Antiviral Defense and Innate Immune Memory in the Oyster
Abstract
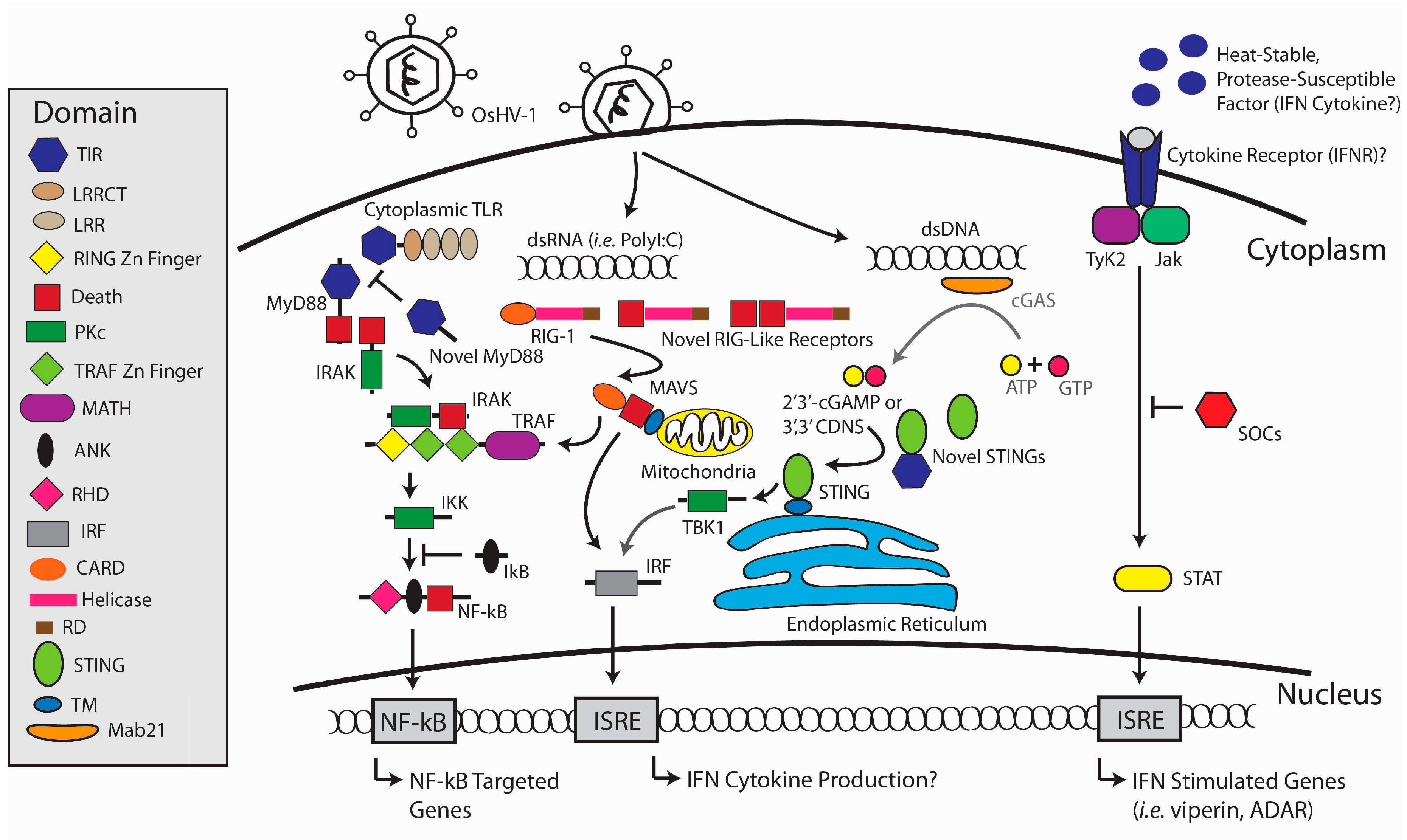
1. Introduction
2. Antiviral Defense in the Animal Kingdom
3. Antiviral Defense in the Oyster
4. Evolutionary Origins of Antiviral Defense Systems
5. Innate Immune Memory and Antiviral Therapeutic Potential for Shellfish Aquaculture
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Escoubas, J.-M.; Destoumieux-Garzon, D.; Montagnani, C.; Gourbal, B.; Duval, D.; Green, T.J.; Charriere, G.M. Immunity in Molluscs. In Encyclopedia of Immunobiology; Ratcliffe, M.J.H., Ed.; Academic Press: Oxford, UK, 2016; pp. 417–436. [Google Scholar]
- Bickham, U.; Bayne, C.J. Molluscan cells in culture: Primary cell cultures and cell lines. Can. J. Zool. 2013, 91, 391–404. [Google Scholar]
- Davison, A.J.; Eberia, R.; Ehlers, B.; Hayward, G.S.; McGeoch, D.J.; Minson, A.C.; Pellet, P.E.; Roizman, B.; Studdert, M.J.; Thiry, E. The Order Herpesvirales. Arch. Virol. 2009, 154, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Segarra, A.; Pepin, J.F.; Arzul, I.; Morga, B.; Faury, N.; Renault, T. Detection and Description of a Particular Ostreid Herpesvirus 1 Genotype Associated with Massive Mortality Outbreaks of Pacific oysters, Crassostrea gigas, in France in 2008. Virus Res. 2010, 153, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, C.; Hick, P.; Gabor, M.; Spiers, Z.; Fell, S.A.; Gu, X.; Read, A.; Go, J.; Dove, M.; O’Connor, W.; et al. Identification and Characterisation of an Ostreid Herpesvirus-1 Microvariant (OsHV-1 μ-Var) in Crassostrea gigas (Pacific Oysters) in Australia. Dis. Aquat. Org. 2013, 105, 109–126. [Google Scholar] [CrossRef] [PubMed]
- Keeling, S.E.; Brosnahan, C.L.; R, W.; Gias, E.; Hannah, M.; Bueno, R.; McDonald, W.L.; Johnston, C. New Zealand juvenile oyster mortality associated with Ostreid herpesvirus 1—An opportunisitic longitudinal study. Dis. Aquat. Org. 2014, 109, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Burge, C.A.; Griffin, F.J.; Friedman, C.S. Mortality and herpesvirus infections of the Pacific oyster Crassostrea gigas in Tomales Bay, California, USA. Dis. Aquat. Org. 2006, 72, 31–43. [Google Scholar] [CrossRef] [PubMed]
- Le Deuff, R.M.; Nicolas, J.L.; Renault, T.; Cochennec, N. Experimental transmission of a Herpes-like virus to axenic Larvae of Pacific oyster, Crassostrea gigas. Bull. Eur. Assoc. Fish Pathol. 1994, 14, 69–72. [Google Scholar]
- Renault, T.; Cochennec, N.; le Deuff, R.; Chollet, B. Herpes-like virus infecting Japanese oyster (Crassostrea gigas) Spat. Bull. Eur. Assoc. Fish Pathol. 1994, 14, 64–66. [Google Scholar]
- Green, T.J.; Raftos, D.A.; Speck, P.; Montagnani, C. Antiviral immunity in marine molluscs. J. Gen. Virol. 2015, 96, 2471–2482. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Ford, S.E. Infectious diseases of marine molluscs and host responses as revealed by genomic tools. Philos. Trans. R. Soc. B 2016. [Google Scholar] [CrossRef] [PubMed]
- Arzul, I.; Corbeil, S.; Morga, B.; Renault, T. Viruses infecting marine molluscs. J. Invertebr. Pathol. 2017, 147, 118–135. [Google Scholar] [CrossRef] [PubMed]
- Mussabekova, A.; Daeffler, L.; Imler, J.-L. Innate and intrinsic antiviral immunity in Drosophila. Cell. Mol. Life Sci. 2017, 74, 2039–2054. [Google Scholar] [CrossRef] [PubMed]
- Sarkies, P.; Miska, E.A. RNAi pathways in the recognition of foreign RNA: Antiviral responses and host-parasite interactions in nematodes. Biochem. Soc. Trans. 2013, 41, 876–880. [Google Scholar] [CrossRef] [PubMed]
- Randall, R.E.; Goodbourn, S. Interferons and viruses: An interplay between induction, signalling, antiviral responses and viral countermeasures. J. Gen. Virol. 2008, 89, 1–47. [Google Scholar] [CrossRef] [PubMed]
- Blair, C.D. Mosquito RNAi is the major innate immune pathway controlling arbovirus infection and transmission. Future Microbiol. 2011, 6, 265–277. [Google Scholar] [CrossRef] [PubMed]
- Wilkins, C.; Dishongh, R.; Moore, S.C.; Whitt, M.A.; Chow, M.; Machaca, K. RNA interference is an antiviral defence mechanism in Caenorhabditis elegans. Nature 2005, 436, 1044–1047. [Google Scholar] [CrossRef] [PubMed]
- Weber, F.; Wagner, V.; Rasmussen, S.B.; Hartmann, R.; Pauldan, S.R. Double-stranded RNA is produced by positive-stranded RNA viruses and DNA viruses but not in detectable amounts by negative-stranded RNA viruses. J. Virol. 2006, 80, 5059–5064. [Google Scholar] [CrossRef] [PubMed]
- Tenoever, B.R. RNA viruses and the host microRNA machinery. Nat. Rev. Microbiol. 2013, 11, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Sabin, L.R.; Cherry, S. Small creature use small RNAs to direct antiviral defenses. Eur. J. Immunol. 2013, 43, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Benitez, A.A.; Spanko, L.A.; Bouhaddou, M.; Sachs, D.; tenOever, B.R. Engineered mammalian RNAi can elicit antiviral protection that Negates the requirement for the interferon response. Cell Rep. 2015, 13, 1456–1466. [Google Scholar] [CrossRef] [PubMed]
- Schoggins, J.W.; Rice, C.M. Interferon-stimulated genes and their antiviral effector functions. Curr. Opin. Virol. 2011, 1, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Aguado, L.C.; tenOever, B.R. RNase III nucleases and the evolution of antiviral systems. BioEssays 2017. [Google Scholar] [CrossRef] [PubMed]
- Dostert, C.; Jouanguy, E.; Irving, P.; Troxler, L.; Galiana-Arnoux, D.; Hetru, C.; Hoffmann, J.A.; Imler, J.-L. The Jak-STAT signaling pathway is required but not sufficient for the antiviral response of Drosophila. Nat. Immunol. 2005, 6, 946–953. [Google Scholar] [CrossRef] [PubMed]
- Paradkar, P.N.; Trinidad, L.; Voysey, R.; Duchemin, J.-B.; Walker, P.J. Secreted Vago restricts West Nile virus infection in Culex mosquito cells by activating the Jak-STAT pathway. Proc. Natl. Acad. Sci. USA 2012, 109, 18915–18920. [Google Scholar] [CrossRef] [PubMed]
- Paradkar, P.N.; Duchemin, J.-B.; Voysey, R.; Walker, P.J. Dicer-2-dependent activation of Culex Vago occurs via the TRAF-Rel2 signaling pathway. PLoS Neg. Trop. Pathog. 2014, 8, e2823. [Google Scholar] [CrossRef] [PubMed]
- Deddouche, S.; Matt, N.; Budd, A.; Mueller, S.; Kemp, C.; Galiana-Arnoux, D.; Dostert, C.; Antoniewski, C.; Hoffmann, J.A.; Imler, J.-L. The Dexd/H-Box helicase Dicer-2 mediates induction of antiviral activity in Drosophila. Nat. Immunol. 2008, 9, 1425–1432. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Li, H.; Chen, Y.; Chen, Y.; Wang, S.; Weng, S.-P.; Xu, X.; He, J. Activation of Vago by interferon regulatory factor (IRF) suggests an interferon system-like antiviral mechanism in shrimp. Sci. Rep. 2015, 5, 15078. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Basavappa, M.; Lu, J.; Dong, S.; Cronkite, D.A.; Prior, J.T.; Reinecker, H.-C.; Hertzog, P.; Han, Y.; Li, W.-X.; et al. Induction and suppression of antiviral RNA interference by influenza A virus in mammalian cells. Nat. Microbiol. 2016, 2, 16250. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Fang, X.; Guo, X.; Li, L.; Luo, R.; Xu, F.; Yang, P.; Zhang, L.; Wang, X.; Qi, H.; et al. The oyster genome reveals stress adaptation and complexity of shell formation. Nature 2012, 490, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Paul-Pont, I.; Evans, O.; Dhand, N.K.; Whittington, R.J. Experimental infections of Pacific oyster Crassostrea gigas Using the Australian Ostreid herpesvirus-1 (OsHV-1) μvar strain. Dis. Aquat. Org. 2015, 113, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Schikorski, D.; Faury, N.; Pepin, J.F.; Saulnier, D.; Tourbiez, D.; Renault, T. Experimental Ostreid herpesvirus 1 Infection of the Pacific oyster Crassostrea gigas: Kinetics of virus DNA detection by q-PCR in seawater and in oyster samples. Virus Res. 2011, 155, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Schikorski, D.; Renault, T.; Saulnier, D.; Faury, N.; Moreau, P.; Pepin, J.F. Experimental infection of Pacific oyster Crassostrea gigas spat by Ostreid herpesvirus 1: Demonstration of oyster spat susceptibility. Vet. Res. 2011, 42, 27. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Jouaux, A.; Ford, S.E.; Lelong, C.; Sourdaine, P.; Mathieu, M.; Guo, X. Transcriptome analysis reveals strong and complex antiviral response in a mollusc. Fish Shellfish Immunol. 2015, 46, 131–144. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, L.; Guo, X.; Litman, G.W.; Dishaw, L.J.; Zhang, G. Massive expansion and functional divergence of innate immune genes in a Protostome. Sci. Rep. 2015, 5, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Green, T.J.; Vergnes, A.; Montagnani, C.; de Lorgeril, J. Distinct immune responses of juvenile and adult oysters (Crassostrea gigas) to viral and bacterial infections. Vet. Res. 2016, 47, 72. [Google Scholar] [CrossRef] [PubMed]
- Rosani, U.; Varotto, L.; Domeneghetti, S.; Arcangeli, G.; Pallavicini, A.; Venier, P. Dual analysis of host and pathogen transcriptomes in Ostreid herpesvirus 1—Positive Crassostrea gigas. Environ. Microbiol. 2015, 17, 4200–4212. [Google Scholar] [CrossRef] [PubMed]
- Renault, T.; Faury, N.; Barbosa-Solomieu, V.; Moreau, K. Suppression substractive hybridisation (SSH) and real time PCR reveal differential gene expression in the Pacific cupped oyster, Crassostrea gigas, challenged with Ostreid herpesvirus 1. Dev. Comp. Immunol. 2011, 35, 725–735. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Huang, B.; Zhang, L.; Li, L.; Zhang, G. Tank-binding kinase-1 broadly affects oyster immune response to bacteria and viruses. Fish Shellfish Immunol. 2016, 56, 330–335. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Trjuillo, A.; Ferro, P.; Garcia-Rosado, E.; Infante, C.; Alonso, M.C.; Bejar, J.; Borrego, J.J.; Manchado, M. Poly I:C induces Mx transcription and promotes an antiviral state against Sole Aquabirnavirus in the flatfish Senegalese sole (Solea Senegalensis Kaup). Fish Shellfish Immunol. 2008, 24, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Plant, K.P.; Thune, R.L. Cloning and characterisation of a channel catfish (Ictalurus Punctatus) Mx gene. Fish Shellfish Immunol. 2004, 16, 391–405. [Google Scholar] [CrossRef] [PubMed]
- Green, T.J.; Montagnani, C. Poly I:C Induces a protective antiviral immune response in the Pacific oyster (Crassostrea gigas) against subsequent challenge with Ostreid herpesvirus (OsHV-1 μVar). Fish Shellfish Immunol. 2013, 35, 382–388. [Google Scholar] [CrossRef] [PubMed]
- Green, T.J.; Rolland, J.-L.; Vergnes, A.; Raftos, D.A.; Montagnani, C. OsHV-1 countermeasures to the Pacific oyster’s anti-viral response. Fish Shellfish Immunol. 2015, 47, 435–443. [Google Scholar] [CrossRef] [PubMed]
- Lafont, M.; Petton, B.; Vergnes, A.; Pauletto, M.; Segarra, A.; Gourbal, B.; Montagnani, C. Long-lasting antiviral innate immune priming in the Lophotrochozoan Pacific oyster, Crassostrea gigas. Sci. Rep. 2017, 7, 13143. [Google Scholar] [CrossRef] [PubMed]
- Pauletto, M.; Segarra, A.; Montagnani, C.; Quillien, V.; Faury, N.; le Grand, J.; Miner, P.; Petton, B.; Labreuche, Y.; Fleury, E.; et al. Long dsRNAs promote an anti-viral response in Pacific oyster hampering Ostreid herpesvirus 1 replication. J. Exp. Biol. 2017, 220, 3671–3685. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Zhang, L.; Du, Y.; Xu, F.; Li, L.; Zhang, G. Characterization of the mollusc RIG-I/MAVS pathway reveals an archaic antiviral signalling framework in invertebrates. Sci. Rep. 2017, 7, 8217. [Google Scholar] [CrossRef] [PubMed]
- Green, T.J.; Speck, P.; Geng, D.; Raftos, D.A.; Beard, M.R.; Helbig, K.J. Oyster viperin retains direct antiviral activity and its transcription occurs via a signalling pathway involving a heat-stable hemolymph protein. J. Gen. Virol. 2015, 96, 3587–3597. [Google Scholar] [CrossRef] [PubMed]
- Helbig, K.J.; Beard, M.R. The Role of viperin in the innate antiviral response. J. Mol. Biol. 2014, 426, 1210–1219. [Google Scholar] [CrossRef] [PubMed]
- Segarra, A.; Baillon, L.; Tourbiez, D.; Benabdelmouna, A.; Faury, N.; Bourgougnon, N.; Renault, T. Ostreid herpesvirus type 1 replication and host response in adult Pacific oysters, Crassostrea gigas. Vet. Res. 2014, 45, 103. [Google Scholar] [CrossRef] [PubMed]
- Moreau, P.; Moreau, K.; Segarra, A.; Tourbiez, D.; Travers, M.-A.; Rubinsztein, D.C.; Renault, T. Autophagy plays an important role in protecting Pacific oysters from OsHV-1 and Vibrio aestuarianus infections. Autophagy 2015, 11, 516–526. [Google Scholar] [CrossRef] [PubMed]
- Martenot, C.; Gervais, O.; Chollet, B.; Houssin, M.; Renault, T. Haemocytes collected from experimentally infected Pacific oysters, Crassostrea gigas: Detection of Ostreid herpesvirus 1 DNA, RNA, proteins in relation with inhibition of apoptosis. PLoS ONE 2017, 12, e0177448. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Zhang, L.; Huang, B.; Guan, X.; Li, L.; Zhang, G. Molecular cloning, characterization, expression of two myeloid differentiation factor 88 (MyD88) in Pacific oyster, Crassostrea gigas. J. World Aquac. Soc. 2013, 44, 759–774. [Google Scholar] [CrossRef]
- Lu, M.; Yang, C.; Li, M.; Yi, Q.; Lu, G.; Wu, Y.; Qu, C.; Wang, L.; Song, L. A Conserved interferon regulation factor 1 (IRF-1) from Pacific oyster Crassostrea gigas functioned as an activator of IFN pathway. Fish Shellfish Immunol. 2018, 76, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Margolis, S.R.; Wilson, S.C.; Vance, R.E. Evolutionary Origins of cGAS-STING Signaling. Trends Immunol. 2017, 38, 733–743. [Google Scholar] [CrossRef] [PubMed]
- Kranzusch, P.J.; Wilson, S.C.; Lee, A.S.Y.; Berger, J.M.; Doudna, J.A.; Vance, R.E. Ancient Origin of cGAS-STING Reveals Mechanism of Universal 2′,3′ Cgamp Signaling. Mol. Cell 2015, 59, 891–903. [Google Scholar] [CrossRef] [PubMed]
- Gerdol, M. Immune-Related Genes in Gastropods and Bivalves: A Comparative Overview. ISJ Invertebr. Surviv. J. 2017, 14, 103–118. [Google Scholar]
- Jenkins, K.A.; Mansell, A. TIR-containing adaptors in Toll-like receptor signalling. Cytokine 2010, 49, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Jeffrey, K.L.; Li, Y.; Ding, S. Reply to Questioning antiviral RNAi in mammals. Nat. Microbiol. 2017, 2, 17053. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gauthier, M.E.A.; Pasquier, L.D.; Degnan, B.M. The genome of the sponge Amphimedon queenslandica provides new perspectives into the origin of Toll-like and interleukin 1 receptor pathways. Evol. Dev. 2010, 12, 519–533. [Google Scholar] [CrossRef] [PubMed]
- Schr¨oder, H.C.; Natalio, F.; Wiens, M.; Tahir, M.N.; Shukoor, M.I.; Tremel, W.; Belikov, S.I.; Krasko, A.; M¨uller, W.E.G. The 2′-5′-Oligoadenylate synthetase in the lowest metazoa: Isolation, cloning, expression and functional activity in the Sponge Lubomirskia baicalensis. Mol. Immunol. 2008, 45, 945–953. [Google Scholar] [CrossRef] [PubMed]
- Kuusksalu, A.; Subbi, J.; Pehk, T.; Reintamm, T.; Muller, W.E.G.; Kelve, M. Identification of the reaction products of (2′-54)oligoadenylate synthetase in the marine sponge. Eur. J. Biochem. 1998, 257, 420–426. [Google Scholar] [CrossRef] [PubMed]
- Owens, L.; Malham, S. Review of the RNA interference pathway in molluscs including some possibilites for use in bivalve aquaculture. J. Mar. Sci. Eng. 2015, 3, 87–99. [Google Scholar] [CrossRef]
- Rosani, U.; Gerdol, M. A bioinformatics approach reveals seven nearly-complete RNA-virus genomes in bivalve RNA-seq data. Virus Res. 2017, 239, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Seo, G.J.; Kincaid, R.P.; Phanaksri, T.; Burke, J.M.; Pare, J.M.; Cox, J.E.; Hsiang, T.; Krug, R.M.; Sullivan, C.S. Reciprocal Inhibition between Intracellular Antiviral Signaling and the RNAi Machinery in Mammalian Cells. Cell Host Microbe 2013, 14, 435–445. [Google Scholar] [CrossRef] [PubMed]
- Girardi, E.; Lefevre, M.; Chane-Woon-Ming, B.; Paro, S.; Claydon, B.; Imler, J.-L.; Meignin, C.; Pfeffer, S. Cross-Species Comparative Analysis of Dicer Proteins During Sindbis Virus Infection. Sci. Rep. 2015, 5, 10693. [Google Scholar] [CrossRef] [PubMed]
- Brubaker, S.W.; Bonham, K.S.; Zanoni, I.; Kagan, J.C. Innate Immune Pattern Recognition: A Cell Biological Perspective. Ann. Rev. Immunol. 2015, 33, 257–290. [Google Scholar] [CrossRef] [PubMed]
- Barber, G.N. STING: Infection, Inflamation and Cancer. Nat. Rev. Immunol. 2015, 15, 760–770. [Google Scholar] [CrossRef] [PubMed]
- Reinert, L.S.; Lopusna, K.; Winther, H.; Sun, C.; Thomsen, M.K.; Nandakumar, R.; Mogensen, T.H.; Meyer, M.; Vaegter, C.; Nyengaard, J.R.; et al. Sensing of HSV-1 by the cGAS-STING pathway in microglia orchestrates antiviral defence in the CNS. Nat. Commun. 2016, 7, 13348. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Wu, F.-H.; Wang, X.; Wang, L.; Siedow, J.N.; Zhang, W.; Pei, Z.-M. Molecular evolutionary and structural analysis of the cytosolic DNA sensor cGAS and STING. Nucleic Acids Res. 2014, 42, 8243–8257. [Google Scholar] [CrossRef] [PubMed]
- Zhong, B.; Yang, Y.; Li, S.; Wang, Y.-Y.; Li, Y.; Diao, F.; Lei, C.; He, X.; Zhang, L.; Tien, P.; Shu, H.-B. The adaptor protein MITA Links virus-sening receptors to IRF3 transcription factor activation. Immunity 2008, 29, 538–550. [Google Scholar] [CrossRef] [PubMed]
- Woodward, J.J.; Iavarone, A.T.; Portnoy, D.A. C-Di-Amp secreted by intracellular Listeria monocytogenes activates a host type I interferon response. Science 2010, 328, 1703–1705. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wang, S.; Lu, K.; Yin, B.; Xiao, B.; Li, S.; He, J.; Li, C. An invertebrate STING from Shrimp activates an innate immune defense against bacterial infection. FEBS Lett. 2017, 591, 1010–1017. [Google Scholar] [CrossRef] [PubMed]
- Green, T.J.; Montagnani, C.; Benkendorff, K.; Robinson, N.; Speck, P. Ontogeny and water temperature influences the antiviral response of the Pacific oyster, Crassostrea gigas. Fish Shellfish Immunol. 2014, 36, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Stetson, D.B.; Medzhitov, R. Recognition of cytosolic DNA activates an IRF3-dependent innate immune response. Immunity 2006, 24, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Hauton, C.; Smith, V.J. Adaptive immunity in invertebrates: A straw house without a mechanistic foundation. BioEssays 2007, 29, 1138–1146. [Google Scholar] [CrossRef] [PubMed]
- Degremont, L.; Lamy, J.-B.; Pepin, J.-F.; Travers, M.-A.; Renault, T. New insight for the genetic evaluation of resistance to Ostreid herpesvirus infection, a worldwide disease, in Crassostrea gigas. PLoS ONE 2015, 10, e0127917. [Google Scholar] [CrossRef] [PubMed]
- Carrasco, N.; Gairin, I.; Perez, J.; Andree, K.B.; Roque, A.; Fernandez-Tejedor, M.; Rodgers, C.J.; Aguilera, C.; Furones, M.D. A production calendar based on water temperature, spat size, husbandry practices reduce OsHV-1 μVar impact on cultured Pacific oyster Crassostrea gigas in the Ebro Delta (Calalonia), Mediterranean coast of Spain. Front. Physiol. 2017, 8, 125. [Google Scholar] [CrossRef] [PubMed]
- Paul-Pont, I.; Dhand, N.K.; Whittington, R.J. Influence of husbandry practices on OsHV-1 associated mortality of Pacific oysters Crassostrea gigas. Aquaculture 2013, 412, 202–214. [Google Scholar] [CrossRef]
- Whittington, R.J.; Dhand, N.K.; Evans, O.; Paul-Pont, I. Further observations on the influence of husbandry practives on OsHV-1 μVar mortality in Pacific oysters Crassostrea gigas: Age, cultivation structures and growing height. Aquaculture 2015, 438, 82–97. [Google Scholar] [CrossRef]
- Whittington, R.J.; Paul, H.M.; Evans, O.; Rubio, A.; Alford, B.; Dhand, N.; Paul-Pont, I. Protection of Pacific oyster (Crassostrea gigas) spat from mortality due to Ostreid herpes virus-1 (OsHV-1 μVar) using simple treatments of incoming seawater in land-based upwellers. Aquaculture 2015, 437, 10–20. [Google Scholar] [CrossRef]
- Pernet, F.; Lupo, C.; Bacher, C.; Whittington, R.J. Infectious disease in oyster aquaculture require a new integrated approach. Philos. Trans. R. Soc. B 2016, 371, 20150213. [Google Scholar] [CrossRef] [PubMed]
- Degremont, L.; Garcia, C.; Allen, S.K., Jr. Genetic improvement for disease resistance in oysters: A review. J. Invertebr. Pathol. 2015, 131, 221–241. [Google Scholar] [CrossRef] [PubMed]
- Pernet, F.; Barret, J.; le Gall, P.; Corporeau, C.; Degremont, L.L.; Lagarde, F.; Pepin, J.F.; Keck, N. Mass mortalities of Pacific oysters Crassostrea gigas reflect infectious diseases and vary with farming practices in the Mediterranean Thau Lagoon, France. Aquac. Environ. Interact. 2012, 2, 215–237. [Google Scholar] [CrossRef]
- Evans, O.; Hick, P.; Whittington, R.J. Detection of Ostreid herpesvirus-1 microvariants in healthy Crassostrea gigas following disease events and Their Possible role as reservoirs of infection. J. Invertebr. Pathol. 2017, 148, 20–33. [Google Scholar] [CrossRef] [PubMed]
- Barbosa-Solomieu, V.; Degremont, L.; Vazquez-Juarez, R.; Ascencio-Valle, F.; Boudry, P.; Renault, T. Ostreid herpesvirus 1 (OsHV-1) detection among three successive generations of Pacific oysters (Crassostrea gigas). Virus Res. 2005, 107, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Little, T.J.; Kraaijeveld, A.R. Ecological and evolutionary implications of immunological priming in invertebrates. Trends Ecol. Evol. 2004, 19, 58–60. [Google Scholar] [CrossRef] [PubMed]
- Contreras-Garduno, J.; Lanz-Mendoza, H.; Franco, B.; Nava, A.; Pedraza-Reyes, M.; Canales-Lazcano, J. Insect immune priming: Ecology and experimental evidences. Ecol. Entomol. 2016, 41, 351–366. [Google Scholar] [CrossRef]
- Green, T.J.; Helbig, K.J.; Speck, P.; Raftos, D.A. Primed for success: Oyster parents treated with Poly(I:C) produce offspring with enhanced protection against Ostreid herpesvirus type I infection. Mol. Immunol. 2016, 78, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Gavery, M.R.; Roberts, S.B. Epigenetic considerations in aquaculture. PeerJ 2017, 5, e4147. [Google Scholar] [CrossRef] [PubMed]
- Granada, L.; Lemos, M.F.L.; Cabral, H.N.; Bossier, P.; Novais, S.C. Epigenetics in aquaculture—The last frontier. Rev. Aquac. 2017. [Google Scholar] [CrossRef]
- Green, T.J.; Benkendorff, K.; Robinson, N.; Raftos, D.; Speck, P. Anti-viral gene induction is absent upon secondary challenge with double-Stranded RNA in the Pacific oyster, Crassostrea gigas. Fish Shellfish Immunol. 2014, 39, 492–497. [Google Scholar] [CrossRef] [PubMed]
- Martins, K.A.O.; Bavari, S.; Salazar, A.M. Vaccine adjuvant uses of poly-IC and derivatives. Expert Rev. Vaccines 2015, 14, 447–459. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Green, T.J.; Speck, P. Antiviral Defense and Innate Immune Memory in the Oyster. Viruses 2018, 10, 133. https://doi.org/10.3390/v10030133
Green TJ, Speck P. Antiviral Defense and Innate Immune Memory in the Oyster. Viruses. 2018; 10(3):133. https://doi.org/10.3390/v10030133
Chicago/Turabian StyleGreen, Timothy J., and Peter Speck. 2018. "Antiviral Defense and Innate Immune Memory in the Oyster" Viruses 10, no. 3: 133. https://doi.org/10.3390/v10030133
APA StyleGreen, T. J., & Speck, P. (2018). Antiviral Defense and Innate Immune Memory in the Oyster. Viruses, 10(3), 133. https://doi.org/10.3390/v10030133





