Preclinical Testing of an Oncolytic Parvovirus in Ewing Sarcoma: Protoparvovirus H-1 Induces Apoptosis and Lytic Infection In Vitro but Fails to Improve Survival In Vivo
Abstract
1. Introduction
2. Materials and Methods
2.1. Sources and Culture Conditions for Human Cell Lines
2.2. Production of Infectious Viral Particles
2.3. Detection of Infectious H-1PV Particles
2.4. Extraction of H-1PV DNA and Qantification by Real-Time PCR
2.5. Conditions of in vitro Infection Experiments
2.6. Microscopy of H-1P-Infected Cells
2.7. Analysis of the Cell Cycle, Including Sub-G1 Apoptotic Cells, during H-1PV Infection
2.8. Quantatitation of Cell Viability and Cell Lysis
2.9. Real-Time Proliferation Measurements
2.10. Animal Experiments
2.11. Statistical Methods
3. Results
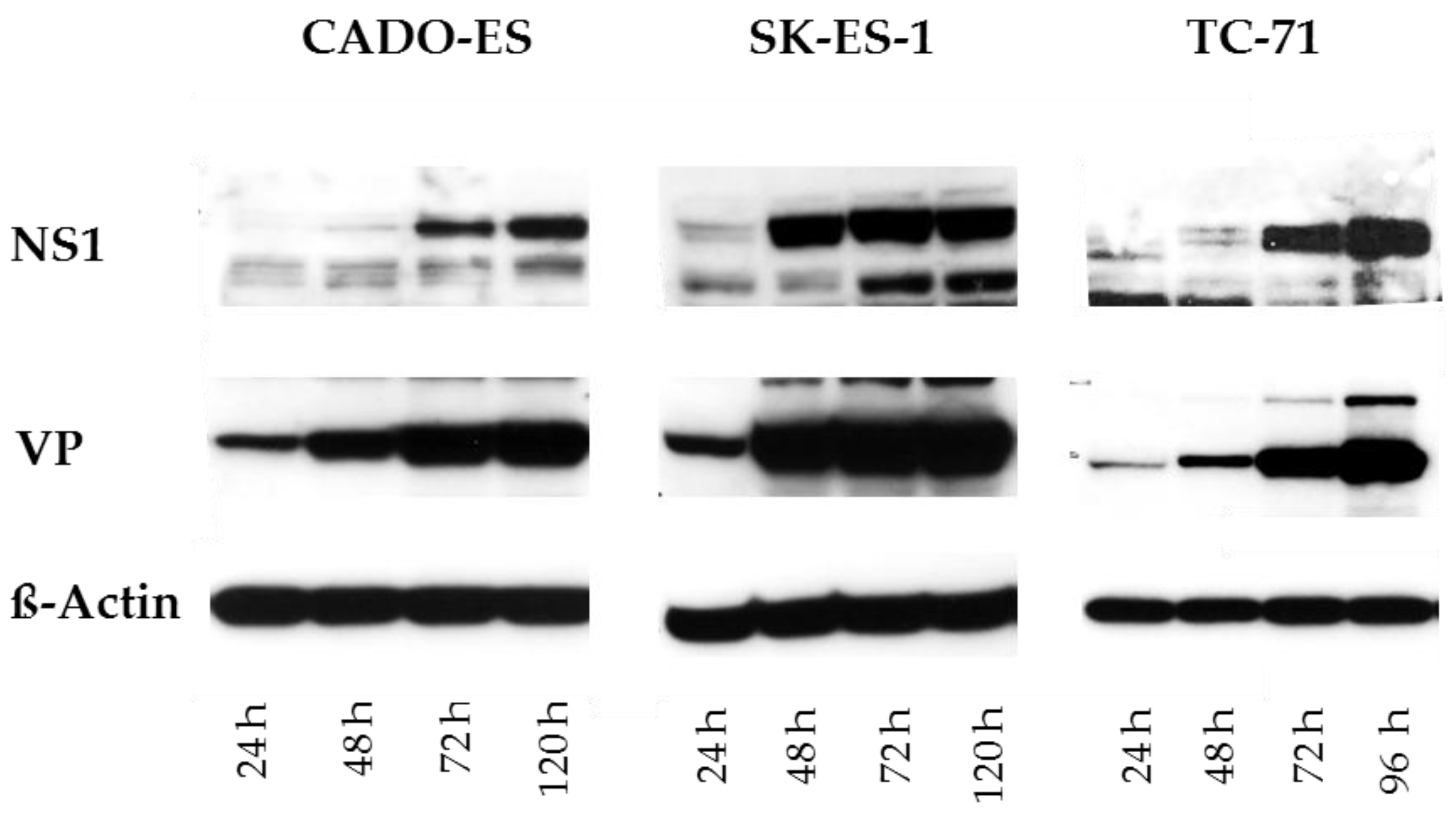
3.1. Ewing Sarcoma (EWS) Cells Are Susceptible to H-1PV Infection and Sustain the Synthesis of Viral Proteins
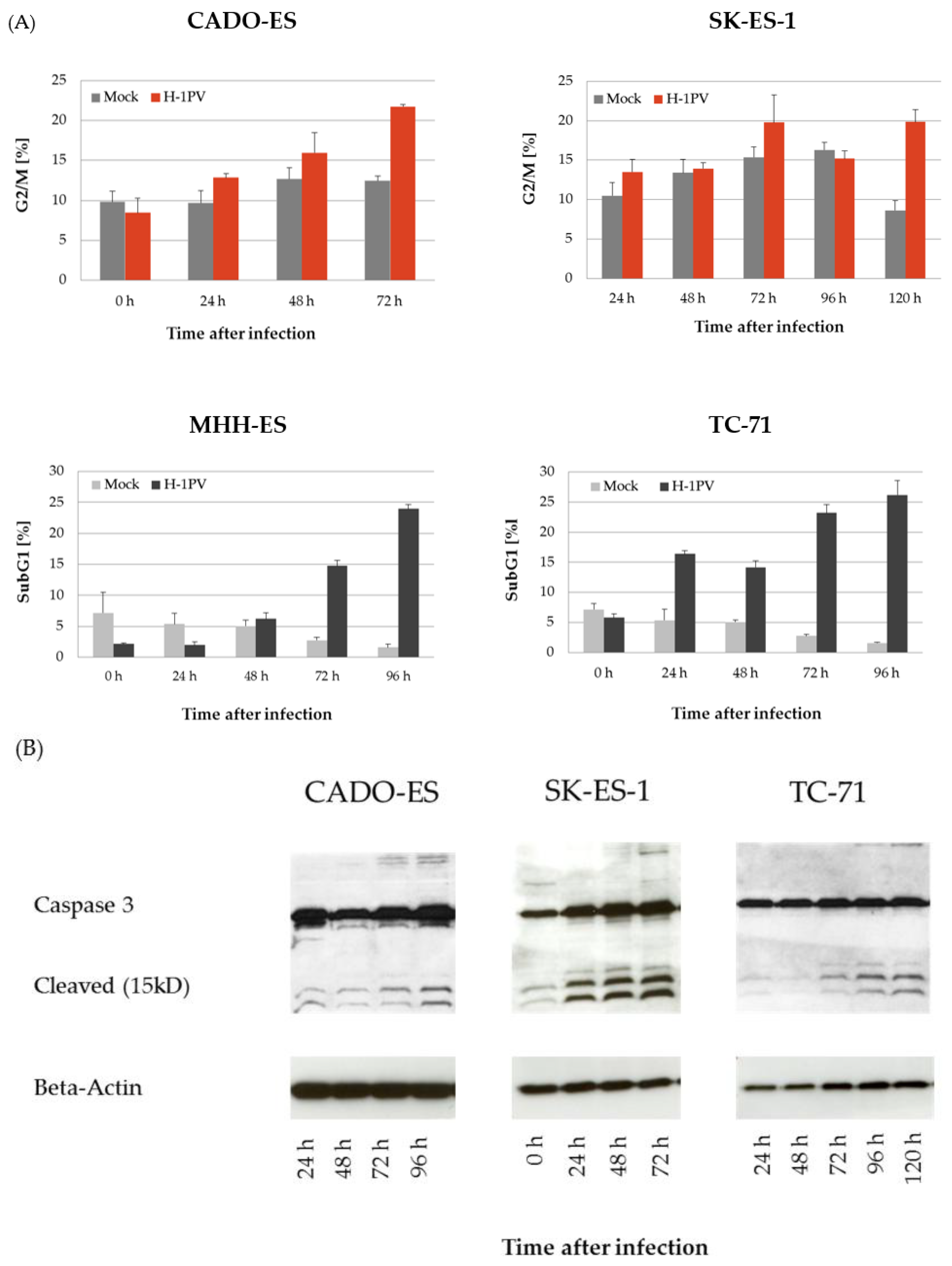
3.2. H-1PV Infection Interferes with the Cell Cycle in Ewing Sarcoma Cell Lines
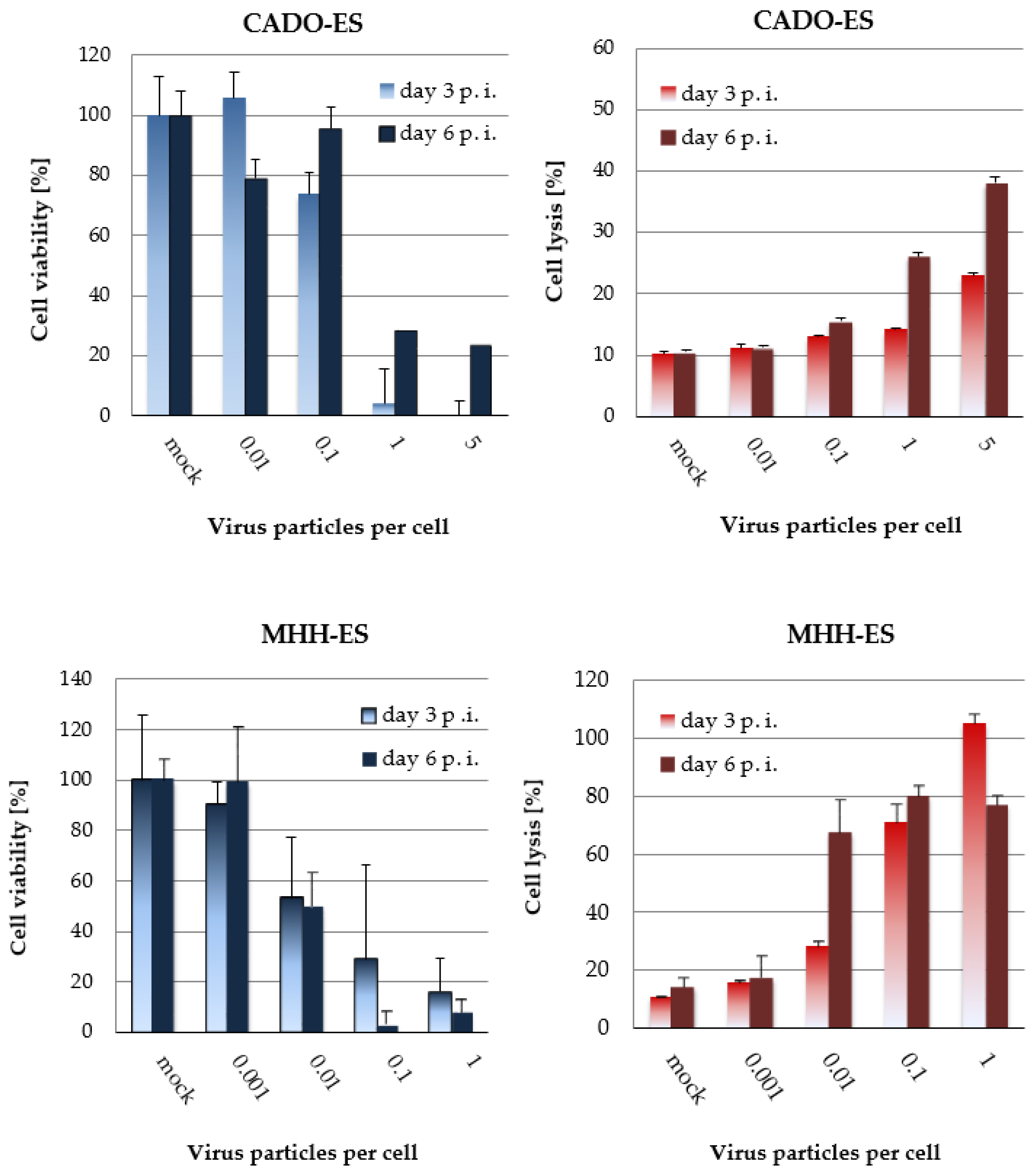
3.3. H-1PV-Induced Cytotoxic Effects in Ewing Sarcoma Cell Lines Correlate with the Dose of Input Virus
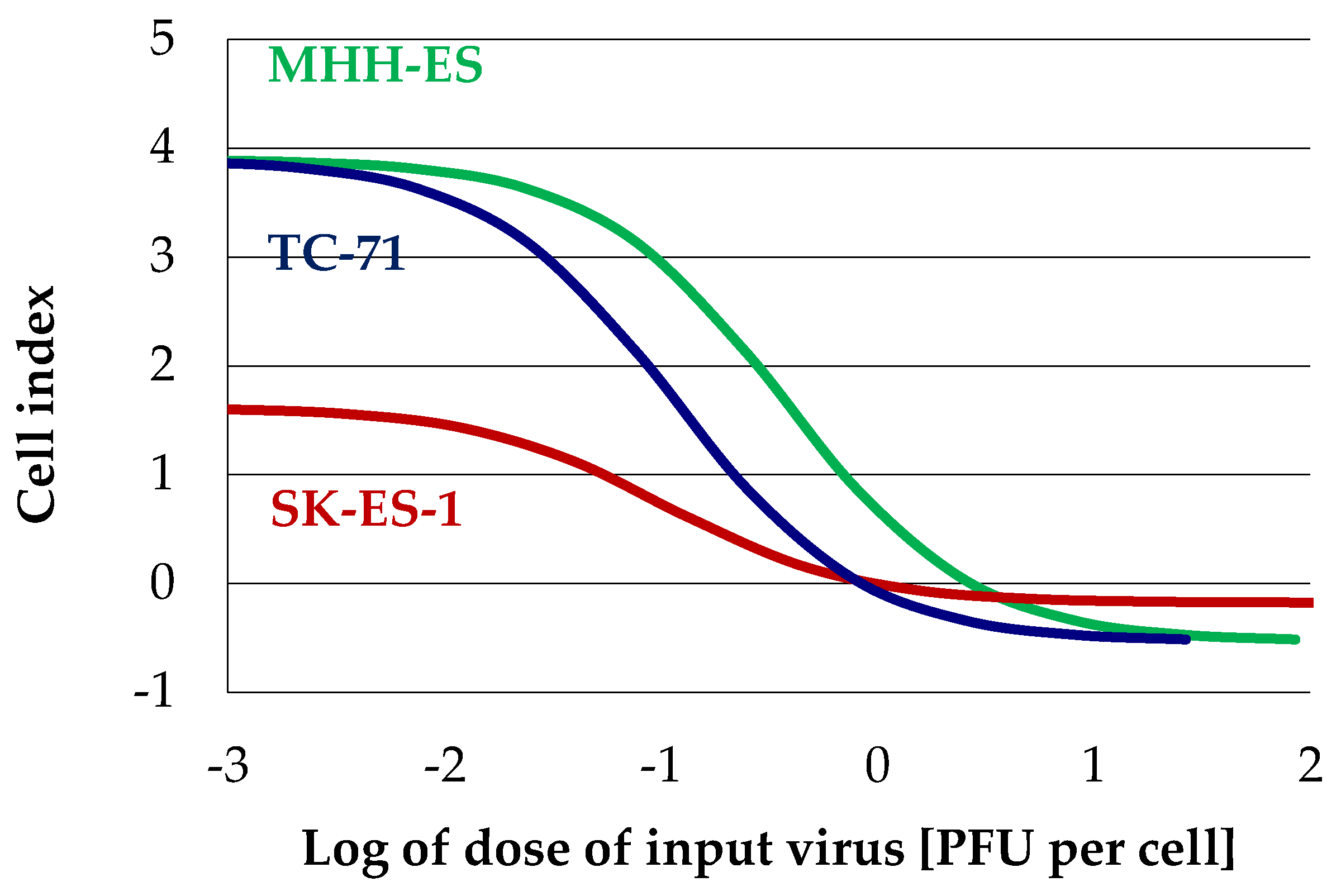
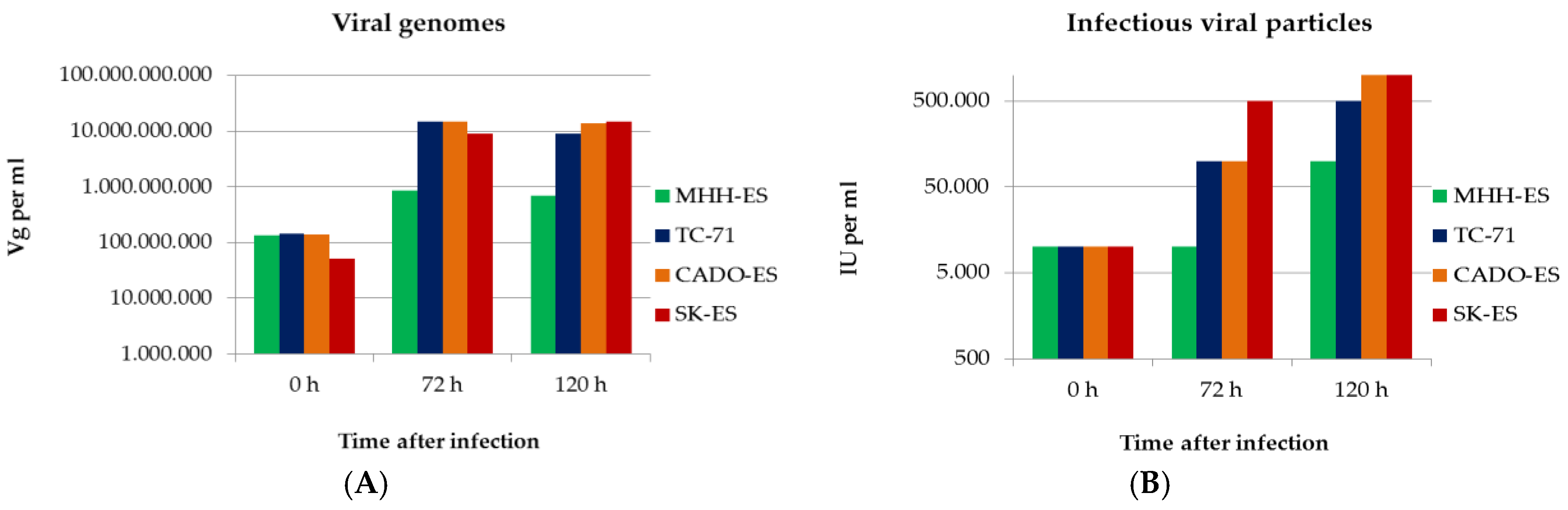
3.4. Ewings’s Sarcoma Cells Efficently Replicate H-1PV
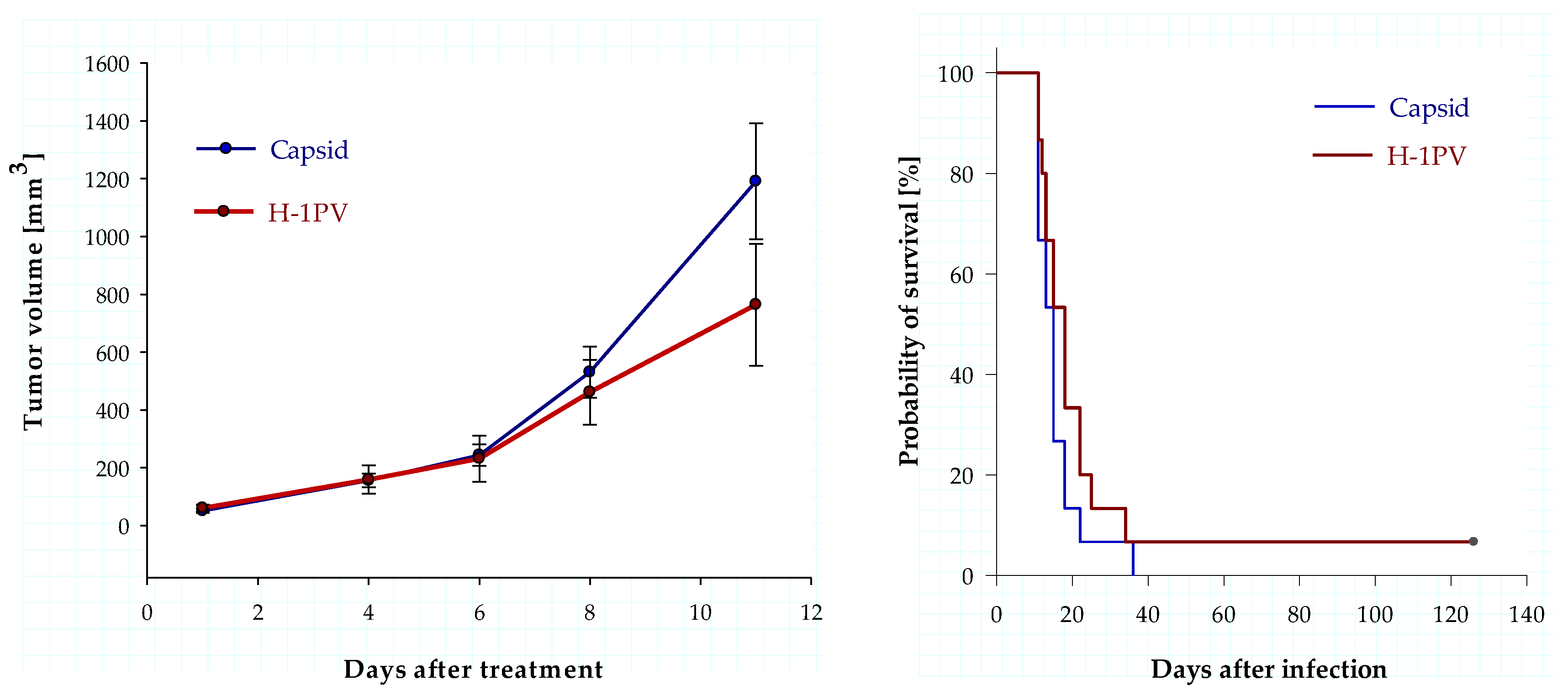
3.5. Intratumoral Infection with H-1PV Represses Human Ewing Sarcoma Cell Proliferation in a Xenograft Mouse Model but Fails to Improve Survival
4. Discussion
4.1. H-1PV Selectively Infects Transformed Human Cells of Mesenchymal Origin
4.2. Ewing Sarcoma Cells Are Fully Permissive to Wild-Type H-1PV Infection
4.3. Animal Data Show Antiproliferative Effects of H-1PV but Fail to Demonstrate Significant Antineoplastic Efficacy in a Ewing Sarcoma Xenograft Model
5. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Esiashvili, N.; Goodman, M.; Marcus, R.B., Jr. Changes in incidence and survival of ewing sarcoma patients over the past 3 decades: Surveillance epidemiology and end results data. J. Pediatr. Hematol. Oncol. 2008, 30, 425–430. [Google Scholar] [CrossRef] [PubMed]
- Miser, J.S.; Goldsby, R.E.; Chen, Z.; Krailo, M.D.; Tarbell, N.J.; Link, M.P.; Fryer, C.J.; Pritchard, D.J.; Gebhardt, M.C.; Dickman, P.S.; et al. Treatment of metastatic ewing sarcoma/primitive neuroectodermal tumor of bone: Evaluation of increasing the dose intensity of chemotherapy—A report from the children’s oncology group. Pediatr. Blood Cancer 2007, 49, 894–900. [Google Scholar] [CrossRef] [PubMed]
- Friedman, G.K.; Pressey, J.G.; Reddy, A.T.; Markert, J.M.; Gillespie, G.Y. Herpes simplex virus oncolytic therapy for pediatric malignancies. Mol. Ther. 2009, 17, 1125–1135. [Google Scholar] [CrossRef] [PubMed]
- Eshun, F.K.; Currier, M.A.; Gillespie, R.A.; Fitzpatrick, J.L.; Baird, W.H.; Cripe, T.P. Vegf blockade decreases the tumor uptake of systemic oncolytic herpes virus but enhances therapeutic efficacy when given after virotherapy. Gene Ther. 2010, 17, 922–929. [Google Scholar] [CrossRef] [PubMed]
- Morton, C.L.; Houghton, P.J.; Kolb, E.A.; Gorlick, R.; Reynolds, C.P.; Kang, M.H.; Maris, J.M.; Keir, S.T.; Wu, J.; Smith, M.A. Initial testing of the replication competent seneca valley virus (NTX-010) by the pediatric preclinical testing program. Pediatr. Blood Cancer 2010, 55, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Hingorani, P.; Zhang, W.; Lin, J.; Liu, L.; Guha, C.; Kolb, E.A. Systemic administration of reovirus (reolysin) inhibits growth of human sarcoma xenografts. Cancer 2011, 117, 1764–1774. [Google Scholar] [CrossRef] [PubMed]
- Lettieri, C.K.; Hingorani, P.; Kolb, E.A. Progress of oncolytic viruses in sarcomas. Expert Rev. Anticancer Ther. 2012, 12, 229–242. [Google Scholar] [CrossRef] [PubMed]
- Nuesch, J.P.; Lacroix, J.; Marchini, A.; Rommelaere, J. Molecular pathways: Rodent parvoviruses—Mechanisms of oncolysis and prospects for clinical cancer treatment. Clin. Cancer Res. 2012, 18, 3516–3523. [Google Scholar] [CrossRef] [PubMed]
- Halder, S.; Nam, H.J.; Govindasamy, L.; Vogel, M.; Dinsart, C.; Salome, N.; McKenna, R.; Agbandje-McKenna, M. Structural characterization of H-1 parvovirus: Comparison of infectious virions to empty capsids. J. Virol. 2013, 87, 5128–5140. [Google Scholar] [CrossRef] [PubMed]
- Zadori, Z.; Szelei, J.; Tijssen, P. Sat: A late NS protein of porcine parvovirus. J. Virol. 2005, 79, 13129–13138. [Google Scholar] [CrossRef] [PubMed]
- Hristov, G.; Kramer, M.; Li, J.; El-Andaloussi, N.; Mora, R.; Daeffler, L.; Zentgraf, H.; Rommelaere, J.; Marchini, A. Through its nonstructural protein NS1, parvovirus H-1 induces apoptosis via accumulation of reactive oxygen species. J. Virol. 2010, 84, 5909–5922. [Google Scholar] [CrossRef] [PubMed]
- Geiss, C.; Kis, Z.; Leuchs, B.; Frank-Stohr, M.; Schlehofer, J.R.; Rommelaere, J.; Dinsart, C.; Lacroix, J. Preclinical testing of an oncolytic parvovirus: Standard protoparvovirus H-1PV efficiently induces osteosarcoma cell lysis in vitro. Viruses 2017, 9, 301. [Google Scholar] [CrossRef] [PubMed]
- Castro, F.; Dirks, W.G.; Fahnrich, S.; Hotz-Wagenblatt, A.; Pawlita, M.; Schmitt, M. High-throughput SNP-based authentication of human cell lines. Int. J. Cancer 2013, 132, 308–314. [Google Scholar] [CrossRef] [PubMed]
- Leuchs, B.; Roscher, M.; Muller, M.; Kurschner, K.; Rommelaere, J. Standardized large-scale h-1pv production process with efficient quality and quantity monitoring. J. Virol. Methods 2016, 229, 48–59. [Google Scholar] [CrossRef] [PubMed]
- Kestler, J.; Neeb, B.; Struyf, S.; van Damme, J.; Cotmore, S.F.; D’Abramo, A.; Tattersall, P.; Rommelaere, J.; Dinsart, C.; Cornelis, J.J. Cis requirements for the efficient production of recombinant DNA vectors based on autonomous parvoviruses. Hum. Gene Ther. 1999, 10, 1619–1632. [Google Scholar] [CrossRef] [PubMed]
- El-Andaloussi, N.; Endele, M.; Leuchs, B.; Bonifati, S.; Kleinschmidt, J.; Rommelaere, J.; Marchini, A. Novel adenovirus-based helper system to support production of recombinant parvovirus. Cancer Gene Ther. 2011, 18, 240–249. [Google Scholar] [CrossRef] [PubMed]
- Lacroix, J.; Leuchs, B.; Li, J.; Hristov, G.; Deubzer, H.E.; Kulozik, A.E.; Rommelaere, J.; Schlehofer, J.R.; Witt, O. Parvovirus H1 selectively induces cytotoxic effects on human neuroblastoma cells. Int. J. Cancer 2010, 127, 1230–1239. [Google Scholar] [CrossRef] [PubMed]
- Geletneky, K.; Hajda, J.; Angelova, A.L.; Leuchs, B.; Capper, D.; Bartsch, A.J.; Neumann, J.O.; Schoning, T.; Husing, J.; Beelte, B.; et al. Oncolytic H-1 parvovirus shows safety and signs of immunogenic activity in a first phase I/IIA glioblastoma trial. Mol. Ther. 2017, 25, 2620–2634. [Google Scholar] [CrossRef] [PubMed]
- Hajda, J.; Lehmann, M.; Krebs, O.; Kieser, M.; Geletneky, K.; Jager, D.; Dahm, M.; Huber, B.; Schoning, T.; Sedlaczek, O.; et al. A non-controlled, single arm, open label, phase II study of intravenous and intratumoral administration of parvoryx in patients with metastatic, inoperable pancreatic cancer: Parvoryx02 protocol. BMC Cancer 2017, 17, 576. [Google Scholar] [CrossRef] [PubMed]
- Toolan, H.W.; Rhode, S.L., III; Gierthy, J.F. Inhibition of 7,12-dimethylbenz(a)anthracene-induced tumors in syrian hamsters by prior infection with H-1 parvovirus. Cancer Res. 1982, 42, 2552–2555. [Google Scholar] [PubMed]
- Angelova, A.L.; Geletneky, K.; Nuesch, J.P.; Rommelaere, J. Tumor selectivity of oncolytic parvoviruses: From in vitro and animal models to cancer patients. Front. Bioeng. Biotechnol. 2015, 3, 55. [Google Scholar] [CrossRef] [PubMed]
- Angelova, A.L.; Aprahamian, M.; Grekova, S.P.; Hajri, A.; Leuchs, B.; Giese, N.A.; Dinsart, C.; Herrmann, A.; Balboni, G.; Rommelaere, J.; et al. Improvement of gemcitabine-based therapy of pancreatic carcinoma by means of oncolytic parvovirus H-1PV. Clin. Cancer Res. 2009, 15, 511–519. [Google Scholar] [CrossRef] [PubMed]
- Geletneky, K.; Kiprianova, I.; Ayache, A.; Koch, R.; Herrero, Y.C.M.; Deleu, L.; Sommer, C.; Thomas, N.; Rommelaere, J.; Schlehofer, J.R. Regression of advanced rat and human gliomas by local or systemic treatment with oncolytic parvovirus H-1 in rat models. Neuro Oncol. 2010, 12, 804–814. [Google Scholar] [CrossRef] [PubMed]

| Cell Line | LD50 [PFU/Cell] | Tumor of Origin | Age of Patient |
|---|---|---|---|
| MHH-ES-1 | 0.01 | Peritoneal metastatic EWS | 12 years |
| TC-71 | 0.1 | Local recurrent EWS | 22 years |
| SK-ES-1 | 5 | Primary Ewing sarcoma | 18 years |
| CADO-ES1 | 5 | Pleural metastatic EWS | 19 years |
| Cell Line | ED50 [PFU/Cell] | R2 | Observation Time |
|---|---|---|---|
| MHH-ES | 0.32 | 0.922 | 256 h |
| SK-ES-1 | 0.11 | 0.972 | 215 h |
| TC-71 | 1.04 | 0.978 | 120 h |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lacroix, J.; Kis, Z.; Josupeit, R.; Schlund, F.; Stroh-Dege, A.; Frank-Stöhr, M.; Leuchs, B.; Schlehofer, J.R.; Rommelaere, J.; Dinsart, C. Preclinical Testing of an Oncolytic Parvovirus in Ewing Sarcoma: Protoparvovirus H-1 Induces Apoptosis and Lytic Infection In Vitro but Fails to Improve Survival In Vivo. Viruses 2018, 10, 302. https://doi.org/10.3390/v10060302
Lacroix J, Kis Z, Josupeit R, Schlund F, Stroh-Dege A, Frank-Stöhr M, Leuchs B, Schlehofer JR, Rommelaere J, Dinsart C. Preclinical Testing of an Oncolytic Parvovirus in Ewing Sarcoma: Protoparvovirus H-1 Induces Apoptosis and Lytic Infection In Vitro but Fails to Improve Survival In Vivo. Viruses. 2018; 10(6):302. https://doi.org/10.3390/v10060302
Chicago/Turabian StyleLacroix, Jeannine, Zoltán Kis, Rafael Josupeit, Franziska Schlund, Alexandra Stroh-Dege, Monika Frank-Stöhr, Barbara Leuchs, Jörg R. Schlehofer, Jean Rommelaere, and Christiane Dinsart. 2018. "Preclinical Testing of an Oncolytic Parvovirus in Ewing Sarcoma: Protoparvovirus H-1 Induces Apoptosis and Lytic Infection In Vitro but Fails to Improve Survival In Vivo" Viruses 10, no. 6: 302. https://doi.org/10.3390/v10060302
APA StyleLacroix, J., Kis, Z., Josupeit, R., Schlund, F., Stroh-Dege, A., Frank-Stöhr, M., Leuchs, B., Schlehofer, J. R., Rommelaere, J., & Dinsart, C. (2018). Preclinical Testing of an Oncolytic Parvovirus in Ewing Sarcoma: Protoparvovirus H-1 Induces Apoptosis and Lytic Infection In Vitro but Fails to Improve Survival In Vivo. Viruses, 10(6), 302. https://doi.org/10.3390/v10060302







