Criteria for Selecting Suitable Infectious Diseases for Phage Therapy
Abstract
1. Introduction
2. Selection of Therapeutic Approaches
2.1. Clinical Need
2.2. Key Elements of the Disease Target
3. Bacteriophages
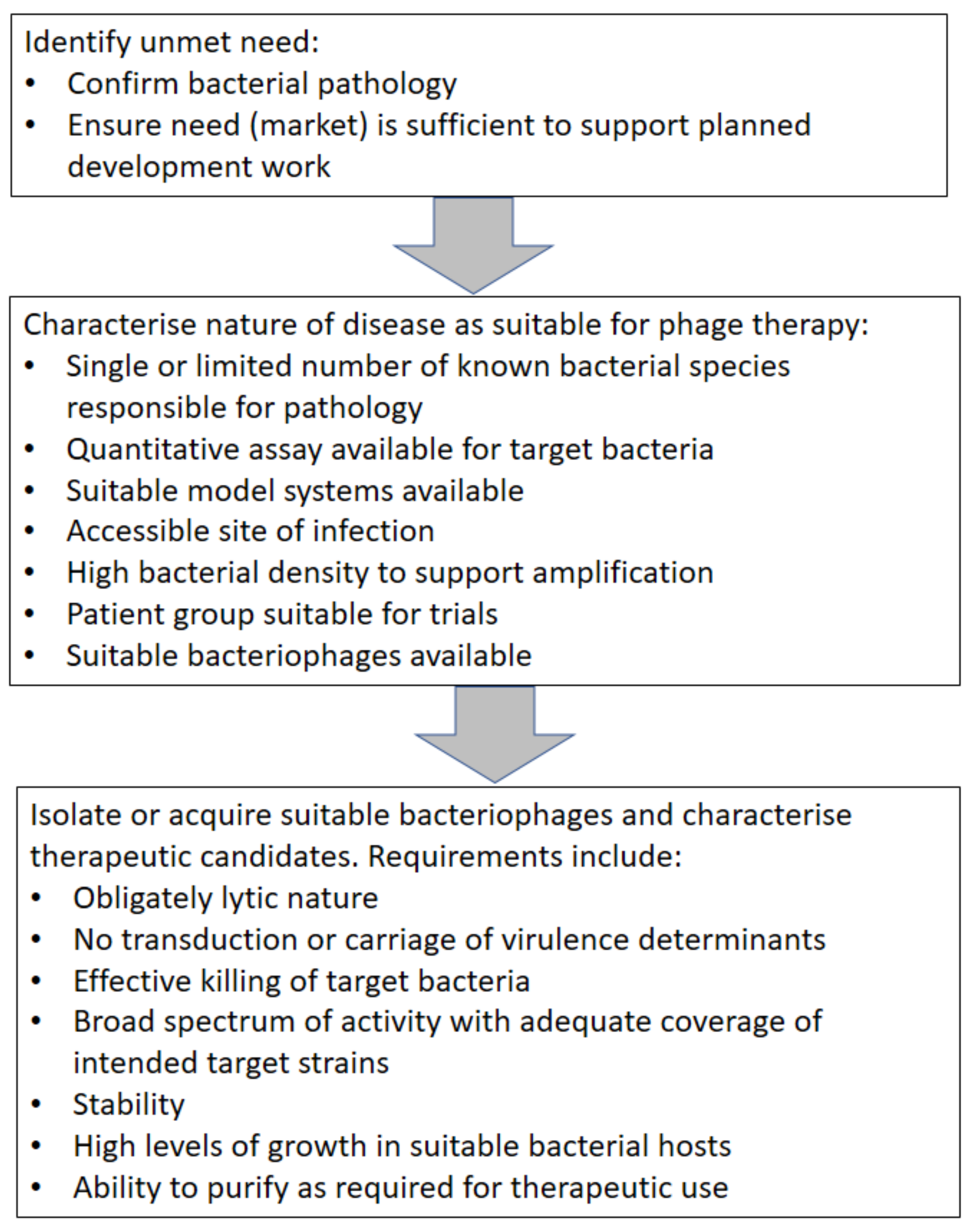
4. Stages of target selection
5. A Worked Example
6. Summary
Acknowledgments
Conflicts of Interest
References
- D’Herelle, F. Sur le role du microbe bacteriophage dans la typhose aviare. Comptes Rendus de l’Académie des Sciences Paris 1919, 169, 932–934. [Google Scholar]
- Eaton, M.D.; Bayne-Jones, S. Bacteriophage therapy: Review of the principles and results of the use of bacteriophage in the treatment of infections. JAMA 1934, 103, 1769–1776, 1847–1853, 1934–1939. [Google Scholar] [CrossRef]
- Krueger, A.P.; Scribner, E.J. The bacteriophage: Its nature and its therapeutic use. JAMA 1941, 116, 2160–2167. [Google Scholar] [CrossRef]
- Chan, M. WHO Director-General briefs UN on Antimicrobial Resistance. 2016. Available online: http://www.who.int/dg/speeches/2016/antimicrobial-resistance-un/en/ (accessed on 9 March 2018).
- Harper, D.R.; Burrowes, B.H.; Kutter, E.M. Bacteriophage: Therapeutic Uses. In Encyclopedia of Life Sciences; John Wiley and Sons: Chichester, UK, 2014. [Google Scholar]
- Harper, D.; Abedon, S.; Burrowes, B.; McConville, M. (Eds.) Bacteriophgages: Biology, Technology, Therapy; Springer: Cham, Switzerland, 2018; ISBN 978-3-319-40598-8. Available online: https://link.springer.com/referencework/10.1007/978-3-319-40598-8#about (accessed on 9 March 2018).
- Czaplewski, L.; Bax, R.; Clokie, M.; Dawson, M.; Fairhead, H.; Fischetti, V.A.; Foster, S.; Gilmore, B.F.; Hancock, R.E.; Harper, D.; et al. Alternatives to antibiotics—A pipeline portfolio review. Lancet Infect. Dis. 2016, 16, 239–251. [Google Scholar] [CrossRef]
- Hagens, S.; Habel, A.; Bläsi, U. Augmentation of the Antimicrobial Efficacy of Antibiotics by Filamentous Phage. Microb. Drug Resist. 2006, 12, 164–168. [Google Scholar] [CrossRef] [PubMed]
- Chan, B.K.; Sistrom, M.; Wertz, J.E.; Kortright, K.E.; Narayan, D.; Turner, P.E. Phage selection restores antibiotic sensitivity in MDR Pseudomonas aeruginosa. Sci. Rep. 2016, 6, 26717. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.K.; Collins, J.J. Engineered bacteriophage targeting gene networks as adjuvants for antibiotic therapy. PNAS 2009, 106, 4629–4634. [Google Scholar] [CrossRef] [PubMed]
- Wright, A.; Hawkins, C.; Ӓnggård, E.; Harper, D.A. Controlled clinical trial of a therapeutic bacteriophage in chronic otitis due to antibiotic-resistant Pseudomonas aeruginosa; A preliminary report of efficacy. Clin. Otolaryngol. 2009, 34, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Blackwell, A. The Potential for Bacteriophage to Control Soft Rot Development in Store. Available online: https://potatoes.ahdb.org.uk/sites/default/files/publication_upload/APS%20Biocontrol%20Ltd.pdf DevelopmentDevelopment (accessed on 9 March 2018).
- Ceri, H.; Olson, M.E.; Stremick, C.; Read, R.R.; Morck, D.; Buret, A. The Calgary biofilm device: New technology for rapid determination of antibiotic susceptibilities of bacterial biofilms. J. Clin. Microbiol. 1999, 37, 1771–1776. [Google Scholar] [PubMed]
- Harper, D.R.; Parracho, H.M.; Walker, J.; Sharp, R.; Hughes, G.; Werthén, M.; Lehman, S.; Morales, S. Bacteriophages and biofilms. Antibiotics 2014, 3, 270–284. [Google Scholar] [CrossRef]
- Kumar, S.R.; Markusic, D.M.; Biswas, M.; High, K.A.; Herzog, R.W. Clinical development of gene therapy: Results and lessons from recent successes. Mol. Ther. Methods Clin. Dev. 2016, 3, 16034. [Google Scholar] [CrossRef] [PubMed]
- FiercePharma. With Its Launch Fizzling Out, UniQure Gives Up on $1M+ Gene Therapy Glybera. Available online: https://www.fiercepharma.com/pharma/uniqure-gives-up-1m-gene-therapy-glybera (accessed on 9 March 2018).
- Brockhurst, M.A.; Koskella, B.; Zhang, Q.G. Bacteria-Phage Antagonistic Coevolution and the Implications for Phage Therapy. In Bacteriophages: Biology, Technology, Therapy; Harper, D., Abedon, S., Burrowes, B., McConville, M., Eds.; Springer Nature: Cham, Switzerland, 2017. [Google Scholar]
- Sillankorva, S.M.; Oliveira, H.; Azeredo, J. Bacteriophages and Their Role in Food Safety. Int. J. Microbiol., 2012, 2012, 863945. [Google Scholar] [CrossRef] [PubMed]
- Sarker, S.A.; Sultana, S.; Reuteler, G.; Moine, D.; Descombes, P.; Charton, F.; Bourdin, G.; McCallin, S.; Ngom-Bru, C.; Neville, T.; et al. Oral Phage Therapy of Acute Bacterial Diarrhea with Two Coliphage Preparations: A Randomized Trial in Children From Bangladesh. EBioMedicine 2016, 4, 124–137. [Google Scholar] [CrossRef] [PubMed]
- Soothill, J.S. Treatment of experimental infections of mice with bacteriophages. J. Med. Microbiol. 1992, 37, 258–261. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, R.; Matsumoto, T.; Sano, G.; Ishii, Y.; Tateda, K.; Sumiyama, Y.; Uchiyama, J.; Sakurai, S.; Matsuzaki, S.; Imai, S. Efficacy of bacteriophage therapy against gut-derived sepsis caused by Pseudomonas aeruginosa in mice. Antimicrob. Agents Chemother. 2007, 51, 446–452. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, C.; Harper, D.; Burch, D.; Änggård, E.; Soothill, J. Topical treatment of Pseudomonas aeruginosa otitis of dogs with a bacteriophage mixture: A before/after clinical trial. Vet. Microbiol. 2010, 146, 309–313. [Google Scholar] [CrossRef] [PubMed]
- Marza, J.A.; Soothill, J.S.; Boydell, P.; Collyns, T.A. Multiplication of therapeutically administered bacteriophages in Pseudomonas aeruginosa infected patients. Burns 2006, 32, 644–646. [Google Scholar] [CrossRef] [PubMed]
- Phagoburn. Available online: http://www.phagoburn.eu/ (accessed on 9 March 2018).
- Rhoads, D.D.; Wolcott, R.D.; Kuskowski, M.A.; Wolcott, B.M.; Ward, L.S.; Sulakvelidze, A. Bacteriophage therapy of venous leg ulcers in humans: Results of a phase I safety trial. J. Wound Care 2009, 18, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Rose, T.; Verbeken, G.; De Vos, D.; Merabishvili, M.; Vaneechoutte, M.; Lavigne, R.; Jennes, S.; Zizi, M.; Pirnay, J.P. Experimental phage therapy of burn wound infection: Difficult first steps. Int. J. Burns Trauma 2014, 4, 66–73. [Google Scholar] [PubMed]
- Cole, S.T.; Eisenach, K.D.; McMurray, D.N.; Jacobs, W.R. (Eds.) Tuberculosis and the Tubercle Bacillus; ASM Press: Washington, DC, USA, 2005; ISBN 1-55581-295-3. [Google Scholar]
- PubMed Health. Closed Comedones. Available online: https://www.ncbi.nlm.nih.gov/pubmedhealth/PMHT0025363/ (accessed on 9 March 2018).
- Payne, R.J.H.; Vincent, A.A. Evidence for a Phage Proliferation Threshold? J. Virol. 2002, 76, 13123–13124. [Google Scholar] [CrossRef] [PubMed]
- Vinner, G.K.; Vladisavljević, G.T.; Clokie, M.R.J.; Malik, D.J. Microencapsulation of Clostridium difficile specific bacteriophages using microfluidic glass capillary devices for colon delivery using pH triggered release. PLoS ONE 2017, 12, e0186239. [Google Scholar] [CrossRef] [PubMed]
- Chibani-Chennoufi, S.; Sidoti, J.; Bruttin, A.; Kutter, E.; Sarker, S.; Brüssow, H. In vitro and in vivo bacteriolytic activities of Escherichia coli phages: Implications for phage therapy. Antimicrob. Agents Chemother. 2004, 48, 2558–2569. [Google Scholar] [CrossRef] [PubMed]
- Ryan, E.M.; Gorman, S.P.; Donnelly, R.F.; Gilmore, B.F. Recent advances in bacteriophage therapy: How delivery routes, formulation, concentration and timing influence the success of phage therapy. J. Pharm. Pharmacol. 2011, 63, 1253–1264. [Google Scholar] [CrossRef] [PubMed]
- Jończyk-Matysiak, E.; Weber-Dąbrowska, B.; Owczarek, B.; Międzybrodzki, R.; Łusiak-Szelachowska, M.; Łodej, N.; Górski, A. Phage-Phagocyte Interactions and Their Implications for Phage Application as Therapeutics. Viruses 2017, 9, 150. [Google Scholar] [CrossRef] [PubMed]
- Łusiak-Szelachowska, M.; Żaczek, M.; Weber-Dąbrowska, B.; Międzybrodzki, R.M.; Letkiewicz, S.; Fortuna, W.; Rogóż, P.; Szufnarowski, K.; Jończyk-Matysiak, E.; Olchawa, E.; et al. Antiphage activity of sera during phage therapy in relation to its outcome. Future Microbiol. 2017, 12, 109–117. [Google Scholar]
- Ledford, H. Myriad ruling causes confusion. Nature 2013, 498, 281–282. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Schooley, R.T.; Biswas, B.; Gill, J.J.; Hernandez-Morales, A.; Lancaster, J.; Lessor, L.; Barr, J.J.; Reed, S.L.; Rohwer, F.; Benler, S.; et al. Development and Use of Personalized Bacteriophage-Based Therapeutic Cocktails to Treat a Patient with a Disseminated Resistant Acinetobacter baumannii Infection. Antimicrob. Agents Chemother. 2017, 61, e00954-17. [Google Scholar] [CrossRef] [PubMed]
- Dalmasso, M.; de Haas, E.; Neve, H. Isolation of a Novel Phage with Activity against Streptococcus mutans Biofilms. PLoS ONE 2015, 10, e0138651. [Google Scholar] [CrossRef] [PubMed]
- Abedon, S.T.; Thomas-Abedon, C. Phage therapy pharmacology. Curr. Pharm. Biotechnol. 2010, 11, 28–47. [Google Scholar] [CrossRef] [PubMed]
- Pirnay, J.P.; De Vos, D.; Verbeken, G.; Merabishvili, M.; Chanishvili, N.; Vaneechoutte, M.; Zizi, M.; Laire, G.; Lavigne, R.; Huys, I.; et al. The phage therapy paradigm: Prêt-à-porter or sur-mesure? Pharm. Res. 2011, 28, 934–937. [Google Scholar] [CrossRef] [PubMed]
- Feiner, R.; Argov, T.; Rabinovich, L.; Sigal, N.; Borovok, I.; Herskovits, A.A. A new perspective on lysogeny: Prophages as active regulatory switches of bacteria. Nat. Rev. Microbiol. 2015, 13, 641–650. [Google Scholar] [CrossRef] [PubMed]
- Hyman, P.; Abedon, S.T. Bacteriophage host range and bacterial resistance. Adv. Appl. Microbiol. 2010, 70, 217–248. [Google Scholar] [PubMed]
- Nale, J.Y.; Spencer, J.; Hargreaves, K.R.; Buckley, A.M.; Trzepiński, P.; Douce, G.R.; Clokie, M.R. Bacteriophage Combinations Significantly Reduce Clostridium difficile Growth in Vitro and Proliferation in Vivo. Antimicrob. Agents Chemother. 2015, 60, 968–981. [Google Scholar] [CrossRef] [PubMed]
- Hargreaves, K.R.; Clokie, M.R. Clostridium difficile phages: Still difficult? Front. Microbiol. 2014, 28, 184. [Google Scholar] [CrossRef] [PubMed]
- Colavecchio, A.; Cadieux, B.; Lo, A.; Goodridge, L.D. Bacteriophages Contribute to the Spread of Antibiotic Resistance Genes among Foodborne Pathogens of the Enterobacteriaceae Family—A Review. Front. Microbiol. 2017, 20, 1108. [Google Scholar] [CrossRef] [PubMed]
- Strauch, E.; Lurz, R.; Beutin, L. Characterization of a Shiga toxin-encoding temperate bacteriophage of Shigella sonnei. Infect. Immun. 2001, 69, 7588–7595. [Google Scholar] [CrossRef] [PubMed]
- Christie, G.E.; Dokland, T. Pirates of the Caudovirales. Virology 2012, 434, 210–221. [Google Scholar] [CrossRef] [PubMed]
- Ackermann, H.-W.; Tremblay, D.; Moineau, S. Long-term bacteriophage preservation. W.F.C.C. Newsl. 2004, 38, 35–40. [Google Scholar]
- European Pharmacopoeia Online. Available online: http://online.edqm.eu/EN/entry.htm (accessed on 9 March 2018).
- USP-NF. Available online: http://www.uspnf.com/?_ga=2.80419031.1739394632.1520820978-1625466886.1520820978 (accessed on 9 March 2018).
- Verbeken, G.; Pirnay, J.P.; De Vos, D.; Jennes, S.; Zizi, M.; Lavigne, R.; Casteels, M.; Huys, I. Optimizing the European regulatory framework for sustainable bacteriophage therapy in human medicine. Arch. Immunol. Ther. Exp. (Warsz.) 2012, 60, 161–172. [Google Scholar] [CrossRef] [PubMed]
- Verbeken, G.; Pirnay, J.P.; Lavigne, R.; Jennes, S.; De Vos, D.; Casteels, M.; Huys, I. Call for a dedicated European legal framework for bacteriophage therapy. Arch. Immunol. Ther. Exp. (Warsz.) 2014, 62, 117–129. [Google Scholar] [CrossRef] [PubMed]
- Huys, I.; Pirnay, J.P.; Lavigne, R.; Jennes, S.; De Vos, D.; Casteels, M.; Verbeken, G. Paving a regulatory pathway for phage therapy. Europe should muster the resources to financially, technically and legally support the introduction of phage therapy. EMBO Rep. 2013, 14, 951–954. [Google Scholar] [CrossRef] [PubMed]
- Defoirdt, T. Quorum-Sensing Systems as Targets for Antivirulence Therapy. Trends Microbiol. 2017, 26, 313–328. [Google Scholar] [CrossRef] [PubMed]
- Harper, D.R.; Enright, M.C. Bacteriophages for the treatment of Pseudomonas aeruginosa infections. J. Appl. Microbiol. 2010, 111, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Soothill, J.S.; Hawkins, C.; Anggard, E.A.; Harper, D.R. Therapeutic use of bacteriophages (letter). Lancet Infect. Dis. 2004, 4, 544–545. [Google Scholar] [CrossRef]

© 2018 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Harper, D.R. Criteria for Selecting Suitable Infectious Diseases for Phage Therapy. Viruses 2018, 10, 177. https://doi.org/10.3390/v10040177
Harper DR. Criteria for Selecting Suitable Infectious Diseases for Phage Therapy. Viruses. 2018; 10(4):177. https://doi.org/10.3390/v10040177
Chicago/Turabian StyleHarper, David R. 2018. "Criteria for Selecting Suitable Infectious Diseases for Phage Therapy" Viruses 10, no. 4: 177. https://doi.org/10.3390/v10040177
APA StyleHarper, D. R. (2018). Criteria for Selecting Suitable Infectious Diseases for Phage Therapy. Viruses, 10(4), 177. https://doi.org/10.3390/v10040177



