A Systematic Review of the Natural Virome of Anopheles Mosquitoes
Abstract
1. Introduction
2. Materials and Methods
3. Results
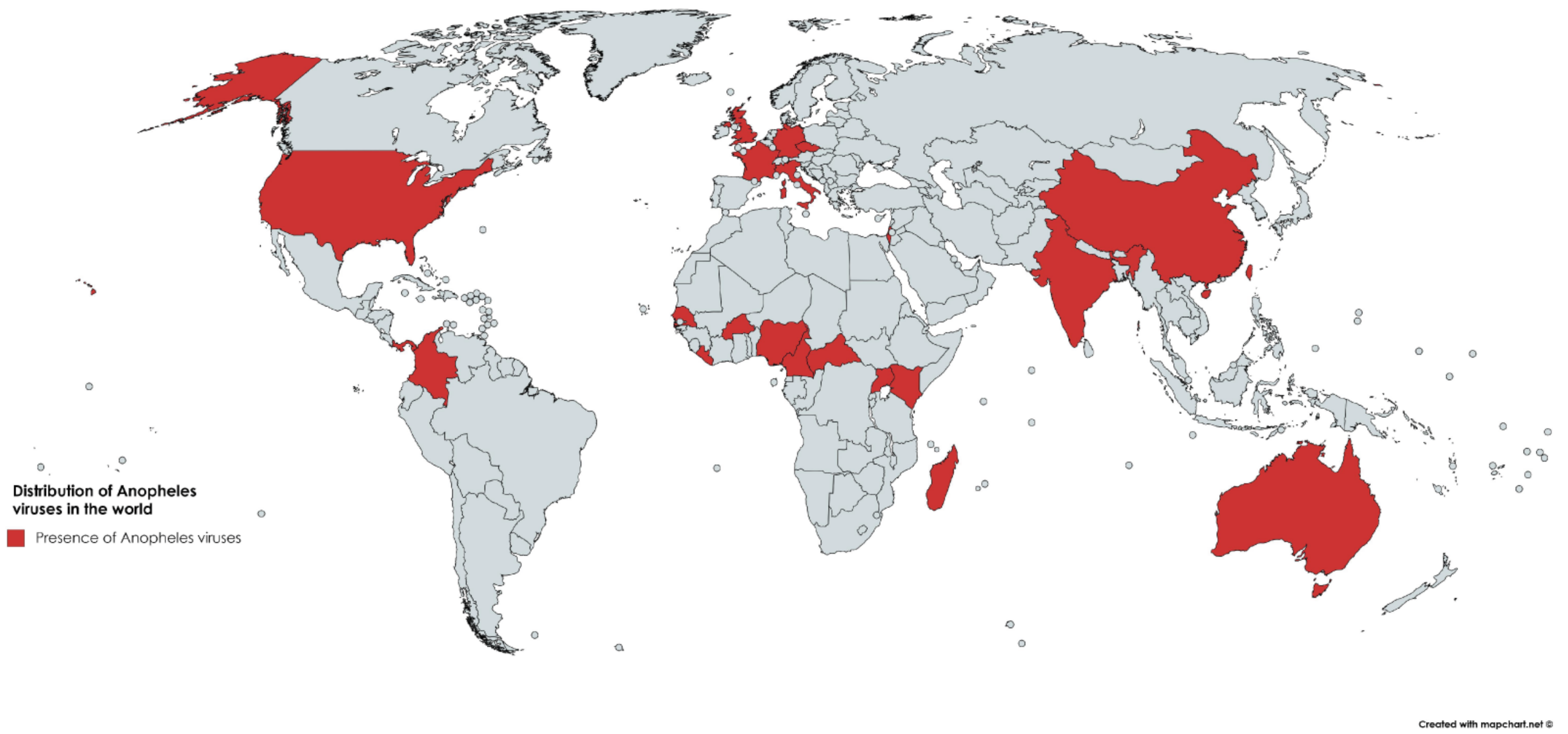
3.1. Bibliographic Search of Publication History on Anopheles Viruses
3.2. Viruses
3.3. DNA Viruses
3.3.1. Densovirus: Anopheles gambiae Densovirus (AgDNV)
3.3.2. Iridovirus: Anopheles minimus Virus (AMIV)
3.3.3. Poxvirus: Myxoma Virus (MYXV)
3.4. RNA Viruses
3.4.1. Alphavirus: O’nyong Nyong Virus (ONNV), Venezuelan Equine Encephalitis Virus (VEEV), Western Equine Encephalitis Virus (WEEV), Sindbis Virus (SINV), Semliki Forest Virus (SFV), and Eilat Virus (EILV)
3.4.2. Flavivirus: West Nile Virus (WNV), Japanese Encephalitis Virus (JEV), Wesselsbron Virus (WSLV), Anopheles Flavivirus (AnFV), Anopheles gambiae Flavivirus (AngFV), Anopheles squamosus Flavivirus (AnsFV), Stratford Virus (STRV), Karumba Virus (KRBV), Haslams Creek Virus (HaCV), Dairy Swamp Virus (DSwV), Mac Peak Virus (McPV), Long Pine Key virus (LPKV), Kampung Karu Virus (KPKV)
3.4.3. Phlebovirus: Rift Valley Fever Virus (RVFV)
3.4.4. Peribunyavirus: Leanyer Virus (LEAV), Ngari Virus (NRIV), Bangui Virus (BGIV), Cache Valley Virus (CVV), Mapputta Virus (MAPV), Tahyna Virus (TAHV), Tataguine Virus (TATV), Batai Virus (BATV), Nyando Virus (NDV), Ilesha Virus (ILEV), Bwamba Virus (BWAV), Anopheles A Virus (ANAV), Anopheles B Virus (ANBV), Tensaw Virus (TENV)
3.4.5. Dicistrovirus: Anopheles C Virus (AnCV) and Anopheles Associated C Virus (AACV)
3.4.6. Cypovirus: Anopheles Cypovirus (AnCPV)
3.4.7. Orbivirus: Tibet Orbivirus (TIBOV), Anopheles annulipes Orbivirus (AAOV) Anopheles hinesorum Orbivirus (AHOV), Orungo Virus (ORUV), Tilligerry Virus (TILV)
3.4.8. Mononegavirus: Bolahun Virus (BOAV) and Gambiae Virus (GAMV)
3.4.9. Almendravirus: Coot Bay Virus (CBV)
3.4.10. Totivirus: Anopheles Totivirus (AToV) and Australian Anopheles Totivirus (AATV)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- World Health Organization. World Malaria Report 2016; World Health Organization: Geneva, Switzerland, 2016. [Google Scholar]
- Sinka, M.E.; Bangs, M.J.; Manguin, S.; Rubio-Palis, Y.; Chareonviriyaphap, T.; Coetzee, M.; Mbogo, C.M.; Hemingway, J.; Patil, A.P.; Temperley, W.H.; et al. A global map of dominant malaria vectors. Parasites Vectors 2012, 5, 69. [Google Scholar] [CrossRef] [PubMed]
- Hay, S.I.; Sinka, M.E.; Okara, R.M.; Kabaria, C.W.; Mbithi, P.M.; Tago, C.C.; Benz, D.; Gething, P.W.; Howes, R.E.; Patil, A.P.; et al. Developing global maps of the dominant Anopheles vectors of human malaria. PLoS Med. 2010, 7, e1000209. [Google Scholar] [CrossRef] [PubMed]
- Hall, R.A.; Bielefeldt-Ohmann, H.; McLean, B.J.; O’Brien, C.A.; Colmant, A.M.; Piyasena, T.B.; Harrison, J.J.; Newton, N.D.; Barnard, R.T.; Prow, N.A.; et al. Commensal viruses of mosquitoes: Host restriction, transmission, and interaction with arboviral pathogens. Evol. Bioinform. Online 2016, 12, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Carissimo, G.; Eiglmeier, K.; Reveillaud, J.; Holm, I.; Diallo, M.; Diallo, D.; Vantaux, A.; Kim, S.; Menard, D.; Siv, S.; et al. Identification and characterization of two novel RNA viruses from Anopheles gambiae species complex mosquitoes. PLoS ONE 2016, 11, e0153881. [Google Scholar] [CrossRef] [PubMed]
- Sow, A.; Faye, O.; Ba, Y.; Ba, H.; Diallo, D.; Faye, O.; Loucoubar, C.; Boushab, M.; Barry, Y.; Diallo, M.; et al. Rift valley fever outbreak, Southern Mauritania, 2012. Emerg. Infect. Dis. 2014, 20, 296–299. [Google Scholar] [CrossRef] [PubMed]
- Rwaguma, E.B.; Lutwama, J.J.; Sempala, S.D.; Kiwanuka, N.; Kamugisha, J.; Okware, S.; Bagambisa, G.; Lanciotti, R.; Roehrig, J.T.; Gubler, D.J. Emergence of epidemic o’nyong-nyong fever in Southwestern Uganda, after an absence of 35 years. Emerg. Infect. Dis. 1997, 3, 77. [Google Scholar] [CrossRef] [PubMed]
- Sanders, E.J.; Rwaguma, E.B.; Kawamata, J.; Kiwanuka, N.; Lutwama, J.J.; Ssengooba, F.P.; Lamunu, M.; Najjemba, R.; Were, W.A.; Bagambisa, G.; et al. O’nyong-nyong fever in South-Central Uganda, 1996–1997: Description of the epidemic and results of a household-based seroprevalence survey. J. Infect. Dis. 1999, 180, 1436–1443. [Google Scholar] [CrossRef] [PubMed]
- Posey, D.L.; O’Rourke, T.; Roehrig, J.T.; Lanciotti, R.S.; Weinberg, M.; Maloney, S. O’nyong-nyong fever in west Africa. Am. J. Trop. Med. Hyg. 2005, 73, 32. [Google Scholar] [PubMed]
- Powers, A.M.; Brault, A.C.; Shirako, Y.; Strauss, E.G.; Kang, W.; Strauss, J.H.; Weaver, S.C. Evolutionary relationships and systematics of the alphaviruses. J. Virol. 2001, 75, 10118–10131. [Google Scholar] [CrossRef] [PubMed]
- Huhtamo, E.; Cook, S.; Moureau, G.; Uzcategui, N.Y.; Sironen, T.; Kuivanen, S.; Putkuri, N.; Kurkela, S.; Harbach, R.E.; Firth, A.E.; et al. Novel flaviviruses from mosquitoes: Mosquito-specific evolutionary lineages within the phylogenetic group of mosquito-borne flaviviruses. Virology 2014, 464–465, 320–329. [Google Scholar] [CrossRef] [PubMed]
- Li, C.X.; Shi, M.; Tian, J.H.; Lin, X.D.; Kang, Y.J.; Chen, L.J.; Qin, X.C.; Xu, J.; Holmes, E.C.; Zhang, Y.Z. Unprecedented genomic diversity of rna viruses in arthropods reveals the ancestry of negative-sense RNA viruses. Elife 2015, 4, e05378. [Google Scholar] [CrossRef] [PubMed]
- Nasar, F.; Palacios, G.; Gorchakov, R.V.; Guzman, H.; Da Rosa, A.P.; Savji, N.; Popov, V.L.; Sherman, M.B.; Lipkin, W.I.; Tesh, R.B.; et al. Eilat virus, a unique alphavirus with host range restricted to insects by RNA replication. Proc. Natl. Acad. Sci. USA 2012, 109, 14622–14627. [Google Scholar] [CrossRef] [PubMed]
- Vasilakis, N.; Forrester, N.L.; Palacios, G.; Nasar, F.; Savji, N.; Rossi, S.L.; Guzman, H.; Wood, T.G.; Popov, V.; Gorchakov, R.; et al. Negevirus: A proposed new taxon of insect-specific viruses with wide geographic distribution. J. Virol. 2013, 87, 2475–2488. [Google Scholar] [CrossRef] [PubMed]
- Zuo, S.; Zhao, Q.; Guo, X.; Zhou, H.; Cao, W.; Zhang, J. Detection of quang binh virus from mosquitoes in China. Virus Res. 2014, 180, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Cook, S.; Chung, B.Y.; Bass, D.; Moureau, G.; Tang, S.; McAlister, E.; Culverwell, C.L.; Glucksman, E.; Wang, H.; Brown, T.D.; et al. Novel virus discovery and genome reconstruction from field RNA samples reveals highly divergent viruses in dipteran hosts. PLoS ONE 2013, 8, e80720. [Google Scholar] [CrossRef] [PubMed]
- Webster, C.L.; Waldron, F.M.; Robertson, S.; Crowson, D.; Ferrari, G.; Quintana, J.F.; Brouqui, J.M.; Bayne, E.H.; Longdon, B.; Buck, A.H.; et al. The discovery, distribution, and evolution of viruses associated with drosophila melanogaster. PLoS Biol. 2015, 13, e1002210. [Google Scholar] [CrossRef] [PubMed]
- Williams, M.C.; Woodall, J.P.; Corbet, P.S. Nyando virus: A hitherto undescribed virus isolated from Anopheles funestus giles collected in Kenya. Archiv für die Gesamte Virusforschung 1965, 15, 422–427. [Google Scholar] [CrossRef] [PubMed]
- Digoutte, J.P.; Gagnard, V.J.M.; Brès, P.; Pajot, F.-X.; Perreau. Infection à virus nyando chez l’homme. Bulletin de la Société de Pathologie Exotique 1972, 65, 751–758. [Google Scholar]
- Jost, H.; Bialonski, A.; Schmetz, C.; Gunther, S.; Becker, N.; Schmidt-Chanasit, J. Isolation and phylogenetic analysis of batai virus, Germany. Am. J. Trop. Med. Hyg. 2011, 84, 241–243. [Google Scholar] [CrossRef] [PubMed]
- Su, C.L.; Yang, C.F.; Teng, H.J.; Lu, L.C.; Lin, C.; Tsai, K.H.; Chen, Y.Y.; Chen, L.Y.; Chang, S.F.; Shu, P.Y. Molecular epidemiology of Japanese encephalitis virus in mosquitoes in Taiwan during 2005–2012. PLoS Negl. Trop. Dis. 2014, 8, e3122. [Google Scholar] [CrossRef] [PubMed]
- Brugman, V.A.; Hernandez-Triana, L.M.; Prosser, S.W.; Weland, C.; Westcott, D.G.; Fooks, A.R.; Johnson, N. Molecular species identification, host preference and detection of myxoma virus in the Anopheles maculipennis complex (diptera: Culicidae) in Southern England, UK. Parasites Vectors 2015, 8, 421. [Google Scholar] [CrossRef] [PubMed]
- Maquart, M.; Boyer, S.; Rakotoharinome, V.M.; Ravaomanana, J.; Tantely, M.L.; Heraud, J.M.; Cardinale, E. High prevalence of west nile virus in domestic birds and detection in 2 new mosquito species in madagascar. PLoS ONE 2016, 11, e0147589. [Google Scholar] [CrossRef] [PubMed]
- Ratovonjato, J.; Olive, M.M.; Tantely, L.M.; Andrianaivolambo, L.; Tata, E.; Razainirina, J.; Jeanmaire, E.; Reynes, J.M.; Elissa, N. Detection, isolation, and genetic characterization of rift valley fever virus from Anopheles (Anopheles) coustani, Anopheles (Anopheles) squamosus, and Culex (Culex) antennatus of the haute matsiatra region, madagascar. Vector Borne Zoonotic Dis. 2011, 11, 753–759. [Google Scholar] [CrossRef] [PubMed]
- Seufi, A.M.; Galal, F.H. Role of Culex and Anopheles mosquito species as potential vectors of rift valley fever virus in sudan outbreak, 2007. BMC Infect. Dis. 2010, 10, 65. [Google Scholar] [CrossRef] [PubMed]
- Carissimo, G.; Pondeville, E.; McFarlane, M.; Dietrich, I.; Mitri, C.; Bischoff, E.; Antoniewski, C.; Bourgouin, C.; Failloux, A.B.; Kohl, A.; et al. Antiviral immunity of Anopheles gambiae is highly compartmentalized, with distinct roles for RNA interference and gut microbiota. Proc. Natl. Acad. Sci. USA 2015, 112, E176–E185. [Google Scholar] [CrossRef] [PubMed]
- Keene, K.M.; Foy, B.D.; Sanchez-Vargas, I.; Beaty, B.J.; Blair, C.D.; Olson, K.E. Rna interference acts as a natural antiviral response to o’nyong-nyong virus (Alphavirus; togaviridae) infection of Anopheles gambiae. Proc. Natl. Acad. Sci. USA 2004, 101, 17240–17245. [Google Scholar] [CrossRef] [PubMed]
- Waldock, J.; Olson, K.E.; Christophides, G.K. Anopheles gambiae antiviral immune response to systemic o’nyong-nyong infection. PLoS Negl. Trop. Dis. 2012, 6, e1565. [Google Scholar] [CrossRef] [PubMed]
- Olival, K.J.; Hosseini, P.R.; Zambrana-Torrelio, C.; Ross, N.; Bogich, T.L.; Daszak, P. Host and viral traits predict zoonotic spillover from mammals. Nature 2017, 546, 646–650. [Google Scholar] [CrossRef] [PubMed]
- Reagan, R.L.; Strand, N.; Brueckner, A.L. Comparison by electron microscopy of anopheles a and anopheles b viruses. Tex. Rep. Biol. Med. 1953, 11, 508–511. [Google Scholar] [PubMed]
- Colmant, A.M.G.; Etebari, K.; Webb, C.E.; Ritchie, S.A.; Jansen, C.C.; van den Hurk, A.F.; Bielefeldt-Ohmann, H.; Hobson-Peters, J.; Asgari, S.; Hall, R.A. Discovery of new orbiviruses and totivirus from Anopheles mosquitoes in Eastern Australia. Arch. Virol. 2017, 162, 3529–3534. [Google Scholar] [CrossRef] [PubMed]
- Fauver, J.R.; Grubaugh, N.D.; Krajacich, B.J.; Weger-Lucarelli, J.; Lakin, S.M.; Fakoli, L.S., 3rd; Bolay, F.K.; Diclaro, J.W., 2nd; Dabire, K.R.; Foy, B.D.; et al. West African Anopheles gambiae mosquitoes harbor a taxonomically diverse virome including new insect-specific flaviviruses, mononegaviruses, and totiviruses. Virology 2016, 498, 288–299. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Hoiczyk, E.; Rasgon, J.L. Viral paratransgenesis in the malaria vector Anopheles gambiae. PLoS Pathog. 2008, 4, e1000135. [Google Scholar] [CrossRef] [PubMed]
- Villinger, J.; Mbaya, M.K.; Ouso, D.; Kipanga, P.N.; Lutomiah, J.; Masiga, D.K. Arbovirus and insect-specific virus discovery in Kenya by novel six genera multiplex high-resolution melting analysis. Mol. Ecol. Resour. 2017, 17, 466–480. [Google Scholar] [CrossRef] [PubMed]
- Trung, H.D.; Van Bortel, W.; Sochantha, T.; Keokenchanh, K.; Quang, N.T.; Cong, L.D.; Coosemans, M. Malaria transmission and major malaria vectors in different geographical areas of Southeast Asia. Trop. Med. Int. Health 2004, 9, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Gordon, S.W.; Tammariello, R.F.; Linthicum, K.J.; Dohm, D.J.; Digoutte, J.P.; Calvo-Wilson, M.A. Arbovirus isolations from mosquitoes collected during 1988 in the Senegal River basin. Am. J. Trop. Med. Hyg. 1992, 47, 742–748. [Google Scholar] [CrossRef] [PubMed]
- Huhtamo, E.; Lambert, A.J.; Costantino, S.; Servino, L.; Krizmancic, L.; Boldorini, R.; Allegrini, S.; Grasso, I.; Korhonen, E.M.; Vapalahti, O.; et al. Isolation and full genomic characterization of batai virus from mosquitoes, Italy 2009. J. Gen. Virol. 2013, 94, 1242–1248. [Google Scholar] [CrossRef] [PubMed]
- Lutwama, J.J.; Rwaguma, E.B.; Nawanga, P.L.; Mukuye, A. Isolations of bwamba virus from South Central Uganda and north Eastern Tanzania. Afr. Health Sci. 2002, 2, 24–28. [Google Scholar] [PubMed]
- Blackmore, C.G.; Blackmore, M.S.; Grimstad, P.R. Role of anopheles quadrimaculatus and coquillettidia perturbans (diptera: Culicidae) in the transmission cycle of cache valley virus (bunyaviridae: Bunyavirus) in the midwest, USA. J. Med. Entomol. 1998, 35, 660–664. [Google Scholar] [CrossRef] [PubMed]
- Contreras, M.A.; Eastwood, G.; Guzman, H.; Popov, V.; Savit, C.; Uribe, S.; Kramer, L.D.; Wood, T.G.; Widen, S.G.; Fish, D.; et al. Almendravirus: A proposed new genus of rhabdoviruses isolated from mosquitoes in tropical regions of the Americas. Am. J. Trop. Med. Hyg. 2017, 96, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Bird, R.G.; Draper, C.C.; Ellis, D.S. A cytoplasmic polyhedrosis virus in midgut cells of Anopheles stephensi and in the sporogonic stages of Plasmodium berghei yoelii. Bull. World Health Organ. 1972, 46, 337–343. [Google Scholar] [PubMed]
- Colmant, A.M.G.; Hobson-Peters, J.; Bielefeldt-Ohmann, H.; van den Hurk, A.F.; Hall-Mendelin, S.; Chow, W.K.; Johansen, C.A.; Fros, J.; Simmonds, P.; Watterson, D.; et al. A new clade of insect-specific flaviviruses from Australian Anopheles mosquitoes displays species-specific host restriction. mSphere 2017, 2, e00262-17. [Google Scholar] [CrossRef] [PubMed]
- Samina, I.; Margalit, J.; Peleg, J. Isolation of viruses from mosquitoes of the Negev, Israel. Trans. R. Soc. Trop. Med. Hyg. 1986, 80, 471–472. [Google Scholar] [CrossRef]
- Digoutte, J.P.; Salaun, J.J.; Robin, Y.; Bres, P.; Cagnard, V.J. minor arboviral diseases in central and west africa (author’s transl). Med. Trop. 1980, 40, 524–533. [Google Scholar]
- Mourya, D.T.; Ilkal, M.A.; Mishra, A.C.; Jacob, P.G.; Pant, U.; Ramanujam, S.; Mavale, M.S.; Bhat, H.R.; Dhanda, V. Isolation of Japanese encephalitis virus from mosquitoes collected in Karnataka state, India from 1985 to 1987. Trans. R. Soc. Trop. Med. Hyg. 1989, 83, 550–552. [Google Scholar] [CrossRef]
- Kuwata, R.; Sugiyama, H.; Yonemitsu, K.; Van Dung, N.; Terada, Y.; Taniguchi, M.; Shimoda, H.; Takano, A.; Maeda, K. Isolation of Japanese encephalitis virus and a novel insect-specific flavivirus from mosquitoes collected in a cowshed in Japan. Arch. Virol. 2015, 160, 2151–2159. [Google Scholar] [CrossRef] [PubMed]
- Guzman, H.; Contreras-Gutierrez, M.A.; Travassos da Rosa, A.P.A.; Nunes, M.R.T.; Cardoso, J.F.; Popov, V.L.; Young, K.I.; Savit, C.; Wood, T.G.; Widen, S.G.; et al. Characterization of three new insect-specific flaviviruses: Their relationship to the mosquito-borne flavivirus pathogens. Am. J. Trop. Med. Hyg. 2018, 98, 410–419. [Google Scholar] [CrossRef] [PubMed]
- Stuckly, K.G.; Wright, P.J. Characterization of leanyer virus: Resemblance to bunyavirus. Aust. J. Exp. Biol. Med. Sci. 1983, 61 Pt 2, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Gauci, P.J.; McAllister, J.; Mitchell, I.R.; Boyle, D.B.; Bulach, D.M.; Weir, R.P.; Melville, L.F.; Gubala, A.J. Genomic characterisation of three mapputta group viruses, a serogroup of Australian and Papua new guinean bunyaviruses associated with human disease. PLoS ONE 2015, 10, e0116561. [Google Scholar] [CrossRef] [PubMed]
- Williams, M.C.; Woodall, J.P.; Corbet, P.S.; Gillett, J.D. O’nyong-nyong fever: An epidemic virus disease in East Africa. 8. Virus isolations from Anopheles mosquitoes. Trans. R. Soc. Trop. Med. Hyg. 1965, 59, 300–306. [Google Scholar] [CrossRef]
- Mohd Jaafar, F.; Belhouchet, M.; Belaganahalli, M.; Tesh, R.B.; Mertens, P.P.; Attoui, H. Full-genome characterisation of orungo, lebombo and changuinola viruses provides evidence for co-evolution of orbiviruses with their arthropod vectors. PLoS ONE 2014, 9, e86392. [Google Scholar]
- Mathiot, C.C.; Grimaud, G.; Garry, P.; Bouquety, J.C.; Mada, A.; Daguisy, A.M.; Georges, A.J. An outbreak of human semliki forest virus infections in Central African Republic. Am. J. Trop. Med. Hyg. 1990, 42, 386–393. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.-Y.; Liang, G.-D. Research on basis of reverse genetics system of a sindbis-like virus xj-160. Virol. J. 2011, 8, 519. [Google Scholar]
- Toi, C.S.; Webb, C.E.; Haniotis, J.; Clancy, J.; Doggett, S.L. Seasonal activity, vector relationships and genetic analysis of mosquito-borne stratford virus. PLoS ONE 2017, 12, e0173105. [Google Scholar] [CrossRef] [PubMed]
- Hubalek, Z.; Sebesta, O.; Pesko, J.; Betasova, L.; Blazejova, H.; Venclikova, K.; Rudolf, I. Isolation of tahyna virus (california encephalitis group) from Anopheles hyrcanus (diptera, culicidae), a mosquito species new to, and expanding in, central Europe. J. Med. Entomol. 2014, 51, 1264–1267. [Google Scholar] [CrossRef] [PubMed]
- Salaun, J.J.; Rickenbach, A.; Bres, P.; Germain, M.; Eouzan, J.P.; Ferrara, L. Isolation in cameroon of 3 strains of tataguine virus. Bulletin de la Societe de Pathologie Exotique et de ses Filiales 1968, 61, 557–564. [Google Scholar] [PubMed]
- Collins, W.E.; Harrison, A.J. Studies of tensaw virus in Anopheles quadrimaculatus, A. albimanus and A. maculatus. Mosq. News 1967, 27, 1–5. [Google Scholar]
- Li, M.; Zheng, Y.; Zhao, G.; Fu, S.; Wang, D.; Wang, Z.; Liang, G. Tibet orbivirus, a novel orbivirus species isolated from Anopheles maculatus mosquitoes in Tibet, China. PLoS ONE 2014, 9, e88738. [Google Scholar] [CrossRef] [PubMed]
- Belaganahalli, M.N.; Maan, S.; Maan, N.S.; Nomikou, K.; Pritchard, I.; Lunt, R.; Kirkland, P.D.; Attoui, H.; Brownlie, J.; Mertens, P.P. Full genome sequencing and genetic characterization of eubenangee viruses identify pata virus as a distinct species within the genus orbivirus. PLoS ONE 2012, 7, e31911. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.R.; Aguilar, P.V.; Coffey, L.L.; Gromowski, G.D.; Wang, E.; Weaver, S.C. Venezuelan equine encephalitis virus transmission and effect on pathogenesis. Emerg. Infect. Dis. 2006, 12, 1190–1196. [Google Scholar] [CrossRef] [PubMed]
- Mancini, G.; Montarsi, F.; Calzolari, M.; Capelli, G.; Dottori, M.; Ravagnan, S.; Lelli, D.; Chiari, M.; Santilli, A.; Quaglia, M.; et al. Mosquito species involved in the circulation of West nile and Usutu viruses in Italy. Vet. Ital. 2017, 53, 97–110. [Google Scholar] [PubMed]
- Kemenesi, G.; Krtinic, B.; Milankov, V.; Kutas, A.; Dallos, B.; Oldal, M.; Somogyi, N.; Nemeth, V.; Banyai, K.; Jakab, F. West nile virus surveillance in mosquitoes, April to October 2013, Vojvodina province, Serbia: Implications for the 2014 season. Eurosurveillance 2014, 19, 20779. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, C.J.; Monath, T.P.; Sabattini, M.S.; Daffner, J.F.; Cropp, C.B.; Calisher, C.H.; Darsie, R.F., Jr.; Jakob, W.L. Arbovirus isolations from mosquitoes collected during and after the 1982–1983 epizootic of western equine encephalitis in Argentina. Am. J. Trop. Med. Hyg. 1987, 36, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Bergoin, M.; Tijssen, P. Densoviruses: A highly diverse group of arthropod parvoviruses. In Insect Virology; Asgari, S., Johnson, K.N., Eds.; Caister Academic Press: Norfolk, UK, 2010; pp. 59–82. [Google Scholar]
- Ren, X.; Rasgon, J.L. Potential for the Anopheles gambiae densonucleosis virus to act as an “evolution-proof” biopesticide. J. Virol. 2010, 84, 7726–7729. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Li, S.; Zhao, Q.; Pei, G.; An, X.; Guo, X.; Zhou, H.; Zhang, Z.; Zhang, J.; Tong, Y. Isolation and characterization of a novel invertebrate iridovirus from adult Anopheles minimus (AMIV) in China. J. Invertebr. Pathol. 2015, 127, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Williams, T.; Ward, V.K. Iridoviruses. In Densoviruses: A Highly Diverse Group of Arthropod Parvoviruses; Asgari, S., Johnson, K.N., Eds.; Caister Academic Press: Norfolk, UK, 2010. [Google Scholar]
- Laboudi, L.; Sadak, A.; Ouahabi, S.; Boccolini, D.; Faraj, C. Molecular characterization of Anopheles maculipennis complex (diptera: Culicidae) in Northern Morocco. Entomol. Faun.-Faun. Entomol. 2014, 67, 37–42. [Google Scholar]
- Perera, S.L.; Pavlik, L.Z.; Arif, B. Entomopoxvirus; Caister Academic: Norfolk, UK, 2010. [Google Scholar]
- Saxton-Shaw, K.D.; Ledermann, J.P.; Borland, E.M.; Stovall, J.L.; Mossel, E.C.; Singh, A.J.; Wilusz, J.; Powers, A.M. O’nyong nyong virus molecular determinants of unique vector specificity reside in non-structural protein 3. PLoS Negl. Trop. Dis. 2013, 7, e1931. [Google Scholar] [CrossRef] [PubMed]
- Arias-Goeta, C.; Mousson, L.; Rougeon, F.; Failloux, A.B. Dissemination and transmission of the E1-226V variant of Chikungunya virus in Aedes albopictus are controlled at the midgut barrier level. PLoS ONE 2013, 8, e57548. [Google Scholar] [CrossRef] [PubMed]
- Weger-Lucarelli, J.; Aliota, M.T.; Wlodarchak, N.; Kamlangdee, A.; Swanson, R.; Osorio, J.E. Dissecting the role of E2 protein domains in alphavirus pathogenicity. J. Virol. 2015, 90, 2418–2433. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A.; Melton, J.V.; Herrero, L.J.; Thaa, B.; Karo-Astover, L.; Gage, P.W.; Nelson, M.A.; Sheng, K.C.; Lidbury, B.A.; Ewart, G.D.; et al. Effects of an in-frame deletion of the 6k gene locus from the genome of ross river virus. J. Virol. 2016, 90, 4150–4159. [Google Scholar] [CrossRef] [PubMed]
- West, J.; Hernandez, R.; Ferreira, D.; Brown, D.T. Mutations in the endodomain of sindbis virus glycoprotein E2 define sequences critical for virus assembly. J. Virol. 2006, 80, 4458–4468. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cohuet, A.; Simard, F.; Wondji, C.S.; Antonio-Nkondjio, C.; Awono-Ambene, P.; Fontenille, D. High malaria transmission intensity due to Anopheles funestus (diptera: Culicidae) in a village of savannah-forest transition area in Cameroon. J. Med. Entomol. 2004, 41, 901–905. [Google Scholar] [CrossRef] [PubMed]
- Derua, Y.A.; Alifrangis, M.; Magesa, S.M.; Kisinza, W.N.; Simonsen, P.E. Sibling species of the Anopheles funestus group, and their infection with malaria and lymphatic filarial parasites, in archived and newly collected specimens from Northeastern Tanzania. Malar. J. 2015, 14, 104. [Google Scholar] [CrossRef] [PubMed]
- Sudia, W.D.; Fernandez, L.; Newhouse, V.F.; Sanz, R.; Calisher, C.H. Arbovirus vector ecology studies in mexico during the 1972 venezuelan equine encephalitis outbreak. Am. J. Epidemiol. 1975, 101, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Rivas, F.; Diaz, L.A.; Cardenas, V.M.; Daza, E.; Bruzon, L.; Alcala, A.; De la Hoz, O.; Caceres, F.M.; Aristizabal, G.; Martinez, J.W.; et al. Epidemic venezuelan equine encephalitis in La Guajira, Colombia, 1995. J. Infect. Dis. 1997, 175, 828–832. [Google Scholar] [CrossRef] [PubMed]
- Hahn, C.S.; Lustig, S.; Strauss, E.G.; Strauss, J.H. Western equine encephalitis virus is a recombinant virus. Proc. Natl. Acad. Sci. USA 1988, 85, 5997–6001. [Google Scholar] [CrossRef] [PubMed]
- Collins, W.E.; Harrison, A.J. Studies of sindbis virus in Anopheles albimanus and Aedes aegypti. Mosq. News 1966, 26, 91–93. [Google Scholar]
- Jupp, P.G. Arboviral zoonoses of Africa. In Handbook of Zoonoses, Section B; Beran, G.W., Steele, J.H., Eds.; CRC Press: Boca Raton, FL, USA; London, UK, 1994; pp. 261–264. [Google Scholar]
- Collins, W.E.; Harrison, A.J.; Skinner, J.C. Studies on the transmission of semliki forest virus by Anopheles freeborni, A. Stephensi, A. Labranchiae atroparvus and A. Sundaicus. Mosq. News 1965, 25, 54–57. [Google Scholar]
- Zou, G.; Puig-Basagoiti, F.; Zhang, B.; Qing, M.; Chen, L.; Pankiewicz, K.W.; Felczak, K.; Yuan, Z.; Shi, P.Y. A single-amino acid substitution in West nile virus 2k peptide between NS4A and NS4B confers resistance to lycorine, a flavivirus inhibitor. Virology 2009, 384, 242–252. [Google Scholar] [CrossRef] [PubMed]
- Larrieu, S.; Cardinale, E.; Ocquidant, P.; Roger, M.; Lepec, R.; Delatte, H.; Camuset, G.; Despres, P.; Brottet, E.; Charlin, C.; et al. A fatal neuroinvasive west nile virus infection in a traveler returning from madagascar: Clinical, epidemiological and veterinary investigations. Am. J. Trop. Med. Hyg. 2013, 89, 211–213. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka, T.; Tsujimura, K.; Kondo, T.; Yasuda, W.; Okada, A.; Noda, K.; Okumura, T.; Matsumura, T. Isolation and genetic analysis of Japanese encephalitis virus from a diseased horse in Japan. J. Vet. Med. Sci. 2006, 68, 293–295. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.R.; Yan, J.Y.; Zhou, J.Y.; Tang, X.W.; He, H.Q.; Xie, R.H.; Mao, H.Y.; Zhang, Y.J.; Xie, S.Y. Sero-molecular epidemiology of Japanese encephalitis in Zhejiang, an eastern province of China. PLoS Negl. Trop. Dis. 2016, 10, e0004936. [Google Scholar] [CrossRef] [PubMed]
- Diagne, M.M.; Faye, M.; Faye, O.; Sow, A.; Balique, F.; Sembene, M.; Granjon, L.; Handschumacher, P.; Faye, O.; Diallo, M.; et al. Emergence of Wesselsbron virus among black rat and humans in Eastern Senegal in 2013. One Health 2017, 3, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Arsevska, E.; Lancelot, R.; El-Mamy, B.; Cêtre-Sossah, C. Situation épidémiologique de la fièvre de la vallée du rift en afrique de l’ouest et du nord. Bulletin Épidemiologique Santé Animale et Alimentation 2016, 74, 25–29. [Google Scholar]
- Savji, N.; Palacios, G.; Travassos da Rosa, A.; Hutchison, S.; Celone, C.; Hui, J.; Briese, T.; Calisher, C.H.; Tesh, R.B.; Lipkin, W.I. Genomic and phylogenetic characterization of leanyer virus, a novel orthobunyavirus isolated in Northern Australia. J. Gen. Virol. 2011, 92, 1676–1687. [Google Scholar] [CrossRef] [PubMed]
- Nashed, N.W.; Olson, J.G.; El-Tigani, A. Isolation of batai virus (bunyaviridae: Bunyavirus) from the blood of suspected malaria patients in Sudan. Am. J. Trop. Med. Hyg. 1993, 48, 676–681. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Shao, X.Q.; Hu, B.; Zhao, J.J.; Zhang, L.; Zhang, H.L.; Bai, X.; Zhang, R.X.; Niu, D.Y.; Sun, Y.G.; et al. Isolation and complete nucleotide sequence of a batai virus strain in Inner Mongolia, China. Virol. J. 2014, 11, 138. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, M.; Wietholter, A.; Blaha, I.; Jost, H.; Heinemann, P.; Lehmann, M.; Miller, T.; Cadar, D.; Yanase, T.; Kley, N.; et al. Surveillance of batai virus in bovines from Germany. Clin. Vaccine Immunol. 2015, 22, 672–673. [Google Scholar] [CrossRef] [PubMed]
- Romi, R.; Boccolini, D.; Hovanesyan, I.; Grigoryan, G.; Di Luca, M.; Sabatinell, G. Anopheles sacharovi (diptera: Culicidae): A reemerging malaria vector in the ararat valley of Armenia. J. Med. Entomol. 2002, 39, 446–450. [Google Scholar] [CrossRef] [PubMed]
- Groseth, A.; Weisend, C.; Ebihara, H. Complete genome sequencing of mosquito and human isolates of ngari virus. J. Virol. 2012, 86, 13846–13847. [Google Scholar] [CrossRef] [PubMed]
- El Mekki, A.A.; Nieuwenhuysen, P.; van der Groen, G.; Pattyn, S.R. Characterization of some ungrouped viruses. Trans. R. Soc. Trop. Med. Hyg. 1981, 75, 799–806. [Google Scholar] [CrossRef]
- Nguyen, N.L.; Zhao, G.; Hull, R.; Shelly, M.A.; Wong, S.J.; Wu, G.; St George, K.; Wang, D.; Menegus, M.A. Cache valley virus in a patient diagnosed with aseptic meningitis. J. Clin. Microbiol. 2013, 51, 1966–1969. [Google Scholar] [CrossRef] [PubMed]
- Cooper, R.D.; Frances, S.P.; Waterson, D.G.; Piper, R.G.; Sweeney, A.W. Distribution of anopheline mosquitoes in northern Australia. J. Am. Mosq. Control Assoc. 1996, 12, 656–663. [Google Scholar] [PubMed]
- Cooper, R.D.; Waterson, D.G.; Frances, S.P.; Beebe, N.W.; Pluess, B.; Sweeney, A.W. Malaria vectors of papua new Guinea. Int. J. Parasitol. 2009, 39, 1495–1501. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Cao, Y.; Fu, S.; Wang, J.; Li, M.; Jiang, S.; Wang, X.; Xing, S.; Feng, L.; Wang, Z.; et al. Tahyna virus infection, a neglected arboviral disease in the qinghai-tibet plateau of China. Vector Borne Zoonotic Dis. 2014, 14, 353–357. [Google Scholar] [CrossRef] [PubMed]
- Bennett, R.S.; Gresko, A.K.; Murphy, B.R.; Whitehead, S.S. Tahyna virus genetics, infectivity, and immunogenicity in mice and monkeys. Virol. J. 2011, 8, 135. [Google Scholar] [CrossRef] [PubMed]
- Djadid, N.D.; Jazayeri, H.; Gholizadeh, S.; Rad Sh, P.; Zakeri, S. First record of a new member of Anopheles hyrcanus group from Iran: Molecular identification, diagnosis, phylogeny, status of kdr resistance and Plasmodium infection. J. Med. Entomol. 2009, 46, 1084–1093. [Google Scholar] [CrossRef] [PubMed]
- Khrabrova, N.V.; Andreeva, Y.V.; Sibataev, A.K.; Alekseeva, S.S.; Esenbekova, P.A. Mosquitoes of Anopheles hyrcanus (diptera, culicidae) group: Species diagnostic and phylogenetic relationships. Am. J. Trop. Med. Hyg. 2015, 93, 619–622. [Google Scholar] [CrossRef] [PubMed]
- Shchetinin, A.M.; Lvov, D.K.; Deriabin, P.G.; Botikov, A.G.; Gitelman, A.K.; Kuhn, J.H.; Alkhovsky, S.V. Genetic and phylogenetic characterization of tataguine and witwatersrand viruses and other orthobunyaviruses of the anopheles a, capim, guama, koongol, mapputta, tete, and turlock serogroups. Viruses 2015, 7, 5987–6008. [Google Scholar] [CrossRef] [PubMed]
- Doherty, R.L.; Whitehead, R.H.; Judith Wetters, E.; Gorman, B.M. Studies of the epidemiology of arthropod-borne virus infections at Mitchell River Mission, Cape York Peninsula, North Queensland. Trans. R. Soc. Trop. Med. Hyg. 1968, 62, 430–438. [Google Scholar] [CrossRef]
- Groseth, A.; Mampilli, V.; Weisend, C.; Dahlstrom, E.; Porcella, S.F.; Russell, B.J.; Tesh, R.B.; Ebihara, H. Molecular characterization of human pathogenic bunyaviruses of the nyando and bwamba/pongola virus groups leads to the genetic identification of mojui dos campos and kaeng khoi virus. PLoS Negl. Trop. Dis. 2014, 8, e3147. [Google Scholar] [CrossRef] [PubMed]
- Morvan, J.M.; Digoutte, J.P.; Marsan, P.; Roux, J.F. Ilesha virus: A new aetiological agent of haemorrhagic fever in Madagascar. Trans. R. Soc. Trop. Med. Hyg. 1994, 88, 205. [Google Scholar] [CrossRef]
- Johnson, B.K.; Chanas, A.C.; Squires, E.J.; Shockley, P.; Simpson, D.I.; Smith, D.H. The isolation of a bwamba virus variant from man in western Kenya. J. Med. Virol. 1978, 2, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Orjuela, L.I.; Ahumada, M.L.; Avila, I.; Herrera, S.; Beier, J.C.; Quinones, M.L. Human biting activity, spatial-temporal distribution and malaria vector role of Anopheles calderoni in the southwest of Colombia. Malar. J. 2015, 14, 256. [Google Scholar] [CrossRef] [PubMed]
- Bridgen, A.; Weber, F.; Fazakerley, J.K.; Elliott, R.M. Bunyamwera bunyavirus nonstructural protein NSs is a nonessential gene product that contributes to viral pathogenesis. Proc. Natl. Acad. Sci. USA 2001, 98, 664–669. [Google Scholar] [CrossRef] [PubMed]
- Ikegami, T.; Narayanan, K.; Won, S.; Kamitani, W.; Peters, C.J.; Makino, S. Rift valley fever virus nss protein promotes post-transcriptional downregulation of protein kinase pkr and inhibits eif2alpha phosphorylation. PLoS Pathog. 2009, 5, e1000287. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, M.; McLees, A.; Elliott, R.M. Viruses in the anopheles a, anopheles b, and tete serogroups in the orthobunyavirus genus (family Bunyaviridae) do not encode an NSs protein. J. Virol. 2009, 83, 7612–7618. [Google Scholar] [CrossRef] [PubMed]
- Bonning, B.C.; Karyn, N.J. Dicistrovirus; Caister Academic: Norfolk, UK, 2010. [Google Scholar]
- Zhang, X.; Ding, K.; Yu, X.; Chang, W.; Sun, J.; Zhou, Z.H. In situ structures of the segmented genome and RNA polymerase complex inside a dsRNA virus. Nature 2015, 527, 531–534. [Google Scholar] [CrossRef] [PubMed]
- Muenworn, V.; Sungvornyothin, S.; Kongmee, M.; Polsomboon, S.; Bangs, M.J.; Akrathanakul, P.; Tanasinchayakul, S.; Prabaripai, A.; Chareonviriyaphap, T. Biting activity and host preference of the malaria vectors Anopheles maculatus and Anopheles sawadwongporni (diptera: Culicidae) in Thailand. J. Vector Ecol. 2009, 34, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Gorman, B.M.; Taylor, J. The rna genome of tilligerry virus. Aust. J. Exp. Biol. Med. Sci. 1978, 56, 369–371. [Google Scholar] [CrossRef] [PubMed]
- Tomori, O.; Fabiyi, A. Neutralizing antibodies to orungo virus in man and animals in Nigeria. Trop. Geogr. Med. 1976, 28, 233–238. [Google Scholar] [PubMed]
- Hartley, M.A.; Ronet, C.; Zangger, H.; Beverley, S.M.; Fasel, N. Leishmania RNA virus: When the host pays the toll. Front. Cell. Infect. Microbiol. 2012, 2, 99. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Cao, L.; Huang, Q.; Qian, Y.; Zhou, X. The complete genome sequence of a novel maize-associated totivirus. Arch. Virol. 2016, 161, 487–490. [Google Scholar] [CrossRef] [PubMed]
- Holmes, E.C. The Evolution and Emergence of RNA Viruses; Oxford University Press: Oxford, UK, 2011. [Google Scholar]
- Campbell, C.L.; Black, W.C.T.; Hess, A.M.; Foy, B.D. Comparative genomics of small RNA regulatory pathway components in vector mosquitoes. BMC Genom. 2008, 9, 425. [Google Scholar] [CrossRef] [PubMed]
- Sow, A.; Loucoubar, C.; Diallo, D.; Faye, O.; Ndiaye, Y.; Senghor, C.S.; Dia, A.T.; Faye, O.; Weaver, S.C.; Diallo, M.; et al. Concurrent malaria and arbovirus infections in Kedougou, southeastern Senegal. Malar. J. 2016, 15, 47. [Google Scholar] [CrossRef] [PubMed]
| Virus Name | Abbreviation | Virus Genus | Anopheles Species | References |
|---|---|---|---|---|
| Anopheles A virus | ANAV | Orthobunyavirus | Anopheles boliviensis | [30] |
| Anopheles annulipes orbivirus | AAOV | Orbivirus | Anopheles annulipes | [31] |
| Anopheles associated C virus | AACV | Cripavirus | Anopheles maculipennis | [16] |
| Anopheles B virus | ANBV | Orthobunyavirus | Anopheles boliviensis | [30] |
| Anopheles C virus | AnCV | Cripavirus | Anopheles gambiae | [5] |
| Anopheles cypovirus | AnCPV | Cypovirus | Anopheles gambiae | [5] |
| Anopheles flavivirus | AnFV | Flavivirus | Anopheles sp. | [32] |
| Anopheles gambiae densovirus | AgDNV | Densovirus | Anopheles gambiae | [33] |
| Anopheles gambiae flavivirus | AngFV | Flavivirus | Anopheles gambiae | [34] |
| Anopheles hinesorum orbivirus | AHOV | Orbivirus | Anopheles hinesorum | [31] |
| Anopheles minimus virus | AMIV | Iridovirus | Anopheles minimus | [35] |
| Anopheles squamosus flavivirus | AnsFV | Flavivirus | Anopheles squamosus | [34] |
| Anopheles totivirus | AToV | Totivirus | Anopheles gambiae | [32] |
| Australian Anopheles totivirus | AATV | Totivirus | Anopheles annulipes | [31] |
| Australian Anopheles totivirus | AATV | Totivirus | Anopheles hinesorum | [31] |
| Bangui virus | BGIV | Orthobunyavirus | Anopheles pharoensis | [36] |
| Batai virus | BATV | Orthobunyavirus | Anopheles maculipennis | [20,37] |
| Bolahum virus | BOAV | Mononegavirus | Anopheles sp. | [32] |
| Bwamba virus | BWAV | Orthobunyavirus | Anopheles funestus | [38] |
| Cache Valley virus | CVV | Orthobunyavirus | Anopheles quadrimaculatus | [39] |
| Coot Bay virus | CBV | Almendravirus | Anopheles quadrimaculatus | [40] |
| Cypovirus | Unnamed | Cypovirus | Anopheles stephensi | [41] |
| Dairy Swamp virus | DSwV | Flavivirus | Anopheles bancrofti | [42] |
| Eliat virus | EILV | Alphavirus | Anopheles coustani | [13,43] |
| Gambiae virus | GAMV | Mononegavirus | Anopheles sp. | [32] |
| Haslams Creek virus | HaCV | Flavivirus | Anopheles annulipes | [42] |
| Ilesha virus | ILEV | Orthobunyavirus | Anopheles gambiae | [44] |
| Japanese encephalitis virus | JEV | Flavivirus | Anopheles peditaeniatus | [45] |
| Japanese encephalitis virus | JEV | Flavivirus | Anopheles sinensis | [46] |
| Kampung karu virus | KPKV | Flavivirus | Anopheles tesselatus | [47] |
| Karumba virus | KRBV | Flavivirus | Anopheles meraukensis | [42] |
| Leanyer virus | LEAV | Orthobunyavirus | Anopheles meraukensis | [48] |
| Long Pine key virus | LPKV | Flavivirus | Anopheles crucians | [47] |
| Mac Peak virus | McPV | Flavivirus | Anopheles farauti | [42] |
| Mapputta virus | MAPV | Orthobunyavirus | Anopheles meraukensis | [49] |
| Myxoma virus | MYXV | Poxvirus | Anopheles maculipennis | [22] |
| Ngari virus | NRIV | Orthobunyavirus | Anopheles gambiae | [36] |
| Nyando virus | NDV | Orthobunyavirus | Anopheles funestus | [18,50] |
| O’nyong nyong virus | ONNV | Alphavirus | Anopheles gambiae | [8,50] |
| O’nyong nyong virus | ONNV | Alphavirus | Anopheles funestus | [8,50] |
| Orungo virus | ORUV | Orbivirus | Anopheles funestus | [51] |
| Rift Valley fever virus | RVFV | Phlebovirus | Anopheles squamosus | [25] |
| Rift Valley fever virus | RVFV | Phlebovirus | Anopheles coustani | [25] |
| Semliki Forest virus | SFV | Alphavirus | Anopheles funestus | [52] |
| Semliki Forest virus | SFV | Alphavirus | Anopheles coustani | [52] |
| Sindbis virus | SINV | Alphavirus | Anopheles pharoensis | [53] |
| Sindbis virus | SINV | Alphavirus | Anopheles albimanus | [53] |
| Stratford virus | STRV | Flavivirus | Anopheles annulipes | [54] |
| Tahyna virus | TAHV | Orthobunyavirus | Anopheles hyrcanus | [55] |
| Tataguine virus | TATV | Orthobunyavirus | Anopheles gambiae | [56] |
| Tensaw virus | TENV | Orthobunyavirus | Anopheles crucians | [57] |
| Tibet orbivirus | TIBOV | Orbivirus | Anopheles maculatus | [58] |
| Tilligerry virus | TILV | Orbivirus | Anopheles annulipes | [59] |
| Venezuelan equine encephalitis virus | VEEV | Alphavirus | Anopheles pseudopunctipennis | [60] |
| Wesselsbron virus | WSLV | Flavivirus | Anopheles coustani | [34] |
| West Nile virus | WNV | Flavivirus | Anopheles pauliani | [23] |
| West Nile virus | WNV | Flavivirus | Anopheles maculipennis | [61,62] |
| Western equine encephalitis virus | WEEV | Alphavirus | Anopheles albitarsis | [63] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nanfack Minkeu, F.; Vernick, K.D. A Systematic Review of the Natural Virome of Anopheles Mosquitoes. Viruses 2018, 10, 222. https://doi.org/10.3390/v10050222
Nanfack Minkeu F, Vernick KD. A Systematic Review of the Natural Virome of Anopheles Mosquitoes. Viruses. 2018; 10(5):222. https://doi.org/10.3390/v10050222
Chicago/Turabian StyleNanfack Minkeu, Ferdinand, and Kenneth D. Vernick. 2018. "A Systematic Review of the Natural Virome of Anopheles Mosquitoes" Viruses 10, no. 5: 222. https://doi.org/10.3390/v10050222
APA StyleNanfack Minkeu, F., & Vernick, K. D. (2018). A Systematic Review of the Natural Virome of Anopheles Mosquitoes. Viruses, 10(5), 222. https://doi.org/10.3390/v10050222





