Ocular Manifestations of Emerging Flaviviruses and the Blood-Retinal Barrier
Abstract
:1. Introduction
Molecular Pathogenesis of Flaviviruses
2. Flaviviruses and Ocular Complications
2.1. Yellow Fever Virus (YFV)
2.2. Japanese Encephalitis Virus (JEV)
2.3. Kyasanur Forest Disease Virus (KFDV)
2.4. West Nile Virus (WNV)
2.5. Dengue Virus (DENV)
2.6. Zika Virus (ZIKV)
3. Experimental Models of Ocular ZIKV Complications
3.1. In Vivo Models
3.2. In Vitro Models
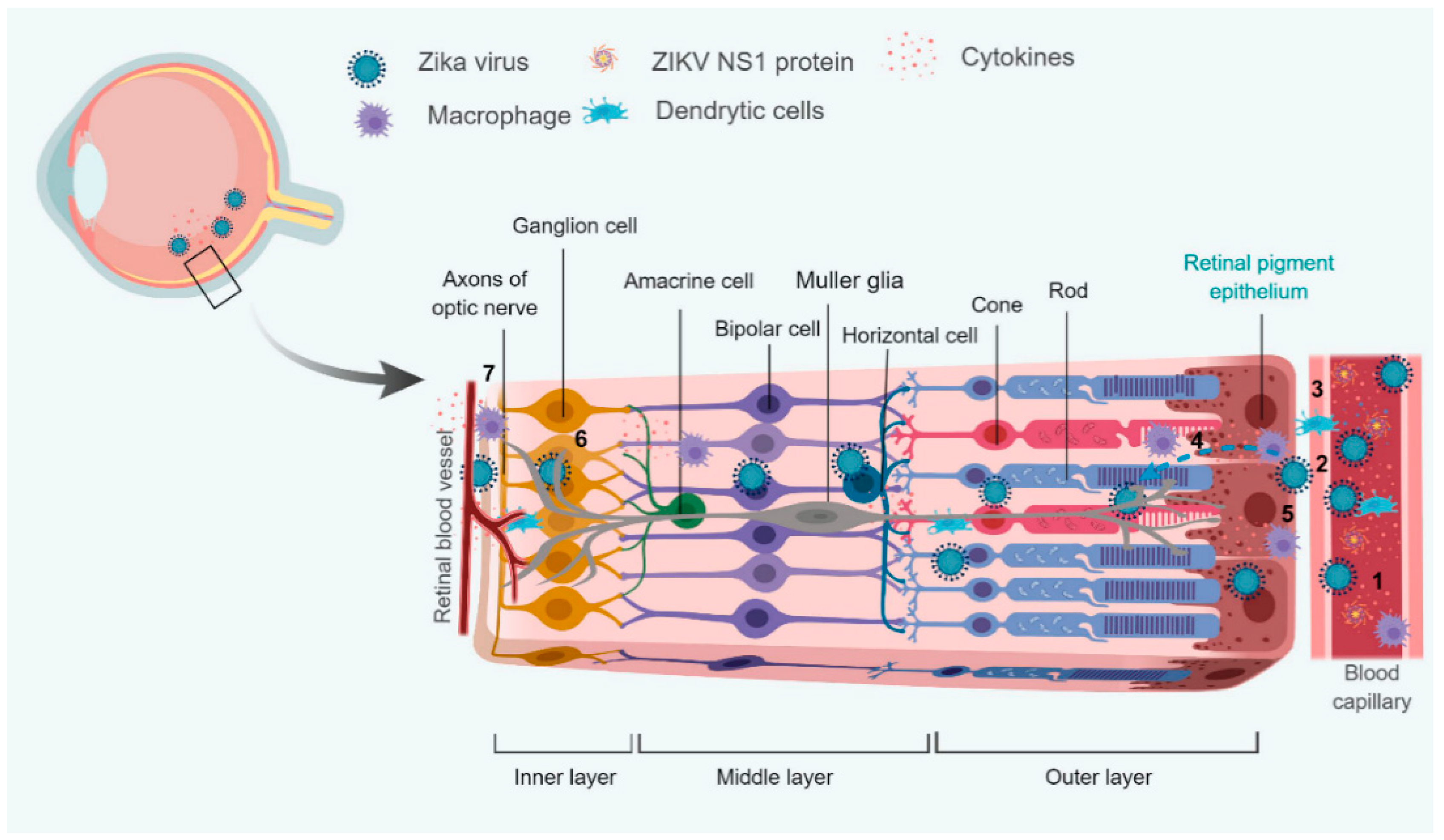
4. Interaction with Blood-Retinal Barrier (BRB)
4.1. Modulation of Retinal Innate and Adaptive Immunity
4.2. Modulation of Cellular Metabolism
5. Conclusions
6. Future Directions
6.1. Possible Involvement of ADE
6.2. Possible Role of Secreted ZIKV NS1 Protein
6.3. Involvement of Immune Response and Cytokine Storm
6.4. Involvement of the Altered Host Machinery
Author Contributions
Funding
Acknowledgments
Disclaimer
Conflicts of Interest
Glossary
| Chorioretinitis | Inflammation of the choroid and retina of the eye. It is a form of posterior uveitis |
| Keratitis | Inflammation of the cornea |
| Iritis | Inflammation of the iris |
| Chorioretinal atrophy | A condition of the eye where both the choroid and retina are damaged. This causes them to wither away and stop working |
| Maculopathy | Any pathological condition of the macula, an area at the center of the retina that is associated with highly sensitive, accurate vision |
| Conjunctivitis | Inflammation of the conjunctiva of the eye |
| Uveitis | Inflammation of the uvea |
| Vitritis | Inflammation of the vitreous body |
| Optic neuritis | Inflammation that damages the optic nerve, a bundle of nerve fibers that transmits visual information from your eye to the brain |
| RPE | Retinal pigment epithelium cells |
| ADE | Antibody-Dependent enhancement |
| Scotoma | A partial loss of vision or a blind spot in an otherwise normal visual field |
| cotton wool spots | Fluffy white patches on the retina. They are caused by damage to nerve fibers and are a result of accumulations of axoplasmic material within the nerve fiber layer |
| Roth’s spot | Retinal hemorrhages with white or pale centers |
| Panophthalmitis | Inflammation of all coats of the eye including intraocular structures |
| iris coloboma | A hole in the iris |
| BRB | Blood-retinal barrier |
References
- Ludwig, G.V.; Iacono-Connors, L.C. Insect-transmitted vertebrate viruses: Flaviviridae. In Vitro Cell. Dev. Biol. Anim. 1993, 29A, 296–309. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Garcia, M.D.; Mazzon, M.; Jacobs, M.; Amara, A. Pathogenesis of flavivirus infections: Using and abusing the host cell. Cell Host Microbe 2009, 5, 318–328. [Google Scholar] [CrossRef] [PubMed]
- Bharucha, T.; Breuer, J. Review: A neglected Flavivirus: An update on Zika virus in 2016 and the future direction of research. Neuropathol. Appl. Neurobiol. 2016, 42, 317–325. [Google Scholar] [CrossRef] [PubMed]
- De Andrade, G.C.; Ventura, C.V.; Mello Filho, P.A.; Maia, M.; Vianello, S.; Rodrigues, E.B. Arboviruses and the eye. Int. J. Retin. Vitreous 2017, 3, 4. [Google Scholar] [CrossRef] [PubMed]
- Sivakumar, R.R.; Prajna, L.; Arya, L.K.; Muraly, P.; Shukla, J.; Saxena, D.; Parida, M. Molecular diagnosis and ocular imaging of West Nile virus retinitis and neuroretinitis. Ophthalmology 2013, 120, 1820–1826. [Google Scholar] [CrossRef] [PubMed]
- Garg, S.; Jampol, L.M. Systemic and intraocular manifestations of West Nile virus infection. Surv. Ophthalmol. 2005, 50, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Alshekhlee, A.; Sultan, B.; Chandar, K. Opsoclonus persisting during sleep in West Nile encephalitis. Arch. Neurol. 2006, 63, 1324–1326. [Google Scholar] [CrossRef] [PubMed]
- Bîrluţiu, V.; Bîrluţiu, R.M. Opsoclonus-myoclonus syndrome attributable to West Nile encephalitis: A case report. J. Med. Case Rep. 2014, 8, 232. [Google Scholar] [CrossRef] [PubMed]
- Monath, T.P.; Vasconcelos, P.F. Yellow fever. J. Clin. Virol. 2015, 64, 160–173. [Google Scholar] [CrossRef] [PubMed]
- Gardner, C.L.; Ryman, K.D. Yellow fever: A reemerging threat. Clin. Lab. Med. 2010, 30, 237–260. [Google Scholar] [CrossRef] [PubMed]
- Voigt, U.; Baum, U.; Behrendt, W.; Hegemann, S.; Terborg, C.; Strobel, J. Neuritis of the optic nerve after vaccinations against hepatitis A.; hepatitis B. and yellow fever. Klinische Monatsblatter Augenheilkunde 2001, 218, 688–690. [Google Scholar] [CrossRef] [PubMed]
- Solomon, T.; Kneen, R.; Dung, N.M.; Khanh, V.C.; Thuy, T.T.; Ha, D.Q.; Day, N.P.; Nisalak, A.; Vaughn, D.W.; White, N.J. Poliomyelitis-like illness due to Japanese encephalitis virus. Lancet 1998, 351, 1094–1097. [Google Scholar] [CrossRef]
- Solomon, T.; Dung, N.M.; Kneen, R.; Gainsborough, M.; Vaughn, D.W.; Khanh, V.T. Japanese encephalitis. J. Neurol. Neurosurg. Psychiatry 2000, 68, 405–415. [Google Scholar] [CrossRef] [PubMed]
- Solomon, T. Flavivirus encephalitis. N. Engl. J. Med. 2004, 351, 370–378. [Google Scholar] [CrossRef] [PubMed]
- Ishii, T.; Matsushita, M.; Hamada, S. Characteristic residual neuropathological features of Japanese, B. encephalitis. Acta Neuropathol. 1977, 38, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, D.; Basu, A. Japanese encephalitis-a pathological and clinical perspective. PLoS Negl. Trop. Dis. 2009, 3, e437. [Google Scholar] [CrossRef] [PubMed]
- Fang, S.T.; Chu, S.Y.; Lee, Y.C. Ischaemic maculopathy in Japanese encephalitis. Eye 2006, 20, 1439–1441. [Google Scholar] [CrossRef] [PubMed]
- Mathur, A.; Khanna, N.; Chaturvedi, U.C. Breakdown of blood-brain barrier by virus-induced cytokine during Japanese encephalitis virus infection. Int. J. Exp. Pathol 1992, 73, 603–611. [Google Scholar] [PubMed]
- Pattnaik, P. Kyasanur forest disease: An epidemiological view in India. Rev. Med. Virol. 2006, 16, 151–165. [Google Scholar] [CrossRef] [PubMed]
- Work, T.H.; Trapido, H.; Murthy, D.P.; Rao, R.L.; Bhatt, P.N.; Kulkarni, K.G. Kyasanur forest disease. III. A preliminary report on the nature of the infection and clinical manifestations in human beings. Indian J. Med. Sci. 1957, 11, 619–645. [Google Scholar] [PubMed]
- Shah, S.Z.; Jabbar, B.; Ahmed, N.; Rehman, A.; Nasir, H.; Nadeem, S.; Jabbar, I.; Rahman, Z.U.; Azam, S. Epidemiology, Pathogenesis, and Control of a Tick-Borne Disease- Kyasanur Forest Disease: Current Status and Future Directions. Front. Cell. Infect. Microbiol. 2018, 8, 149. [Google Scholar] [CrossRef] [PubMed]
- Grard, G.; Moureau, G.; Charrel, R.N.; Lemasson, J.J.; Gonzalez, J.P.; Gallian, P.; Gritsun, T.S.; Holmes, E.C.; Gould, E.A.; de Lamballerie, X. Genetic characterization of tick-borne flaviviruses: New insights into evolution, pathogenetic determinants and taxonomy. Virology 2007, 361, 80–92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rao, R.L. Clinical observations on Kyasanur Forest disease cases. J. Indian Med. Assoc. 1958, 31, 113–116. [Google Scholar] [PubMed]
- Ocular manifestations of Kyasanur forest disease (a clinical study). Indian J. Ophthalmol. 1983, 31, 700–702.
- Boo, Y.L.; Aris, M.A.M.; Chin, P.W.; Sulaiman, W.A.W.; Basri, H.; Hoo, F.K. Guillain-Barré syndrome complicating dengue fever: Two case reports. Ci Ji Yi Xue Za Zhi 2016, 28, 157–159. [Google Scholar] [CrossRef] [PubMed]
- Su, D.H.; Bacsal, K.; Chee, S.P.; Flores, J.V.; Lim, W.K.; Cheng, B.C.; Jap, A.H.; Group, D.M.S. Prevalence of dengue maculopathy in patients hospitalized for dengue fever. Ophthalmology 2007, 114, 1743–1747. [Google Scholar] [CrossRef] [PubMed]
- Chan, D.P.; Teoh, S.C.; Tan, C.S.; Nah, G.K.; Rajagopalan, R.; Prabhakaragupta, M.K.; Chee, C.K.; Lim, T.H.; Goh, K.Y.; Eye Institute Dengue-Related Ophthalmic Complications Workgroup. Ophthalmic complications of dengue. Emerg. Infect. Dis. 2006, 12, 285–289. [Google Scholar] [CrossRef] [PubMed]
- Tabbara, K. Dengue retinochoroiditis. Ann. Saudi Med. 2012, 32, 530–533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beral, L.; Laurence, B.; Merle, H.; Harold, M.; David, T.; Thierry, D. Ocular complications of Dengue fever. Ophthalmology 2008, 115, 1100–1101. [Google Scholar] [PubMed]
- Lei, H.Y.; Yeh, T.M.; Liu, H.S.; Lin, Y.S.; Chen, S.H.; Liu, C.C. Immunopathogenesis of dengue virus infection. J. Biomed. Sci. 2001, 8, 377–388. [Google Scholar] [CrossRef] [PubMed]
- Lim, W.K.; Mathur, R.; Koh, A.; Yeoh, R.; Chee, S.P. Ocular manifestations of dengue fever. Ophthalmology 2004, 111, 2057–2064. [Google Scholar] [CrossRef] [PubMed]
- Donnio, A.; Béral, L.; Olindo, S.; Cabie, A.; Merle, H. Dengue, a new etiology in oculomotor paralysis. Can. J. Ophthalmol. 2010, 45, 183–184. [Google Scholar] [CrossRef] [PubMed]
- Aragão, R.E.; Barreira, I.M.; Lima, L.N.; Rabelo, L.P.; Pereira, F.B. Bilateral optic neuritis after dengue viral infection: Case report. Arq. Bras. Oftalmol. 2010, 73, 175–178. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Villegas, V.; Berrocal, A.M.; Davis, J.L. Bilateral choroidal effusions associated with dengue fever. Retina 2003, 23, 576–578. [Google Scholar] [CrossRef] [PubMed]
- Saranappa, S.B.S.; Sowbhagya, H.N. Panophthalmitis in dengue fever. Indian Pediatr. 2012, 49, 760. [Google Scholar] [CrossRef]
- Ventura, C.V.; Maia, M.; Ventura, B.V.; Linden, V.V.; Araújo, E.B.; Ramos, R.C.; Rocha, M.A.; Carvalho, M.D.; Belfort, R.; Ventura, L.O. Ophthalmological findings in infants with microcephaly and presumable intra-uterus Zika virus infection. Arq. Bras. Oftalmol. 2016, 79, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Ventura, L.O.; Ventura, C.V.; Lawrence, L.; van der Linden, V.; van der Linden, A.; Gois, A.L.; Cavalcanti, M.M.; Barros, E.A.; Dias, N.C.; Berrocal, A.M.; et al. Visual impairment in children with congenital Zika syndrome. J. AAPOS 2017, 21, 295–299. [Google Scholar] [CrossRef] [PubMed]
- Sarno, M.; Sacramento, G.A.; Khouri, R.; do Rosário, M.S.; Costa, F.; Archanjo, G.; Santos, L.A.; Nery, N.; Vasilakis, N.; Ko, A.I.; et al. Zika Virus Infection and Stillbirths: A Case of Hydrops Fetalis, Hydranencephaly and Fetal Demise. PLoS Negl. Trop. Dis. 2016, 10, e0004517. [Google Scholar] [CrossRef] [PubMed]
- Miranda, H.A.; Costa, M.C.; Frazão, M.A.M.; Simão, N.; Franchischini, S.; Moshfeghi, D.M. Expanded Spectrum of Congenital Ocular Findings in Microcephaly with Presumed Zika Infection. Ophthalmology 2016, 123, 1788–1794. [Google Scholar] [CrossRef] [PubMed]
- Miranda-Filho, D.E.B.; Martelli, C.M.; Ximenes, R.A.; Araújo, T.V.; Rocha, M.A.; Ramos, R.C.; Dhalia, R.; França, R.F.; Marques Júnior, E.T.; Rodrigues, L.C. Initial Description of the Presumed Congenital Zika Syndrome. Am. J. Public Health 2016, 106, 598–600. [Google Scholar] [CrossRef] [PubMed]
- Furtado, J.M.; Espósito, D.L.; Klein, T.M.; Teixeira-Pinto, T.; da Fonseca, B.A. Uveitis Associated with Zika Virus Infection. N. Engl. J. Med. 2016, 375, 394–396. [Google Scholar] [CrossRef] [PubMed]
- Kodati, S.; Palmore, T.N.; Spellman, F.A.; Cunningham, D.; Weistrop, B.; Sen, H.N. Bilateral posterior uveitis associated with Zika virus infection. Lancet 2017, 389, 125–126. [Google Scholar] [CrossRef]
- Merle, H.; Najioullah, F.; Chassery, M.; Césaire, R.; Hage, R. Zika-Related Bilateral Hypertensive Anterior Acute Uveitis. JAMA Ophthalmol. 2017, 135, 284–285. [Google Scholar] [CrossRef] [PubMed]
- Monath, T.P. Treatment of yellow fever. Antivir. Res. 2008, 78, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Callender, D.M. Management and control of yellow fever virus: Brazilian outbreak January–April, 2018. Glob. Public Health 2018, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Sanna, A.; Andrieu, A.; Carvalho, L.; Mayence, C.; Tabard, P.; Hachouf, M.; Cazaux, C.M.; Enfissi, A.; Rousset, D.; Kallel, H. Yellow fever cases in French Guiana, evidence of an active circulation in the Guiana Shield, 2017 and 2018. Eurosurveill 2018, 23, 1800471. [Google Scholar] [CrossRef] [PubMed]
- Erlanger, T.E.; Weiss, S.; Keiser, J.; Utzinger, J.; Wiedenmayer, K. Past, present, and future of Japanese encephalitis. Emerg. Infect. Dis. 2009, 15, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, A. Control of Japanese encephalitis in Japan: Immunization of humans and animals, and vector control. Curr. Top. Microbiol. Immunol. 2002, 267, 139–152. [Google Scholar] [PubMed]
- Work, T.H.; Roderiguez, F.R.; Bhatt, P.N. Virological epidemiology of the 1958 epidemic of Kyasanur Forest disease. Am. J. Public Health Nations Health 1959, 49, 869–874. [Google Scholar] [CrossRef] [PubMed]
- Mourya, D.T.; Yadav, P.D.; Mehla, R.; Barde, P.V.; Yergolkar, P.N.; Kumar, S.R.; Thakare, J.P.; Mishra, A.C. Diagnosis of Kyasanur forest disease by nested RT-PCR, real-time RT-PCR and IgM capture ELISA. J. Virol. Methods 2012, 186, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Smithburn, K.C.; Hughes, T.P.; Burke, A.W.; Paul, J.H. A Neurotropic Virus Isolated from the Blood of a Native of Uganda1. Am. J. Trop. Med. Hyg. 1940, 1, 471–492. [Google Scholar] [CrossRef]
- Merle, H.; Donnio, A.; Jean-Charles, A.; Guyomarch, J.; Hage, R.; Najioullah, F.; Césaire, R.; Cabié, A. Ocular manifestations of emerging arboviruses: Dengue fever, Chikungunya, Zika virus, West Nile virus, and yellow fever. J. Fr. Ophtalmol. 2018, 41, e235–e243. [Google Scholar] [CrossRef] [PubMed]
- Abroug, F.; Ouanes-Besbes, L.; Letaief, M.; Ben Romdhane, F.; Khairallah, M.; Triki, H.; Bouzouiaia, N. A cluster study of predictors of severe West Nile virus infection. Mayo Clin. Proc. 2006, 81, 12–16. [Google Scholar] [CrossRef] [PubMed]
- Khairallah, M.; Ben Yahia, S.; Ladjimi, A.; Zeghidi, H.; Ben Romdhane, F.; Besbes, L.; Zaouali, S.; Messaoud, R. Chorioretinal involvement in patients with West Nile virus infection. Ophthalmology 2004, 111, 2065–2070. [Google Scholar] [CrossRef] [PubMed]
- Khairallah, M.; Ben Yahia, S.; Attia, S.; Jelliti, B.; Zaouali, S.; Ladjimi, A. Severe ischemic maculopathy in a patient with West Nile virus infection. Ophthalmic Surg. Lasers Imaging 2006, 37, 240–242. [Google Scholar] [PubMed]
- Yahia, S.B.; Khairallah, M. Ocular manifestations of West Nile virus infection. Int. J. Med. Sci. 2009, 6, 114–115. [Google Scholar] [CrossRef] [PubMed]
- Holmes, E.C.; Twiddy, S.S. The origin, emergence and evolutionary genetics of dengue virus. Infect. Genet. Evol. 2003, 3, 19–28. [Google Scholar] [CrossRef]
- Bhatt, S.; Gething, P.W.; Brady, O.J.; Messina, J.P.; Farlow, A.W.; Moyes, C.L.; Drake, J.M.; Brownstein, J.S.; Hoen, A.G.; Sankoh, O.; et al. The global distribution and burden of dengue. Nature 2013, 496, 504–507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rudolph, K.E.; Lessler, J.; Moloney, R.M.; Kmush, B.; Cummings, D.A. Incubation periods of mosquito-borne viral infections: A systematic review. Am. J. Trop. Med. Hyg. 2014, 90, 882–891. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, M.S.; Rasotgi, V.; Jain, S.; Gupta, V. Discovery of fifth serotype of dengue virus (DENV-5): A new public health dilemma in dengue control. Med. J. Armed Forces India 2015, 71, 67–70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dejnirattisai, W.; Jumnainsong, A.; Onsirisakul, N.; Fitton, P.; Vasanawathana, S.; Limpitikul, W.; Puttikhunt, C.; Edwards, C.; Duangchinda, T.; Supasa, S.; et al. Cross-reacting antibodies enhance dengue virus infection in humans. Science 2010, 328, 745–748. [Google Scholar] [CrossRef] [PubMed]
- Glasner, D.R.; Ratnasiri, K.; Puerta-Guardo, H.; Espinosa, D.A.; Beatty, P.R.; Harris, E. Dengue virus NS1 cytokine-independent vascular leak is dependent on endothelial glycocalyx components. PLoS Pathog. 2017, 13, e1006673. [Google Scholar] [CrossRef] [PubMed]
- Beatty, P.R.; Puerta-Guardo, H.; Killingbeck, S.S.; Glasner, D.R.; Hopkins, K.; Harris, E. Dengue virus NS1 triggers endothelial permeability and vascular leak that is prevented by NS1 vaccination. Sci. Transl. Med. 2015, 7, 304ra141. [Google Scholar] [CrossRef] [PubMed]
- Avirutnan, P.; Punyadee, N.; Noisakran, S.; Komoltri, C.; Thiemmeca, S.; Auethavornanan, K.; Jairungsri, A.; Kanlaya, R.; Tangthawornchaikul, N.; Puttikhunt, C.; et al. Vascular leakage in severe dengue virus infections: A potential role for the nonstructural viral protein NS1 and complement. J. Infect. Dis. 2006, 193, 1078–1088. [Google Scholar] [CrossRef] [PubMed]
- Sirisena, N.; Noordeen, F.; Fernando, L. NS 1 lasts longer than the dengue virus nucleic acid in the clinically suspected patients with dengue fever and dengue haemorrhagic fever. Virusdisease 2017, 28, 341–344. [Google Scholar] [CrossRef] [PubMed]
- Seet, R.C.; Quek, A.M.; Lim, E.C. Symptoms and risk factors of ocular complications following dengue infection. J. Clin. Virol. 2007, 38, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Chee, E.; Sims, J.L.; Jap, A.; Tan, B.H.; Oh, H.; Chee, S.P. Comparison of prevalence of dengue maculopathy during two epidemics with differing predominant serotypes. Am. J. Ophthalmol. 2009, 148, 910–913. [Google Scholar] [CrossRef] [PubMed]
- Carr, J.M.; Ashander, L.M.; Calvert, J.K.; Ma, Y.; Aloia, A.; Bracho, G.G.; Chee, S.P.; Appukuttan, B.; Smith, J.R. Molecular Responses of Human Retinal Cells to Infection with Dengue Virus. Mediat. Inflamm. 2017, 2017, 3164375. [Google Scholar] [CrossRef] [PubMed]
- Guy, B.; Jackson, N. Dengue vaccine: Hypotheses to understand CYD-TDV-induced protection. Nat. Rev. Microbiol. 2016, 14, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Murphy, B.R.; Whitehead, S.S. Immune response to dengue virus and prospects for a vaccine. Annu. Rev. Immunol. 2011, 29, 587–619. [Google Scholar] [CrossRef] [PubMed]
- De Silva, A.M.; Harris, E. Which Dengue Vaccine Approach Is the Most Promising, and Should We Be Concerned about Enhanced Disease after Vaccination? The Path to a Dengue Vaccine: Learning from Human Natural Dengue Infection Studies and Vaccine Trials. Cold Spring Harb. Perspect. Biol. 2018, 10, a029371. [Google Scholar] [CrossRef] [PubMed]
- Dick, G.W.; Kitchen, S.F.; Haddow, A.J. Zika virus. I. Isolations and serological specificity. Trans. R. Soc. Trop. Med. Hyg. 1952, 46, 509–520. [Google Scholar] [CrossRef]
- Macnamara, F.N. Zika virus: A report on three cases of human infection during an epidemic of jaundice in Nigeria. Trans. R. Soc. Trop. Med. Hyg. 1954, 48, 139–145. [Google Scholar] [CrossRef]
- Faye, O.; Freire, C.C.; Iamarino, A.; de Oliveira, J.V.; Diallo, M.; Zanotto, P.M.; Sall, A.A. Molecular evolution of Zika virus during its emergence in the 20(th) century. PLoS Negl. Trop. Dis. 2014, 8, e2636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manangeeswaran, M.; Kielczewski, J.L.; Sen, H.N.; Xu, B.C.; Ireland, D.D.C.; McWilliams, I.L.; Chan, C.C.; Caspi, R.R.; Verthelyi, D. ZIKA virus infection causes persistent chorioretinal lesions. Emerg. Microbes Infect. 2018, 7, 96. [Google Scholar] [CrossRef] [PubMed]
- Miner, J.J.; Diamond, M.S. Zika Virus Pathogenesis and Tissue Tropism. Cell Host Microbe 2017, 21, 134–142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Henry, C.R.; Al-Attar, L.; Cruz-Chacón, A.M.; Davis, J.L. Chorioretinal Lesions Presumed Secondary to Zika Virus Infection in an Immunocompromised Adult. JAMA Ophthalmol. 2017, 135, 386–389. [Google Scholar] [CrossRef] [PubMed]
- Fontes, B.M. Zika virus-related hypertensive iridocyclitis. Arq. Bras. Oftalmol. 2016, 79, 63. [Google Scholar] [CrossRef] [PubMed]
- De Paula Freitas, B.; de Oliveira Dias, J.R.; Prazeres, J.; Sacramento, G.A.; Ko, A.I.; Maia, M.; Belfort, R., Jr. Ocular Findings in Infants With Microcephaly Associated With Presumed Zika Virus Congenital Infection in Salvador, Brazil. JAMA Ophthalmol. 2016, 134, 529–535. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, M.P.; Parra Saad, E.; Ospina Martinez, M.; Corchuelo, S.; Mercado Reyes, M.; Herrera, M.J.; Parra Saavedra, M.; Rico, A.; Fernandez, A.M.; Lee, R.K.; et al. Ocular Histopathologic Features of Congenital Zika Syndrome. JAMA Ophthalmol. 2017, 135, 1163–1169. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Wu, D.; Zhong, H.; Guan, D.; Zhang, H.; Tan, Q.; Ke, C. Presence of Zika Virus in Conjunctival Fluid. JAMA Ophthalmol. 2016, 134, 1330–1332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abbink, P.; Stephenson, K.E.; Barouch, D.H. Zika virus vaccines. Nat. Rev. Microbiol. 2018, 16, 594–600. [Google Scholar] [CrossRef] [PubMed]
- Modjarrad, K.; Lin, L.; George, S.L.; Stephenson, K.E.; Eckels, K.H.; De La Barrera, R.A.; Jarman, R.G.; Sondergaard, E.; Tennant, J.; Ansel, J.L.; et al. Preliminary aggregate safety and immunogenicity results from three trials of a purified inactivated Zika virus vaccine candidate: Phase 1, randomised, double-blind, placebo-controlled clinical trials. Lancet 2018, 391, 563–571. [Google Scholar] [CrossRef]
- Larocca, R.A.; Abbink, P.; Peron, J.P.; Zanotto, P.M.; Iampietro, M.J.; Badamchi-Zadeh, A.; Boyd, M.; Nganga, D.; Kirilova, M.; Nityanandam, R.; et al. Vaccine protection against Zika virus from Brazil. Nature 2016, 536, 474–478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lazear, H.M.; Govero, J.; Smith, A.M.; Platt, D.J.; Fernandez, E.; Miner, J.J.; Diamond, M.S. A Mouse Model of Zika Virus Pathogenesis. Cell Host Microbe 2016, 19, 720–730. [Google Scholar] [CrossRef] [PubMed]
- Rossi, S.L.; Tesh, R.B.; Azar, S.R.; Muruato, A.E.; Hanley, K.A.; Auguste, A.J.; Langsjoen, R.M.; Paessler, S.; Vasilakis, N.; Weaver, S.C. Characterization of a Novel Murine Model to Study Zika Virus. Am. J. Trop. Med. Hyg. 2016, 94, 1362–1369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aliota, M.T.; Caine, E.A.; Walker, E.C.; Larkin, K.E.; Camacho, E.; Osorio, J.E. Characterization of Lethal Zika Virus Infection in AG129 Mice. PLoS Negl. Trop. Dis. 2016, 10, e0004682. [Google Scholar] [CrossRef] [PubMed]
- Miner, J.J.; Sene, A.; Richner, J.M.; Smith, A.M.; Santeford, A.; Ban, N.; Weger-Lucarelli, J.; Manzella, F.; Rückert, C.; Govero, J.; et al. Zika Virus Infection in Mice Causes Panuveitis with Shedding of Virus in Tears. Cell Rep. 2016, 16, 3208–3218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Julander, J.G.; Siddharthan, V.; Evans, J.; Taylor, R.; Tolbert, K.; Apuli, C.; Stewart, J.; Collins, P.; Gebre, M.; Neilson, S.; et al. Efficacy of the broad-spectrum antiviral compound BCX4430 against Zika virus in cell culture and in a mouse model. Antivir. Res. 2017, 137, 14–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zmurko, J.; Marques, R.E.; Schols, D.; Verbeken, E.; Kaptein, S.J.; Neyts, J. The Viral Polymerase Inhibitor 7-Deaza-2′-C-Methyladenosine Is a Potent Inhibitor of In Vitro Zika Virus Replication and Delays Disease Progression in a Robust Mouse Infection Model. PLoS Negl. Trop. Dis. 2016, 10, e0004695. [Google Scholar] [CrossRef] [PubMed]
- Weger-Lucarelli, J.; Duggal, N.K.; Bullard-Feibelman, K.; Veselinovic, M.; Romo, H.; Nguyen, C.; Rückert, C.; Brault, A.C.; Bowen, R.A.; Stenglein, M.; et al. Development and Characterization of Recombinant Virus Generated from a New World Zika Virus Infectious Clone. J. Virol. 2017, 91. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Xu, D.; Ye, Q.; Hong, S.; Jiang, Y.; Liu, X.; Zhang, N.; Shi, L.; Qin, C.F.; Xu, Z. Zika Virus Disrupts Neural Progenitor Development and Leads to Microcephaly in Mice. Cell Stem Cell 2016, 19, 672. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.Y.; Zuo, G.L.; Li, X.F.; Ye, Q.; Deng, Y.Q.; Huang, X.Y.; Cao, W.C.; Qin, C.F.; Luo, Z.G. Vertical transmission of Zika virus targeting the radial glial cells affects cortex development of offspring mice. Cell Res. 2016, 26, 645–654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, P.K.; Guest, J.M.; Kanwar, M.; Boss, J.; Gao, N.; Juzych, M.S.; Abrams, G.W.; Yu, F.S.; Kumar, A. Zika virus infects cells lining the blood-retinal barrier and causes chorioretinal atrophy in mouse eyes. JCI Insight 2017, 2, e92340. [Google Scholar] [CrossRef] [PubMed]
- Van den Pol, A.N.; Mao, G.; Yang, Y.; Ornaghi, S.; Davis, J.N. Zika Virus Targeting in the Developing Brain. J. Neurosci. 2017, 37, 2161–2175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aleman, T.S.; Ventura, C.V.; Cavalcanti, M.M.; Serrano, L.W.; Traband, A.; Nti, A.A.; Gois, A.L.; Bravo-Filho, V.; Martins, T.T.; Nichols, C.W.; et al. Quantitative Assessment of Microstructural Changes of the Retina in Infants With Congenital Zika Syndrome. JAMA Ophthalmol. 2017, 135, 1069–1076. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira Dias, J.R.; Ventura, C.V.; de Paula Freitas, B.; Prazeres, J.; Ventura, L.O.; Bravo-Filho, V.; Aleman, T.; Ko, A.I.; Zin, A.; Belfort, R.; et al. Zika and the Eye: Pieces of a Puzzle. Prog. Retin. Eye Res. 2018. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Yang, M.; Azar, S.R.; Soong, L.; Weaver, S.C.; Sun, J.; Chen, Y.; Rossi, S.L.; Cai, J. Viral Retinopathy in Experimental Models of Zika Infection. Investig. Ophthalmol. Vis. Sci. 2017, 58, 4355–4365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salinas, S.; Erkilic, N.; Damodar, K.; Moles, J.P.; Fournier-Wirth, C.; Van de Perre, P.; Kalatzis, V.; Simonin, Y. Zika Virus Efficiently Replicates in Human Retinal Epithelium and Disturbs Its Permeability. J. Virol. 2017, 91, e02144-16. [Google Scholar] [CrossRef] [PubMed]
- Contreras, D.; Jones, M.; Martinez, L.E.; Gangalapudi, V.; Tang, J.; Wu, Y.; Zhao, J.J.; Chen, Z.; Wang, S.; Arumugaswami, V. Modeling Zika Virus Congenital Eye Disease: Differential Susceptibility of Fetal Retinal Progenitor Cells and iPSC-Derived Retinal Stem Cells to Zika Virus Infection. bioRxiv 2017. [Google Scholar] [CrossRef]
- Fronk, A.H.; Vargis, E. Methods for culturing retinal pigment epithelial cells: A review of current protocols and future recommendations. J. Tissue Eng. 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Coránguez, M.; Ramos, C.; Antonetti, D.A. The inner blood-retinal barrier: Cellular basis and development. Vis. Res. 2017, 139, 123–137. [Google Scholar] [CrossRef] [PubMed]
- Campbell, M.; Humphries, P. The blood-retina barrier: Tight junctions and barrier modulation. Adv. Exp. Med. Biol. 2012, 763, 70–84. [Google Scholar] [PubMed]
- Roach, T.; Alcendor, D.J. Zika virus infection of cellular components of the blood-retinal barriers: Implications for viral associated congenital ocular disease. J. Neuroinflamm. 2017, 14, 43. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.V.; Nagineni, C.N.; Chin, M.S.; Hooks, J.J.; Detrick, B. Innate immunity in the retina: Toll-like receptor (TLR) signaling in human retinal pigment epithelial cells. J. Neuroimmunol. 2004, 153, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Chin, M.S.; Nagineni, C.N.; Hooper, L.C.; Detrick, B.; Hooks, J.J. Cyclooxygenase-2 gene expression and regulation in human retinal pigment epithelial cells. Investig. Ophthalmol. Vis. Sci. 2001, 42, 2338–2346. [Google Scholar]
- Momma, Y.; Nagineni, C.N.; Chin, M.S.; Srinivasan, K.; Detrick, B.; Hooks, J.J. Differential expression of chemokines by human retinal pigment epithelial cells infected with cytomegalovirus. Investig. Ophthalmol. Vis. Sci. 2003, 44, 2026–2033. [Google Scholar] [CrossRef]
- Percopo, C.M.; Hooks, J.J.; Shinohara, T.; Caspi, R.; Detrick, B. Cytokine-mediated activation of a neuronal retinal resident cell provokes antigen presentation. J. Immunol. 1990, 145, 4101–4107. [Google Scholar] [PubMed]
- Perez, V.L.; Caspi, R.R. Immune mechanisms in inflammatory and degenerative eye disease. Trends Immunol. 2015, 36, 354–363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taylor, A.W. Ocular Immune Privilege and Transplantation. Front. Immunol. 2016, 7, 37. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Dawson, R.; Forrester, J.V.; Liversidge, J. Identification of novel dendritic cell populations in normal mouse retina. Investig. Ophthalmol. Vis. Sci. 2007, 48, 1701–1710. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Luo, H.; Liu, H.; Ha, Y.; Mays, E.R.; Lawrence, R.E.; Winkelmann, E.; Barrett, A.D.; Smith, S.B.; Wang, M.; et al. p38MAPK plays a critical role in induction of a pro-inflammatory phenotype of retinal Müller cells following Zika virus infection. Antivir. Res. 2017, 145, 70–81. [Google Scholar] [CrossRef] [PubMed]
- Casazza, R.L.; Lazear, H.M. Antiviral immunity backfires: Pathogenic effects of type I interferon signaling in fetal development. Sci. Immunol. 2018, 3, eaar3446. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.K.; Khatri, I.; Jha, A.; Pretto, C.D.; Spindler, K.R.; Arumugaswami, V.; Giri, S.; Kumar, A.; Bhasin, M.K. Determination of system level alterations in host transcriptome due to Zika virus (ZIKV) Infection in retinal pigment epithelium. Sci. Rep. 2018, 8, 11209. [Google Scholar] [CrossRef] [PubMed]
- Coyaud, E.; Ranadheera, C.; Cheng, D.T.; Goncalves, J.; Dyakov, B.; Laurent, E.; St-Germain, J.R.; Pelletier, L.; Gingras, A.C.; Brumell, J.H.; et al. Global interactomics uncovers extensive organellar targeting by Zika virus. Mol. Cell Proteom. 2018. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Giri, S. 5-Aminoimidazole-4-carboxamide ribonucleoside-mediated adenosine monophosphate-activated protein kinase activation induces protective innate responses in bacterial endophthalmitis. Cell. Microbiol. 2016, 18, 1815–1830. [Google Scholar] [CrossRef] [PubMed]
- Mankouri, J.; Harris, M. Viruses and the fuel sensor: The emerging link between AMPK and virus replication. Rev. Med. Virol. 2011, 21, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, J.; Young, L.H.; Caplan, M.J. AMP-activated protein kinase regulates the assembly of epithelial tight junctions. Proc. Natl. Acad. Sci. USA 2006, 103, 17272–17277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yano, T.; Matsui, T.; Tamura, A.; Uji, M.; Tsukita, S. The association of microtubules with tight junctions is promoted by cingulin phosphorylation by AMPK. J. Cell Biol. 2013, 203, 605–614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jampol, L.M.; Ferris, F.L.; Bishop, R.J. Ebola and the eye. JAMA Ophthalmol. 2015, 133, 1105–1106. [Google Scholar] [CrossRef] [PubMed]
- Dejnirattisai, W.; Supasa, P.; Wongwiwat, W.; Rouvinski, A.; Barba-Spaeth, G.; Duangchinda, T.; Sakuntabhai, A.; Cao-Lormeau, V.M.; Malasit, P.; Rey, F.A.; et al. Dengue virus sero-cross-reactivity drives antibody-dependent enhancement of infection with zika virus. Nat. Immunol. 2016, 17, 1102–1108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawiecki, A.B.; Christofferson, R.C. Zika Virus-Induced Antibody Response Enhances Dengue Virus Serotype 2 Replication In Vitro. J. Infect. Dis. 2016, 214, 1357–1360. [Google Scholar] [CrossRef] [PubMed]
- George, J.; Valiant, W.G.; Mattapallil, M.J.; Walker, M.; Huang, Y.S.; Vanlandingham, D.L.; Misamore, J.; Greenhouse, J.; Weiss, D.E.; Verthelyi, D.; et al. Prior Exposure to Zika Virus Significantly Enhances Peak Dengue-2 Viremia in Rhesus Macaques. Sci. Rep. 2017, 7, 10498. [Google Scholar] [CrossRef] [PubMed]
- Modhiran, N.; Watterson, D.; Muller, D.A.; Panetta, A.K.; Sester, D.P.; Liu, L.; Hume, D.A.; Stacey, K.J.; Young, P.R. Dengue virus NS1 protein activates cells via Toll-like receptor 4 and disrupts endothelial cell monolayer integrity. Sci. Transl. Med. 2015, 7, 304ra142. [Google Scholar] [CrossRef] [PubMed]
- Guabiraba, R.; Ryffel, B. Dengue virus infection: Current concepts in immune mechanisms and lessons from murine models. Immunology 2014, 141, 143–156. [Google Scholar] [CrossRef] [PubMed]
- Mangione, J.N.; Huy, N.T.; Lan, N.T.; Mbanefo, E.C.; Ha, T.T.; Bao, L.Q.; Nga, C.T.; Tuong, V.V.; Dat, T.V.; Thuy, T.T.; et al. The association of cytokines with severe dengue in children. Trop. Med. Health 2014, 42, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Appanna, R.; Wang, S.M.; Ponnampalavanar, S.A.; Lum, L.C.; Sekaran, S.D. Cytokine factors present in dengue patient sera induces alterations of junctional proteins in human endothelial cells. Am. J. Trop. Med. Hyg. 2012, 87, 936–942. [Google Scholar] [CrossRef] [PubMed]
- Rothwell, C.; Lebreton, A.; Young Ng, C.; Lim, J.Y.; Liu, W.; Vasudevan, S.; Labow, M.; Gu, F.; Gaither, L.A. Cholesterol biosynthesis modulation regulates dengue viral replication. Virology 2009, 389, 8–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Apte-Sengupta, S.; Sirohi, D.; Kuhn, R.J. Coupling of replication and assembly in flaviviruses. Curr. Opin. Virol. 2014, 9, 134–142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
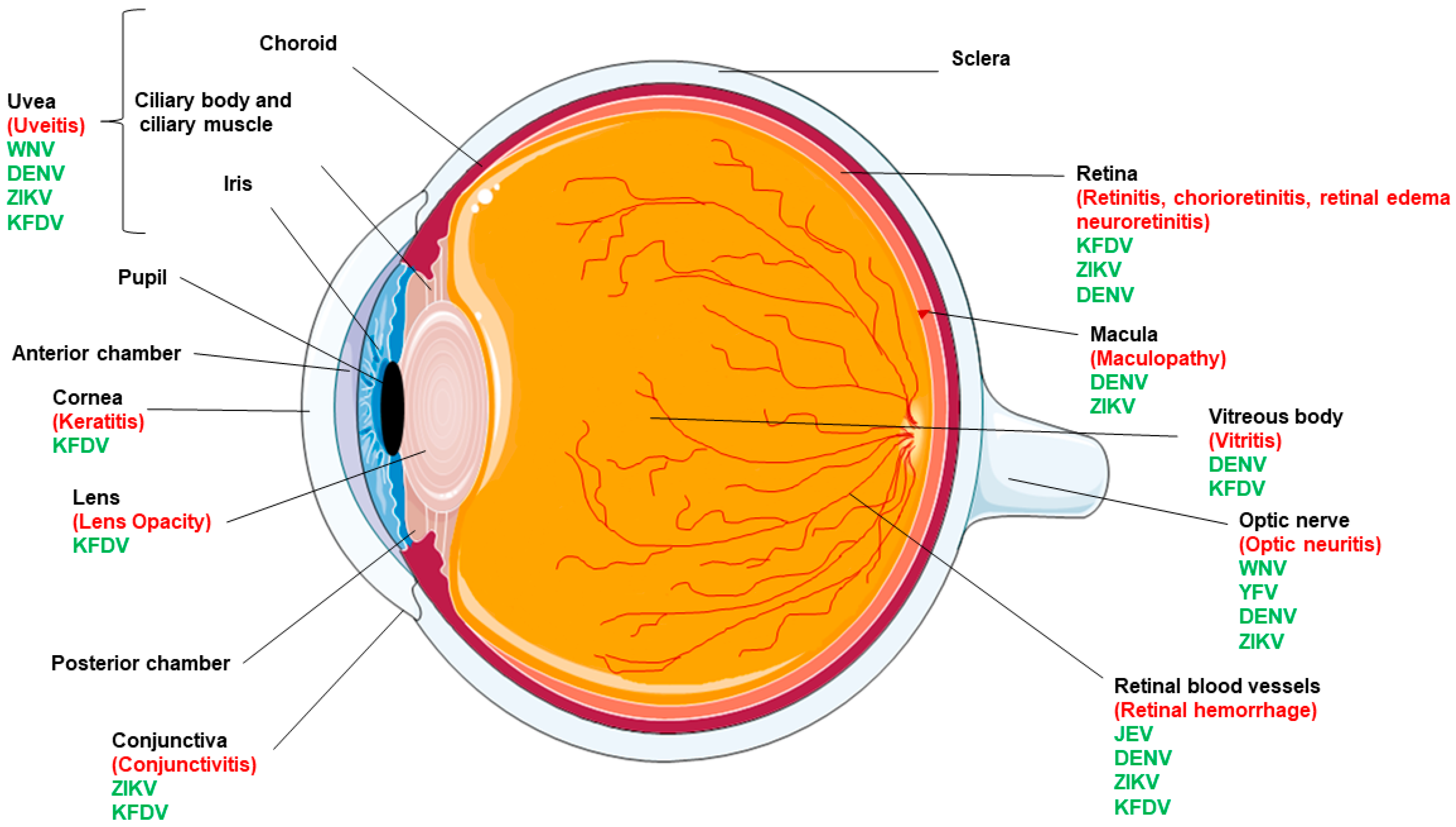
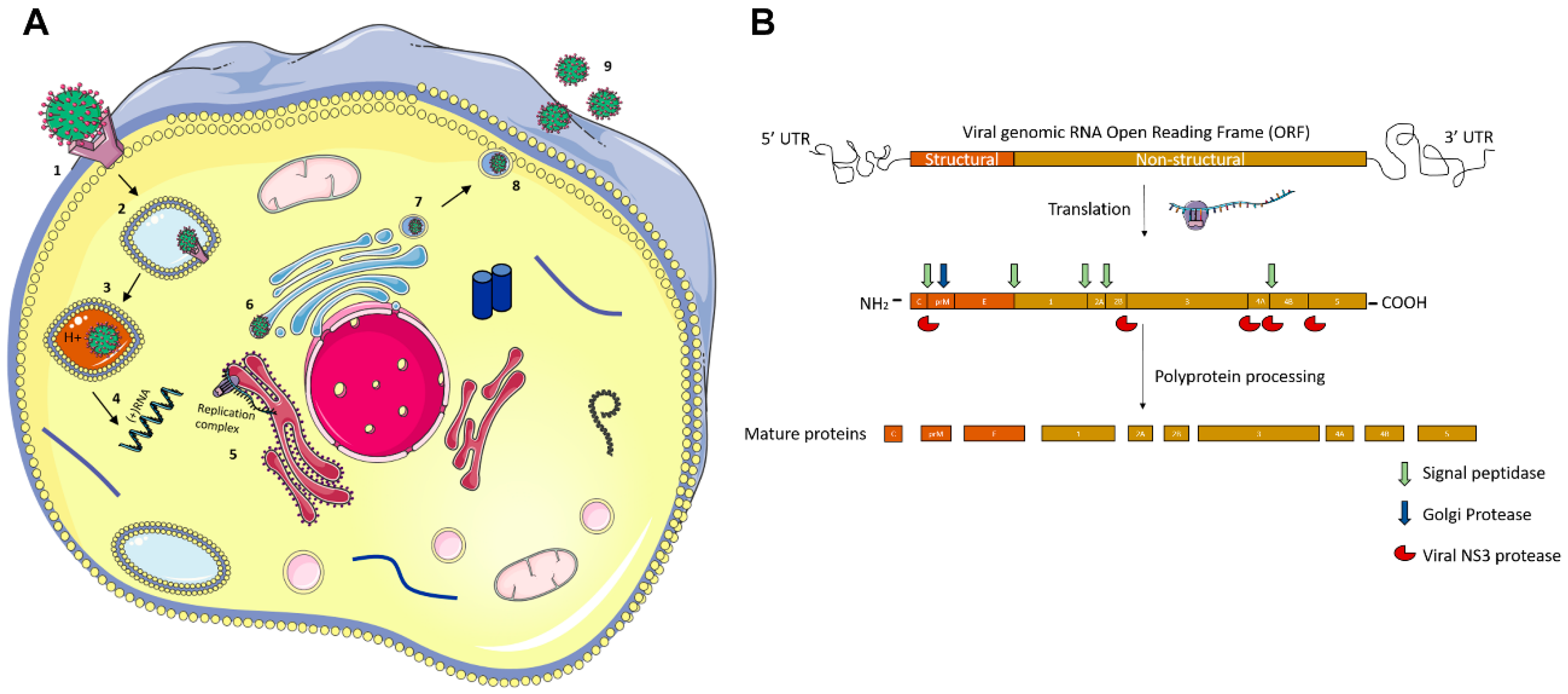
| Ocular Complication | ZIKV | DENV | JEV | WNV | YFV | KFDV |
|---|---|---|---|---|---|---|
| Conjunctivitis/keratitis | + | + | − | − | − | + |
| Macular mottling | + | + | − | − | − | − |
| Chorioretinal atrophy | + focal pigmentary clumping | + | − | − | − | + |
| Optic nerve abnormalities | + | + | − | + | + | − |
| Cataract | Hypoplasia, cupping, pallor | − | − | − | − | − |
| Microphthalmia | + | − | − | − | − | − |
| Iris coloboma | + | − | − | − | − | − |
| Uveitis | + | + | − | + | − | + |
| Chorioretinitis | + | + | − | − | − | + |
| Retinal hemorrhage | + | + | + | − | − | + |
| Virus | General Symptoms | Ocular Disease in Humans | References |
|---|---|---|---|
| West Nile Virus | headache, photophobia, back pain, confusion, fever, encephalitis, meningoencephalitis, acute flaccid paralysis—poliomyelitis-like, Guillain–Barré syndrome | chorioretinitis, anterior uveitis, retinal vasculitis, optic neuritis, and congenital chorioretinal scarring | [5,6,7,8] |
| Yellow Fever Virus | fever, chills, malaise, headache, lower back pain, generalized myalgia, nausea, and dizziness, vomiting, epigastric pain, prostration, and dehydration, petechiae, ecchymoses, epistaxis (bleeding of the gums), and the characteristic “black vomit” (gastrointestinal bleeding) | loss of vision, optic neuritis | [9,10,11] |
| Japanese Encephalitis Virus | Fever, headache, vomiting, fits, encephalitis, and coma | Blurred vision, retinal hemorrhage, ocular fundus | [12,13,14,15,16,17,18] |
| Kyasanur Forest Disease Virus | Frontal headache, fever, hemorrhagic pneumonitis, hepatomegaly and parenchymatic degeneration, nephrosis, characteristic reticulo-endothelial cells in spleen and liver along with leucopenia, thrombocytopenia, reduced red blood cells, bradycardia, meningoencephalitis, hemorrhagic fever manifestations, coma, mental disturbance, giddiness, stiff neck, abnormality of reflexes | hemorrhages in the conjunctiva, vitreous humor, and retina, mild iritis, the opacity of lens and keratitis | [19,20,21,22,23,24] |
| Dengue Virus | Fever, retro-orbital pain, myalgia, thrombocytopenia, severe abdominal pain, persistent vomiting, bleeding gums, restlessness | maculopathy, blurred vision, scotoma, floaters, subconjunctival hemorrhage, uveitis, vitritis, retinal hemorrhaging, retinal venular widening, higher retinal vascular dimension, retinal vascular sheathing, RPE mottling, tortuous vessels, acute macular neuroretinopathy, intraretinal macular, retinal edema, cotton wool spots, Roth’s spot, retinal detachment, retinochoroiditis, neuroretinitis, choroidal effusions, choroidal neovascularization, optic disc swelling and optic disc neuropathy, oculomotor nerve palsy, and panophthalmitis | [25,26,27,28,29,30,31,32,33,34,35] |
| Zika Virus | fever, rash, headache, joint pain, conjunctivitis, muscle pain, and may result in Guillain-Barre syndrome, microcephaly, hearing loss, seizures, impaired joint movement, facial deformities | gross macular pigment mottling, foveal reflex loss, and macular neuroretinal atrophy, chorioretinal atrophy, optic neuritis, retinal hemorrhaging, retinal mottling, iris coloboma, lens subluxation, gross macular pigment mottling, optic nerve hyperplasia, macular chorioretinal atrophy, anterior uveitis, and non-purulent conjunctivitis | [36,37,38,39,40,41,42,43] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, S.; Farr, D.; Kumar, A. Ocular Manifestations of Emerging Flaviviruses and the Blood-Retinal Barrier. Viruses 2018, 10, 530. https://doi.org/10.3390/v10100530
Singh S, Farr D, Kumar A. Ocular Manifestations of Emerging Flaviviruses and the Blood-Retinal Barrier. Viruses. 2018; 10(10):530. https://doi.org/10.3390/v10100530
Chicago/Turabian StyleSingh, Sneha, Dustin Farr, and Ashok Kumar. 2018. "Ocular Manifestations of Emerging Flaviviruses and the Blood-Retinal Barrier" Viruses 10, no. 10: 530. https://doi.org/10.3390/v10100530
APA StyleSingh, S., Farr, D., & Kumar, A. (2018). Ocular Manifestations of Emerging Flaviviruses and the Blood-Retinal Barrier. Viruses, 10(10), 530. https://doi.org/10.3390/v10100530





