirical evidence that the individual phages can complement resistance that may arise to another component. This is somewhat analogous to antibiotics that target multiple critical points in bacterial metabolism. The frequency of spontaneous resistance to AB-SA01 was measured as no greater than 3 × 10−9. This value is less frequent than for rifampicin [22] and approximately 10-fold higher than for daptomycin and linezolid [46,47], though it may be an overestimate since none of the counted colonies proved to be heritably resistant to AB-SA01. Unlike static small molecules, phages also have the potential to evolve in situ, adapting to local bacterial populations and undergoing antagonistic co-evolution to bypass newly developed resistance [48,49]. How this will play out clinically remains to be seen. In vitro, mutual adaptation often leads to long-term maintenance of both phage and bacterial populations [49], but patterns of in vitro and in vivo mutation have been shown to differ [48]. The collective global experience treating single patients, including with AB-SA01 [50], strongly suggests that phage administration can lead to clinical resolution of infection, sometimes with confirmed pathogen eradication [51,52,53,54,55,56,57,58,59,60].
The rare instances in which AB-SA01 formed plaques on a S. aureus strain, even though the individually tested component phages did not, are intriguing. Between-phage synergy has not been extensively studied. Commonly proposed mechanisms tend to focus on combinations of unrelated or distantly related phages in which, for example, two phages use different receptors [61] or one phage has a tailspike protein with depolymerase activity that degrades bacterial capsule and increases the accessibility of a cell surface receptor to a second phage that does possess such enzymatic activity [62]. This type of mechanism seems unlikely for AB-SA01 given the high degree of relatedness among its component phages. Our observations could conceivably be the result of interactions downstream of phage adsorption, e.g. an in trans effect in which each phage in a co-infected cell expresses gene(s) necessary for both phages to bypass an intracellular resistance mechanism that would otherwise have prevented the second phage from completing replication and lysis. However, this is hypothetical and would need to be investigated further.
A frequent point of discussion for phage therapy is whether a fixed composition phage product will remain active against globally circulating strains of bacteria for long enough to be useful. It has sometimes been postulated that the rapid pace of bacterial evolution might cause the clinical populations of a target pathogen to change rapidly enough that a phage product might no longer be relevant by the time it obtains market approval, or that once in use, resistance may develop too quickly for the phage product to remain useful. While resistance development is a relevant issue for any novel antibacterial, we are not aware of evidence that this risk or rate would be higher for phage products than for other antibacterial agents being developed with a similar focus on novel mechanisms of action and resistance management. On the contrary, traits such as complementation among component phages and phage evolution offer a means of combating this and the evolution of phage resistance often carries other fitness costs [63]. The data presented here show that, at least for S. aureus, it appears possible to create a fixed-composition phage product that has activity against the vast majority of circulating clinical strains over several years, including MDR strains. When looking only at in vitro AB-SA01 activity on the 2013, 2015, and 2016 Global Panels, it is possible to suppose that activity has been gradually decreasing over time. However, it is equally possible, especially considering the aggregate results shown in Table 1, that the three Global Panels represent a mean of approximately 94% with one result each above and below this percentage. Notably, 96.3% of the 27 contemporary S. aureus isolates received between 2017 and 2018 by AmpliPhi from physicians requesting AB-SA01 to treat individual patients with refractory S. aureus infections were sensitive to AB-SA01, offering “real-world” support for the expectation that AB-SA01 will be active against the isolates of patients not responding to antibiotics.
Murine models of acute pneumonia showed that AB-SA01 exhibits antibacterial activity in a vertebrate infection. Both the prototype product and AB-SA01 were as effective as vancomycin in reducing lung bacterial burdens. The efficacy of S. aureus phages was observed in both neutropenic and immunocompetent mice. In Pseudomonas aeruginosa pneumonia models, phages were observed to be ineffective in neutropenic mice even if the same phages had successfully controlled a similar infection in mice with different or no immune deficiencies [64]. This likely reflects a genuine difference between S. aureus and P. aeruginosa pathogenesis. Skerrett et al [65] reported that myeloid differentiation factor 88, which is required for neutrophil production, is essential for host defense against P. aeruginosa but not S. aureus pneumonia. Neutrophil elastase is important for eradication of P. aeruginosa by the host’s innate immune system [66], whereas S. aureus produces neutrophil elastase inhibitors and appears particularly resistant to neutrophil killing [67,68].
Most staphylococcal phages fall into three broad categories, temperate siphoviruses, obligately lytic myoviruses, and obligately lytic podoviruses [69]. The myoviruses have historically been grouped together and described as K-like or Twort-like [69], though recent taxonomic proposals divide them into four genera within a proposed Twortvirinae subfamily [70,71]. Collectively the staphylococcus myoviruses tend to have broad host ranges and are frequently discussed as actual and proposed components of therapeutic phage preparations [72,73]. Previous studies have also shown the potential of K-like S. aureus phages to treat biofilm-associated infections. Guimin et al. [44] studied two of the three AB-SA01 component phages and showed that they can reduce in vitro S. aureus biofilm. A four-phage mix containing the precursor of an AB-SA01 component phage also significantly degraded in vitro biofilm [74] and was used to treat mature S. aureus biofilm in a sheep sinus infection model [75]. After 3 days of treatment, the phage-treated sheep had significantly lower mucosal biofilm mass. Compared to the controls, the sheep were healthy, showed comparable levels of sinus mucosal inflammation and had healthy looking cilia.
Randomized, controlled clinical trials are needed to show that single-patient clinical observations and systematic preclinical data collected in a research environment will translate into broad clinical efficacy. The chemistry, manufacturing, and control aspects of AB-SA01 production (including but not limited to production, purification, quality control, storage, and stability) are beyond the scope of this manuscript. However, when added to the preclinical characterization data presented here, AB-SA01 and its associated data package enabled clinical studies under the oversight of the U.S. Food and Drug Administration and Australia’s Therapeutic Goods Administration (TGA). In 2016, the safety and tolerability of AB-SA01 was tested in two clinical trials: one healthy volunteer study in the United States under an Investigational New Drug (IND) application (NCT02757755) and one open-label investigator-initiated study in Australia among post-rhinoplasty CRS patients (ACTRN12616000002482). AB-SA01 was safe and well tolerated in both study populations. Among CRS patients, there were preliminary indications of efficacy that will need to be confirmed in placebo-controlled studies, such as reductions in sinus bacterial load, improved endoscopic findings, and general symptom improvement [76]. Finally, 15 patients with serious or life-threatening S. aureus infections not responding to antibiotics have received a cumulative total of more than 400 doses AB-SA01, including more than 300 administered intravenously under Individual Patient Expanded Access INDs in the United States or Australia’s Special Access Scheme. No serious adverse events attributed to AB-SA01 were reported and observations from these patients suggest that it may be fruitful to investigate the efficacy of AB-SA01 in randomized controlled trials involving indications such as bacteremia, native and prosthetic valve endocarditis, prosthetic joint infections, and ventricular assist device infections.
AB-SA01 is a well-characterized phage investigational product that has entered clinical development for the treatment of S. aureus infections. While it is frequently suggested that existing regulatory structures are not compatible with the clinical development of phage products or with the timely emergency treatment of patients not responding to antibiotics, AB-SA01 has thus far satisfied FDA and TGA requirements to conduct clinical trials and single-patient emergency treatment. As with any antibacterial, epidemiological shifts might eventually necessitate a compositional update. At that point, the accumulated clinical and regulatory experience that will hopefully have been established with fixed-composition products should pave the way for data-driven strategies to streamline updates to phage products.
Supplementary Materials
The following are available online at http://www.mdpi.com/1999-4915/11/1/88/s1, Table S1: Phage sensitivity of S. aureus strains with known MLST.
Author Contributions
Conceptualization, S.M., S.M.L.; Methodology, S.M., S.M.L., G.M., D.R., R.A.C., F.S., S.D.B.; Writing—Original Draft Preparation, S.M.L., S.M.; Writing—Review and Editing, S.M.L., G.M., D.R., R.A.C., F.S., S.D.B., S.M.
Acknowledgments
We thank Mikeljon Nikolich, Andrey Filippov, and Kirill Sergueev at Walter Reed Army Institute of Research, as well as Joseph Bertsche and Sam Boundy at AmpliPhi Biosciences for their contributions to host range testing. We thank Jon Iredell, Bernard Hudson, Peter Hawkey, and H.C. Claus Bachert for generously sharing clinical bacterial isolates. We thank Anthony Smithyman for his insightful scientific discussions and input over the years. We thank the University of North Texas Health Sciences Center pre-clinical services lab (Fort Worth, TX) and Karen J Shaw (Hearts Consulting) for contributions to the neutropenic mouse study and KWS Biotest (Bristol, UK) for the immunocompetent mouse study.
Conflicts of Interest
The authors are employees of AmpliPhi Biosciences, which is developing AB-SA01. Following collaborative study design, all animal studies were independently conducted and analyzed by external vendors.
References
- Summers, W.C. The strange history of phage therapy. Bacteriophage 2012, 2, 130–133. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, J. Tackling Drug-Resistance Infections Globally: Final Report and Reccomendations; Review on Antimicrobial Resistance: London, UK, 2016. [Google Scholar]
- Rice, L.B. Federal funding for the study of antimicrobial resistance in nosocomial pathogens: No ESKAPE. J. Infect. Dis. 2008, 197, 1079–1081. [Google Scholar] [CrossRef] [PubMed]
- Stefani, S.; Chung, D.R.; Lindsay, J.A.; Friedrich, A.W.; Kearns, A.M.; Westh, H.; Mackenzie, F.M. Methicillin-resistant Staphylococcus aureus (MRSA): Global epidemiology and harmonisation of typing methods. Int. J. Antimicrob. Agents 2012, 39, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Otter, J.A.; French, G.L. Molecular epidemiology of community-associated meticillin-resistant Staphylococcus aureus in Europe. Lancet Infect. Dis. 2010, 10, 227–239. [Google Scholar] [CrossRef]
- WHO. Prioritization of Pathogens to Guide Discovery, Research and Development Of New Antibiotics for Drug-Resistant Bacterial Infections, Including Tuberculosis; WHO: Geneva, Switzerland, 2017. [Google Scholar]
- CDC. Antibiotic Resistance Threats in the United States; CDC: Atlanta, GA, USA, 2013. [Google Scholar]
- Kallberg, C.; Ardal, C.; Salvesen Blix, H.; Klein, E.; Martinez, E.M.; Lindbaek, M.; Outterson, K.; Rottingen, J.A.; Laxminarayan, R. Introduction and geographic availability of new antibiotics approved between 1999 and 2014. PLoS ONE 2018, 13, e0205166. [Google Scholar] [CrossRef] [PubMed]
- Senior, K. FDA approves first drug in new class of antibiotics. Lancet 2000, 355, 1523. [Google Scholar] [CrossRef]
- Ikeda-Dantsuji, Y.; Hanaki, H.; Nakae, T.; Takesue, Y.; Tomono, K.; Honda, J.; Yanagihara, K.; Mikamo, H.; Fukuchi, K.; Kaku, M.; et al. Emergence of Linezolid-Resistant Mutants in a Susceptible-Cell Population of Methicillin-Resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 2011, 55, 2466–2468. [Google Scholar] [CrossRef] [PubMed]
- Morales, G.; Picazo, J.J.; Baos, E.; Candel, F.J.; Arribi, A.; Peláez, B.; Andrade, R.; de la Torre, M.-Á.; Fereres, J.; Sánchez-García, M. Resistance to Linezolid Is Mediated by the cfr Gene in the First Report of an Outbreak of Linezolid-Resistant Staphylococcus aureus. Clin. Infect. Dis. 2010, 50, 821–825. [Google Scholar] [CrossRef] [PubMed]
- Bayer, A.S.; Schneider, T.; Sahl, H.-G. Mechanisms of daptomycin resistance in Staphylococcus aureus: Role of the cell membrane and cell wall. Ann. N. Y. Acad. Sci. 2013, 1277, 139–158. [Google Scholar] [CrossRef]
- Dortet, L.; Anguel, N.; Fortineau, N.; Richard, C.; Nordmann, P. In vivo acquired daptomycin resistance during treatment of methicillin-resistant Staphylococcus aureus endocarditis. Int. J. Infect. Dis. 2013, 17, e1076–e1077. [Google Scholar] [CrossRef]
- Stein, G.E.; Babinchak, T. Tigecycline: An update. Diagn. Microbiol. Infect. Dis. 2013, 75, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Long, S.W.; Olsen, R.J.; Mehta, S.C.; Palzkill, T.; Cernoch, P.L.; Perez, K.K.; Musick, W.L.; Rosato, A.E.; Musser, J.M. PBP2a mutations causing high-level ceftaroline resistance in clinical methicillin-resistant Staphylococcus aureus isolates. Antimicrob. Agents Chemother. 2014, 58, 6668–6674. [Google Scholar] [CrossRef] [PubMed]
- Karlowsky, J.A.; Nichol, K.; Zhanel, G.G. Telavancin: Mechanisms of action, in vitro activity, and mechanisms of resistance. Clin. Infect. Dis 2015, 61 (Suppl. 2), S58–S68. [Google Scholar] [CrossRef]
- Jones, R.N.; Moeck, G.; Arhin, F.F.; Dudley, M.N.; Rhomberg, P.R.; Mendes, R.E. Results from Oritavancin Resistance Surveillance Programs (2011 to 2014): Clarification for Using Vancomycin as a Surrogate To Infer Oritavancin Susceptibility. Antimicrob. Agents Chemother. 2016, 60, 3174–3177. [Google Scholar] [CrossRef] [PubMed]
- Morroni, G.; Brenciani, A.; Brescini, L.; Fioriti, S.; Simoni, S.; Pocognoli, A.; Mingoia, M.; Giovanetti, E.; Barchiesi, F.; Giacometti, A.; et al. A high rate of ceftobiprole resistance among clinical MRSA from a hospital in central Italy. Antimicrob. Agents Chemother. 2018, 62, e01663-1810. [Google Scholar] [CrossRef] [PubMed]
- Werth, B.J.; Jain, R.; Hahn, A.; Cummings, L.; Weaver, T.; Waalkes, A.; Sengupta, D.; Salipante, S.J.; Rakita, R.M.; Butler-Wu, S.M. Emergence of dalbavancin non-susceptible, vancomycin-intermediate Staphylococcus aureus (VISA) after treatment of MRSA central line-associated bloodstream infection with a dalbavancin- and vancomycin-containing regimen. Clin. Microbiol. Infect. 2018, 24, e421–e429. [Google Scholar] [CrossRef] [PubMed]
- Molina-Manso, D.; del Prado, G.; Ortiz-Perez, A.; Manrubia-Cobo, M.; Gomez-Barrena, E.; Cordero-Ampuero, J.; Esteban, J. In vitro susceptibility to antibiotics of staphylococci in biofilms isolated from orthopaedic infections. Int. J. Antimicrob. Agents 2013, 41, 521–523. [Google Scholar] [CrossRef] [PubMed]
- Czaplewski, L.; Bax, R.; Clokie, M.; Dawson, M.; Fairhead, H.; Fischetti, V.A.; Foster, S.; Gilmore, B.F.; Hancock, R.E.; Harper, D.; et al. Alternatives to antibiotics-a pipeline portfolio review. Lancet Infect. Dis. 2016, 16, 239–251. [Google Scholar] [CrossRef]
- O’Neill, A.J.; Cove, J.H.; Chopra, I. Mutation frequencies for resistance to fusidic acid and rifampicin in Staphylococcus aureus. J. Antimicrob. Chemother. 2001, 47, 647–650. [Google Scholar] [CrossRef] [PubMed]
- Gottlieb, S. (Washington, DC, USA). FDA’s Strategic Approach for Combating Antimicrobial Rsistance. Personal Communication, 2018. [Google Scholar]
- Loc-Carrillo, C.; Abedon, S.T. Pros and cons of phage therapy. Bacteriophage 2011, 1, 111–114. [Google Scholar] [CrossRef]
- Gorski, A.; Dabrowska, K.; Switala-Jelen, K.; Nowaczyk, M.; Weber-Dabrowska, B.; Boratynski, J.; Wietrzyk, J.; Opolski, A. New insights into the possible role of bacteriophages in host defense and disease. Med. Immunol. 2003, 2, 2. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, S.; Baker, K.; Padman, B.S.; Patwa, R.; Dunstan, R.A.; Weston, T.A.; Schlosser, K.; Bailey, B.; Lithgow, T.; Lazarou, M.; et al. Bacteriophage Transcytosis Provides a Mechanism To Cross Epithelial Cell Layers. mBio 2017, 8, e01874–e01817. [Google Scholar] [CrossRef] [PubMed]
- Gill, J.J.; Hyman, P. Phage choice, isolation, and preparation for phage therapy. Curr. Pharm. Biotechnol. 2010, 11, 2–14. [Google Scholar] [CrossRef] [PubMed]
- Merabishvili, M.; Pirnay, J.P.; Verbeken, G.; Chanishvili, N.; Tediashvili, M.; Lashkhi, N.; Glonti, T.; Krylov, V.; Mast, J.; Van Parys, L.; et al. Quality-controlled small-scale production of a well-defined bacteriophage cocktail for use in human clinical trials. PLoS ONE 2009, 4, e4944. [Google Scholar] [CrossRef] [PubMed]
- Casey, E.; van Sinderen, D.; Mahony, J. In Vitro Characteristics of Phages to Guide ‘Real Life’ Phage Therapy Suitability. Viruses 2018, 10, 163. [Google Scholar] [CrossRef] [PubMed]
- Reindel, R.; Fiore, C.R. Phage Therapy: Considerations and Challenges for Development. Clin. Infect. Dis. 2017, 64, 1589–1590. [Google Scholar] [CrossRef] [PubMed]
- Carlton, R.M. Phage therapy: Past history and future prospects. Arch. Immunol. Ther. Exp. 1999, 47, 267–274. [Google Scholar]
- Magiorakos, A.P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clinic. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef] [PubMed]
- Mazzocco, A.; Waddell, T.E.; Lingohr, E.; Johnson, R.P. Enumeration of bacteriophages using the small drop plaque assay system. Methods mol. Biol. 2009, 501, 81–85. [Google Scholar] [PubMed]
- O’Flynn, G.; Ross, R.P.; Fitzgerald, G.F.; Coffey, A. Evaluation of a cocktail of three bacteriophages for biocontrol of Escherichia coli O157:H7. Appl. Environ. Microbiol. 2004, 70, 3417–3424. [Google Scholar] [CrossRef]
- Soding, J. Protein homology detection by HMM-HMM comparison. Bioinformatics 2005, 21, 951–960. [Google Scholar] [CrossRef] [PubMed]
- Soding, J.; Biegert, A.; Lupas, A.N. The HHpred interactive server for protein homology detection and structure prediction. Nucleic Acids Res. 2005, 33, W244–W248. [Google Scholar] [CrossRef] [PubMed]
- Darling, A.C.E.; Mau, B.; Blattner, F.R.; Perna, N.T. Mauve: Multiple alignment of conserved genomic sequence with rearrangements. Genome Res. 2004, 14, 1394–1403. [Google Scholar] [CrossRef] [PubMed]
- Kadurugamuwa, J.L.; Sin, L.V.; Yu, J.; Francis, K.P.; Kimura, R.; Purchio, T.; Contag, P.R. Rapid Direct Method for Monitoring Antibiotics in a Mouse Model of Bacterial Biofilm Infection. Antimicrob. Agents Chemother. 2003, 47, 3130–3137. [Google Scholar] [CrossRef] [PubMed]
- Carlson, K. Working with bacteriophages: Common techniques and methodological approaches. In Bacteriophages: Biology and Applications; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Tattevin, P.; Diep, B.A.; Jula, M.; Perdreau-Remington, F. Long-term follow-up of methicillin-resistant Staphylococcus aureus molecular epidemiology after emergence of clone USA300 in San Francisco jail populations. J. Clin. Microbiol. 2008, 46, 4056–4057. [Google Scholar] [CrossRef]
- Diep, B.A.; Carleton, H.A.; Chang, R.F.; Sensabaugh, G.F.; Perdreau-Remington, F. Roles of 34 virulence genes in the evolution of hospital- and community-associated strains of methicillin-resistant Staphylococcus aureus. J. Infect. Dis. 2006, 193, 1495–1503. [Google Scholar] [CrossRef] [PubMed]
- Diep, B.A.; Chambers, H.F.; Graber, C.J.; Szumowski, J.D.; Miller, L.G.; Han, L.L.; Chen, J.H.; Lin, F.; Lin, J.; Phan, T.H.; et al. Emergence of multidrug-resistant, community-associated, methicillin-resistant Staphylococcus aureus clone USA300 in men who have sex with men. Ann. Intern. Med. 2008, 148, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Diep, B.A.; Gill, S.R.; Chang, R.F.; Phan, T.H.; Chen, J.H.; Davidson, M.G.; Lin, F.; Lin, J.; Carleton, H.A.; Mongodin, E.F.; et al. Complete genome sequence of USA300, an epidemic clone of community-acquired meticillin-resistant Staphylococcus aureus. Lancet 2006, 367, 731–739. [Google Scholar] [CrossRef]
- Guimin, Z.; Yin, Z.; Paramasivan, S.; Richter, K.; Morales, S.; Wormald, P.J.; Vreugde, S. Bacteriophage effectively kills multidrug resistant Staphylococcus aureus clinical isolates from chronic rhinosinusitis patients. Int. Forum Allergy Rhinol. 2018, 8, 406–414. [Google Scholar]
- Labrie, S.J.; Samson, J.E.; Moineau, S. Bacteriophage resistance mechanisms. Nat. Rev. Microbiol. 2010, 8, 317–327. [Google Scholar] [CrossRef]
- Silverman, J.A.; Oliver, N.; Andrew, T.; Li, T. Resistance Studies with Daptomycin. Antimicrob. Agents Chemother. 2001, 45, 1799–1802. [Google Scholar] [CrossRef] [PubMed]
- Zurenko, G.E.; Yagi, B.H.; Schaadt, R.D.; Allison, J.W.; Kilburn, J.O.; Glickman, S.E.; Hutchinson, D.K.; Barbachyn, M.R.; Brickner, S.J. In vitro activities of U-100592 and U-100766, novel oxazolidinone antibacterial agents. Antimicrob. Agents Chemother. 1996, 40, 839–845. [Google Scholar] [CrossRef] [PubMed]
- De Sordi, L.; Khanna, V.; Debarbieux, L. The Gut Microbiota Facilitates Drifts in the Genetic Diversity and Infectivity of Bacterial Viruses. Cell Host Microbe 2017, 22, 801–808. [Google Scholar] [CrossRef] [PubMed]
- Buckling, A.; Rainey, P.B. Antagonistic coevolution between a bacterium and a bacteriophage. Proc. Biol. Sci. 2002, 269, 931–936. [Google Scholar] [CrossRef] [PubMed]
- Fabijan, A.; Ho, J.; Lin, R.C.Y.; Maddocks, S.; Gilbey, T.; Sandaradura, I.; Chan, S.; Morales, S.; Venturini, C.; Branston, S.; et al. Safety and tolerability of bacteriophage therapy in the treatment of severe Staphylococcus aureus bacteremia. Manuscript in preparation.
- Slopek, S.; Weber-Dabrowska, B.; Dabrowski, M.; Kucharewicz-Krukowska, A. Results of bacteriophage treatment of suppurative bacterial infections in the years 1981-1986. Arch. Immunol. Ther. Exp. 1987, 35, 569–583. [Google Scholar]
- Weber-Dabrowska, B.; Mulczyk, M.; Gorski, A. Bacteriophage therapy of bacterial infections: An update of our institute’s experience. Arch. Immunol. Ther. Exp. 2000, 48, 547–551. [Google Scholar]
- Kutateladze, M.; Adamia, R. Phage therapy experience at the Eliava Institute. Med. Mal. Infect. 2008, 38, 426–430. [Google Scholar] [CrossRef]
- Wright, A.; Hawkins, C.H.; Anggard, E.E.; Harper, D.R. A controlled clinical trial of a therapeutic bacteriophage preparation in chronic otitis due to antibiotic-resistant Pseudomonas aeruginosa; a preliminary report of efficacy. Clin. Otolaryngol. 2009, 34, 349–357. [Google Scholar] [CrossRef]
- Chanishvili, N. Phage therapy–history from Twort and d’Herelle through Soviet experience to current approaches. Adv. Virus Res. 2012, 83, 3–40. [Google Scholar]
- Miedzybrodzki, R.; Borysowski, J.; Weber-Dabrowska, B.; Fortuna, W.; Letkiewicz, S.; Szufnarowski, K.; Pawelczyk, Z.; Rogoz, P.; Klak, M.; Wojtasik, E.; et al. Clinical aspects of phage therapy. Adv. Virus Res. 2012, 83, 73–121. [Google Scholar]
- Schooley, R.T.; Biswas, B.; Gill, J.J.; Hernandez-Morales, A.; Lancaster, J.; Lessor, L.; Barr, J.J.; Reed, S.L.; Rohwer, F.; Benler, S.; et al. Development and Use of Personalized Bacteriophage-Based Therapeutic Cocktails To Treat a Patient with a Disseminated Resistant Acinetobacter baumannii Infection. Antimicrob. Agents Chemother. 2017, 61, e00954-17. [Google Scholar] [CrossRef] [PubMed]
- Jennes, S.; Merabishvili, M.; Soentjens, P.; Pang, K.W.; Rose, T.; Keersebilck, E.; Soete, O.; François, P.-M.; Teodorescu, S.; Verween, G.; et al. Use of bacteriophages in the treatment of colistin-only-sensitive Pseudomonas aeruginosa septicaemia in a patient with acute kidney injury-a case report. Crit. Care 2017, 21, 129. [Google Scholar] [CrossRef] [PubMed]
- Chan, B.K.; Turner, P.E.; Kim, S.; Mojibian, H.R.; Elefteriades, J.A.; Narayan, D. Phage treatment of an aortic graft infected with Pseudomonas aeruginosa. Evolut. Med. Public Health 2018, 2018, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Gorski, A.; Miedzybrodzki, R.; Borysowski, J.; Weber-Dabrowska, B.; Lobocka, M.; Fortuna, W.; Letkiewicz, S.; Zimecki, M.; Filby, G. Bacteriophage therapy for the treatment of infections. Curr. Opin. Investig. Drugs 2009, 10, 766–774. [Google Scholar] [PubMed]
- Chaudhry, W.N.; Concepcion-Acevedo, J.; Park, T.; Andleeb, S.; Bull, J.J.; Levin, B.R. Synergy and Order Effects of Antibiotics and Phages in Killing Pseudomonas aeruginosa Biofilms. PLoS ONE 2017, 12, e0168615. [Google Scholar] [CrossRef] [PubMed]
- Schmerer, M.; Molineux, I.J.; Bull, J.J. Synergy as a rationale for phage therapy using phage cocktails. PeerJ 2014, 2, e590. [Google Scholar] [CrossRef] [PubMed]
- Oechslin, F. Resistance Development to Bacteriophages Occurring during Bacteriophage Therapy. Viruses 2018, 10, 351. [Google Scholar] [CrossRef]
- Roach, D.R.; Leung, C.Y.; Henry, M.; Morello, E.; Singh, D.; Di Santo, J.P.; Weitz, J.S.; Debarbieux, L. Synergy between the Host Immune System and Bacteriophage Is Essential for Successful Phage Therapy against an Acute Respiratory Pathogen. Cell Host Microbe 2017, 22, 38–47.e4. [Google Scholar] [CrossRef]
- Skerrett, S.J.; Liggitt, H.D.; Hajjar, A.M.; Wilson, C.B. Cutting edge: Myeloid differentiation factor 88 is essential for pulmonary host defense against Pseudomonas aeruginosa but not Staphylococcus aureus. J. Immunol. 2004, 172, 3377–3381. [Google Scholar] [CrossRef]
- Hirche, T.O.; Benabid, R.; Deslee, G.; Gangloff, S.; Achilefu, S.; Guenounou, M.; Lebargy, F.; Hancock, R.E.; Belaaouaj, A. Neutrophil elastase mediates innate host protection against Pseudomonas aeruginosa. J. Immunol. 2008, 181, 4945–4954. [Google Scholar] [CrossRef]
- Stapels, D.A.; Geisbrecht, B.V.; Rooijakkers, S.H. Neutrophil serine proteases in antibacterial defense. Curr. Opin. Microbiol. 2015, 23, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Stapels, D.A.; Ramyar, K.X.; Bischoff, M.; von Kockritz-Blickwede, M.; Milder, F.J.; Ruyken, M.; Eisenbeis, J.; McWhorter, W.J.; Herrmann, M.; van Kessel, K.P.; et al. Staphylococcus aureus secretes a unique class of neutrophil serine protease inhibitors. Proc. Natl. Acad. Sci. USA 2014, 111, 13187–13192. [Google Scholar] [CrossRef] [PubMed]
- Ajuebor, J.; Buttimer, C.; Arroyo-Moreno, S.; Chanishvili, N.; Gabriel, E.M.; O’Mahony, J.; McAuliffe, O.; Neve, H.; Franz, C.; Coffey, A. Comparison of Staphylococcus Phage K with Close Phage Relatives Commonly Employed in Phage Therapeutics. Antibiotics 2018, 7, 37. [Google Scholar] [CrossRef] [PubMed]
- McCallin, S.; Sarker, S.A.; Sultana, S.; Oechslin, F.; Brussow, H. Metagenome analysis of Russian and Georgian Pyophage cocktails and a placebo-controlled safety trial of single phage versus phage cocktail in healthy Staphylococcus aureus carriers. Environ. Microbiol. 2018, 20, 3278–3293. [Google Scholar] [CrossRef] [PubMed]
- Łobocka, M.; Hejnowicz, M.S.; Dabrowski, K.; Gozdek, A.; Kosakowski, J.; Witkowska, M.; Ulatowska, M.I.; Weber-Dabrowska, B.; Kwiatek, M.; Parasion, S.; et al. Genomics of staphylococcal Twort-like phages--potential therapeutics of the post-antibiotic era. Adv. Virus Res. 2012, 83, 143–216. [Google Scholar] [PubMed]
- Adriaenssens, E.M.; Wittmann, J.; Kuhn, J.H.; Turner, D.; Sullivan, M.B.; Dutilh, B.E.; Jang, H.B.; van Zyl, L.J.; Klumpp, J.; Lobocka, M.; et al. Taxonomy of prokaryotic viruses: 2017 update from the ICTV Bacterial and Archaeal Viruses Subcommittee. Arch. Virol. 2018, 163, 1125–1129. [Google Scholar] [CrossRef] [PubMed]
- Barylski, J.; Enault, F.; Dutilh, B.E.; Schuller, M.B.P.; Edwards, R.A.; Gillis, A.; Klumpp, J.; Knezevic, P.; Krupovic, M.; Kuhn, J.H.; et al. Analysis of Spounaviruses as a Case 3 Study for the Overdue Reclassification of 4 Tailed Bacteriophages. bioRxiv 2017. [Google Scholar] [CrossRef]
- Drilling, A.; Morales, S.; Jardeleza, C.; Vreugde, S.; Speck, P.; Wormald, P.J. Bacteriophage reduces biofilm of Staphylococcus aureus ex vivo isolates from chronic rhinosinusitis patients. Am. J. Rhinol. Allergy 2014, 28, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Drilling, A.; Morales, S.; Boase, S.; Jervis-Bardy, J.; James, C.; Jardeleza, C.; Tan, N.C.; Cleland, E.; Speck, P.; Vreugde, S.; et al. Safety and efficacy of topical bacteriophage and ethylenediaminetetraacetic acid treatment of Staphylococcus aureus infection in a sheep model of sinusitis. Int. Forum Allergy Rhinol. 2014, 4, 176–186. [Google Scholar] [CrossRef]
- Ooi MLD, A.J.; Morales, S.; Fong, S.; Moraitis, S.; Macias-Valle, L.; Vreugde, S.; Psaltis, A.; Wormald, P.-J. Phage Therapy for S. aureus Chronic Rhinosinusitis: A Phase 1 First-in-Human Study. Manuscript in preparation.
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).