Cytokine Networks Dysregulation during HTLV-1 Infection and Associated Diseases
Abstract
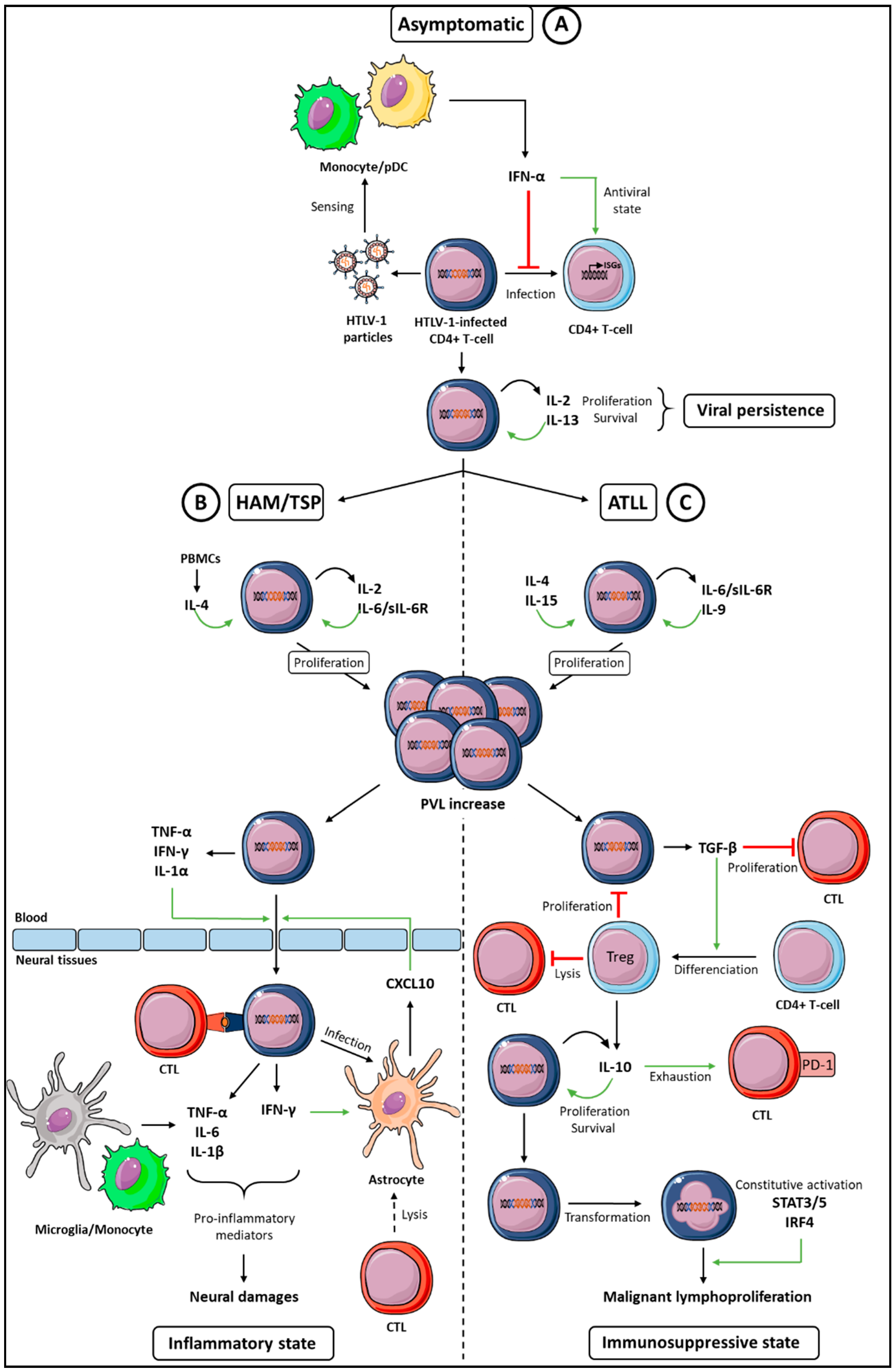
1. Introduction
2. Cytokines Supporting HTLV-1-Infected T-Cells Proliferation and Survival
2.1. IL-2
2.2. IL-4
2.3. IL-6
3. Paradoxical Functions of the IFN-I Antiviral Cytokine
4. Cytokines Promoting the HTLV-1-Induced Diseases Development
4.1. Cytokine Signature in HAM-TSP
4.2. The Interplay between IFN-γ and CXCL10 in the Model of the HAM/TSP Development
5. Cytokine Signature in ATLL
5.1. Low IFN-γ Expression
5.2. TGF-β Expression and Treg Development
5.3. IL-10 and T-Cell Exhaustion
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Futsch, N.; Mahieux, R.; Dutartre, H. HTLV-1, the Other Pathogenic Yet Neglected Human Retrovirus: From Transmission to Therapeutic Treatment. Viruses 2017, 10, 1. [Google Scholar] [CrossRef] [PubMed]
- Bangham, C.R.; Cook, L.B.; Melamed, A. HTLV-1 clonality in adult T-cell leukaemia and non-malignant HTLV-1 infection. Semin. Cancer Biol. 2014, 26, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Rizkallah, G.; Alais, S.; Futsch, N.; Tanaka, Y.; Journo, C.; Mahieux, R.; Dutartre, H. Dendritic cell maturation, but not type I interferon exposure, restricts infection by HTLV-1, and viral transmission to T-cells. PLoS Pathog. 2017, 13, e1006353. [Google Scholar] [CrossRef]
- Furuta, R.; Yasunaga, J.I.; Miura, M.; Sugata, K.; Saito, A.; Akari, H.; Ueno, T.; Takenouchi, N.; Fujisawa, J.I.; Koh, K.R.; et al. Human T-cell leukemia virus type 1 infects multiple lineage hematopoietic cells in vivo. PLoS Pathog. 2017, 13, e1006722. [Google Scholar] [CrossRef] [PubMed]
- Kannian, P.; Yin, H.; Doueiri, R.; Lairmore, M.D.; Fernandez, S.; Green, P.L. Distinct Transformation Tropism Exhibited by Human T Lymphotropic Virus Type 1 (HTLV-1) and HTLV-2 Is the Result of Postinfection T Cell Clonal Expansion. J. Virol. 2012, 86, 3757–3766. [Google Scholar] [CrossRef] [PubMed]
- Jones, K.S.; Petrow-Sadowski, C.; Huang, Y.K.; Bertolette, D.C.; Ruscetti, F.W. Cell-free HTLV-1 infects dendritic cells leading to transmission and transformation of CD4(+) T cells. Nat. Med. 2008, 14, 429–436. [Google Scholar] [CrossRef] [PubMed]
- Alais, S.; Mahieux, R.; Dutartre, H. Viral Source-Independent High Susceptibility of Dendritic Cells to Human T-Cell Leukemia Virus Type 1 Infection Compared to That of T Lymphocytes. J. Virol. 2015, 89, 10580–10590. [Google Scholar] [CrossRef]
- Gessain, A.; Cassar, O. Epidemiological Aspects and World Distribution of HTLV-1 Infection. Front. Microbiol. 2012, 3, 388. [Google Scholar] [CrossRef]
- Tsukasaki, K.; Tobinai, K. Clinical Trials and Treatment of ATL. Leuk. Res. Treat. 2012, 2012, 101754. [Google Scholar] [CrossRef]
- Osame, M.; Janssen, R.; Kubota, H.; Nishitani, H.; Igata, A.; Nagataki, S.; Mori, M.; Goto, I.; Shimabukuro, H.; Khabbaz, R.; et al. Nationwide survey of HTLV-I-associated myelopathy in Japan: Association with blood transfusion. Ann. Neurol. 1990, 28, 50–56. [Google Scholar] [CrossRef]
- Bangham, C.R.; Araujo, A.; Yamano, Y.; Taylor, G.P. HTLV-1-associated myelopathy/tropical spastic paraparesis. Nat. Rev. Dis. Primers 2015, 1, 15012. [Google Scholar] [CrossRef] [PubMed]
- Hodson, A.; Laydon, D.J.; Bain, B.J.; Fields, P.A.; Taylor, G.P. Pre-morbid human T-lymphotropic virus type I proviral load, rather than percentage of abnormal lymphocytes, is associated with an increased risk of aggressive adult T-cell leukemia/lymphoma. Haematologica 2013, 98, 385–388. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, P.D.; Kachimarek, A.C.; Bittencourt, A.L. Early Onset of HTLV-1 Associated Myelopathy/Tropical Spastic Paraparesis (HAM/TSP) and Adult T-cell Leukemia/Lymphoma (ATL): Systematic Search and Review. J. Trop. Pediatr. 2018, 64, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Umehara, F.; Izumo, S.; Nakagawa, M.; Ronquillo, A.T.; Takahashi, K.; Matsumuro, K.; Sato, E.; Osame, M. Immunocytochemical analysis of the cellular infiltrate in the spinal cord lesions in HTLV-I-associated myelopathy. J. Neuropathol. Exp. Neurol. 1993, 52, 424–430. [Google Scholar] [CrossRef] [PubMed]
- Kubota, R.; Soldan, S.S.; Martin, R.; Jacobson, S. Selected cytotoxic T lymphocytes with high specificity for HTLV-I in cerebrospinal fluid from a HAM/TSP patient. J. Neurovirol. 2002, 8, 53–57. [Google Scholar] [CrossRef] [PubMed]
- Nagai, M.; Yamano, Y.; Brennan, M.B.; Mora, C.A.; Jacobson, S. Increased HTLV-I proviral load and preferential expansion of HTLV-I Tax-specific CD8+ T cells in cerebrospinal fluid from patients with HAM/TSP. Ann. Neurol. 2001, 50, 807–812. [Google Scholar] [CrossRef] [PubMed]
- Matsuura, E.; Kubota, R.; Tanaka, Y.; Takashima, H.; Izumo, S. Visualization of HTLV-1–Specific Cytotoxic T Lymphocytes in the Spinal Cords of Patients With HTLV-1–Associated Myelopathy/Tropical Spastic Paraparesis. J. Neuropathol. Exp. Neurol. 2015, 74, 2–14. [Google Scholar] [CrossRef]
- Shimoyama, M. Diagnostic criteria and classification of clinical subtypes of adult T-cell leukaemia-lymphoma. A report from the Lymphoma Study Group (1984–87). Br. J. Haematol. 1991, 79, 428–437. [Google Scholar] [CrossRef]
- Enose-Akahata, Y.; Vellucci, A.; Jacobson, S. Role of HTLV-1 Tax and HBZ in the Pathogenesis of HAM/TSP. Front. Microbiol. 2017, 8, 2563. [Google Scholar] [CrossRef]
- Giam, C.-Z.; Semmes, O.J. HTLV-1 Infection and Adult T-Cell Leukemia/Lymphoma—A Tale of Two Proteins: Tax and HBZ. Viruses 2016, 8, 161. [Google Scholar] [CrossRef]
- Arenas-Ramirez, N.; Woytschak, J.; Boyman, O. Interleukin-2: Biology, Design and Application. Trends Immunol. 2015, 36, 763–777. [Google Scholar] [CrossRef]
- Leung, K.; Nabel, G.J. HTLV-1 transactivator induces interleukin-2 receptor expression through an NF-κB-like factor. Nature 1988, 333, 776–778. [Google Scholar] [CrossRef] [PubMed]
- Tendler, C.L.; Greenberg, S.J.; Blattner, W.A.; Manns, A.; Murphy, E.; Fleisher, T.; Hanchard, B.; Morgan, O.; Burton, J.D.; Nelson, D.L. Transactivation of interleukin 2 and its receptor induces immune activation in human T-cell lymphotropic virus type I-associated myelopathy: Pathogenic implications and a rationale for immunotherapy. Proc. Natl. Acad. Sci. USA 1990, 87, 5218–5222. [Google Scholar] [CrossRef] [PubMed]
- Itoyama, Y.; Minato, S.; Kira, J.; Goto, I.; Sato, H.; Okochi, K.; Yamamoto, N. Spontaneous proliferation of peripheral blood lymphocytes increased in patients with HTLV-I-associated myelopathy. Neurology 1988, 38, 1302–1307. [Google Scholar] [CrossRef]
- Collins, N.D.; D’Souza, C.; Albrecht, B.; Robek, M.D.; Ratner, L.; Ding, W.; Green, P.L.; Lairmore, M.D. Proliferation Response to Interleukin-2 and Jak/Stat Activation of T Cells Immortalized by Human T-Cell Lymphotropic Virus Type 1 Is Independent of Open Reading Frame I Expression. J. Virol. 1999, 73, 9642–9649. [Google Scholar]
- Hori, T.; Uchiyama, T.; Umadome, H.; Tamori, S.; Tsudo, M.; Araki, K.; Uchino, H. Dissociation of interleukin-2-mediated cell proliferation and interleukin-2 receptor upregulation in adult T-cell leukemia cells. Leuk. Res. 1986, 10, 1447–1453. [Google Scholar] [CrossRef]
- Uchiyama, T.; Hori, T.; Tsudo, M.; Wano, Y.; Umadome, H.; Tamori, S.; Yodoi, J.; Maeda, M.; Sawami, H.; Uchino, H. Interleukin-2 receptor (Tac antigen) expressed on adult T cell leukemia cells. J. Clin. Investig. 1985, 76, 446–453. [Google Scholar] [CrossRef]
- Uchiyama, T.; Kamio, M.; Kodaka, T.; Tamori, S.; Fukuhara, S.; Amakawa, R.; Uchino, H.; Araki, K. Leukemic cells from some adult T-cell leukemia patients proliferate in response to interleukin-4. Blood 1988, 72, 1182–1186. [Google Scholar] [PubMed]
- Arya, S.K.; Wong-Staal, F.; Gallo, R.C. T-cell growth factor gene: Lack of expression in human T-cell leukemia-lymphoma virus-infected cells. Science 1984, 223, 1086–1087. [Google Scholar] [CrossRef] [PubMed]
- Migone, T.S.; Lin, J.X.; Cereseto, A.; Mulloy, J.C.; O’Shea, J.J.; Franchini, G.; Leonard, W.J. Constitutively activated Jak-STAT pathway in T cells transformed with HTLV-I. Science 1995, 269, 79–81. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Lee, B.; Korecka, M.; Li, G.; Weyland, C.; Eck, S.; Gessain, A.; Arima, N.; Lessin, S.R.; Shaw, L.M.; et al. Differences in phosphorylation of the IL-2R associated JAK/STAT proteins between HTLV-I (+), IL-2-independent and IL-2-dependent cell lines and uncultured leukemic cells from patients with adult T-cell lymphoma/leukemia. Leuk. Res. 1999, 23, 373–384. [Google Scholar] [CrossRef]
- Takemoto, S.; Mulloy, J.C.; Cereseto, A.; Migone, T.-S.; Patel, B.K.R.; Matsuoka, M.; Yamaguchi, K.; Takatsuki, K.; Kamihira, S.; White, J.D.; et al. Proliferation of adult T cell leukemia/lymphoma cells is associated with the constitutive activation of JAK/STAT proteins. Proc. Natl. Acad. Sci. USA 1997, 94, 13897–13902. [Google Scholar] [CrossRef]
- Huey, D.D.; Bolon, B.; Perle, K.M.D.L.; Kannian, P.; Jacobson, S.; Ratner, L.; Green, P.L.; Niewiesk, S. Role of Wild-type and Recombinant Human T-cell Leukemia Viruses in Lymphoproliferative Disease in Humanized NSG Mice. Comp. Med. 2018, 68, 4–14. [Google Scholar]
- Chen, J.; Petrus, M.; Bryant, B.R.; Nguyen, V.P.; Goldman, C.K.; Bamford, R.; Morris, J.C.; Janik, J.E.; Waldmann, T.A. Autocrine/paracrine cytokine stimulation of leukemic cell proliferation in smoldering and chronic adult T-cell leukemia. Blood 2010, 116, 5948–5956. [Google Scholar] [CrossRef]
- Fung, M.M.; Chu, Y.-L.; Fink, J.L.; Wallace, A.; McGuire, K.L. IL-2- and STAT5-regulated cytokine gene expression in cells expressing the Tax protein of HTLV-1. Oncogene 2005, 24, 4624–4633. [Google Scholar] [CrossRef]
- Yamada, Y.; Sugawara, K.; Hata, T.; Tsuruta, K.; Moriuchi, R.; Maeda, T.; Atogami, S.; Murata, K.; Fujimoto, K.; Kohno, T.; et al. Interleukin-15 (IL-15) Can Replace the IL-2 Signal in IL-2–Dependent Adult T-Cell Leukemia (ATL) Cell Lines: Expression of IL-15 Receptor α on ATL Cells. Blood 1998, 91, 4265–4272. [Google Scholar]
- Nakagawa, M.; Shaffer, A.L.; Ceribelli, M.; Zhang, M.; Wright, G.; Huang, D.W.; Xiao, W.; Powell, J.; Petrus, M.N.; Yang, Y.; et al. Targeting the HTLV-I-Regulated BATF3/IRF4 Transcriptional Network in Adult T Cell Leukemia/Lymphoma. Cancer Cell 2018, 34, 286–297.e10. [Google Scholar] [CrossRef]
- Mori, N.; Yamashita, U.; Tanaka, Y.; Nakata, K.; Oda, S.; Morimoto, I.; Eto, S. Interleukin-4 induces proliferation of adult T-cell leukemia cells. Eur. J. Haematol. 1993, 50, 133–140. [Google Scholar] [CrossRef]
- Mori, N.; Shirakawa, F.; Murakami, S.; Oda, S.; Eto, S. Characterization and regulation of interleukin-4 receptor in adult T-cell leukemia cells. Eur. J. Haematol. 1996, 56, 241–247. [Google Scholar] [CrossRef]
- Yamada, Y.; Ohmoto, Y.; Hata, T.; Yamamura, M.; Murata, K.; Tsukasaki, K.; Kohno, T.; Chen, Y.; Kamihira, S.; Tomonaga, M. Features of the Cytokines Secreted by Adult T Cell Leukemia (ATL) Cells. Leuk. Lymphoma 1996, 21, 443–447. [Google Scholar] [CrossRef]
- Gupta, S.; Jiang, M.; Anthony, A.; Pernis, A.B. Lineage-Specific Modulation of Interleukin 4 Signaling by Interferon Regulatory Factor 4. J. Exp. Med. 1999, 190, 1837–1848. [Google Scholar] [CrossRef]
- Ramos, J.C.; Ruiz, P.; Ratner, L.; Reis, I.M.; Brites, C.; Pedroso, C.; Byrne, G.E.; Toomey, N.L.; Andela, V.; Harhaj, E.W.; et al. IRF-4 and c-Rel expression in antiviral-resistant adult T-cell leukemia/lymphoma. Blood 2007, 109, 3060–3068. [Google Scholar] [CrossRef]
- Kataoka, K.; Nagata, Y.; Kitanaka, A.; Shiraishi, Y.; Shimamura, T.; Yasunaga, J.; Totoki, Y.; Chiba, K.; Sato-Otsubo, A.; Nagae, G.; et al. Integrated molecular analysis of adult T cell leukemia/lymphoma. Nat. Genet. 2015, 47, 1304–1315. [Google Scholar] [CrossRef]
- Cherian, M.A.; Olson, S.; Sundaramoorthi, H.; Cates, K.; Cheng, X.; Harding, J.; Martens, A.; Challen, G.A.; Tyagi, M.; Ratner, L.; et al. An activating mutation of interferon regulatory factor 4 (IRF4) in adult T-cell leukemia. J. Biol. Chem. 2018, 293, 6844–6858. [Google Scholar] [CrossRef]
- Matsuoka, M.; Jeang, K.T. Human T-cell leukaemia virus type 1 (HTLV-1) infectivity and cellular transformation. Nat. Rev. Cancer 2007, 7, 270–280. [Google Scholar] [CrossRef]
- Starling, A.L.B.; Coelho-dos-Reis, J.G.A.; Peruhype-Magalhães, V.; Pascoal-Xavier, M.A.; Gonçalves, D.U.; Béla, S.R.; Lambertucci, J.R.; Labanca, L.; Pereira, S.R.S.; Teixeira-Carvalho, A.; et al. Immunological signature of the different clinical stages of the HTLV-1 infection: Establishing serum biomarkers for HTLV-1-associated disease morbidity. Biomarkers 2015, 20, 502–512. [Google Scholar] [CrossRef]
- Furukawa, Y.; Saito, M.; Matsumoto, W.; Usuku, K.; Tanaka, Y.; Izumo, S.; Osame, M. Different Cytokine Production in Tax-Expressing Cells between Patients with Human T Cell Lymphotropic Virus Type I (HTLV-I)–Associated Myelopathy/Tropical Spastic Paraparesis and Asymptomatic HTLV-I Carriers. J. Infect. Dis. 2003, 187, 1116–1125. [Google Scholar] [CrossRef]
- Wang, T.; Secombes, C.J. The evolution of IL-4 and IL-13 and their receptor subunits. Cytokine 2015, 75, 8–13. [Google Scholar] [CrossRef]
- Wäldele, K.; Schneider, G.; Ruckes, T.; Grassmann, R. Interleukin-13 Overexpression by Tax Transactivation: A Potential Autocrine Stimulus in Human T-Cell Leukemia Virus-Infected Lymphocytes. J. Virol. 2004, 78, 6081–6090. [Google Scholar] [CrossRef]
- Chung, H.-K.; Young, H.A.; Goon, P.K.C.; Heidecker, G.; Princler, G.L.; Shimozato, O.; Taylor, G.P.; Bangham, C.R.M.; Derse, D. Activation of interleukin-13 expression in T cells from HTLV-1-infected individuals and in chronically infected cell lines. Blood 2003, 102, 4130–4136. [Google Scholar] [CrossRef]
- Silbermann, K.; Schneider, G.; Grassmann, R. Stimulation of interleukin-13 expression by human T-cell leukemia virus type 1 oncoprotein Tax via a dually active promoter element responsive to NF-κB and NFAT. J. Gen. Virol. 2008, 89, 2788–2798. [Google Scholar] [CrossRef]
- Nishimoto, N.; Yoshizaki, K.; Eiraku, N.; Machigashira, K.; Tagoh, H.; Ogata, A.; Kuritani, T.; Osame, M.; Kishimoto, T. Elevated levels of interleukin-6 in serum and cerebrospinal fluid of HTLV-I-associated myelopathy/tropical spastic paraparesis. J. Neurol. Sci. 1990, 97, 183–193. [Google Scholar] [CrossRef]
- Inagaki, A.; Ishida, T.; Ishii, T.; Komatsu, H.; Iida, S.; Ding, J.; Yonekura, K.; Takeuchi, S.; Takatsuka, Y.; Utsunomiya, A.; et al. Clinical significance of serum Th1-, Th2- and regulatory T cells-associated cytokines in adult T-cell leukemia/lymphoma: High interleukin-5 and -10 levels are significant unfavorable prognostic factors. Int. J. Cancer 2006, 118, 3054–3061. [Google Scholar] [CrossRef]
- Yamamura, M.; Yamada, Y.; Momita, S.; Kamihira, S.; Tomonaga, M. Circulating interleukin-6 levels are elevated in adult T-cell leukaemia/lymphoma patients and correlate with adverse clinical features and survival. Br. J. Haematol. 1998, 100, 129–134. [Google Scholar] [CrossRef]
- Kagdi, H.; Demontis, M.A.; Ramos, J.C.; Taylor, G.P. Switching and loss of cellular cytokine producing capacity characterize in vivo viral infection and malignant transformation in human T- lymphotropic virus type 1 infection. PLoS Pathog. 2018, 14, e1006861. [Google Scholar] [CrossRef]
- Sawada, L.; Nagano, Y.; Hasegawa, A.; Kanai, H.; Nogami, K.; Ito, S.; Sato, T.; Yamano, Y.; Tanaka, Y.; Masuda, T.; et al. IL-10-mediated signals act as a switch for lymphoproliferation in Human T-cell leukemia virus type-1 infection by activating the STAT3 and IRF4 pathways. PLoS Pathog. 2017, 13, e1006597. [Google Scholar] [CrossRef]
- Horiuchi, S.; Yamamoto, N.; Dewan, M.Z.; Takahashi, Y.; Yamashita, A.; Yoshida, T.; Nowell, M.A.; Richards, P.J.; Jones, S.A.; Yamamoto, N. Human T-cell leukemia virus type-I Tax induces expression of interleukin-6 receptor (IL-6R): Shedding of soluble IL-6R and activation of STAT3 signaling. Int. J. Cancer 2006, 119, 823–830. [Google Scholar] [CrossRef]
- Rose-John, S.; Neurath, M.F. IL-6 trans-Signaling: The Heat Is On. Immunity 2004, 20, 2–4. [Google Scholar] [CrossRef]
- McNab, F.; Mayer-Barber, K.; Sher, A.; Wack, A.; O’Garra, A. Type I interferons in infectious disease. Nat. Rev. Immunol. 2015, 15, 87–103. [Google Scholar] [CrossRef]
- Brubaker, S.W.; Bonham, K.S.; Zanoni, I.; Kagan, J.C. Innate Immune Pattern Recognition: A Cell Biological Perspective. Annu. Rev. Immunol. 2015, 33, 257–290. [Google Scholar] [CrossRef]
- Ivashkiv, L.B.; Donlin, L.T. Regulation of type I interferon responses. Nat. Rev. Immunol. 2014, 14, 36–49. [Google Scholar] [CrossRef]
- Tomasello, E.; Pollet, E.; Vu Manh, T.-P.; Uzé, G.; Dalod, M. Harnessing Mechanistic Knowledge on Beneficial Versus Deleterious IFN-I Effects to Design Innovative Immunotherapies Targeting Cytokine Activity to Specific Cell Types. Front. Immunol. 2014, 5, 526. [Google Scholar] [CrossRef]
- Journo, C.; Mahieux, R. HTLV-1 and innate immunity. Viruses 2011, 3, 1374–1394. [Google Scholar] [CrossRef]
- Kannagi, M.; Hasegawa, A.; Kinpara, S.; Shimizu, Y.; Takamori, A.; Utsunomiya, A. Double control systems for human T-cell leukemia virus type 1 by innate and acquired immunity. Cancer Sci. 2011, 102, 670–676. [Google Scholar] [CrossRef]
- Bazarbachi, A.; Plumelle, Y.; Carlos Ramos, J.; Tortevoye, P.; Otrock, Z.; Taylor, G.; Gessain, A.; Harrington, W.; Panelatti, G.; Hermine, O. Meta-analysis on the use of zidovudine and interferon-alfa in adult T-cell leukemia/lymphoma showing improved survival in the leukemic subtypes. J. Clin. Oncol. 2010, 28, 4177–4183. [Google Scholar] [CrossRef]
- Kuroda, Y.; Kurohara, K.; Fujiyama, F.; Takashima, H.; Endo, C.; Matsui, M.; Neshige, R.; Kakigi, R. Systemic interferon-α in the treatment of HTLV-I-associated myelopathy. Acta Neurol. Scand. 1992, 86, 82–86. [Google Scholar] [CrossRef]
- Nakamura, T.; Shibayama, K.; Nagasato, K.; Matsuo, H.; Tsujihata, M.; Nagataki, S. The efficacy of interferon-α treatment in human T-lymphotropic virus type-I-associated myelopathy. Jpn. J. Med. 1990, 29, 362–367. [Google Scholar] [CrossRef]
- Yamasaki, K.; Kira, J.; Koyanagi, Y.; Kawano, Y.; Miyano-Kurosaki, N.; Nakamura, M.; Baba, E.; Suzuki, J.; Yamamoto, A.; Yamamoto, N.; et al. Long-term, high dose interferon-α treatment in HTLV-I-associated myelopathy/tropical spastic paraparesis: A combined clinical, virological and immunological study. J. Neurol. Sci. 1997, 147, 135–144. [Google Scholar] [CrossRef]
- Rafatpanah, H.; Rezaee, A.; Etemadi, M.M.; Hosseini, R.F.; Khorram, B.; Afsahr, L.; Taylor, G.; Mokhber, N.; Mahmoudi, M.; Abbaszadegan, M.R.; et al. The impact of interferon-α treatment on clinical and immunovirological aspects of HTLV-1-associated myelopathy in northeast of Iran. J. Neuroimmunol. 2012, 250, 87–93. [Google Scholar] [CrossRef]
- Ijichi, S.; Izumo, S.; Nagai, M.; Shinmyozu, K.; Hall, W.W. Osame, mitsuhiro Anti-viral and immunomodulatory effects of interferon-α on cultured lymphocytes from patients with human T lymphotropic virus type I-associated myelopathy (HAM/TSP). J. Neuroimmunol. 1995, 61, 213–221. [Google Scholar] [CrossRef]
- Tattermusch, S.; Skinner, J.A.; Chaussabel, D.; Banchereau, J.; Berry, M.P.; McNab, F.W.; O’Garra, A.; Taylor, G.P.; Bangham, C.R. Systems biology approaches reveal a specific interferon-inducible signature in HTLV-1 associated myelopathy. PLoS Pathog. 2012, 8, e1002480. [Google Scholar] [CrossRef]
- Macchi, B.; D’Onofrio, C.; Labianca, R.A.; Bonmassar, E. Mononuclear cells from peripheral blood of adult donors and from cord blood are equally protected by α- and β-interferons against infection with HTLV-I. Pharmacol. Res. 1990, 22, 503–514. [Google Scholar] [CrossRef]
- Cachat, A.; Chevalier, S.A.; Alais, S.; Ko, N.L.; Ratner, L.; Journo, C.; Dutartre, H.; Mahieux, R. Alpha interferon restricts human T-lymphotropic virus type 1 and 2 de novo infection through PKR activation. J. Virol. 2013, 87, 13386–13396. [Google Scholar] [CrossRef]
- Kinpara, S.; Kijiyama, M.; Takamori, A.; Hasegawa, A.; Sasada, A.; Masuda, T.; Tanaka, Y.; Utsunomiya, A.; Kannagi, M. Interferon-α (IFN-α) suppresses HTLV-1 gene expression and cell cycling, while IFN-α combined with zidovudine induces p53 signaling and apoptosis in HTLV-1-infected cells. Retrovirology 2013, 10, 52. [Google Scholar] [CrossRef]
- Cachat, A.; Alais, S.; Chevalier, S.A.; Journo, C.; Fusil, F.; Dutartre, H.; Boniface, A.; Ko, N.L.; Gessain, A.; Cosset, F.L.; et al. ADAR1 enhances HTLV-1 and HTLV-2 replication through inhibition of PKR activity. Retrovirology 2014, 11, 93. [Google Scholar] [CrossRef]
- Feng, X.; Ratner, L. Human T-cell leukemia virus type 1 blunts signaling by interferon α. Virology 2008, 374, 210–216. [Google Scholar] [CrossRef]
- Oliere, S.; Hernandez, E.; Lezin, A.; Arguello, M.; Douville, R.; Nguyen, T.L.; Olindo, S.; Panelatti, G.; Kazanji, M.; Wilkinson, P.; et al. HTLV-1 evades type I interferon antiviral signaling by inducing the suppressor of cytokine signaling 1 (SOCS1). PLoS Pathog. 2010, 6, e1001177. [Google Scholar] [CrossRef]
- Smith, D.; Buckle, G.J.; Hafler, D.A.; Frank, D.A.; Hollsberg, P. HTLV-I-infected T cells evade the antiproliferative action of IFN-β. Virology 1999, 257, 314–321. [Google Scholar] [CrossRef]
- Charoenthongtrakul, S.; Zhou, Q.; Shembade, N.; Harhaj, N.S.; Harhaj, E.W. Human T cell leukemia virus type 1 Tax inhibits innate antiviral signaling via NF-kappaB-dependent induction of SOCS1. J. Virol. 2011, 85, 6955–6962. [Google Scholar] [CrossRef]
- Zhang, J.; Yamada, O.; Kawagishi, K.; Araki, H.; Yamaoka, S.; Hattori, T.; Shimotohno, K. Human T-cell leukemia virus type 1 Tax modulates interferon-α signal transduction through competitive usage of the coactivator CBP/p300. Virology 2008, 379, 306–313. [Google Scholar] [CrossRef]
- Haller, O.; Kochs, G.; Weber, F. The interferon response circuit: Induction and suppression by pathogenic viruses. Virology 2006, 344, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Narulla, M.S.; Alsairi, A.; Charmier, L.; Noonan, S.; Conroy, D.; Hall, W.W.; Sheehy, N. Positive and Negative Regulation of Type I Interferons by the Human T Cell Leukemia Virus Antisense Protein HBZ. J. Virol. 2017, 91, e00853-17. [Google Scholar] [CrossRef] [PubMed]
- Hyun, J.; Ramos, J.C.; Toomey, N.; Balachandran, S.; Lavorgna, A.; Harhaj, E.; Barber, G.N. Oncogenic human T-cell lymphotropic virus type 1 tax suppression of primary innate immune signaling pathways. J. Virol. 2015, 89, 4880–4893. [Google Scholar] [CrossRef] [PubMed]
- Yuen, C.K.; Chan, C.P.; Fung, S.Y.; Wang, P.H.; Wong, W.M.; Tang, H.M.; Yuen, K.S.; Jin, D.Y.; Kok, K.H. Suppression of Type I Interferon Production by Human T-Cell Leukemia Virus Type 1 Oncoprotein Tax through Inhibition of IRF3 Phosphorylation. J. Virol. 2016, 90, 3902–3912. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yang, S.; Liu, L.; Wang, H.; Yang, B. HTLV-1 Tax impairs K63-linked ubiquitination of STING to evade host innate immunity. Virus Res. 2017, 232, 13–21. [Google Scholar] [CrossRef]
- Suzuki, S.; Zhou, Y.; Refaat, A.; Takasaki, I.; Koizumi, K.; Yamaoka, S.; Tabuchi, Y.; Saiki, I.; Sakurai, H. Human T cell lymphotropic virus 1 manipulates interferon regulatory signals by controlling the TAK1-IRF3 and IRF4 pathways. J. Biol. Chem. 2010, 285, 4441–4446. [Google Scholar] [CrossRef]
- Diani, E.; Avesani, F.; Bergamo, E.; Cremonese, G.; Bertazzoni, U.; Romanelli, M.G. HTLV-1 Tax protein recruitment into IKKε and TBK1 kinase complexes enhances IFN-I expression. Virology 2015, 476, 92–99. [Google Scholar] [CrossRef]
- Sze, A.; Belgnaoui, S.M.; Olagnier, D.; Lin, R.; Hiscott, J.; van Grevenynghe, J. Host restriction factor SAMHD1 limits human T cell leukemia virus type 1 infection of monocytes via STING-mediated apoptosis. Cell Host Microbe 2013, 14, 422–434. [Google Scholar] [CrossRef]
- Wang, J.; Kang, L.; Song, D.; Liu, L.; Yang, S.; Ma, L.; Guo, Z.; Ding, H.; Wang, H.; Yang, B. Ku70 Senses HTLV-1 DNA and Modulates HTLV-1 Replication. J. Immunol. 2017, 199, 2475–2482. [Google Scholar] [CrossRef]
- Yang, B.; Song, D.; Liu, Y.; Cui, Y.; Lu, G.; Di, W.; Xing, H.; Ma, L.; Guo, Z.; Guan, Y.; et al. IFI16 regulates HTLV-1 replication through promoting HTLV-1 RTI-induced innate immune responses. FEBS Lett. 2018, 592, 1693–1704. [Google Scholar] [CrossRef]
- Colisson, R.; Barblu, L.; Gras, C.; Raynaud, F.; Hadj-Slimane, R.; Pique, C.; Hermine, O.; Lepelletier, Y.; Herbeuval, J.P. Free HTLV-1 induces TLR7-dependent innate immune response and TRAIL relocalization in killer plasmacytoid dendritic cells. Blood 2010, 115, 2177–2185. [Google Scholar] [CrossRef] [PubMed]
- Hishizawa, M.; Imada, K.; Kitawaki, T.; Ueda, M.; Kadowaki, N.; Uchiyama, T. Depletion and impaired interferon-α-producing capacity of blood plasmacytoid dendritic cells in human T-cell leukaemia virus type I-infected individuals. Br. J. Haematol. 2004, 125, 568–575. [Google Scholar] [CrossRef] [PubMed]
- Azakami, K.; Sato, T.; Araya, N.; Utsunomiya, A.; Kubota, R.; Suzuki, K.; Hasegawa, D.; Izumi, T.; Fujita, H.; Aratani, S.; et al. Severe loss of invariant NKT cells exhibiting anti-HTLV-1 activity in patients with HTLV-1-associated disorders. Blood 2009, 114, 3208–3215. [Google Scholar] [CrossRef] [PubMed]
- Manuel, S.L.; Sehgal, M.; Khan, Z.K.; Goedert, J.J.; Betts, M.R.; Jain, P. An Altered Maturation and Adhesion Phenotype of Dendritic Cells in Diseased Individuals Compared to Asymptomatic Carriers of Human T Cell Leukemia Virus Type 1. AIDS Res. Hum. Retroviruses 2013, 29, 1273–1285. [Google Scholar] [CrossRef] [PubMed]
- Neco, H.V.P.D.C.; Teixeira, V.G.D.S.; da Trindade, A.C.L.; Magalhães, P.M.R.; de Lorena, V.M.B.; Castellano, L.R.C.; de Souza, J.R.; Vasconcelos, L.R.; de Moura, P.M.M.F.; de Morais, C.N.L. Mediators Go Together: High Production of CXCL9, CXCL10, IFN-γ, and TNF-α in HTLV-1-Associated Myelopathy/Tropical Spastic Paraparesis. AIDS Res. Hum. Retroviruses 2017, 33, 1134–1139. [Google Scholar] [CrossRef]
- Starling, A.L.B.; Martins-Filho, O.A.; Lambertucci, J.R.; Labanca, L.; de Souza Pereira, S.R.; Teixeira-Carvalho, A.; Martins, M.L.; Ribas, J.G.; Carneiro-Proietti, A.B.F.; Gonçalves, D.U. Proviral load and the balance of serum cytocines in HTLV-1-asymptomatic infection and in HTLV-1-associated myelopathy/tropical spastic paraparesis (HAM/TSP). Acta Trop. 2013, 125, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Tendler, C.L.; Greenberg, S.J.; Burton, J.D.; Danielpour, D.; Kim, S.-J.; Blattner, W.A.; Manns, A.; Waldmann, T.A. Cytokine induction in HTLV-I associated myelopathy and adult T-cell leukemia: Alternate molecular mechanisms underlying retroviral pathogenesis. J. Cell. Biochem. 1991, 46, 302–311. [Google Scholar] [CrossRef]
- Goncalves, D.U.; Proietti, F.A.; Barbosa-Stancioli, E.F.; Martins, M.L.; Ribas, J.G.; Martins-Filho, O.A.; Teixeira-Carvalho, A.; Peruhype-Magalhães, V.; Carneiro-Proietti, A.B. HTLV-1-associated myelopathy/tropical spastic paraparesis (HAM/TSP) inflammatory network. Inflamm. Allergy Drug Targets 2008, 7, 98–107. [Google Scholar] [CrossRef]
- Uchiyama, T. Human T Cell Leukemia Virus Type I (htlv-I) and Human Diseases. Annu. Rev. Immunol. 1997, 15, 15–37. [Google Scholar] [CrossRef]
- Sonar, S.A.; Shaikh, S.; Joshi, N.; Atre, A.N.; Lal, G. IFN-γ promotes transendothelial migration of CD4+ T cells across the blood–brain barrier. Immunol. Cell Biol. 2017, 95, 843–853. [Google Scholar] [CrossRef]
- Yamano, Y.; Coler-Reilly, A. HTLV-1 induces a Th1-like state in CD4+CCR4+ T cells that produces an inflammatory positive feedback loop via astrocytes in HAM/TSP. J. Neuroimmunol. 2017, 304, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Afonso, P.V.; Ozden, S.; Prevost, M.-C.; Schmitt, C.; Seilhean, D.; Weksler, B.; Couraud, P.-O.; Gessain, A.; Romero, I.A.; Ceccaldi, P.-E. Human Blood-Brain Barrier Disruption by Retroviral-Infected Lymphocytes: Role of Myosin Light Chain Kinase in Endothelial Tight-Junction Disorganization. J. Immunol. 2007, 179, 2576–2583. [Google Scholar] [CrossRef]
- Afonso, P.V.; Ozden, S.; Cumont, M.-C.; Seilhean, D.; Cartier, L.; Rezaie, P.; Mason, S.; Lambert, S.; Huerre, M.; Gessain, A.; et al. Alteration of blood-brain barrier integrity by retroviral infection. PLoS Pathog. 2008, 4, e1000205. [Google Scholar] [CrossRef] [PubMed]
- Montanheiro, P.; Vergara, M.P.P.; Smid, J.; da Silva Duarte, A.J.; de Oliveira, A.C.P.; Casseb, J. High production of RANTES and MIP-1α in the tropical spastic paraparesis/HTLV-1-associated myelopathy (TSP/HAM). J. Neuroimmunol. 2007, 188, 138–142. [Google Scholar] [CrossRef]
- Brito-Melo, G.E.A.; Peruhype-Magalhães, V.; Teixeira-Carvalho, A.; Barbosa-Stancioli, E.F.; Carneiro-Proietti, A.B.F.; Catalan-Soares, B.; Ribas, J.G.; Martins-Filho, O.A. IL-10 produced by CD4+ and CD8+ T cells emerge as a putative immunoregulatory mechanism to counterbalance the monocyte-derived TNF-α and guarantee asymptomatic clinical status during chronic HTLV-I infection. Clin. Exp. Immunol. 2007, 147, 35–44. [Google Scholar] [CrossRef]
- Fujimoto, T.; Nakamura, T.; Furuya, T.; Nakane, S.; Shirabe, S.; Kambara, C.; Hamasaki, S.; Yoshimura, T.; Eguchi, K. Relationship between the clinical efficacy of pentoxifylline treatment and elevation of serum T helper type 2 cytokine levels in patients with human T-lymphotropic virus type I-associated myelopathy. Intern. Med. 1999, 38, 717–721. [Google Scholar] [CrossRef]
- Ali, A.; Rudge, P.; Dalgleish, A.G. Neopterin concentrations in serum and cerebrospinal fluid in HTLV-I infected individuals. J. Neurol. 1992, 239, 270–272. [Google Scholar]
- Tarokhian, H.; Taghadosi, M.; Rafatpanah, H.; Rajaei, T.; Azarpazhooh, M.R.; Valizadeh, N.; Rezaee, S.A.R. The effect of HTLV-1 virulence factors (HBZ, Tax, proviral load), HLA class I and plasma neopterin on manifestation of HTLV-1 associated myelopathy tropical spastic paraparesis. Virus Res. 2017, 228, 1–6. [Google Scholar] [CrossRef]
- Sato, T.; Coler-Reilly, A.; Utsunomiya, A.; Araya, N.; Yagishita, N.; Ando, H.; Yamauchi, J.; Inoue, E.; Ueno, T.; Hasegawa, Y.; et al. CSF CXCL10, CXCL9, and neopterin as candidate prognostic biomarkers for HTLV-1-associated myelopathy/tropical spastic paraparesis. PLoS Negl. Trop. Dis. 2013, 7, e2479. [Google Scholar] [CrossRef]
- Ando, H.; Sato, T.; Tomaru, U.; Yoshida, M.; Utsunomiya, A.; Yamauchi, J.; Araya, N.; Yagishita, N.; Coler-Reilly, A.; Shimizu, Y.; et al. Positive feedback loop via astrocytes causes chronic inflammation in virus-associated myelopathy. Brain 2013, 136, 2876–2887. [Google Scholar] [CrossRef]
- Chaves, D.G.; Sales, C.C.; de Cássia Gonçalves, P.; da Silva-Malta, M.C.F.; Romanelli, L.C.; Ribas, J.G.; de Freitas Carneiro-Proietti, A.B.; Martins, M.L. Plasmatic proinflammatory chemokines levels are tricky markers to monitoring HTLV-1 carriers. J. Med. Virol. 2016, 88, 1438–1447. [Google Scholar] [CrossRef] [PubMed]
- Araya, N.; Sato, T.; Ando, H.; Tomaru, U.; Yoshida, M.; Coler-Reilly, A.; Yagishita, N.; Yamauchi, J.; Hasegawa, A.; Kannagi, M.; et al. HTLV-1 induces a Th1-like state in CD4+CCR4+ T cells. J. Clin. Investig. 2014, 124, 3431–3442. [Google Scholar] [CrossRef] [PubMed]
- Lehky, T.J.; Fox, C.H.; Koenig, S.; Levin, M.C.; Flerlage, N.; Izumo, S.; Sato, E.; Raine, C.S.; Osame, M.; Jacobson, S. Detection of human T-lymphotropic virus type I (HTLV-I) tax RNA in the central nervous system of HTLV-I-associated myelopathy/tropical spastic paraparesis patients by in situ hybridization. Ann. Neurol. 1995, 37, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Levin, M.C.; Rosenblum, M.K.; Fox, C.H.; Jacobson, S. Localization of retrovirus in the central nervous system of a patient co-infected with HTLV-1 and HIV with HAM/TSP and HIV-associated dementia. J. Neurovirol. 2001, 7, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Méndez, E.; Kawanishi, T.; Clemens, K.; Siomi, H.; Soldan, S.S.; Calabresi, P.; Brady, J.; Jacobson, S. Astrocyte-specific expression of human T-cell lymphotropic virus type 1 (HTLV-1) Tax: Induction of tumor necrosis factor α and susceptibility to lysis by CD8+ HTLV-1-specific cytotoxic T cells. J. Virol. 1997, 71, 9143–9149. [Google Scholar] [PubMed]
- Szymocha, R.; Brisson, C.; Bernard, A.; Akaoka, H.; Belin, M.F.; Giraudon, P. Long-term effects of HTLV-1 on brain astrocytes: Sustained expression of Tax-1 associated with synthesis of inflammatory mediators. J. Neurovirol. 2000, 6, 350–357. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, P.; Rochford, R.; Antel, J.; Canute, G.; Wrzesinski, S.; Sieburg, M.; Feuer, G. Proinflammatory cytokine gene induction by human T-cell leukemia virus type 1 (HTLV-1) and HTLV-2 Tax in primary human glial cells. J. Virol. 2007, 81, 1690–1700. [Google Scholar] [CrossRef]
- Iwatsuki, K.; Harada, H.; Motoki, Y.; Kaneko, F.; Jin, F.; Takigawa, M. Diversity of immunobiological functions of T-cell lines established from patients with adult T-cell leukaemia. Br. J. Dermatol. 1995, 133, 861–867. [Google Scholar] [CrossRef]
- Micallef, M.; Ariyasu, T.; Dao, T.; Matsuo, Y.; Minowada, J. Constitutive expression of immunosuppression-associated cytokine genes in a panel of human T-leukemia-cell lines—High-incidence of transforming growth-factor-Beta gene-expression. Int. J. Oncol. 1994, 4, 633–638. [Google Scholar] [CrossRef]
- Mitre, E.; Thompson, R.W.; Carvalho, E.M.; Nutman, T.B.; Neva, F.A. Majority of interferon-gamma-producing CD4+ cells in patients infected with human T cell lymphotrophic virus do not express tax protein. J. Infect. Dis. 2003, 188, 428–432. [Google Scholar] [CrossRef]
- Brown, D.A.; Nelson, F.B.; Reinherz, E.L.; Diamond, D.J. The human interferon-gamma gene contains an inducible promoter that can be transactivated by tax I and II. Eur. J. Immunol. 1991, 21, 1879–1885. [Google Scholar] [CrossRef] [PubMed]
- Kannagi, M.; Harashima, N.; Kurihara, K.; Ohashi, T.; Utsunomiya, A.; Tanosaki, R.; Masuda, M.; Tomonaga, M.; Okamura, J. Tumor immunity against adult T-cell leukemia. Cancer Sci. 2005, 96, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Mitra-Kaushik, S.; Harding, J.; Hess, J.; Schreiber, R.; Ratner, L. Enhanced tumorigenesis in HTLV-1 tax-transgenic mice deficient in interferon-gamma. Blood 2004, 104, 3305–3311. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Kehrl, J.H.; Burton, J.; Tendler, C.L.; Jeang, K.T.; Danielpour, D.; Thevenin, C.; Kim, K.Y.; Sporn, M.B.; Roberts, A.B. Transactivation of the transforming growth factor β 1 (TGF-β 1) gene by human T lymphotropic virus type 1 tax: A potential mechanism for the increased production of TGF-β 1 in adult T cell leukemia. J. Exp. Med. 1990, 172, 121–129. [Google Scholar] [CrossRef]
- Niitsu, Y.; Urushizaki, Y.; Koshida, Y.; Terui, K.; Mahara, K.; Kohgo, Y.; Urushizaki, I. Expression of TGF-β gene in adult T cell leukemia. Blood 1988, 71, 263–266. [Google Scholar]
- Huang, S.S.; Huang, J.S. TGF-β control of cell proliferation. J. Cell. Biochem. 2005, 96, 447–462. [Google Scholar] [CrossRef]
- Höllsberg, P.; Ausubel, L.J.; Hafler, D.A. Human T cell lymphotropic virus type I-induced T cell activation. Resistance to TGF-β 1-induced suppression. J. Immunol. 1994, 153, 566–573. [Google Scholar]
- Grant, C.; Oh, U.; Yao, K.; Yamano, Y.; Jacobson, S. Dysregulation of TGF-β signaling and regulatory and effector T-cell function in virus-induced neuroinflammatory disease. Blood 2008, 111, 5601–5609. [Google Scholar] [CrossRef]
- Mori, N.; Morishita, M.; Tsukazaki, T.; Giam, C.Z.; Kumatori, A.; Tanaka, Y.; Yamamoto, N. Human T-cell leukemia virus type I oncoprotein Tax represses Smad-dependent transforming growth factor β signaling through interaction with CREB-binding protein/p300. Blood 2001, 97, 2137–2144. [Google Scholar] [CrossRef]
- Lee, D.K.; Kim, B.-C.; Brady, J.N.; Jeang, K.-T.; Kim, S.-J. Human T-cell lymphotropic virus type 1 tax inhibits transforming growth factor-β signaling by blocking the association of Smad proteins with Smad-binding element. J. Biol. Chem. 2002, 277, 33766–33775. [Google Scholar] [CrossRef]
- Arnulf, B.; Villemain, A.; Nicot, C.; Mordelet, E.; Charneau, P.; Kersual, J.; Zermati, Y.; Mauviel, A.; Bazarbachi, A.; Hermine, O. Human T-cell lymphotropic virus oncoprotein Tax represses TGF-β 1 signaling in human T cells via c-Jun activation: A potential mechanism of HTLV-I leukemogenesis. Blood 2002, 100, 4129–4138. [Google Scholar] [CrossRef]
- Zhao, T.; Satou, Y.; Sugata, K.; Miyazato, P.; Green, P.L.; Imamura, T.; Matsuoka, M. HTLV-1 bZIP factor enhances TGF-β signaling through p300 coactivator. Blood 2011, 118, 1865–1876. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, A.; Taylor, G.P.; Klose, R.J.; Schofield, C.J.; Bangham, C.R. Histone H2A monoubiquitylation and p38-MAPKs regulate immediate-early gene-like reactivation of latent retrovirus HTLV-1. JCI Insight 2018, 3, e123196. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Jin, W.; Hardegen, N.; Lei, K.-J.; Li, L.; Marinos, N.; McGrady, G.; Wahl, S.M. Conversion of peripheral CD4+CD25- naive T cells to CD4+CD25+ regulatory T cells by TGF-β induction of transcription factor Foxp3. J. Exp. Med. 2003, 198, 1875–1886. [Google Scholar] [CrossRef] [PubMed]
- Satou, Y.; Utsunomiya, A.; Tanabe, J.; Nakagawa, M.; Nosaka, K.; Matsuoka, M. HTLV-1 modulates the frequency and phenotype of FoxP3+CD4+ T cells in virus-infected individuals. Retrovirology 2012, 9, 46. [Google Scholar] [CrossRef] [PubMed]
- Toulza, F.; Nosaka, K.; Tanaka, Y.; Schioppa, T.; Balkwill, F.; Taylor, G.P.; Bangham, C.R.M. Human T-lymphotropic virus type 1-induced CC chemokine ligand 22 maintains a high frequency of functional FoxP3+ regulatory T cells. J. Immunol. 2010, 185, 183–189. [Google Scholar] [CrossRef]
- Toulza, F.; Nosaka, K.; Takiguchi, M.; Pagliuca, T.; Mitsuya, H.; Tanaka, Y.; Taylor, G.P.; Bangham, C.R.M. FoxP3+ regulatory T cells are distinct from leukemia cells in HTLV-1-associated adult T-cell leukemia. Int. J. Cancer 2009, 125, 2375–2382. [Google Scholar] [CrossRef]
- Mori, N.; Gill, P.S.; Mougdil, T.; Murakami, S.; Eto, S.; Prager, D. Interleukin-10 gene expression in adult T-cell leukemia. Blood 1996, 88, 1035–1045. [Google Scholar] [CrossRef]
- Kchour, G.; Rezaee, R.; Farid, R.; Ghantous, A.; Rafatpanah, H.; Tarhini, M.; Kooshyar, M.M.; El Hajj, H.; Berry, F.; Mortada, M.; et al. The combination of arsenic, interferon-α, and zidovudine restores an “immunocompetent-like” cytokine expression profile in patients with adult T-cell leukemia lymphoma. Retrovirology 2013, 10, 91. [Google Scholar] [CrossRef]
- Yasuma, K.; Yasunaga, J.; Takemoto, K.; Sugata, K.; Mitobe, Y.; Takenouchi, N.; Nakagawa, M.; Suzuki, Y.; Matsuoka, M. HTLV-1 bZIP Factor Impairs Anti-viral Immunity by Inducing Co-inhibitory Molecule, T Cell Immunoglobulin and ITIM Domain (TIGIT). PLoS Pathog. 2016, 12, e1005372. [Google Scholar] [CrossRef]
- Datta, A.; Sinha-Datta, U.; Dhillon, N.K.; Buch, S.; Nicot, C. The HTLV-I p30 interferes with TLR4 signaling and modulates the release of pro- and anti-inflammatory cytokines from human macrophages. J. Biol. Chem. 2006, 281, 23414–23424. [Google Scholar] [CrossRef] [PubMed]
- Sabat, R.; Grütz, G.; Warszawska, K.; Kirsch, S.; Witte, E.; Wolk, K.; Geginat, J. Biology of interleukin-10. Cytokine Growth Fact. Rev. 2010, 21, 331–344. [Google Scholar] [CrossRef] [PubMed]
- Blackburn, S.D.; Wherry, E.J. IL-10, T cell exhaustion and viral persistence. Trends Microbiol. 2007, 15, 143–146. [Google Scholar] [CrossRef] [PubMed]
- Lamichhane, P.; Karyampudi, L.; Shreeder, B.; Krempski, J.; Bahr, D.; Daum, J.; Kalli, K.R.; Goode, E.L.; Block, M.S.; Cannon, M.J.; et al. IL10 Release upon PD-1 Blockade Sustains Immunosuppression in Ovarian Cancer. Cancer Res. 2017, 77, 6667–6678. [Google Scholar] [CrossRef] [PubMed]
- Kozako, T.; Yoshimitsu, M.; Fujiwara, H.; Masamoto, I.; Horai, S.; White, Y.; Akimoto, M.; Suzuki, S.; Matsushita, K.; Uozumi, K.; et al. PD-1/PD-L1 expression in human T-cell leukemia virus type 1 carriers and adult T-cell leukemia/lymphoma patients. Leukemia 2009, 23, 375–382. [Google Scholar] [CrossRef]
- Shimauchi, T.; Kabashima, K.; Nakashima, D.; Sugita, K.; Yamada, Y.; Hino, R.; Tokura, Y. Augmented expression of programmed death-1 in both neoplastic and non-neoplastic CD4+ T-cells in adult T-cell leukemia/lymphoma. Int. J. Cancer 2007, 121, 2585–2590. [Google Scholar] [CrossRef]
- Masaki, A.; Ishida, T.; Suzuki, S.; Ito, A.; Narita, T.; Kinoshita, S.; Ri, M.; Kusumoto, S.; Komatsu, H.; Inagaki, H.; et al. Human T-cell lymphotropic/leukemia virus type 1 (HTLV-1) Tax-specific T-cell exhaustion in HTLV-1-infected individuals. Cancer Sci. 2018, 109, 2383–2390. [Google Scholar] [CrossRef]
- Kozako, T.; Arima, N.; Toji, S.; Masamoto, I.; Akimoto, M.; Hamada, H.; Che, X.-F.; Fujiwara, H.; Matsushita, K.; Tokunaga, M.; et al. Reduced frequency, diversity, and function of human T cell leukemia virus type 1-specific CD8+ T cell in adult T cell leukemia patients. J. Immunol. 2006, 177, 5718–5726. [Google Scholar] [CrossRef]
- Espíndola, O.M.; Oliveira, L.C.; Ferreira, P.M.S.; Leite, A.C.C.B.; Lima, M.A.S.D.; Andrada-Serpa, M.J. High IFN-γ/IL-10 expression ratio and increased frequency of persistent human T-cell lymphotropic virus type 1-infected clones are associated with human T-cell lymphotropic virus type 1-associated myelopathy/tropical spastic paraparesis development. Intervirology 2015, 58, 106–114. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Futsch, N.; Prates, G.; Mahieux, R.; Casseb, J.; Dutartre, H. Cytokine Networks Dysregulation during HTLV-1 Infection and Associated Diseases. Viruses 2018, 10, 691. https://doi.org/10.3390/v10120691
Futsch N, Prates G, Mahieux R, Casseb J, Dutartre H. Cytokine Networks Dysregulation during HTLV-1 Infection and Associated Diseases. Viruses. 2018; 10(12):691. https://doi.org/10.3390/v10120691
Chicago/Turabian StyleFutsch, Nicolas, Gabriela Prates, Renaud Mahieux, Jorge Casseb, and Hélène Dutartre. 2018. "Cytokine Networks Dysregulation during HTLV-1 Infection and Associated Diseases" Viruses 10, no. 12: 691. https://doi.org/10.3390/v10120691
APA StyleFutsch, N., Prates, G., Mahieux, R., Casseb, J., & Dutartre, H. (2018). Cytokine Networks Dysregulation during HTLV-1 Infection and Associated Diseases. Viruses, 10(12), 691. https://doi.org/10.3390/v10120691




