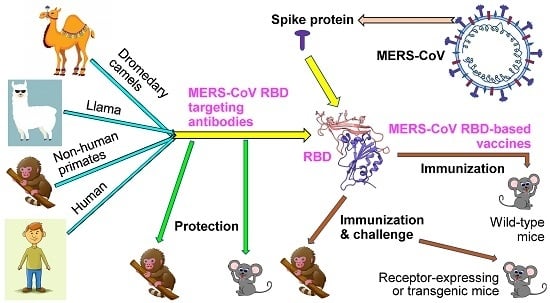
Advances in MERS-CoV Vaccines and Therapeutics Based on the Receptor-Binding Domain
Abstract
1. Introduction
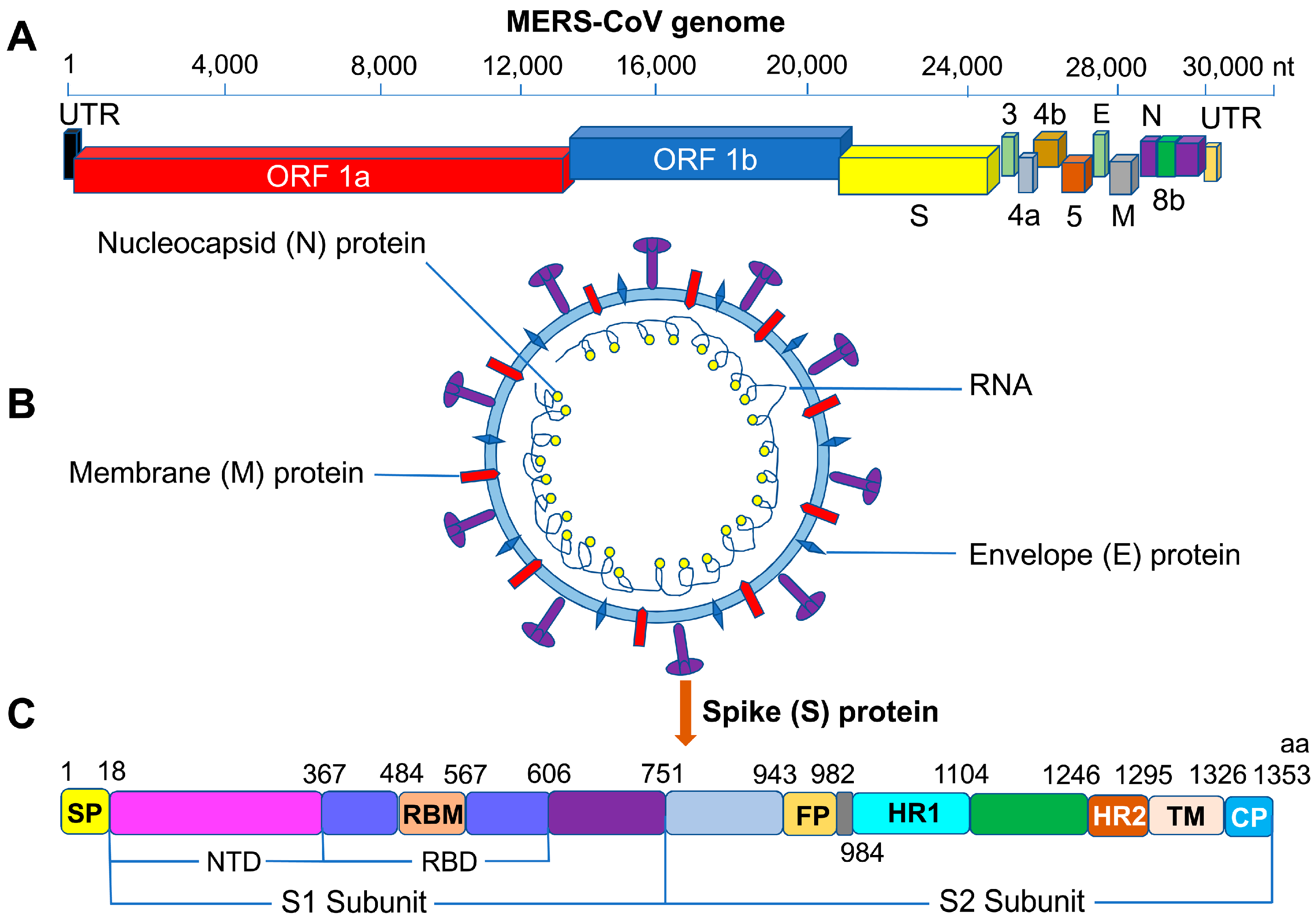
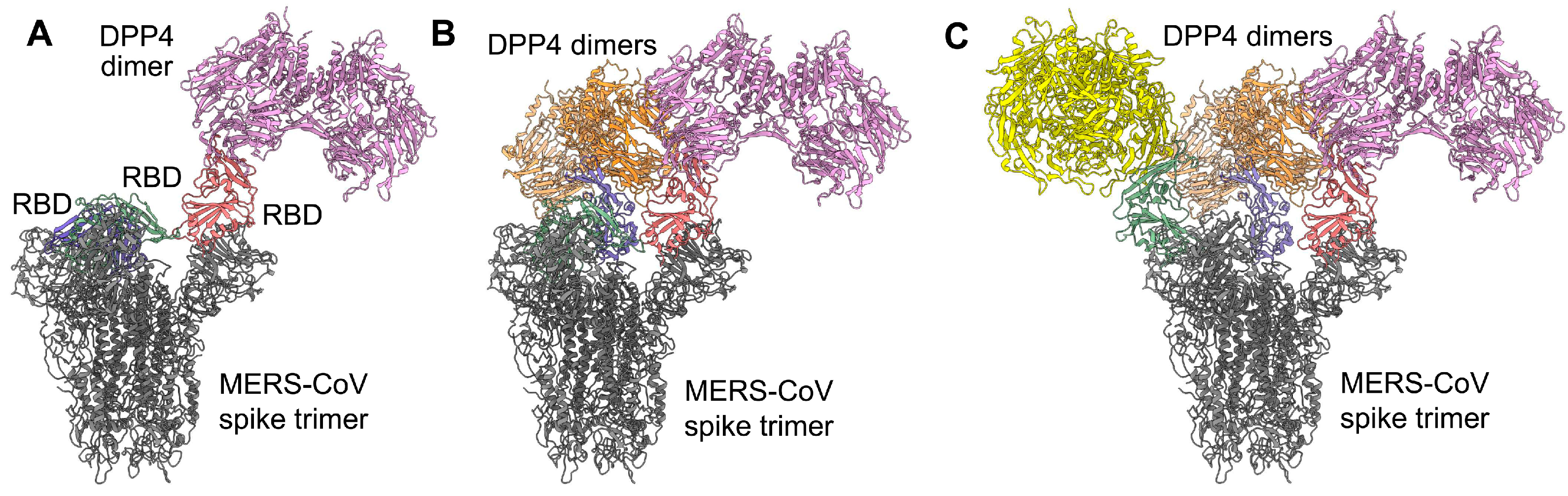
2. MERS-CoV S Protein RBD
3. Recent Advances in the Development of Vaccines Based on the MERS-CoV S-Protein RBD
4. Recent Advances in the Development of Therapeutics Based on the MERS-CoV S-Protein RBD
4.1. MERS-CoV S-Protein RBD-Targeting mAbs
4.2. Nanobodies Targeting the MERS-CoV S-protein RBD
5. Potential Challenges and Future Perspectives
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Zaki, A.M.; van Boheemen, S.; Bestebroer, T.M.; Osterhaus, A.D.; Fouchier, R.A. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N. Engl. J. Med. 2012, 367, 1814–1820. [Google Scholar] [CrossRef] [PubMed]
- Sha, J.; Li, Y.; Chen, X.; Hu, Y.; Ren, Y.; Geng, X.; Zhang, Z.; Liu, S. Fatality risks for nosocomial outbreaks of Middle East respiratory syndrome coronavirus in the Middle East and South Korea. Arch. Virol. 2017, 162, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Rivers, C.M.; Majumder, M.S.; Lofgren, E.T. Risks of death and severe disease in patients with Middle East respiratory syndrome coronavirus, 2012-2015. Am. J. Epidemiol. 2016, 184, 460–464. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.M.; Hsu, C.Y.; Lai, C.C.; Yen, M.F.; Wikramaratna, P.S.; Chen, H.H.; Wang, T.H. Impact of comorbidity on fatality rate of patients with Middle East respiratory syndrome. Sci. Rep. 2017, 7, 11307. [Google Scholar] [CrossRef] [PubMed]
- Matsuyama, R.; Nishiura, H.; Kutsuna, S.; Hayakawa, K.; Ohmagari, N. Clinical determinants of the severity of Middle East respiratory syndrome (MERS): A systematic review and meta-analysis. BMC Public Health 2016, 16, 1203. [Google Scholar] [CrossRef] [PubMed]
- Badawi, A.; Ryoo, S.G. Prevalence of comorbidities in the Middle East respiratory syndrome coronavirus (MERS-CoV): A systematic review and meta-analysis. Int. J. Infect. Dis. 2016, 49, 129–133. [Google Scholar] [CrossRef] [PubMed]
- Assiri, A.; Al-Tawfiq, J.A.; Al-Rabeeah, A.A.; Al-Rabiah, F.A.; Al-Hajjar, S.; Al-Barrak, A.; Flemban, H.; Al-Nassir, W.N.; Balkhy, H.H.; Al-Hakeem, R.F.; et al. Epidemiological, demographic, and clinical characteristics of 47 cases of Middle East respiratory syndrome coronavirus disease from Saudi Arabia: A descriptive study. Lancet Infect. Dis. 2013, 13, 752–761. [Google Scholar] [CrossRef]
- Jansen, A.; Chiew, M.; Konings, F.; Lee, C.K.; Ailan, L. Sex matters - a preliminary analysis of Middle East respiratory syndrome in the Republic of Korea, 2015. West. Pac. Surveill Response J. 2015, 6, 68–71. [Google Scholar] [CrossRef]
- Kim, K.H.; Tandi, T.E.; Choi, J.W.; Moon, J.M.; Kim, M.S. Middle East respiratory syndrome coronavirus (MERS-CoV) outbreak in South Korea, 2015: Epidemiology, characteristics and public health implications. J. Hosp. Infect. 2017, 95, 207–213. [Google Scholar] [CrossRef]
- Assiri, A.; McGeer, A.; Perl, T.M.; Price, C.S.; Al Rabeeah, A.A.; Cummings, D.A.; Alabdullatif, Z.N.; Assad, M.; Almulhim, A.; Makhdoom, H.; et al. Hospital outbreak of Middle East respiratory syndrome coronavirus. N. Engl. J. Med. 2013, 369, 407–416. [Google Scholar] [CrossRef]
- Alfaraj, S.H.; Al-Tawfiq, J.A.; Memish, Z.A. Middle East respiratory syndrome coronavirus (MERS-CoV) infection during pregnancy: Report of two cases & review of the literature. J. Microbiol. Immunol. Infect. 2018. [Google Scholar] [CrossRef]
- Jeong, S.Y.; Sung, S.I.; Sung, J.H.; Ahn, S.Y.; Kang, E.S.; Chang, Y.S.; Park, W.S.; Kim, J.H. MERS-CoV infection in a pregnant woman in Korea. J. Korean Med. Sci. 2017, 32, 1717–1720. [Google Scholar] [CrossRef] [PubMed]
- Alfaraj, S.H.; Al-Tawfiq, J.A.; Altuwaijri, T.A.; Memish, Z.A. Middle East respiratory syndrome coronavirus in pediatrics: A report of seven cases from Saudi Arabia. Front. Med. 2018. [Google Scholar] [CrossRef] [PubMed]
- Assiri, A.; Abedi, G.R.; Al, M.M.; Bin, S.A.; Gerber, S.I.; Watson, J.T. Middle East respiratory syndrome coronavirus infection during pregnancy: A Report of 5 cases from Saudi Arabia. Clin. Infect. Dis. 2016, 63, 951–953. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.Y. An outbreak of Middle East respiratory syndrome coronavirus infection in South Korea, 2015. Yonsei Med. J. 2015, 56, 1174–1176. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.S.; Wong, N.S. Probable transmission chains of Middle East respiratory syndrome coronavirus and the multiple generations of secondary infection in South Korea. Int. J. Infect. Dis. 2015, 38, 65–67. [Google Scholar] [CrossRef] [PubMed]
- Anthony, S.J.; Gilardi, K.; Menachery, V.D.; Goldstein, T.; Ssebide, B.; Mbabazi, R.; Navarrete-Macias, I.; Liang, E.; Wells, H.; Hicks, A.; et al. Further evidence for bats as the evolutionary source of Middle East respiratory syndrome coronavirus. MBio 2017, 8, e00373-17. [Google Scholar] [CrossRef]
- Wang, Q.; Qi, J.; Yuan, Y.; Xuan, Y.; Han, P.; Wan, Y.; Ji, W.; Li, Y.; Wu, Y.; Wang, J.; et al. Bat origins of MERS-CoV supported by bat coronavirus HKU4 usage of human receptor CD26. Cell Host Microbe 2014, 16, 328–337. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Du, L.; Liu, C.; Wang, L.; Ma, C.; Tang, J.; Baric, R.S.; Jiang, S.; Li, F. Receptor usage and cell entry of bat coronavirus HKU4 provide insight into bat-to-human transmission of MERS coronavirus. Proc. Natl. Acad. Sci. USA 2014, 111, 12516–12521. [Google Scholar] [CrossRef]
- Munster, V.J.; Adney, D.R.; Van Doremalen, N.; Brown, V.R.; Miazgowicz, K.L.; Milne-Price, S.; Bushmaker, T.; Rosenke, R.; Scott, D.; Hawkinson, A.; et al. Replication and shedding of MERS-CoV in Jamaican fruit bats (Artibeus jamaicensis). Sci. Rep. 2016, 6, 21878. [Google Scholar] [CrossRef]
- Luo, C.M.; Wang, N.; Yang, X.L.; Liu, H.Z.; Zhang, W.; Li, B.; Hu, B.; Peng, C.; Geng, Q.B.; Zhu, G.J.; et al. Discovery of novel bat coronaviruses in south China that use the same receptor as Middle East respiratory syndrome coronavirus. J. Virol. 2018, 92, e00116-18. [Google Scholar] [CrossRef]
- Woo, P.C.Y.; Lau, S.K.P.; Chen, Y.; Wong, E.Y.M.; Chan, K.H.; Chen, H.; Zhang, L.; Xia, N.; Yuen, K.Y. Rapid detection of MERS coronavirus-like viruses in bats: Pote1ntial for tracking MERS coronavirus transmission and animal origin. Emerg. Microbes Infect. 2018, 7, 18. [Google Scholar] [CrossRef] [PubMed]
- Lau, S.K.P.; Zhang, L.; Luk, H.K.H.; Xiong, L.; Peng, X.; Li, K.S.M.; He, X.; Zhao, P.S.; Fan, R.Y.Y.; Wong, A.C.P.; et al. Receptor usage of a novel bat lineage C Betacoronavirus reveals evolution of Middle East respiratory syndrome-related coronavirus spike proteins for human dipeptidyl peptidase 4 binding. J. Infect. Dis. 2018, 218, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Dudas, G.; Carvalho, L.M.; Rambaut, A.; Bedford, T. MERS-CoV spillover at the camel-human interface. Elife 2018, 7, e31257. [Google Scholar] [CrossRef]
- Yusof, M.F.; Eltahir, Y.M.; Serhan, W.S.; Hashem, F.M.; Elsayed, E.A.; Marzoug, B.A.; Abdelazim, A.S.; Bensalah, O.K.; Al Muhairi, S.S. Prevalence of Middle East respiratory syndrome coronavirus (MERS-CoV) in dromedary camels in Abu Dhabi Emirate, United Arab Emirates. Virus Genes 2015, 50, 509–513. [Google Scholar] [CrossRef] [PubMed]
- Haagmans, B.L.; Al Dhahiry, S.H.; Reusken, C.B.; Raj, V.S.; Galiano, M.; Myers, R.; Godeke, G.J.; Jonges, M.; Farag, E.; Diab, A.; et al. Middle East respiratory syndrome coronavirus in dromedary camels: An outbreak investigation. Lancet Infect. Dis. 2014, 14, 140–145. [Google Scholar] [CrossRef]
- Alshukairi, A.N.; Zheng, J.; Zhao, J.; Nehdi, A.; Baharoon, S.A.; Layqah, L.; Bokhari, A.; Al Johani, S.M.; Samman, N.; Boudjelal, M.; et al. High prevalence of MERS-CoV infection in camel workers in Saudi Arabia. MBio 2018, 9, e01985-18. [Google Scholar] [CrossRef]
- Harcourt, J.L.; Rudoler, N.; Tamin, A.; Leshem, E.; Rasis, M.; Giladi, M.; Haynes, L.M. The prevalence of Middle East respiratory syndrome coronavirus (MERS-CoV) antibodies in dromedary camels in Israel. Zoonoses Public Health 2018. [Google Scholar] [CrossRef] [PubMed]
- Saqib, M.; Sieberg, A.; Hussain, M.H.; Mansoor, M.K.; Zohaib, A.; Lattwein, E.; Muller, M.A.; Drosten, C.; Corman, V.M. Serologic evidence for MERS-CoV infection in dromedary camels, Punjab, Pakistan, 2012-2015. Emerg. Infect. Dis. 2017, 23, 550–551. [Google Scholar] [CrossRef] [PubMed]
- Harrath, R.; Abu Duhier, F.M. Sero-prevalence of Middle East respiratory syndrome coronavirus (MERS-CoV) specific antibodies in dromedary camels in Tabuk, Saudi Arabia. J. Med. Virol. 2018, 90, 1285–1289. [Google Scholar] [CrossRef] [PubMed]
- Falzarano, D.; Kamissoko, B.; de Wit, E.; Maiga, O.; Cronin, J.; Samake, K.; Traore, A.; Milne-Price, S.; Munster, V.J.; Sogoba, N.; et al. Dromedary camels in northern Mali have high seropositivity to MERS-CoV. One Health 2017, 3, 41–43. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.; El-Shesheny, R.; Kandeil, A.; Shehata, M.; Elsokary, B.; Gomaa, M.; Hassan, N.; El, S.A.; El-Taweel, A.; Sobhy, H.; et al. Cross-sectional surveillance of Middle East respiratory syndrome coronavirus (MERS-CoV) in dromedary camels and other mammals in Egypt, August 2015 to January 2016. Euro. Surveill 2017, 22, 30487. [Google Scholar] [CrossRef]
- Ali, M.A.; Shehata, M.M.; Gomaa, M.R.; Kandeil, A.; El-Shesheny, R.; Kayed, A.S.; El-Taweel, A.N.; Atea, M.; Hassan, N.; Bagato, O.; et al. Systematic, active surveillance for Middle East respiratory syndrome coronavirus in camels in Egypt. Emerg. Microbes Infect. 2017, 6. [Google Scholar] [CrossRef] [PubMed]
- Deem, S.L.; Fevre, E.M.; Kinnaird, M.; Browne, A.S.; Muloi, D.; Godeke, G.J.; Koopmans, M.; Reusken, C.B. Serological evidence of MERS-CoV antibodies in dromedary camels (Camelus dromedaries) in Laikipia county, Kenya. PLoS ONE 2015, 10, e0140125. [Google Scholar] [CrossRef] [PubMed]
- Conzade, R.; Grant, R.; Malik, M.R.; Elkholy, A.; Elhakim, M.; Samhouri, D.; Ben Embarek, P.K.; Van Kerkhove, M.D. Reported direct and indirect contact with dromedary camels among laboratory-confirmed MERS-CoV cases. Viruses 2018, 10, 425. [Google Scholar] [CrossRef]
- Hui, D.S.; Azhar, E.I.; Kim, Y.J.; Memish, Z.A.; Oh, M.D.; Zumla, A. Middle East respiratory syndrome coronavirus: Risk factors and determinants of primary, household, and nosocomial transmission. Lancet Infect. Dis. 2018, 18, e217–e227. [Google Scholar] [CrossRef]
- Amer, H.; Alqahtani, A.S.; Alzoman, H.; Aljerian, N.; Memish, Z.A. Unusual presentation of Middle East respiratory syndrome coronavirus leading to a large outbreak in Riyadh during 2017. Am. J. Infect. Control. 2018, 46, 1022–1025. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Kim, Y.S.; Jung, Y.; Choi, S.Y.; Cho, N.H.; Jeong, H.W.; Heo, J.Y.; Yoon, J.H.; Lee, J.; Cheon, S.; et al. Outbreaks of Middle East respiratory syndrome in two hospitals initiated by a single patient in Daejeon, South Korea. Infect. Chemother. 2016, 48, 99–107. [Google Scholar] [CrossRef]
- Cho, S.Y.; Kang, J.M.; Ha, Y.E.; Park, G.E.; Lee, J.Y.; Ko, J.H.; Lee, J.Y.; Kim, J.M.; Kang, C.I.; Jo, I.J.; et al. MERS-CoV outbreak following a single patient exposure in an emergency room in South Korea: An epidemiological outbreak study. Lancet 2016, 388, 994–1001. [Google Scholar] [CrossRef]
- Hastings, D.L.; Tokars, J.I.; Abdel Aziz, I.Z.; Alkhaldi, K.Z.; Bensadek, A.T.; Alraddadi, B.M.; Jokhdar, H.; Jernigan, J.A.; Garout, M.A.; Tomczyk, S.M.; et al. Outbreak of Middle East respiratory syndrome at tertiary care hospital, Jeddah, Saudi Arabia, 2014. Emerg. Infect. Dis. 2016, 22, 794–801. [Google Scholar] [CrossRef]
- Hunter, J.C.; Nguyen, D.; Aden, B.; Al, B.Z.; Al, D.W.; Abu, E.K.; Khudair, A.; Al, M.M.; El, S.F.; Imambaccus, H.; et al. Transmission of Middle East respiratory syndrome coronavirus infections in healthcare settings, Abu Dhabi. Emerg. Infect. Dis. 2016, 22, 647–656. [Google Scholar] [CrossRef]
- Harriman, K.; Brosseau, L.; Trivedi, K. Hospital-associated Middle East respiratory syndrome coronavirus infections. N. Engl. J. Med. 2013, 369, 1761. [Google Scholar] [PubMed]
- Memish, Z.A.; Al-Tawfiq, J.A.; Assiri, A. Hospital-associated Middle East respiratory syndrome coronavirus infections. N. Engl. J. Med. 2013, 369, 1761–1762. [Google Scholar] [CrossRef] [PubMed]
- Memish, Z.A.; Zumla, A.I.; Al-Hakeem, R.F.; Al-Rabeeah, A.A.; Stephens, G.M. Family cluster of Middle East respiratory syndrome coronavirus infections. N. Engl. J. Med. 2013, 368, 2487–2494. [Google Scholar] [CrossRef] [PubMed]
- Al-Tawfiq, J.A.; Auwaerter, P.G. Healthcare-associated infections: The hallmark of Middle East respiratory syndrome coronavirus with review of the literature. J. Hosp. Infect. 2019, 101, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Guery, B.; Poissy, J.; El, M.L.; Sejourne, C.; Ettahar, N.; Lemaire, X.; Vuotto, F.; Goffard, A.; Behillil, S.; Enouf, V.; et al. Clinical features and viral diagnosis of two cases of infection with Middle East respiratory syndrome coronavirus: A report of nosocomial transmission. Lancet 2013, 381, 2265–2272. [Google Scholar] [CrossRef]
- Arabi, Y.M.; Arifi, A.A.; Balkhy, H.H.; Najm, H.; Aldawood, A.S.; Ghabashi, A.; Hawa, H.; Alothman, A.; Khaldi, A.; Al, R.B. Clinical course and outcomes of critically ill patients with Middle East respiratory syndrome coronavirus infection. Ann. Intern. Med. 2014, 160, 389–397. [Google Scholar] [CrossRef]
- Zhou, J.; Li, C.; Zhao, G.; Chu, H.; Wang, D.; Yan, H.H.; Poon, V.K.; Wen, L.; Wong, B.H.; Zhao, X.; et al. Human intestinal tract serves as an alternative infection route for Middle East respiratory syndrome coronavirus. Sci. Adv. 2017, 3, eaao4966. [Google Scholar] [CrossRef]
- Payne, D.C.; Iblan, I.; Rha, B.; Alqasrawi, S.; Haddadin, A.; Al, N.M.; Alsanouri, T.; Ali, S.S.; Harcourt, J.; Miao, C.; et al. Persistence of antibodies against Middle East respiratory syndrome coronavirus. Emerg. Infect. Dis. 2016, 22, 1824–1826. [Google Scholar] [CrossRef]
- Muller, M.A.; Meyer, B.; Corman, V.M.; Al-Masri, M.; Turkestani, A.; Ritz, D.; Sieberg, A.; Aldabbagh, S.; Bosch, B.J.; Lattwein, E.; et al. Presence of Middle East respiratory syndrome coronavirus antibodies in Saudi Arabia: A nationwide, cross-sectional, serological study. Lancet Infect. Dis. 2015, 15, 559–564. [Google Scholar] [CrossRef]
- Arabi, Y.M.; Hajeer, A.H.; Luke, T.; Raviprakash, K.; Balkhy, H.; Johani, S.; Al-Dawood, A.; Al-Qahtani, S.; Al-Omari, A.; Al-Hameed, F.; et al. Feasibility of using convalescent plasma immunotherapy for MERS-CoV infection, Saudi Arabia. Emerg. Infect. Dis. 2016, 22, 1554–1561. [Google Scholar] [CrossRef] [PubMed]
- Arabi, Y.; Balkhy, H.; Hajeer, A.H.; Bouchama, A.; Hayden, F.G.; Al-Omari, A.; Al-Hameed, F.M.; Taha, Y.; Shindo, N.; Whitehead, J.; et al. Feasibility, safety, clinical, and laboratory effects of convalescent plasma therapy for patients with Middle East respiratory syndrome coronavirus infection: A study protocol. Springerplus 2015, 4, 709. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.H.; Seok, H.; Cho, S.Y.; Ha, Y.E.; Baek, J.Y.; Kim, S.H.; Kim, Y.J.; Park, J.K.; Chung, C.R.; Kang, E.S.; et al. Challenges of convalescent plasma infusion therapy in Middle East respiratory coronavirus infection: A single centre experience. Antivir. Ther. 2018, 23, 617–622. [Google Scholar] [CrossRef] [PubMed]
- Beigel, J.H.; Voell, J.; Kumar, P.; Raviprakash, K.; Wu, H.; Jiao, J.A.; Sullivan, E.; Luke, T.; Davey, R.T., Jr. Safety and tolerability of a novel, polyclonal human anti-MERS coronavirus antibody produced from transchromosomic cattle: A phase 1 randomised, double-blind, single-dose-escalation study. Lancet Infect. Dis. 2018, 18, 410–418. [Google Scholar] [CrossRef]
- Zhou, Y.; Jiang, S.; Du, L. Prospects for a MERS-CoV spike vaccine. Expert. Rev. Vaccines 2018, 17, 677–686. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Jiang, S.; Du, L. Current advancements and potential strategies in the development of MERS-CoV vaccines. Expert. Rev. Vaccines 2014, 13, 761–774. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.F.; Li, K.S.; To, K.K.; Cheng, V.C.; Chen, H.; Yuen, K.Y. Is the discovery of the novel human betacoronavirus 2c EMC/2012 (HCoV-EMC) the beginning of another SARS-like pandemic? J. Infect. 2012, 65, 477–489. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Yang, Y.; Zhou, Y.; Lu, L.; Li, F.; Jiang, S. MERS-CoV spike protein: A key target for antivirals. Expert. Opin. Ther. Targets 2017, 21, 131–143. [Google Scholar] [CrossRef]
- Van Boheemen, S.; de Graaf, M.; Lauber, C.; Bestebroer, T.M.; Raj, V.S.; Zaki, A.M.; Osterhaus, A.D.; Haagmans, B.L.; Gorbalenya, A.E.; Snijder, E.J.; et al. Genomic characterization of a newly discovered coronavirus associated with acute respiratory distress syndrome in humans. MBio 2012, 3, e00473-12. [Google Scholar] [CrossRef]
- Almazan, F.; DeDiego, M.L.; Sola, I.; Zuniga, S.; Nieto-Torres, J.L.; Marquez-Jurado, S.; Andres, G.; Enjuanes, L. Engineering a replication-competent, propagation-defective Middle East respiratory syndrome coronavirus as a vaccine candidate. MBio 2013, 4, e00650-13. [Google Scholar] [CrossRef]
- Scobey, T.; Yount, B.L.; Sims, A.C.; Donaldson, E.F.; Agnihothram, S.S.; Menachery, V.D.; Graham, R.L.; Swanstrom, J.; Bove, P.F.; Kim, J.D.; et al. Reverse genetics with a full-length infectious cDNA of the Middle East respiratory syndrome coronavirus. Proc. Natl. Acad. Sci. USA 2013, 110, 16157–16162. [Google Scholar] [CrossRef]
- Nakagawa, K.; Narayanan, K.; Wada, M.; Popov, V.L.; Cajimat, M.; Baric, R.S.; Makino, S. The endonucleolytic RNA cleavage function of nsp1 of Middle East respiratory syndrome coronavirus promotes the production of infectious virus particles in specific human cell lines. J. Virol. 2018, 92, e01157-18. [Google Scholar] [CrossRef]
- Terada, Y.; Kawachi, K.; Matsuura, Y.; Kamitani, W. MERS coronavirus nsp1 participates in an efficient propagation through a specific interaction with viral RNA. Virology 2017, 511, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Menachery, V.D.; Gralinski, L.E.; Mitchell, H.D.; Dinnon, K.H., 3rd; Leist, S.R.; Yount, B.L., Jr.; Graham, R.L.; McAnarney, E.T.; Stratton, K.G.; Cockrell, A.S.; et al. Middle East respiratory syndrome coronavirus nonstructural protein 16 Is necessary for interferon resistance and viral pathogenesis. mSphere 2017, 2, e00346-17. [Google Scholar] [CrossRef]
- Zhang, L.; Li, L.; Yan, L.; Ming, Z.; Jia, Z.; Lou, Z.; Rao, Z. Structural and biochemical characterization of endoribonuclease Nsp15 encoded by Middle East respiratory syndrome coronavirus. J. Virol. 2018, 92, e00893-18. [Google Scholar] [CrossRef]
- Batool, M.; Shah, M.; Patra, M.C.; Yesudhas, D.; Choi, S. Structural insights into the Middle East respiratory syndrome coronavirus 4a protein and its dsRNA binding mechanism. Sci. Rep. 2017, 7, 11362. [Google Scholar] [CrossRef] [PubMed]
- Rabouw, H.H.; Langereis, M.A.; Knaap, R.C.; Dalebout, T.J.; Canton, J.; Sola, I.; Enjuanes, L.; Bredenbeek, P.J.; Kikkert, M.; de Groot, R.J.; et al. Middle East respiratory coronavirus accessory protein 4a inhibits PKR-mediated antiviral stress responses. PLoS Pathog. 2016, 12, e1005982. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, K.; Narayanan, K.; Wada, M.; Makino, S. Inhibition of stress granule formation by Middle East respiratory syndrome coronavirus 4a accessory protein facilitates viral translation, leading to efficient virus replication. J. Virol. 2018, 92, e00902-18. [Google Scholar] [CrossRef]
- Canton, J.; Fehr, A.R.; Fernandez-Delgado, R.; Gutierrez-Alvarez, F.J.; Sanchez-Aparicio, M.T.; Garcia-Sastre, A.; Perlman, S.; Enjuanes, L.; Sola, I. MERS-CoV 4b protein interferes with the NF-kappaB-dependent innate immune response during infection. PLoS Pathog. 2018, 14, e1006838. [Google Scholar] [CrossRef]
- Park, J.E.; Li, K.; Barlan, A.; Fehr, A.R.; Perlman, S.; McCray, P.B., Jr.; Gallagher, T. Proteolytic processing of Middle East respiratory syndrome coronavirus spikes expands virus tropism. Proc. Natl. Acad. Sci. USA 2016, 113, 12262–12267. [Google Scholar] [CrossRef]
- Millet, J.K.; Whittaker, G.R. Host cell entry of Middle East respiratory syndrome coronavirus after two-step, furin-mediated activation of the spike protein. Proc. Natl. Acad. Sci. USA 2014, 111, 15214–15219. [Google Scholar] [CrossRef] [PubMed]
- Li, F. Structure, function, and evolution of coronavirus spike proteins. Annu. Rev. Virol. 2016, 3, 237–261. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Wong, G.; Lu, G.; Yan, J.; Gao, G.F. MERS-CoV spike protein: Targets for vaccines and therapeutics. Antiviral Res. 2016, 133, 165–177. [Google Scholar] [CrossRef] [PubMed]
- Raj, V.S.; Mou, H.; Smits, S.L.; Dekkers, D.H.; Muller, M.A.; Dijkman, R.; Muth, D.; Demmers, J.A.; Zaki, A.; Fouchier, R.A.; et al. Dipeptidyl peptidase 4 is a functional receptor for the emerging human coronavirus-EMC. Nature 2013, 495, 251–254. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Lu, G.; Qi, J.; Li, Y.; Wu, Y.; Deng, Y.; Geng, H.; Li, H.; Wang, Q.; Xiao, H.; et al. Structure of the fusion core and inhibition of fusion by a heptad repeat peptide derived from the S protein of Middle East respiratory syndrome coronavirus. J. Virol. 2013, 87, 13134–13140. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Liu, Q.; Zhu, Y.; Chan, K.H.; Qin, L.; Li, Y.; Wang, Q.; Chan, J.F.; Du, L.; Yu, F.; et al. Structure-based discovery of Middle East respiratory syndrome coronavirus fusion inhibitor. Nat. Commun. 2014, 5, 3067. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Rajashankar, K.R.; Yang, Y.; Agnihothram, S.S.; Liu, C.; Lin, Y.L.; Baric, R.S.; Li, F. Crystal structure of the receptor-binding domain from newly emerged Middle East respiratory syndrome coronavirus. J. Virol. 2013, 87, 10777–10783. [Google Scholar] [CrossRef] [PubMed]
- Matsuyama, S.; Shirato, K.; Kawase, M.; Terada, Y.; Kawachi, K.; Fukushi, S.; Kamitani, W. Middle East respiratory syndrome coronavirus spike protein is not activated directly by cellular furin during viral entry into target cells. J. Virol. 2018, 92, e00683-18. [Google Scholar] [CrossRef]
- Barlan, A.; Zhao, J.; Sarkar, M.K.; Li, K.; McCray, P.B., Jr.; Perlman, S.; Gallagher, T. Receptor variation and susceptibility to Middle East respiratory syndrome coronavirus infection. J. Virol. 2014, 88, 4953–4961. [Google Scholar] [CrossRef]
- Van Doremalen, N.; Miazgowicz, K.L.; Milne-Price, S.; Bushmaker, T.; Robertson, S.; Scott, D.; Kinne, J.; McLellan, J.S.; Zhu, J.; Munster, V.J. Host species restriction of Middle East respiratory syndrome coronavirus through its receptor, dipeptidyl peptidase 4. J. Virol. 2014, 88, 9220–9232. [Google Scholar]
- Letko, M.; Miazgowicz, K.; McMinn, R.; Seifert, S.N.; Sola, I.; Enjuanes, L.; Carmody, A.; van Doremalen, N.; Munster, V. Adaptive evolution of MERS-CoV to species variation in DPP4. Cell Rep. 2018, 24, 1730–1737. [Google Scholar] [CrossRef]
- Chu, H.; Chan, C.M.; Zhang, X.; Wang, Y.; Yuan, S.; Zhou, J.; Au-Yeung, R.K.; Sze, K.H.; Yang, D.; Shuai, H.; et al. Middle East respiratory syndrome coronavirus and bat coronavirus HKU9 both can utilize GRP78 for attachment onto host cells. J. Biol. Chem. 2018, 293, 11709–11726. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Hulswit, R.J.G.; Widjaja, I.; Raj, V.S.; McBride, R.; Peng, W.; Widagdo, W.; Tortorici, M.A.; van Dieren, B.; Lang, Y.; et al. Identification of sialic acid-binding function for the Middle East respiratory syndrome coronavirus spike glycoprotein. Proc. Natl. Acad. Sci. USA 2017, 114, E8508–E8517. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Shi, X.; Jiang, L.; Zhang, S.; Wang, D.; Tong, P.; Guo, D.; Fu, L.; Cui, Y.; Liu, X.; et al. Structure of MERS-CoV spike receptor-binding domain complexed with human receptor DPP4. Cell Res. 2013, 23, 986–993. [Google Scholar] [CrossRef] [PubMed]
- Lu, G.; Hu, Y.; Wang, Q.; Qi, J.; Gao, F.; Li, Y.; Zhang, Y.; Zhang, W.; Yuan, Y.; Bao, J.; et al. Molecular basis of binding between novel human coronavirus MERS-CoV and its receptor CD26. Nature 2013, 500, 227–231. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Cao, D.; Zhang, Y.; Ma, J.; Qi, J.; Wang, Q.; Lu, G.; Wu, Y.; Yan, J.; Shi, Y.; et al. Cryo-EM structures of MERS-CoV and SARS-CoV spike glycoproteins reveal the dynamic receptor binding domains. Nat. Commun. 2017, 8, 15092. [Google Scholar] [CrossRef]
- Pallesen, J.; Wang, N.; Corbett, K.S.; Wrapp, D.; Kirchdoerfer, R.N.; Turner, H.L.; Cottrell, C.A.; Becker, M.M.; Wang, L.; Shi, W.; et al. Immunogenicity and structures of a rationally designed prefusion MERS-CoV spike antigen. Proc. Natl Acad. Sci. USA 2017, 114, E7348–E7357. [Google Scholar] [CrossRef]
- Lan, J.; Deng, Y.; Chen, H.; Lu, G.; Wang, W.; Guo, X.; Lu, Z.; Gao, G.F.; Tan, W. Tailoring subunit vaccine immunity with adjuvant combinations and delivery routes using the Middle East respiratory coronavirus (MERS-CoV) receptor-binding domain as an antigen. PLoS ONE 2014, 9, e112602. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Son, A.; Kim, J.; Kwon, S.B.; Kim, M.H.; Kim, P.; Kim, J.; Byun, Y.H.; Sung, J.; Lee, J.; et al. Chaperna-mediated assembly of ferritin-based Middle East respiratory syndrome-coronavirus nanoparticles. Front. Immunol. 2018, 9, 1093. [Google Scholar] [CrossRef]
- Wang, C.; Zheng, X.; Gai, W.; Wong, G.; Wang, H.; Jin, H.; Feng, N.; Zhao, Y.; Zhang, W.; Li, N.; et al. Novel chimeric virus-like particles vaccine displaying MERS-CoV receptor-binding domain induce specific humoral and cellular immune response in mice. Antiviral Res. 2017, 140, 55–61. [Google Scholar] [CrossRef]
- Lan, J.; Yao, Y.; Deng, Y.; Chen, H.; Lu, G.; Wang, W.; Bao, L.; Deng, W.; Wei, Q.; Gao, G.F.; et al. Recombinant receptor binding domain protein induces partial protective immunity in Rhesus Macaques against Middle East respiratory syndrome coronavirus challenge. EBioMedicine 2015, 2, 1438–1446. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Channappanavar, R.; Ma, C.; Wang, L.; Tang, J.; Garron, T.; Tao, X.; Tasneem, S.; Lu, L.; Tseng, C.T.; et al. Identification of an ideal adjuvant for receptor-binding domain-based subunit vaccines against Middle East respiratory syndrome coronavirus. Cell Mol. Immunol. 2016, 13, 180–190. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Tai, W.; Yang, Y.; Zhao, G.; Zhu, Q.; Sun, S.; Liu, C.; Tao, X.; Tseng, C.K.; Perlman, S.; et al. Introduction of neutralizing immunogenicity index to the rational design of MERS coronavirus subunit vaccines. Nat. Commun. 2016, 7, 13473. [Google Scholar] [CrossRef] [PubMed]
- Tai, W.; Zhao, G.; Sun, S.; Guo, Y.; Wang, Y.; Tao, X.; Tseng, C.K.; Li, F.; Jiang, S.; Du, L.; et al. A recombinant receptor-binding domain of MERS-CoV in trimeric form protects human dipeptidyl peptidase 4 (hDPP4) transgenic mice from MERS-CoV infection. Virology 2016, 499, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Wang, L.; Tao, X.; Zhang, N.; Yang, Y.; Tseng, C.T.; Li, F.; Zhou, Y.; Jiang, S.; Du, L. Searching for an ideal vaccine candidate among different MERS coronavirus receptor-binding fragments--the importance of immunofocusing in subunit vaccine design. Vaccine 2014, 32, 6170–6176. [Google Scholar] [CrossRef]
- Zhang, N.; Tang, J.; Lu, L.; Jiang, S.; Du, L. Receptor-binding domain-based subunit vaccines against MERS-CoV. Virus Res. 2015, 202, 151–159. [Google Scholar] [CrossRef]
- Tang, J.; Zhang, N.; Tao, X.; Zhao, G.; Guo, Y.; Tseng, C.T.; Jiang, S.; Du, L.; Zhou, Y. Optimization of antigen dose for a receptor-binding domain-based subunit vaccine against MERS coronavirus. Hum. Vaccin Immunother. 2015, 11, 1244–1250. [Google Scholar] [CrossRef]
- Wang, Y.; Tai, W.; Yang, J.; Zhao, G.; Sun, S.; Tseng, C.K.; Jiang, S.; Zhou, Y.; Du, L.; Gao, J. Receptor-binding domain of MERS-CoV with optimal immunogen dosage and immunization interval protects human transgenic mice from MERS-CoV infection. Hum. Vaccin Immunother. 2017, 13, 1615–1624. [Google Scholar] [CrossRef]
- Nyon, M.P.; Du, L.; Tseng, C.K.; Seid, C.A.; Pollet, J.; Naceanceno, K.S.; Agrawal, A.; Algaissi, A.; Peng, B.H.; Tai, W.; et al. Engineering a stable CHO cell line for the expression of a MERS-coronavirus vaccine antigen. Vaccine 2018, 36, 1853–1862. [Google Scholar] [CrossRef]
- Tai, W.; Wang, Y.; Fett, C.A.; Zhao, G.; Li, F.; Perlman, S.; Jiang, S.; Zhou, Y.; Du, L. Recombinant receptor-binding domains of multiple Middle East respiratory syndrome coronaviruses (MERS-CoVs) induce cross-neutralizing antibodies against divergent human and camel MERS-CoVs and antibody escape mutants. J. Virol. 2017, 91, e01651-16. [Google Scholar] [CrossRef]
- Ma, C.; Li, Y.; Wang, L.; Zhao, G.; Tao, X.; Tseng, C.T.; Zhou, Y.; Du, L.; Jiang, S. Intranasal vaccination with recombinant receptor-binding domain of MERS-CoV spike protein induces much stronger local mucosal immune responses than subcutaneous immunization: Implication for designing novel mucosal MERS vaccines. Vaccine 2014, 32, 2100–2108. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Shi, W.; Chappell, J.D.; Joyce, M.G.; Zhang, Y.; Kanekiyo, M.; Becker, M.M.; van Doremalen, N.; Fischer, R.; Wang, N.; et al. Importance of neutralizing monoclonal antibodies targeting multiple antigenic sites on MERS-CoV Spike to avoid neutralization escape. J. Virol 2018, JVI.02002-17. [Google Scholar]
- Li, Y.; Wan, Y.; Liu, P.; Zhao, J.; Lu, G.; Qi, J.; Wang, Q.; Lu, X.; Wu, Y.; Liu, W.; et al. A humanized neutralizing antibody against MERS-CoV targeting the receptor-binding domain of the spike protein. Cell. Res. 2015, 25, 1237–1249. [Google Scholar] [CrossRef] [PubMed]
- Corti, D.; Zhao, J.; Pedotti, M.; Simonelli, L.; Agnihothram, S.; Fett, C.; Fernandez-Rodriguez, B.; Foglierini, M.; Agatic, G.; Vanzetta, F.; et al. Prophylactic and postexposure efficacy of a potent human monoclonal antibody against MERS coronavirus. Proc. Natl. Acad. Sci. USA 2015, 112, 10473–10478. [Google Scholar] [CrossRef]
- Pascal, K.E.; Coleman, C.M.; Mujica, A.O.; Kamat, V.; Badithe, A.; Fairhurst, J.; Hunt, C.; Strein, J.; Berrebi, A.; Sisk, J.M.; et al. Pre- and postexposure efficacy of fully human antibodies against Spike protein in a novel humanized mouse model of MERS-CoV infection. Proc. Natl. Acad. Sci. USA 2015, 112, 8738–8743. [Google Scholar] [CrossRef] [PubMed]
- Ying, T.; Prabakaran, P.; Du, L.; Shi, W.; Feng, Y.; Wang, Y.; Wang, L.; Li, W.; Jiang, S.; Dimitrov, D.S.; et al. Junctional and allele-specific residues are critical for MERS-CoV neutralization by an exceptionally potent germline-like antibody. Nat. Commun. 2015, 6, 8223. [Google Scholar] [CrossRef] [PubMed]
- Qiu, H.; Sun, S.; Xiao, H.; Feng, J.; Guo, Y.; Tai, W.; Wang, Y.; Du, L.; Zhao, G.; Zhou, Y. Single-dose treatment with a humanized neutralizing antibody affords full protection of a human transgenic mouse model from lethal Middle East respiratory syndrome (MERS)-coronavirus infection. Antiviral Res. 2016, 132, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Zhao, G.; Yang, Y.; Qiu, H.; Wang, L.; Kou, Z.; Tao, X.; Yu, H.; Sun, S.; Tseng, C.T.; et al. A conformation-dependent neutralizing monoclonal antibody specifically targeting receptor-binding domain in Middle East respiratory syndrome coronavirus spike protein. J. Virol. 2014, 88, 7045–7053. [Google Scholar] [CrossRef] [PubMed]
- Ying, T.; Du, L.; Ju, T.W.; Prabakaran, P.; Lau, C.C.; Lu, L.; Liu, Q.; Wang, L.; Feng, Y.; Wang, Y.; et al. Exceptionally potent neutralization of middle East respiratory syndrome coronavirus by human monoclonal antibodies. J. Virol. 2014, 88, 7796–7805. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Jiang, S. Middle East respiratory syndrome: Current status and future prospects for vaccine development. Expert. Opin. Biol. Ther. 2015, 15, 1647–1651. [Google Scholar] [CrossRef]
- Wang, L.; Shi, L.; Joyce, M.G.; Modjarrad, K.; Zhang, Y.; Leung, K.; Lees, C.R.; Zhou, T.; Yassine, H.M.; Kanekiyo, M.; et al. Evaluation of candidate vaccine approaches for MERS-CoV. Nat. Commun. 2015, 6, 7712. [Google Scholar] [CrossRef] [PubMed]
- Niu, P.; Zhao, G.; Deng, Y.; Sun, S.; Wang, W.; Zhou, Y.; Tan, W. A novel human mAb (MERS-GD27) provides prophylactic and postexposure efficacy in MERS-CoV susceptible mice. Sci. China Life Sci. 2018, 61, 1280–1282. [Google Scholar] [CrossRef] [PubMed]
- Niu, P.; Zhang, S.; Zhou, P.; Huang, B.; Deng, Y.; Qin, K.; Wang, P.; Wang, W.; Wang, X.; Zhou, J.; et al. Ultrapotent human neutralizing antibody repertoires against Middle East respiratory syndrome coronavirus from a recovered patient. J. Infect Dis. 2018, 218, 1249–1260. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Bao, L.; Chen, C.; Zou, T.; Xue, Y.; Li, F.; Lv, Q.; Gu, S.; Gao, X.; Cui, S.; et al. Human neutralizing monoclonal antibody inhibition of Middle East respiratory syndrome coronavirus replication in the common marmoset. J. Infect Dis. 2017, 215, 1807–1815. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhou, P.; Wang, P.; Li, Y.; Jiang, L.; Jia, W.; Wang, H.; Fan, A.; Wang, D.; Shi, X.; et al. Structural definition of a unique neutralization epitope on the receptor-binding domain of MERS-CoV spike glycoprotein. Cell Rep. 2018, 24, 441–452. [Google Scholar] [CrossRef] [PubMed]
- Raj, V.S.; Okba, N.M.A.; Gutierrez-Alvarez, J.; Drabek, D.; van Dieren, B.; Widagdo, W.; Lamers, M.M.; Widjaja, I.; Fernandez-Delgado, R.; Sola, I.; et al. Chimeric camel/human heavy-chain antibodies protect against MERS-CoV infection. Sci. Adv. 2018, 4, eaas9667. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; He, L.; Sun, S.; Qiu, H.; Tai, W.; Chen, J.; Li, J.; Chen, Y.; Guo, Y.; Wang, Y.; et al. A novel nanobody targeting Middle East respiratory syndrome coronavirus (MERS-CoV) receptor-binding domain has potent cross-neutralizing activity and protective efficacy against MERS-CoV. J. Virol 2018, 92, e00837-18. [Google Scholar] [CrossRef]
- Yu, X.; Zhang, S.; Jiang, L.; Cui, Y.; Li, D.; Wang, D.; Wang, N.; Fu, L.; Shi, X.; Li, Z.; et al. Structural basis for the neutralization of MERS-CoV by a human monoclonal antibody MERS-27. Sci Rep. 2015, 5, 13133. [Google Scholar] [CrossRef]
- Wilken, L.; McPherson, A. Application of camelid heavy-chain variable domains (VHHs) in prevention and treatment of bacterial and viral infections. Int. Rev. Immunol. 2018, 37, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Van Heeke, G.; Allosery, K.; De Brabandere, V.; De Smedt, T.; Detalle, L.; de Fougerolles, A. Nanobodies(R) as inhaled biotherapeutics for lung diseases. Pharmacol. Ther. 2017, 169, 47–56. [Google Scholar]
- Detalle, L.; Stohr, T.; Palomo, C.; Piedra, P.A.; Gilbert, B.E.; Mas, V.; Millar, A.; Power, U.F.; Stortelers, C.; Allosery, K.; et al. Generation and characterization of ALX-0171, a potent novel therapeutic nanobody for the treatment of respiratory syncytial virus infection. Antimicrob. Agents Chemother. 2015, 60, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Steeland, S.; Vandenbroucke, R.E.; Libert, C. Nanobodies as therapeutics: Big opportunities for small antibodies. Drug Discov. Today 2016, 21, 1076–1113. [Google Scholar] [CrossRef] [PubMed]
- Muyldermans, S. Nanobodies: Natural single-domain antibodies. Annu. Rev. Biochem. 2013, 82, 775–797. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Tai, W.; Zhou, Y.; Jiang, S. Vaccines for the prevention against the threat of MERS-CoV. Expert Rev. Vaccines 2016, 15, 1123–1134. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Shen, L.; Gu, X. Evolutionary dynamics of MERS-CoV: Potential recombination, positive selection and transmission. Sci. Rep. 2016, 6, 25049. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Cheon, S.; Min, C.K.; Sohn, K.M.; Kang, Y.J.; Cha, Y.J.; Kang, J.I.; Han, S.K.; Ha, N.Y.; Kim, G.; et al. Spread of mutant Middle East respiratory syndrome coronavirus with reduced affinity to human CD26 during the South Korean outbreak. MBio 2016, 7, e00019. [Google Scholar] [CrossRef] [PubMed]
- Kleine-Weber, H.; Elzayat, M.T.; Wang, L.; Graham, B.S.; Muller, M.A.; Drosten, C.; Pohlmann, S.; Hoffmann, M. Mutations in the spike protein of Middle East respiratory syndrome coronavirus transmitted in Korea increase resistance to antibody-mediated neutralization. J. Virol. 2019, 93, e01381-18. [Google Scholar] [CrossRef]
- Wang, C.; Hua, C.; Xia, S.; Li, W.; Lu, L.; Jiang, S. Combining a fusion inhibitory peptide targeting the MERS-CoV S2 protein HR1 domain and a neutralizing antibody specific for the S1 protein receptor-binding domain (RBD) showed potent synergism against pseudotyped MERS-CoV with or without mutations in RBD. Viruses 2019, 11, 31. [Google Scholar] [CrossRef]
- Galasiti Kankanamalage, A.C.; Kim, Y.; Damalanka, V.C.; Rathnayake, A.D.; Fehr, A.R.; Mehzabeen, N.; Battaile, K.P.; Lovell, S.; Lushington, G.H.; Perlman, S.; et al. Structure-guided design of potent and permeable inhibitors of MERS coronavirus 3CL protease that utilize a piperidine moiety as a novel design element. Eur. J. Med. Chem 2018, 150, 334–346. [Google Scholar] [CrossRef]
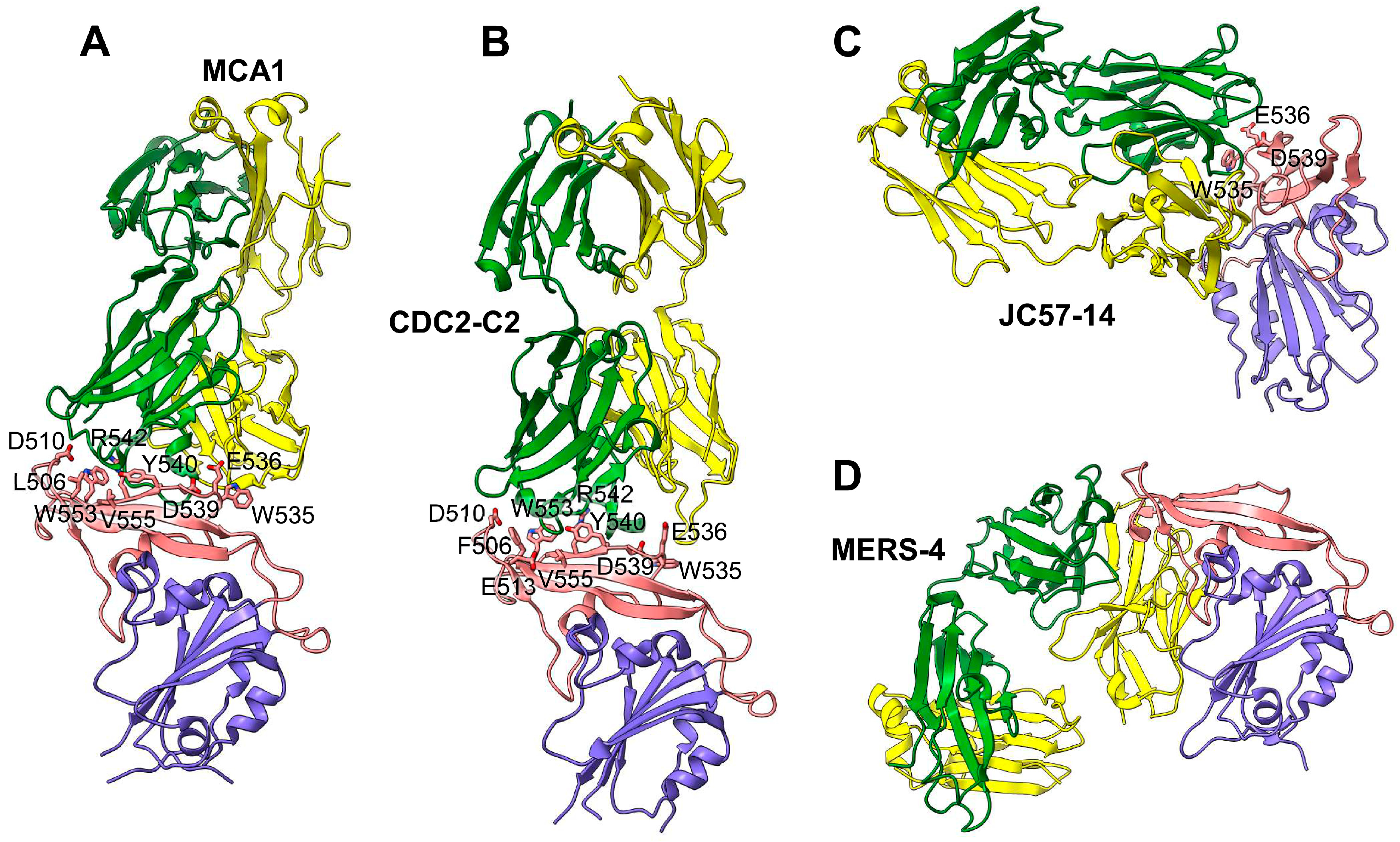
| Name | Functionality and Antigenicity | Immunogenicity in Induction of Antibody Response | Immunogenicity in Induction of Cellular Immune Response | Protective Immunity | Ref. |
|---|---|---|---|---|---|
| RBD-[SSG]-FR and RBD-FR nanoparticles | Bind to DPP4 receptor; antisera block RBD-hDPP4 binding | Induce MERS-CoV RBD-specific antibodies (IgG, IgG1, IgG2a, IgG2b, IgA) in mice | Elicit MERS-CoV RBD-specific T-cell responses (IFN-γ, TNF-α) in mouse splenocytes | N/A | [89] |
| sVLP (spherical virus-like particle) | N/A | Induces MERS-CoV RBD-specific antibodies (IgG) in mice, neutralizing pseudotyped MERS-CoV (1:320) | Elicits MERS-CoV RBD-specific cellular immune response (IFN-γ, IL-2, IL-4) in mouse splenocytes | N/A | [90] |
| rRBD (recombinant RBD) | N/A | Induces MERS-CoV RBD-specific antibodies (IgG, IgG1, IgG2a) in mice or NHPs, neutralizing pseudotyped (1:800 to 1:1,600) and live (EMC2012: 1:269 to 1:363) MERS-CoV | Elicits MERS-CoV RBD-specific cellular immune response (TNF-α, IFN-γ, IL-2, IL-4, IL-6) in mouse splenocytes or monkey PBMCs | Partially protects NHPs from MERS-CoV (EMC2012: 6.5 × 107 TCID50) infection with alleviated pneumonia and decreased viral load | [88,91] |
| RBD (S377-588)-Fc | Binds strongly to soluble and cell-associated hDPP4 or cDPP4 receptors and MERS-CoV RBD-specific neutralizing mAbs (Mersmab1, m336, m337, m338) | Induces MERS-CoV S1-specific antibodies (IgG, IgG1, IgG2a) in mice and rabbits, cross-neutralizing 17 pseudotyped (>1:104), 2 live (EMC2012, London1-2012: ≥1:103) MERS-CoV, and 5 mAb escape mutants (>1:104) | Elicits MERS-CoV S1-specific cellular immune responses (IFN-γ, IL-2) in mouse splenocytes | Protects Ad5/hDPP4-transduced BALB/c mice and hDPP4-Tg mice (67% survival rate) from challenge by MERS-CoV (EMC2012: 105 PFU for BALB/c; 103–104 TCID50 for Tg), without toxicity or immune enhancement | [92,95,96,97,98,99] |
| 2012-RBD 2013-RBD 2014-RBD 2015-RBD Camel-RBD | Bind strongly to hDPP4 and cDPP4 receptors and MERS-CoV RBD-specific mAbs (Mersmab1, m336, m337, m338) with high affinity | Induce MERS-CoV S1-specific antibodies (IgG, IgG1, IgG2a) in mice, potently cross-neutralizing 17 pseudotyped (≥1:104), 2 live (EMC2012, London1-2012: >1:102) MERS-CoV, and 5 mAb escape mutants (≥1:104) | N/A | N/A | [100] |
| RBD-Fd | Binds strongly to soluble and cell-associated hDPP4 receptors and MERS-CoV RBD-specific mAbs (Mersmab1, m336, m337, m338) | Induces robust and long-term MERS-CoV S1-specific antibodies (IgG, IgG1, IgG2a) in mice, neutralizing at least 9 pseudotyped (>1:104) and live (EMC2012: >1:103) MERS-CoV | N/A | Protects hDPP4-Tg mice (83% survival rate) from lethal MERS-CoV (EMC2012: 104 TCID50) challenge | [94] |
| RBD (T579N) | Binds strongly to soluble and cell-associated hDPP4 receptors and MERS-CoV RBD-specific mAbs (hHS-1, m336, m337, m338) | Induces highly potent neutralizing antibodies in mice against live MERS-CoV (EMC2012: >1:3 × 103) | N/A | Significantly enhances efficacy in fully protecting hDPP4-Tg mice (100% survival rate) from lethal MERS-CoV (EMC2012: 104 TCID50) challenge | [93] |
| Name | Source | Binding MERS-CoV RBD | Structure Available | In vitro Anti-MERS-CoV Activity | In vivo Protection | Ref. |
|---|---|---|---|---|---|---|
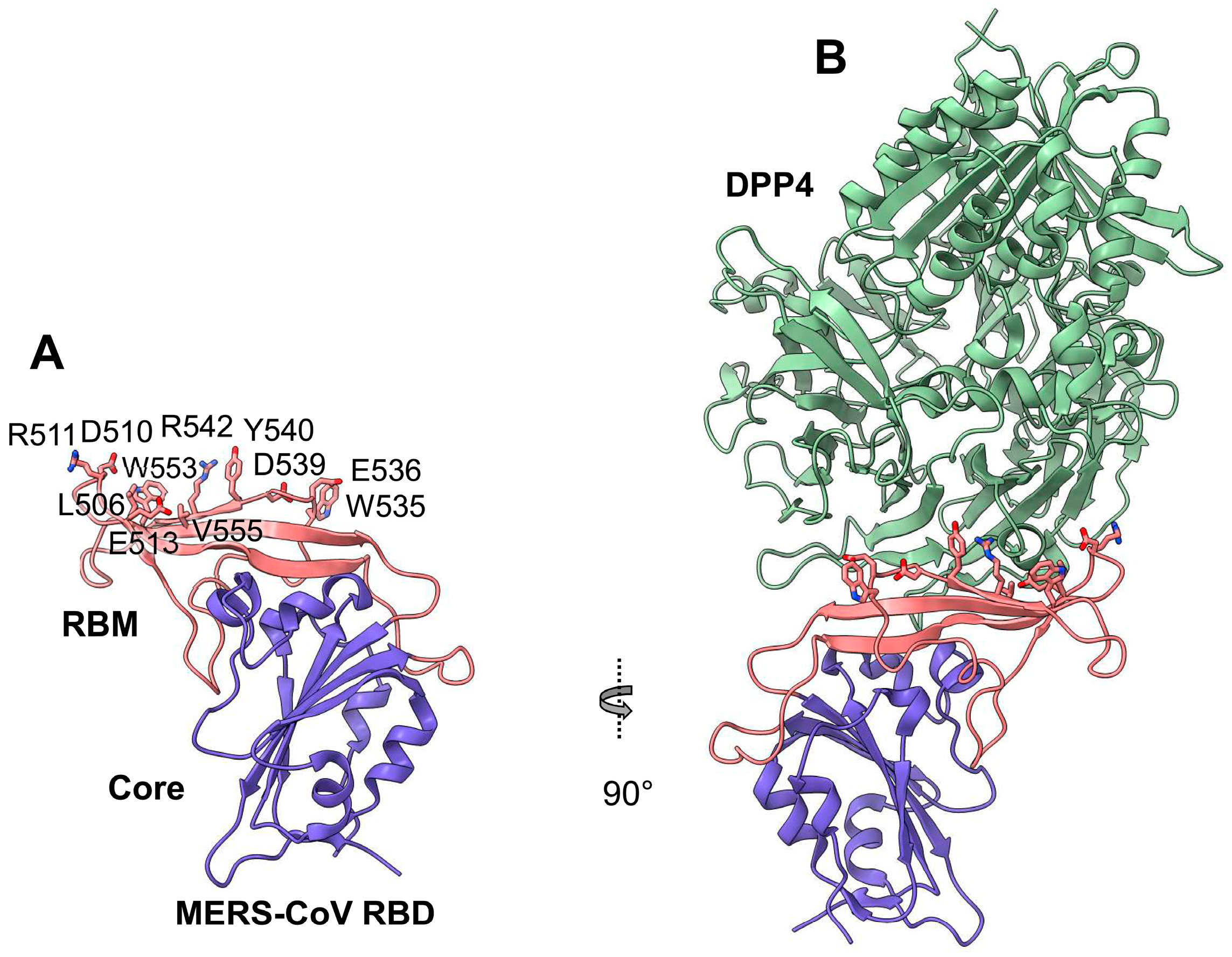
| MERS-GD27 MERS-GD33 mAbs | Human | Kd: 0.775 nM (for MERS-GD27) and 0.575 nM (for MERS-GD33). Recognize RBD residues L506, D510, E513, W535, E536, D539, E540, W553 (for MERS-GD27), R511, and A556 (for MERS-GD33) | Yes, crystal structure for the Fab–RBD complex | IC50: 0.001 µg/mL against pseudotyped MERS-CoV; 0.001 µg/mL against live MERS-CoV; both mAbs have synergistic effect against pseudotyped MERS-CoV with reduced IC50 by 0.499-fold (for MERS-GD27) or 6.05-fold (for MERS-GD33) vs individual mAbs | MERS-GD27 prophylactically and therapeutically protects hDPP4-Tg mice against MERS-CoV (EMC2012: 3 LD50) with 60% and 40% survival rates, respectively | [112,113] |
| MCA1 mAb | Human | Recognizes RBD residues D510, W535, E536, D539, Y540, R542, and Q544 | Yes, crystal structure for the Fab–RBD complex | IC50: 0.39 µg/mL against live MERS-CoV (EMC2012) | Prophylactically and therapeutically (5–20 mg/kg) inhibits MERS-CoV (EMC2012: 5 × 106 TCID50) replication in common marmosets, improving clinical outcomes and reducing lung disease and viral replication | [114] |
| JC57-14 mAb | Macaque | Recognizes RBD residues W535, E536, D539, Y540, and R542 | Yes, crystal structure for the Fab–RBD complex | IC50: 0.0084 µg/mL against pseudotyped MERS-CoV and 0.07 µg/mL against live MERS-CoV (EMC2012), cross-neutralizing 8 pseudotyped MERS-CoVs | N/A | [102] |
| CDC2-C2 mAb | Human | Recognizes RBD residues F506, D509, W535, E536, D539, Y540, and R542 | Yes, crystal structure for the Fab–RBD complex | IC50: 0.0057 µg/mL against pseudotyped MERS-CoV and 0.058 µg/mL against live MERS-CoV (EMC2012), cross-neutralizing 10 pseudotyped MERS-CoVs | Prophylactically (20 mg/kg) protects hDPP4-Tg mice against MERS-CoV (EMC2012: 106 TCID50) in lungs with 100% survival rate | [102] |
| MERS-4 mAb | Human | Recognizes RBD residues L507, L545, S546, P547, G549; binds RBD from outside of the RBD DPP4-binding interface | Yes, crystal structure for the Fab–RBD complex | Has synergistic neutralization effect with MERS-27, m336, and 5F9 mAbs against pseudotyped MERS-CoV, with the reduction of IC50 by 2.6-fold (for MERS-4 + m336) and 15.21-fold (for MERS-4 + 5F9) | N/A | [115] |
| VHH-83 HCAb-83 VHHs | Dromedary | Kd: 0.1 nM (for VHH-83) and 2.5 pM (for HCAb-83). Recognizes RBD residue D539 | N/A | PRNT50: 0.0012–0.0014 µg/mL against live MERS-CoV (EMC2012) | HCAb-83 (200 µg) prophylactically protects hDPP4-Tg mice (K18) against MERS-CoV (EMC2012: 105 PFU) in lungs with 100% survival rate | [116] |
| NbMS10 NbMS10-Fc VHHs | Llama | Kd: 0.87 nM (for NbMS10) and 0.35 nM (for NbMS10-Fc). Recognize RBD residue D539 | N/A | IC50: 0.003–0.979 µg/ml (for NbMS10) and 0.003–0.067 µg/ml (for NbMS10-Fc) in cross-neutralizing ≥11 pseudotyped MERS-CoVs | NbMS10-Fc (10 mg/kg) prophylactically and therapeutically protects hDPP4-Tg mice against MERS-CoV (EMC2012, 105.3 TCID50) with 100% survival rate | [117] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Y.; Yang, Y.; Huang, J.; Jiang, S.; Du, L. Advances in MERS-CoV Vaccines and Therapeutics Based on the Receptor-Binding Domain. Viruses 2019, 11, 60. https://doi.org/10.3390/v11010060
Zhou Y, Yang Y, Huang J, Jiang S, Du L. Advances in MERS-CoV Vaccines and Therapeutics Based on the Receptor-Binding Domain. Viruses. 2019; 11(1):60. https://doi.org/10.3390/v11010060
Chicago/Turabian StyleZhou, Yusen, Yang Yang, Jingwei Huang, Shibo Jiang, and Lanying Du. 2019. "Advances in MERS-CoV Vaccines and Therapeutics Based on the Receptor-Binding Domain" Viruses 11, no. 1: 60. https://doi.org/10.3390/v11010060
APA StyleZhou, Y., Yang, Y., Huang, J., Jiang, S., & Du, L. (2019). Advances in MERS-CoV Vaccines and Therapeutics Based on the Receptor-Binding Domain. Viruses, 11(1), 60. https://doi.org/10.3390/v11010060