Bovine Lactoferrin Prevents Influenza A Virus Infection by Interfering with the Fusogenic Function of Viral Hemagglutinin
Abstract
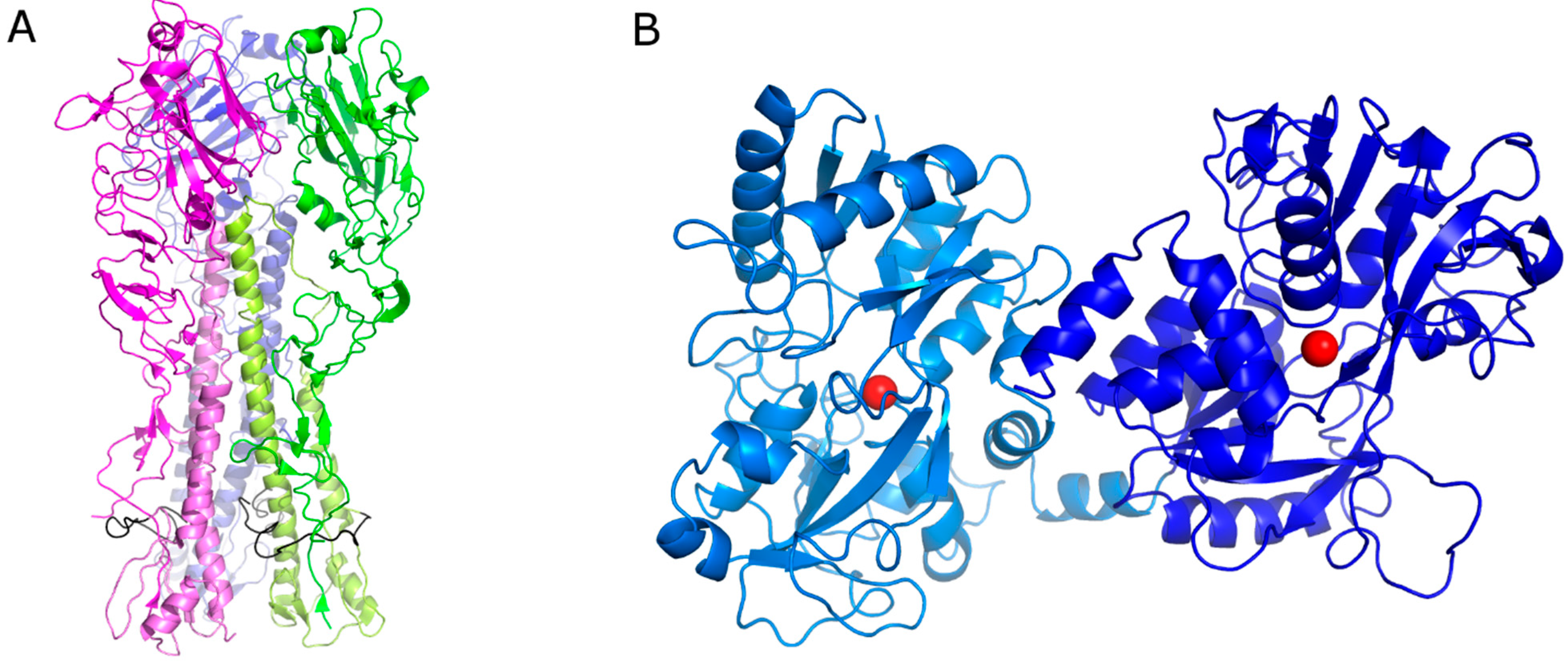
1. Introduction
2. Materials and Methods
2.1. Cells
2.2. Virus
2.3. Lactoferrin and Ammonium Chloride
2.4. Hydrolysis of bLf and Characterization of Its C-lobe
2.5. Effect of Lactoferrin on Influenza Virus Infection: Time Course Assay
2.6. Indirect Immunofluorescence Staining
2.7. Enzyme-Linked Immunosorbent Assay (ELISA)
2.8. Transmission Electron Microscopy (TEM)
2.9. Hemolysis Inhibition Assay
2.10. Statistical Analysis
3. Results
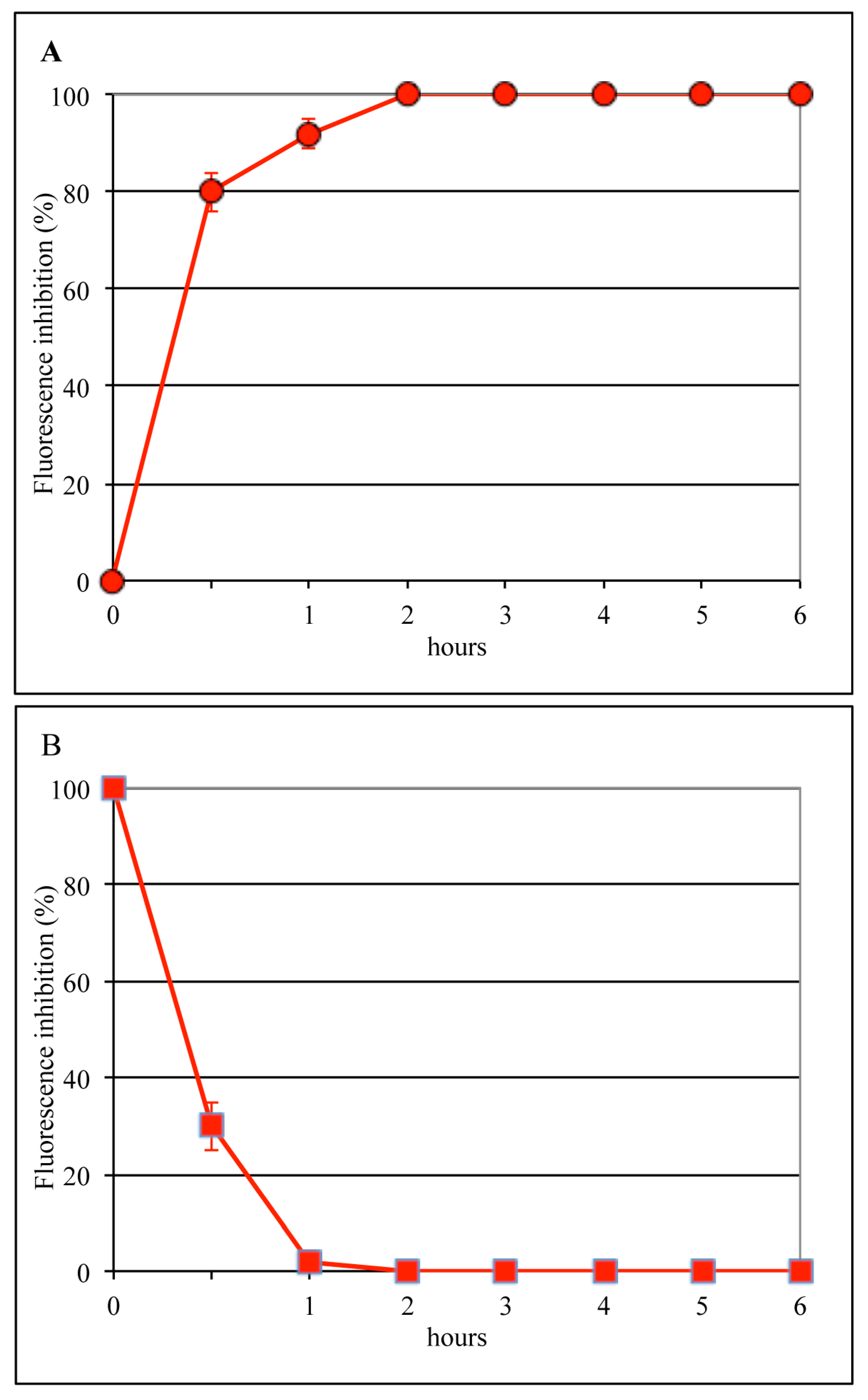
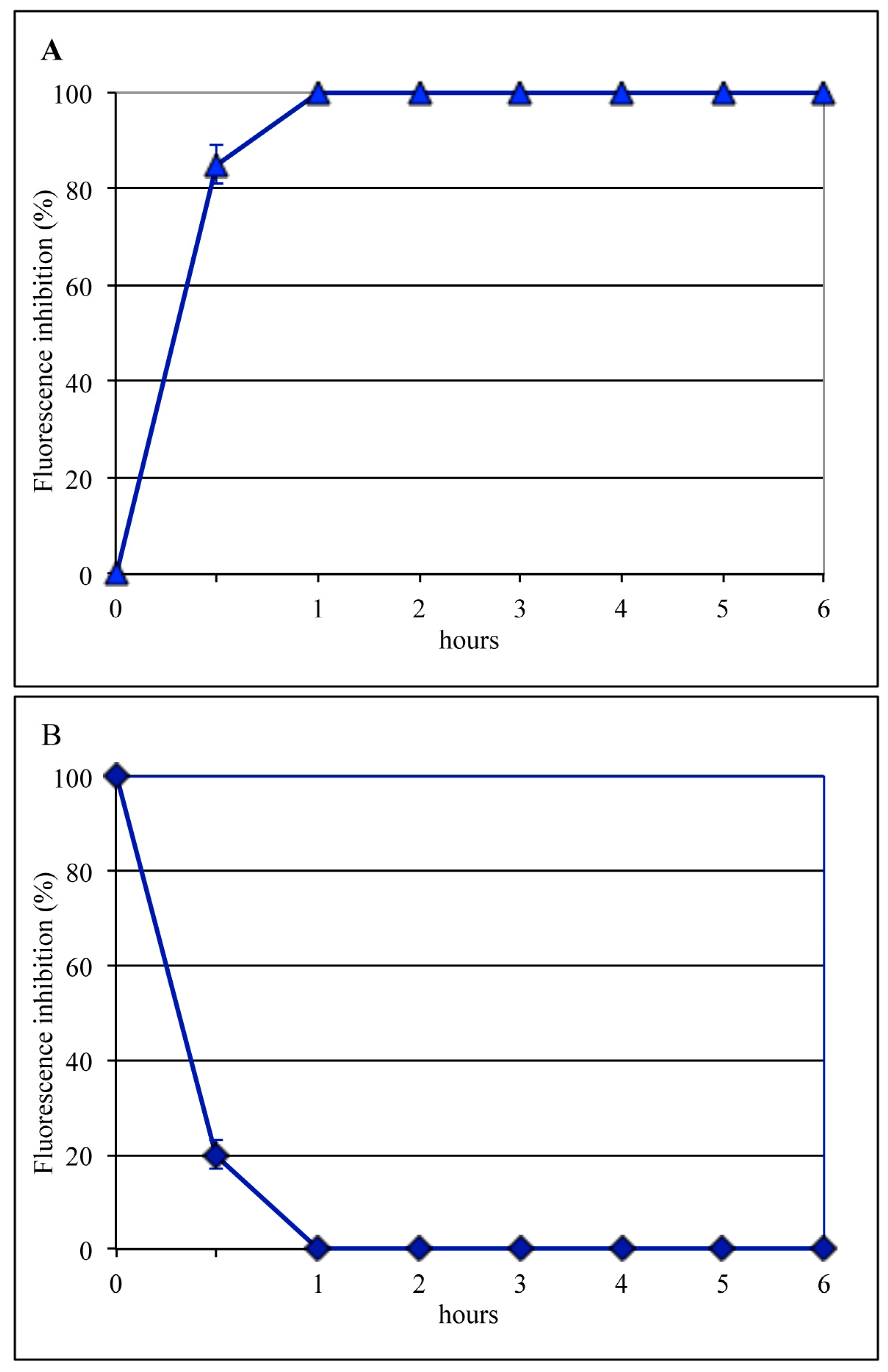
3.1. Lactoferrin Inhibits the Early Phases of Influenza Virus Infection
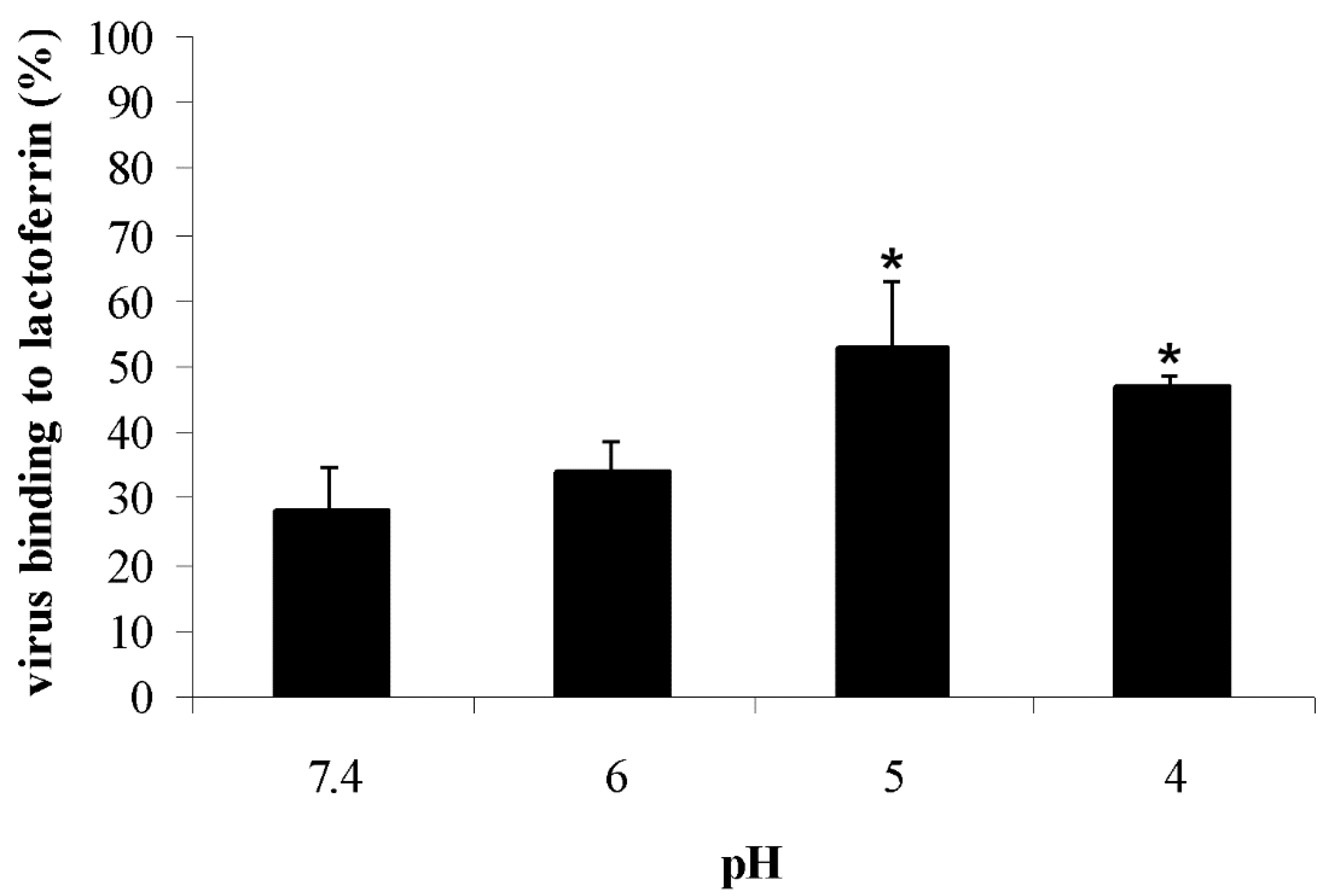
3.2. Direct Lactoferrin Binding Assay at Low pH
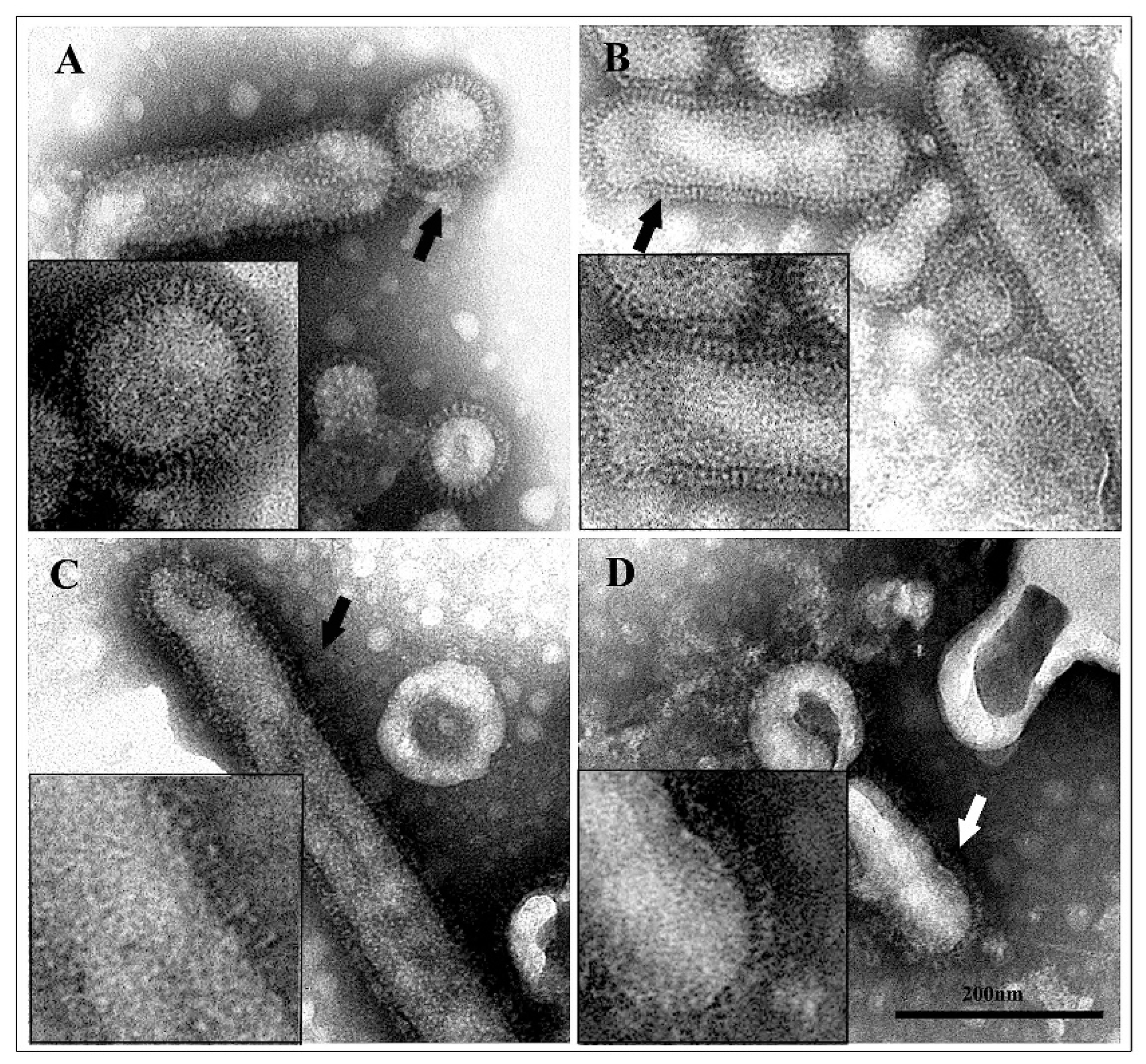
3.3. Transmission Electron Microscopy of Untreated and Acid-Treated Virus Particles
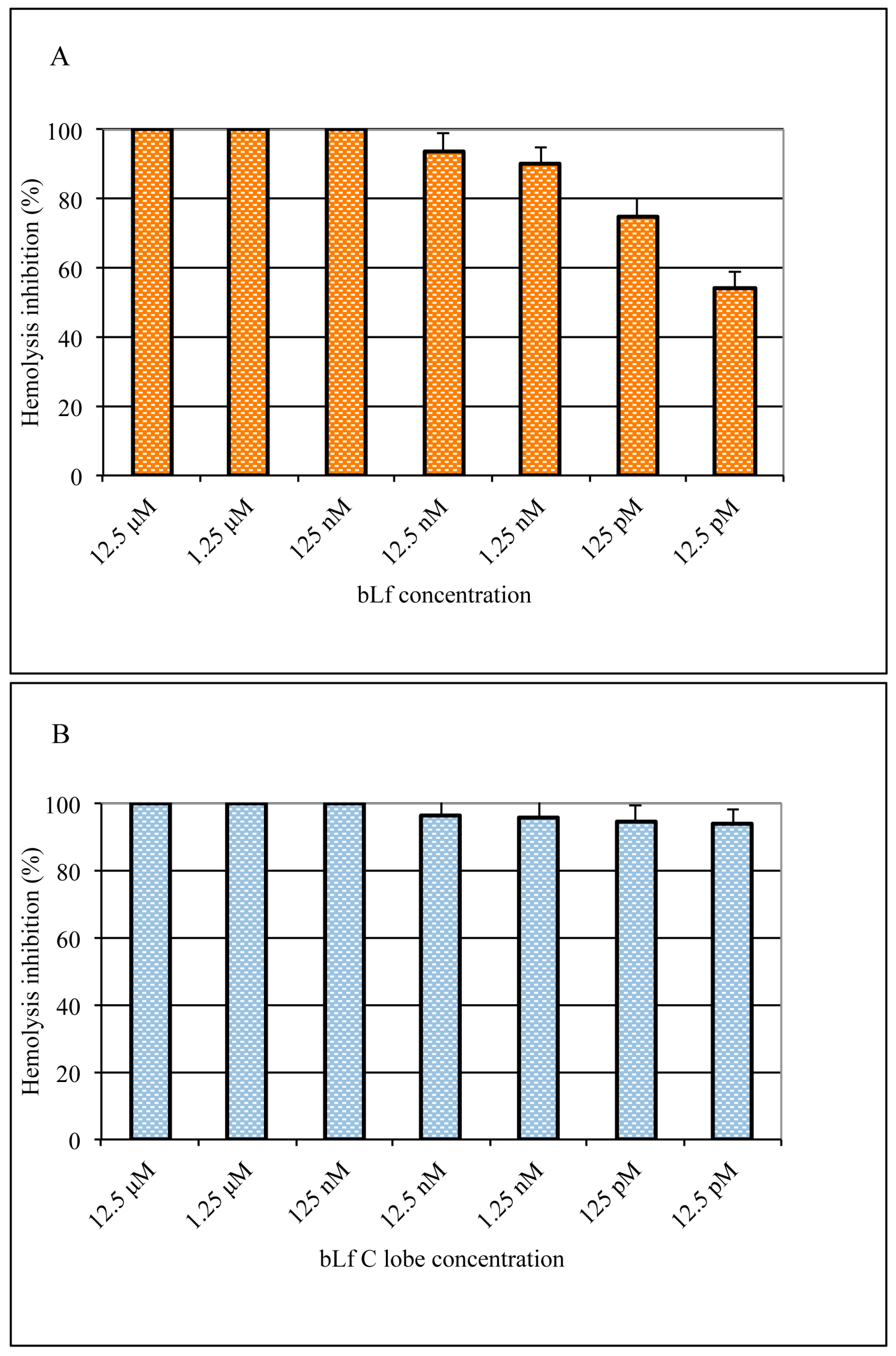
3.4. Inhibitory Effect of Lactoferrin and Lactoferrin C-lobe on Hemolysis
4. Discussion
Patent
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Deyde, V.M.; Xu, X.; Bright, R.A.; Shaw, M.; Smith, C.B.; Zhang, Y.; Shu, Y.; Gubareva, L.V.; Cox, N.J.; Klimov, A.I. Surveillance of resistance to adamantanes among influenza A(H3N2) and A(H1N1) viruses isolated worldwide. J. Infect. Dis. 2007, 196, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Hay, A.; Collins, P.J.; Russell, R.J. Antivirals and resistance. In Avian Influenza (Monographs in Virology Volume 27); Klenk, H.-D., Matrosovich, M.N., Stech, J., Eds.; Publisher Karger: Basel, Switzerland, 2008; pp. 252–271. ISBN 978-3-8055-8501-9. [Google Scholar]
- Gubareva, L.; Okomo-Adhiambo, M.; Deyde, V.; Sheu, T.G.; Garten, R.; Smith, C.; Barnes, J.; Myrick, A.; Hillman, M.; Shaw, M.; et al. Centers for Disease Control and Prevention (CDC). Update: Drug susceptibility of swine-origin influenza A (H1N1) viruses. Morb. Mortal. Wkly. Rep. 2009, 58, 433–435. [Google Scholar]
- Cheng, P.K.C.; To, A.P.C.; Leung, T.W.C.; Leung, P.C.K.; Lee, C.W.C.; Lim, W.W.L. Oseltamivir- and Amantadine-Resistant Influenza Virus A (H1N1). Emerg. Infect. Dis. 2010, 16, 155–156. [Google Scholar] [CrossRef] [PubMed]
- Van der Vries, E.; Stelma, F.F.; Boucher, C.A. Emergence of a multidrug- resistant pandemic influenza A (H1N1) virus. N. Engl. J. Med. 2010, 363, 1381–1382. [Google Scholar] [CrossRef] [PubMed]
- Das, K.; Aramini, J.M.; Ma, L.-C.; Krug, R.M.; Arnold, E. Structures of influenza A proteins and insights into antiviral drug targets. Nat. Struct. Mol. Biol. 2010, 17, 530–538. [Google Scholar] [CrossRef] [PubMed]
- Hartshorn, K.L.; Liou, L.S.; White, M.R.; Kazhdan, M.M.; Tauber, J.L.; Tauber, A.I. Neutrophil deactivation by influenza A virus. Role of hemagglutinin binding to specific sialic acid-bearing cellular proteins. J. Immunol. 1995, 154, 3952–3960. [Google Scholar] [PubMed]
- Skehel, J.J.; Bayley, P.M.; Brown, E.B.; Martin, S.R.; Waterfield, M.D.; White, J.M.; Wilson, I.A.; Wiley, D.C. Changes in the conformation of influenza virus hemagglutinin at the pH optimum of virus-mediated membrane fusion. Proc. Natl. Acad. Sci. USA 1982, 79, 968–972. [Google Scholar] [CrossRef]
- Wilson, I.A.; Skehel, J.J.; Wiley, D.C. Structure of the haemagglutinin membrane glycoprotein of influenza virus at 3 A resolution. Nature 1981, 289, 366–373. [Google Scholar] [CrossRef]
- Wiley, D.C.; Skehel, J.J. The structure and function of the hemagglutinin membrane glycoprotein of influenza virus. Annu. Rev. Biochem. 1987, 56, 365–394. [Google Scholar] [CrossRef]
- Skehel, J.; Wiley, D. Influenza haemagglutinin. Vaccine 2002, 20, S51–S54. [Google Scholar] [CrossRef]
- Levay, P.F.; Viljoen, M. Lactoferrin: A general review. Haematologica 1995, 80, 252–267. [Google Scholar] [PubMed]
- Marchetti, M.; Superti, F. Antiviral activity of lactoferrin. In Recent Developments in Antiviral Research; Pandalai, S.G., Ed.; Publisher Transworld Research Network: Trivandrum, India, 2001; Volume 1, pp. 193–203. [Google Scholar]
- González-Chávez, S.A.; Arévalo-Gallegos, S.; Rascón-Cruz, Q. Lactoferrin: Structure, function and applications. Int. J. Antimicrob. Agents 2009, 33, 301.e1–301.e8. [Google Scholar] [CrossRef] [PubMed]
- Seganti, L.; Di Biase, A.M.; Marchetti, M.; Pietrantoni, A.; Tinari, A.; Superti, F. Antiviral activity of lactoferrin towards naked viruses. Biometals 2004, 3, 295–299. [Google Scholar] [CrossRef]
- Uperti, F.; Berlutti, F.; Paesano, R.; Valenti, P. Structure and activity of lactoferrin—A multi functional protective agent for human health. In Iron Metabolism and Disease; Fuchs, H., Ed.; Publisher Research Signpost: Trivandrum, India, 2008; Volume 8, pp. 1–32. [Google Scholar]
- Moore, S.A.; Anderson, B.F.; Groom, C.R.; Haridas, M.; Baker, E.N. Three-dimensional structure of diferric bovine lactoferrin at 2.8 Å resolution. J. Mol. Biol. 1997, 274, 222–236. [Google Scholar] [CrossRef] [PubMed]
- Anderson, B.F.; Baker, H.M.; Dodson, E.J.; Norris, G.E.; Rumball, S.V.; Waters, J.M.; Baker, E.N. Structure of human lactoferrin at 3.2-A resolution. Proc. Natl. Acad. Sci. USA 1987, 84, 1769–1773. [Google Scholar] [CrossRef]
- Pietrantoni, A.; Dofrelli, E.; Tinari, A.; Ammendolia, M.G.; Puzelli, S.; Fabiani, C.; Donatelli, I.; Superti, F. Bovine lactoferrin inhibits influenza A virus induced programmed cell death in vitro. Biometals 2010, 23, 465–475. [Google Scholar] [CrossRef]
- Pietrantoni, A.; Ammendolia, M.G.; Superti, F. Bovine lactoferrin: Involvement of metal saturation and carbohydrates in the inhibition of influenza virus infection. Biochem. Cell Biol. 2012, 90, 442–448. [Google Scholar] [CrossRef]
- Ammendolia, M.G.; Agamennone, M.; Pietrantoni, A.; Lannutti, F.; Siciliano, R.A.; De Giulio, B.; Amici, C.; Superti, F. Bovine lactoferrin-derived peptides as novel broad-spectrum inhibitors of influenza virus. Pathog. Glob. Health 2012, 106, 12–19. [Google Scholar] [CrossRef]
- Klimov, A.; Balish, A.; Veguilla, V.; Sun, H.; Schiffer, J.; Lu, X.; Katz, J.M.; Hancock, K. Influenza virus titration, antigenic characterization, and serological methods for antibody detection. Methods Mol. Biol. 2012, 865, 25–51. [Google Scholar] [CrossRef]
- Superti, F.; Agamennone, M.; Ammendolia, M.G.; Pietrantoni, A.; Lannutti, F. Lactoferrin Derived Peptides for Use as Broadspectrum Inhibitors of Influenza virus Infection. European Patent Number EP 2780365, 22 February 2017. [Google Scholar]
- Pietrantoni, A.; Ammendolia, M.G.; Tinari, A.; Siciliano, R.; Valenti, P.; Superti, F. Bovine lactoferrin peptidic fragments involved in inhibition of Echovirus 6 in vitro infection. Antivir. Res. 2006, 69, 98–106. [Google Scholar] [CrossRef]
- Groves, M.L. The isolation of a red protein from milk. J. Am. Chem. Soc. 1960, 82, 3345–3350. [Google Scholar] [CrossRef]
- Superti, F.; Siciliano, R.; Rega, B.; Giansanti, F.; Valenti, P.; Antonini, G. Involvement of bovine lactoferrin metal saturation, sialic acid and protein fragments in the inhibition of rotavirus infection. Biochim. Biophys. Acta 2001, 1528, 107–115. [Google Scholar] [CrossRef]
- Bodian, D.L.; Yamasaki, R.B.; Buswell, R.L.; Stearns, J.F.; White, J.M.; Kuntz, I.D. Inhibition of the fusion-inducing conformational change of influenza hemagglutinin by benzoquinones and hydroquinones. Biochemistry 1993, 32, 2967–2978. [Google Scholar] [CrossRef]
- Ruigrok, R.W.; Wrigley, N.G.; Calder, L.J.; Cusack, S.; Wharton, S.A.; Brown, E.B.; Skehel, J.J. Electron microscopy of the low pH structure of influenza virus haemagglutinin. EMBO J. 1986, 5, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Lamb, R.A.; Krug, R.M. Orthomyxoviridae: The viruses and their replication. In Fields Virology, 4th ed.; Knipe, D.M., Howley, P.M., Eds.; Publisher Lippincott Williams and Wilkins: Philadelphia, PA, USA, 2001; pp. 1487–1532. [Google Scholar]
- Kiso, M.; Mitamura, K.; Sakai-Tagawa, Y.; Shiraishi, K.; Kawakami, C.; Kimura, K.; Hayden, F.G.; Sugaya, N.; Kawaoka, Y. Resistant influenza A viruses in children treated with oseltamivir: Descriptive study. Lancet 2004, 364, 759–765. [Google Scholar] [CrossRef]
- Hayden, F.G. Antiviral resistance in influenza viruses—Implications for management and pandemic response. N. Engl. J. Med. 2006, 354, 785–788. [Google Scholar] [CrossRef] [PubMed]
- Meijer, A.; Lackenby, A.; Hungnes, O.; Lina, B.; van-der-Werf, S.; Schweiger, B.; Opp, M.; Paget, J.; van-de-Kassteele, J.; Hay, A.; et al. European Influenza Surveillance Scheme Oseltamivir-resistant influenza virus A (H1N1), Europe, 2007–2008 season. Emerg. Infect. Dis. 2009, 15, 552–560. [Google Scholar] [CrossRef]
- Matlin, K.S.; Reggio, H.; Helenius, A.; Simons, K. Infectious entry pathway of influenza-virus in a canine kidney-cell line. J. Cell Biol. 1981, 91, 601–613. [Google Scholar] [CrossRef]
- Yoshimura, A.; Ohnishi, S. Uncoating of influenza viruses in endosomes. J. Virol. 1984, 51, 497–504. [Google Scholar]
- Lakadamyali, M.; Rust, M.J.; Zhuang, X. Endocytosis of influenza viruses. Microbes Infect. 2004, 6, 929–936. [Google Scholar] [CrossRef]
- Sieczkarski, S.B.; Whittaker, G.R. Differential requirements of Rab5 and Rab7 for endocytosis of influenza and other enveloped viruses. Traffic 2003, 4, 333–343. [Google Scholar] [CrossRef] [PubMed]
- Ohkuma, S.; Poole, B. Fluorescence probe measurement of the intralysosomal pH in living cells and the perturbation of pH by various agents. Proc. Natl. Acad. Sci. USA 1978, 75, 3327–3331. [Google Scholar] [CrossRef]
- Superti, F.; Derer, M.; Tsiang, H. Mechanism of rabies virus entry into CER cells. J. Gen. Virol. 1984, 65, 781–789. [Google Scholar] [CrossRef] [PubMed]
- Marsh, M.; Helenius, A. Virus entry into animal cells. Adv. Virus Res. 1989, 36, 107–151. [Google Scholar] [CrossRef] [PubMed]
- Ammendolia, M.G.; Pietrantoni, A.; Tinari, A.; Valenti, P.; Superti, F. Bovine lactoferrin inhibits echovirus endocytic pathway by interacting with viral structural polypeptides. Antivir. Res. 2007, 73, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Jiang, R.; Lopez, V.; Kelleher, S.L.; Lönnerdal, B. Apo- and holo-lactoferrin are both internalized by lactoferrin receptor via clathrin-mediated endocytosis but differentially affect ERK-signaling and cell proliferation in Caco-2 cells. J. Cell. Physiol. 2011, 226, 3022–3031. [Google Scholar] [CrossRef] [PubMed]
- Florian, P.; Macovei, A.; Sima, L.; Nichita, N.; Mattsby-Baltzer, I.; Roseanu, A. Endocytosis and trafficking of human lactoferrin in macrophage-like human THP-1 cells (1). Biochem. Cell Biol. 2012, 90, 449–455. [Google Scholar] [CrossRef]
- Bi, B.Y.; Liu, J.L.; Legrand, D.; Roche, A.C.; Capron, M.; Spik, G.; Mazurier, J. Internalization of human lactoferrin by the Jurkat human lymphoblastic T-cell line. Eur. J. Cell Biol. 1996, 69, 288–296. [Google Scholar]
- Fontana, J.; Steven, A.C. Influenza virus-mediated membrane fusion: Structural insights from electron microscopy. Arch. Biochem. Biophys. 2015, 581, 86–97. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Superti, F.; Agamennone, M.; Pietrantoni, A.; Ammendolia, M.G. Bovine Lactoferrin Prevents Influenza A Virus Infection by Interfering with the Fusogenic Function of Viral Hemagglutinin. Viruses 2019, 11, 51. https://doi.org/10.3390/v11010051
Superti F, Agamennone M, Pietrantoni A, Ammendolia MG. Bovine Lactoferrin Prevents Influenza A Virus Infection by Interfering with the Fusogenic Function of Viral Hemagglutinin. Viruses. 2019; 11(1):51. https://doi.org/10.3390/v11010051
Chicago/Turabian StyleSuperti, Fabiana, Mariangela Agamennone, Agostina Pietrantoni, and Maria Grazia Ammendolia. 2019. "Bovine Lactoferrin Prevents Influenza A Virus Infection by Interfering with the Fusogenic Function of Viral Hemagglutinin" Viruses 11, no. 1: 51. https://doi.org/10.3390/v11010051
APA StyleSuperti, F., Agamennone, M., Pietrantoni, A., & Ammendolia, M. G. (2019). Bovine Lactoferrin Prevents Influenza A Virus Infection by Interfering with the Fusogenic Function of Viral Hemagglutinin. Viruses, 11(1), 51. https://doi.org/10.3390/v11010051





