HTLV-1, the Other Pathogenic Yet Neglected Human Retrovirus: From Transmission to Therapeutic Treatment
Abstract
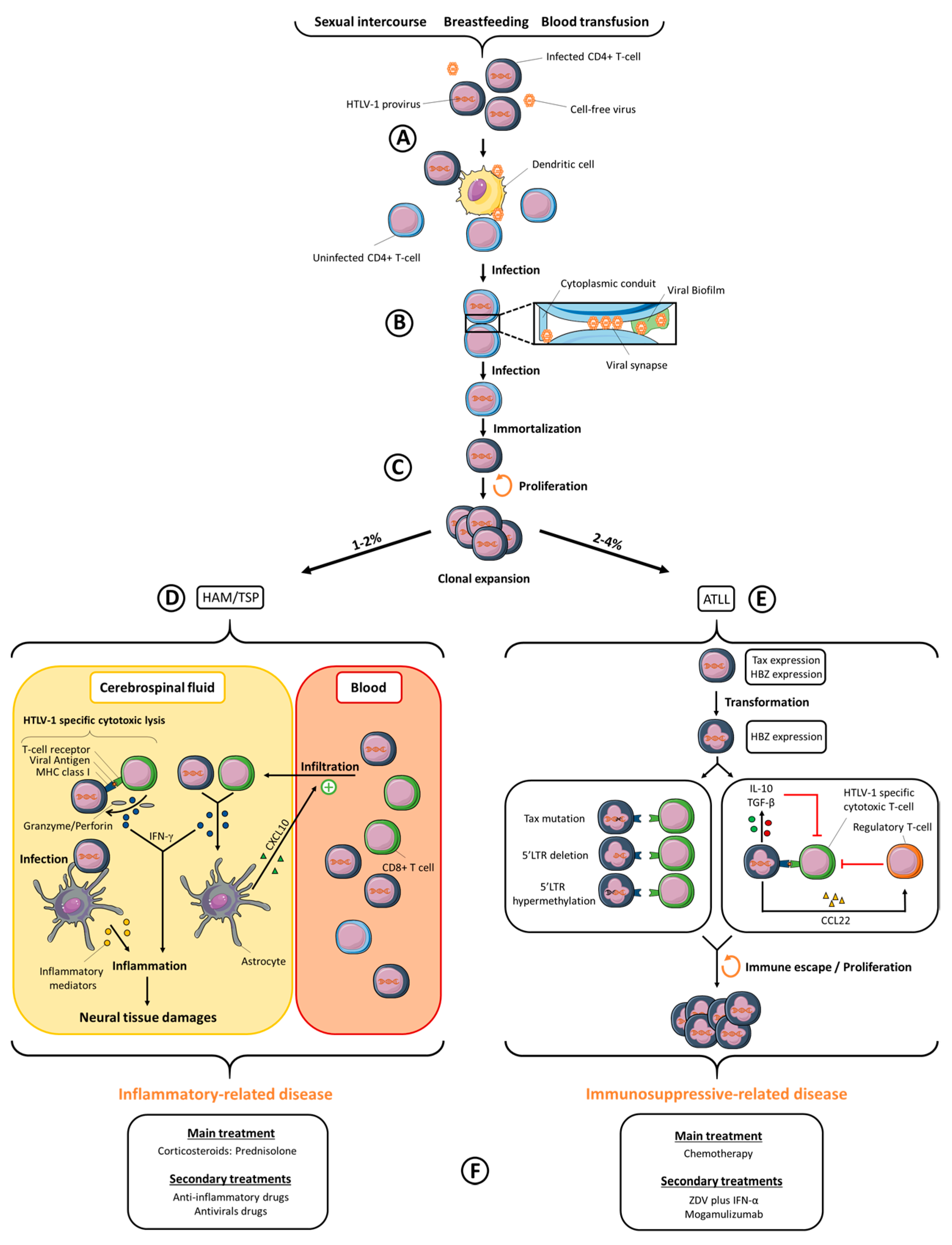
:1. Introduction
2. Inter-Individual Viral Transmission
3. Cellular Transmission of HTLV-1
4. Viral Dissemination
5. HTLV-1-Associated Diseases
5.1. ATLL
5.2. TSP/HAM
5.3. Other Syndromes
6. HIV-1 and HTLV-1 Co-Infections
7. HTLV-1 Treatment and Drug Development
7.1. ATLL
7.2. TSP/HAM
7.3. Other Symptoms
7.4. HIV-1/HTLV-1 Co-Infection
8. Vaccine
9. Conclusions
Acknowledgments
Conflicts of Interest
References
- Poiesz, B.J.; Ruscetti, F.W.; Gazdar, A.F.; Bunn, P.A.; Minna, J.D.; Gallo, R.C. Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc. Natl. Acad. Sci. USA 1980, 77, 7415–7419. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, M.; Miyoshi, I.; Hinuma, Y. Isolation and characterization of retrovirus from cell lines of human adult T-cell leukemia and its implication in the disease. Proc. Natl. Acad. Sci. USA 1982, 79, 2031–2035. [Google Scholar] [CrossRef] [PubMed]
- Barre-Sinoussi, F.; Chermann, J.C.; Rey, F.; Nugeyre, M.T.; Chamaret, S.; Gruest, J.; Dauguet, C.; Axler-Blin, C.; Vezinet-Brun, F.; Rouzioux, C.; et al. Isolation of a T-lymphotropic retrovirus from a patient at risk for acquired immune deficiency syndrome (AIDS). Science 1983, 220, 868–871. [Google Scholar] [CrossRef] [PubMed]
- Keele, B.F.; Van Heuverswyn, F.; Li, Y.; Bailes, E.; Takehisa, J.; Santiago, M.L.; Bibollet-Ruche, F.; Chen, Y.; Wain, L.V.; Liegeois, F.; et al. Chimpanzee reservoirs of pandemic and nonpandemic HIV-1. Science 2006, 313, 523–526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vandamme, A.M.; Salemi, M.; Desmyter, J. The simian origins of the pathogenic human T-cell lymphotropic virus type I. Trends Microbiol. 1998, 6, 477–483. [Google Scholar] [CrossRef]
- De The, G.; Bomford, R. An HTLV-I vaccine: Why, how, for whom? AIDS Res. Hum. Retrovir. 1993, 9, 381–386. [Google Scholar] [CrossRef] [PubMed]
- Gessain, A.; Cassar, O. Epidemiological aspects and world distribution of HTLV-1 infection. Front. Microbiol. 2012, 3, 388. [Google Scholar] [CrossRef] [PubMed]
- Deeks, S.G.; Overbaugh, J.; Phillips, A.; Buchbinder, S. HIV infection. Nat. Rev. Dis. Primers 2015, 1, 15035. [Google Scholar] [CrossRef] [PubMed]
- Becerra, J.C.; Bildstein, L.S.; Gach, J.S. Recent insights into the HIV/AIDS pandemic. Microb. Cell 2016, 3, 451–475. [Google Scholar] [CrossRef] [PubMed]
- Hino, S. Establishment of the milk-borne transmission as a key factor for the peculiar endemicity of human T-lymphotropic virus type 1 (HTLV-1): The ATL prevention program nagasaki. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2011, 87, 152–166. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, J.E.; Khabbaz, R.F.; Murphy, E.L.; Hermansen, S.; Roberts, C.; Lal, R.; Heneine, W.; Wright, D.; Matijas, L.; Thomson, R.; et al. Male-to-female transmission of human T-cell lymphotropic virus types I and II: Association with viral load. The retrovirus epidemiology donor study group. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 1996, 12, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Okochi, K.; Sato, H. Transmission of ATLV (HTLV-I) through blood transfusion. Princess Takamatsu Symp. 1984, 15, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Murphy, E.L. Infection with human T-lymphotropic virus types-1 and -2 (HTLV-1 and -2): Implications for blood transfusion safety. Transfus. Clin. Biol. 2016, 23, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Demontis, M.A.; Sadiq, M.T.; Golz, S.; Taylor, G.P. HTLV-1 viral RNA is detected rarely in plasma of HTLV-1 infected subjects. J. Med. Virol. 2015, 87, 2130–2134. [Google Scholar] [CrossRef] [PubMed]
- Pique, C.; Jones, K.S. Pathways of cell-cell transmission of HTLV-1. Front. Microbiol. 2012, 3, 378. [Google Scholar] [CrossRef] [PubMed]
- Sobata, R.; Matsumoto, C.; Uchida, S.; Suzuki, Y.; Satake, M.; Tadokoro, K. Estimation of the infectious viral load required for transfusion-transmitted human T-lymphotropic virus type 1 infection (TT-HTLV-1) and of the effectiveness of leukocyte reduction in preventing TT-HTLV-1. Vox Sang. 2015, 109, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Satake, M.; Iwanaga, M.; Sagara, Y.; Watanabe, T.; Okuma, K.; Hamaguchi, I. Incidence of human T-lymphotropic virus 1 infection in adolescent and adult blood donors in japan: A nationwide retrospective cohort analysis. Lancet Infect. Dis. 2016, 16, 1246–1254. [Google Scholar] [CrossRef]
- Richardson, J.H.; Edwards, A.J.; Cruickshank, J.K.; Rudge, P.; Dalgleish, A.G. In vivo cellular tropism of human T-cell leukemia virus type 1. J. Virol. 1990, 64, 5682–5687. [Google Scholar] [PubMed]
- Koyanagi, Y.; Itoyama, Y.; Nakamura, N.; Takamatsu, K.; Kira, J.; Iwamasa, T.; Goto, I.; Yamamoto, N. In vivo infection of human T-cell leukemia virus type I in non-T cells. Virology 1993, 196, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Macatonia, S.E.; Cruickshank, J.K.; Rudge, P.; Knight, S.C. Dendritic cells from patients with tropical spastic paraparesis are infected with HTLV-1 and stimulate autologous lymphocyte proliferation. AIDS Res. Hum. Retrovir. 1992, 8, 1699–1706. [Google Scholar] [CrossRef] [PubMed]
- Nagai, M.; Brennan, M.B.; Sakai, J.A.; Mora, C.A.; Jacobson, S. CD8(+) T cells are an in vivo reservoir for human T-cell lymphotropic virus type I. Blood 2001, 98, 1858–1861. [Google Scholar] [CrossRef] [PubMed]
- Campbell, J.H.; Hearps, A.C.; Martin, G.E.; Williams, K.C.; Crowe, S.M. The importance of monocytes and macrophages in HIV pathogenesis, treatment, and cure. AIDS 2014, 28, 2175–2187. [Google Scholar] [CrossRef] [PubMed]
- Kandathil, A.J.; Sugawara, S.; Balagopal, A. Are T cells the only HIV-1 reservoir? Retrovirology 2016, 13, 86. [Google Scholar] [CrossRef] [PubMed]
- McIlroy, D.; Autran, B.; Cheynier, R.; Wain-Hobson, S.; Clauvel, J.P.; Oksenhendler, E.; Debre, P.; Hosmalin, A. Infection frequency of dendritic cells and CD4+ T lymphocytes in spleens of human immunodeficiency virus-positive patients. J. Virol. 1995, 69, 4737–4745. [Google Scholar] [PubMed]
- Pope, M.; Gezelter, S.; Gallo, N.; Hoffman, L.; Steinman, R.M. Low levels of HIV-1 infection in cutaneous dendritic cells promote extensive viral replication upon binding to memory CD4+ T cells. J. Exp. Med. 1995, 182, 2045–2056. [Google Scholar] [CrossRef] [PubMed]
- Dutartre, H.; Claviere, M.; Journo, C.; Mahieux, R. Cell-free versus cell-to-cell infection by human immunodeficiency virus type 1 and human T-lymphotropic virus type 1: Exploring the link among viral source, viral trafficking, and viral replication. J. Virol. 2016, 90, 7607–7617. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Cao, S.; Martin, J.L.; Mueller, J.D.; Mansky, L.M. Morphology and ultrastructure of retrovirus particles. AIMS Biophys. 2015, 2, 343–369. [Google Scholar] [CrossRef] [PubMed]
- Fogarty, K.H.; Berk, S.; Grigsby, I.F.; Chen, Y.; Mansky, L.M.; Mueller, J.D. Interrelationship between cytoplasmic retroviral Gag concentration and Gag-membrane association. J. Mol. Biol. 2014, 426, 1611–1624. [Google Scholar] [CrossRef] [PubMed]
- Alais, S.; Mahieux, R.; Dutartre, H. Viral source-independent high susceptibility of dendritic cells to human T-cell leukemia virus type 1 infection compared to that of T lymphocytes. J. Virol. 2015, 89, 10580–10590. [Google Scholar] [CrossRef] [PubMed]
- Jones, K.S.; Petrow-Sadowski, C.; Huang, Y.K.; Bertolette, D.C.; Ruscetti, F.W. Cell-free HTLV-1 infects dendritic cells leading to transmission and transformation of CD4(+) T cells. Nat. Med. 2008, 14, 429–436. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Zhang, S.; Kronqvist, M.; Wallin, M.; Ekstrom, M.; Derse, D.; Garoff, H. Intersubunit disulfide isomerization controls membrane fusion of human T-cell leukemia virus env. J. Virol. 2008, 82, 7135–7143. [Google Scholar] [CrossRef] [PubMed]
- Shinagawa, M.; Jinno-Oue, A.; Shimizu, N.; Roy, B.B.; Shimizu, A.; Hoque, S.A.; Hoshino, H. Human T-cell leukemia viruses are highly unstable over a wide range of temperatures. J. Gen. Virol. 2012, 93, 608–617. [Google Scholar] [CrossRef] [PubMed]
- Derse, D.; Hill, S.A.; Lloyd, P.A.; Chung, H.; Morse, B.A. Examining human T-lymphotropic virus type 1 infection and replication by cell-free infection with recombinant virus vectors. J. Virol. 2001, 75, 8461–8468. [Google Scholar] [CrossRef] [PubMed]
- Gross, C.; Thoma-Kress, A.K. Molecular mechanisms of HTLV-1 cell-to-cell transmission. Viruses 2016, 8, 74. [Google Scholar] [CrossRef] [PubMed]
- Iwami, S.; Takeuchi, J.S.; Nakaoka, S.; Mammano, F.; Clavel, F.; Inaba, H.; Kobayashi, T.; Misawa, N.; Aihara, K.; Koyanagi, Y.; et al. Cell-to-cell infection by HIV contributes over half of virus infection. Elife 2015, 4. [Google Scholar] [CrossRef] [PubMed]
- Igakura, T.; Stinchcombe, J.C.; Goon, P.K.; Taylor, G.P.; Weber, J.N.; Griffiths, G.M.; Tanaka, Y.; Osame, M.; Bangham, C.R. Spread of HTLV-I between lymphocytes by virus-induced polarization of the cytoskeleton. Science 2003, 299, 1713–1716. [Google Scholar] [CrossRef] [PubMed]
- Pais-Correia, A.M.; Sachse, M.; Guadagnini, S.; Robbiati, V.; Lasserre, R.; Gessain, A.; Gout, O.; Alcover, A.; Thoulouze, M.I. Biofilm-like extracellular viral assemblies mediate HTLV-1 cell-to-cell transmission at virological synapses. Nat. Med. 2010, 16, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Van Prooyen, N.; Gold, H.; Andresen, V.; Schwartz, O.; Jones, K.; Ruscetti, F.; Lockett, S.; Gudla, P.; Venzon, D.; Franchini, G. Human T-cell leukemia virus type 1 p8 protein increases cellular conduits and virus transmission. Proc. Natl. Acad. Sci. USA 2010, 107, 20738–20743. [Google Scholar] [CrossRef] [PubMed]
- Nejmeddine, M.; Barnard, A.L.; Tanaka, Y.; Taylor, G.P.; Bangham, C.R. Human T-lymphotropic virus, type 1, tax protein triggers microtubule reorientation in the virological synapse. J. Biol. Chem. 2005, 280, 29653–29660. [Google Scholar] [CrossRef] [PubMed]
- Chevalier, S.A.; Durand, S.; Dasgupta, A.; Radonovich, M.; Cimarelli, A.; Brady, J.N.; Mahieux, R.; Pise-Masison, C.A. The transcription profile of Tax-3 is more similar to Tax-1 than Tax-2: Insights into HTLV-3 potential leukemogenic properties. PLoS ONE 2012, 7, e41003. [Google Scholar] [CrossRef] [PubMed]
- Mori, N.; Murakami, S.; Oda, S.; Eto, S. Human T-cell leukemia virus type I Tax induces intracellular adhesion molecule-1 expression in T cells. Blood 1994, 84, 350–351. [Google Scholar] [PubMed]
- Nejmeddine, M.; Bangham, C.R. The HTLV-1 virological synapse. Viruses 2010, 2, 1427–1447. [Google Scholar] [CrossRef] [PubMed]
- Hubner, W.; McNerney, G.P.; Chen, P.; Dale, B.M.; Gordon, R.E.; Chuang, F.Y.; Li, X.D.; Asmuth, D.M.; Huser, T.; Chen, B.K. Quantitative 3D video microscopy of hiv transfer across T cell virological synapses. Science 2009, 323, 1743–1747. [Google Scholar] [CrossRef] [PubMed]
- Sattentau, Q.J. Cell-to-cell spread of retroviruses. Viruses 2010, 2, 1306–1321. [Google Scholar] [CrossRef] [PubMed]
- Rudnicka, D.; Feldmann, J.; Porrot, F.; Wietgrefe, S.; Guadagnini, S.; Prevost, M.C.; Estaquier, J.; Haase, A.T.; Sol-Foulon, N.; Schwartz, O. Simultaneous cell-to-cell transmission of human immunodeficiency virus to multiple targets through polysynapses. J. Virol. 2009, 83, 6234–6246. [Google Scholar] [CrossRef] [PubMed]
- Ivanchenko, S.; Godinez, W.J.; Lampe, M.; Krausslich, H.G.; Eils, R.; Rohr, K.; Brauchle, C.; Muller, B.; Lamb, D.C. Dynamics of HIV-1 assembly and release. PLoS Pathog. 2009, 5, e1000652. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jolly, C.; Welsch, S.; Michor, S.; Sattentau, Q.J. The regulated secretory pathway in CD4(+) t cells contributes to human immunodeficiency virus type-1 cell-to-cell spread at the virological synapse. PLoS Pathog. 2011, 7, e1002226. [Google Scholar] [CrossRef] [PubMed]
- Rustom, A.; Saffrich, R.; Markovic, I.; Walther, P.; Gerdes, H.H. Nanotubular highways for intercellular organelle transport. Science 2004, 303, 1007–1010. [Google Scholar] [CrossRef] [PubMed]
- Sherer, N.M.; Lehmann, M.J.; Jimenez-Soto, L.F.; Horensavitz, C.; Pypaert, M.; Mothes, W. Retroviruses can establish filopodial bridges for efficient cell-to-cell transmission. Nat. Cell Biol. 2007, 9, 310–315. [Google Scholar] [CrossRef] [PubMed]
- Malbec, M.; Roesch, F.; Schwartz, O. A new role for the HTLV-1 p8 protein: Increasing intercellular conduits and viral cell-to-cell transmission. Viruses 2011, 3, 254–259. [Google Scholar] [CrossRef] [PubMed]
- Wattel, E.; Cavrois, M.; Gessain, A.; Wain-Hobson, S. Clonal expansion of infected cells: A way of life for HTLV-I. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 1996, 13, S92–S99. [Google Scholar] [CrossRef] [PubMed]
- Matsuoka, M.; Jeang, K.T. Human T-cell leukaemia virus type 1 (HTLV-1) infectivity and cellular transformation. Nat. Rev. Cancer 2007, 7, 270–280. [Google Scholar] [CrossRef] [PubMed]
- Hanon, E.; Hall, S.; Taylor, G.P.; Saito, M.; Davis, R.; Tanaka, Y.; Usuku, K.; Osame, M.; Weber, J.N.; Bangham, C.R. Abundant Tax protein expression in CD4+ T cells infected with human T-cell lymphotropic virus type I (HTLV-I) is prevented by cytotoxic T lymphocytes. Blood 2000, 95, 1386–1392. [Google Scholar] [PubMed]
- Kinpara, S.; Hasegawa, A.; Utsunomiya, A.; Nishitsuji, H.; Furukawa, H.; Masuda, T.; Kannagi, M. Stromal cell-mediated suppression of human T-cell leukemia virus type 1 expression in vitro and in vivo by type I interferon. J. Virol. 2009, 83, 5101–5108. [Google Scholar] [CrossRef] [PubMed]
- Cachat, A.; Chevalier, S.A.; Alais, S.; Ko, N.L.; Ratner, L.; Journo, C.; Dutartre, H.; Mahieux, R. α interferon restricts human T-lymphotropic virus type 1 and 2 de novo infection through PKR activation. J. Virol. 2013, 87, 13386–13396. [Google Scholar] [CrossRef] [PubMed]
- Ilinskaya, A.; Derse, D.; Hill, S.; Princler, G.; Heidecker, G. Cell-cell transmission allows human T-lymphotropic virus 1 to circumvent tetherin restriction. Virology 2013, 436, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Taylor, G.P.; Goon, P.; Furukawa, Y.; Green, H.; Barfield, A.; Mosley, A.; Nose, H.; Babiker, A.; Rudge, P.; Usuku, K.; et al. Zidovudine plus lamivudine in human T-lymphotropic virus type-I-associated myelopathy: A randomised trial. Retrovirology 2006, 3, 63. [Google Scholar] [CrossRef] [PubMed]
- Fox, J.M.; Hilburn, S.; Demontis, M.A.; Brighty, D.W.; Rios Grassi, M.F.; Galvao-Castro, B.; Taylor, G.P.; Martin, F. Long terminal repeat circular DNA as markers of active viral replication of human T lymphotropic virus-1 in vivo. Viruses 2016, 8, 80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Afonso, P.V.; Mekaouche, M.; Mortreux, F.; Toulza, F.; Moriceau, A.; Wattel, E.; Gessain, A.; Bangham, C.R.; Dubreuil, G.; Plumelle, Y.; et al. Highly active antiretroviral treatment against STLV-1 infection combining reverse transcriptase and HDAC inhibitors. Blood 2010, 116, 3802–3808. [Google Scholar] [CrossRef] [PubMed]
- Macchi, B.; Balestrieri, E.; Ascolani, A.; Hilburn, S.; Martin, F.; Mastino, A.; Taylor, G.P. Susceptibility of primary HTLV-1 isolates from patients with HTLV-1-associated myelopathy to reverse transcriptase inhibitors. Viruses 2011, 3, 469–483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Macchi, B.; Faraoni, I.; Zhang, J.; Grelli, S.; Favalli, C.; Mastino, A.; Bonmassar, E. Azt inhibits the transmission of human T cell leukaemia/lymphoma virus type I to adult peripheral blood mononuclear cells in vitro. J. Gen. Virol. 1997, 78, 1007–1016. [Google Scholar] [CrossRef] [PubMed]
- Agosto, L.M.; Uchil, P.D.; Mothes, W. HIV cell-to-cell transmission: Effects on pathogenesis and antiretroviral therapy. Trends Microbiol. 2015, 23, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Turpin, J.; Alais, S.; Marcais, A.; Bruneau, J.; Melamed, A.; Gadot, N.; Tanaka, Y.; Hermine, O.; Melot, S.; Lacoste, R.; et al. Whole body clonality analysis in an aggressive STLV-1 associated leukemia (ATLL) reveals an unexpected clonal complexity. Cancer Lett. 2017, 389, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Villaudy, J.; Wencker, M.; Gadot, N.; Gillet, N.A.; Scoazec, J.Y.; Gazzolo, L.; Manz, M.G.; Bangham, C.R.; Dodon, M.D. HTLV-1 propels thymic human T cell development in “human immune system” Rag2(−)/(−) γ c(−)/(−) mice. PLoS Pathog. 2011, 7, e1002231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Furuta, R.; Yasunaga, J.I.; Miura, M.; Sugata, K.; Saito, A.; Akari, H.; Ueno, T.; Takenouchi, N.; Fujisawa, J.I.; Koh, K.R.; et al. Human T-cell leukemia virus type 1 infects multiple lineage hematopoietic cells in vivo. PLoS Pathog. 2017, 13, e1006722. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo-Redondo, R.; Fryer, H.R.; Bedford, T.; Kim, E.Y.; Archer, J.; Pond, S.L.K.; Chung, Y.S.; Penugonda, S.; Chipman, J.; Fletcher, C.V.; et al. Persistent HIV-1 replication maintains the tissue reservoir during therapy. Nature 2016, 530, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Gillet, N.A.; Malani, N.; Melamed, A.; Gormley, N.; Carter, R.; Bentley, D.; Berry, C.; Bushman, F.D.; Taylor, G.P.; Bangham, C.R. The host genomic environment of the provirus determines the abundance of HTLV-1-infected T-cell clones. Blood 2011, 117, 3113–3122. [Google Scholar] [CrossRef] [PubMed]
- Cook, L.B.; Melamed, A.; Demontis, M.A.; Laydon, D.J.; Fox, J.M.; Tosswill, J.H.; de Freitas, D.; Price, A.D.; Medcalf, J.F.; Martin, F.; et al. Rapid dissemination of human T-lymphotropic virus type 1 during primary infection in transplant recipients. Retrovirology 2016, 13, 3. [Google Scholar] [CrossRef] [PubMed]
- Jain, P.; Manuel, S.L.; Khan, Z.K.; Ahuja, J.; Quann, K.; Wigdahl, B. DC-sign mediates cell-free infection and transmission of human T-cell lymphotropic virus type 1 by dendritic cells. J. Virol. 2009, 83, 10908–10921. [Google Scholar] [CrossRef] [PubMed]
- Rizkallah, G.; Alais, S.; Futsch, N.; Tanaka, Y.; Journo, C.; Mahieux, R.; Dutartre, H. Dendritic cell maturation, but not type I interferon exposure, restricts infection by HTLV-1, and viral transmission to T-cells. PLoS Pathog. 2017, 13, e1006353. [Google Scholar] [CrossRef]
- Ali, A.; Patterson, S.; Cruickshank, K.; Rudge, P.; Dalgleish, A.G.; Knight, S.C. Dendritic cells infected in vitro with human T cell leukaemia/lymphoma virus type-1 (HTLV-1); enhanced lymphocytic proliferation and tropical spastic paraparesis. Clin. Exp. Immunol. 1993, 94, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Makino, M.; Shimokubo, S.; Wakamatsu, S.I.; Izumo, S.; Baba, M. The role of human T-lymphotropic virus type 1 (HTLV-1)-infected dendritic cells in the development of HTLV-1-associated myelopathy/tropical spastic paraparesis. J. Virol. 1999, 73, 4575–4581. [Google Scholar] [PubMed]
- Rahman, S.; Manuel, S.L.; Khan, Z.K.; Wigdahl, B.; Acheampong, E.; Tangy, F.; Jain, P. Depletion of dendritic cells enhances susceptibility to cell-free infection of human t cell leukemia virus type 1 in CD11C-diphtheria toxin receptor transgenic mice. J. Immunol. 2010, 184, 5553–5561. [Google Scholar] [CrossRef] [PubMed]
- Valeri, V.W.; Hryniewicz, A.; Andresen, V.; Jones, K.; Fenizia, C.; Bialuk, I.; Chung, H.K.; Fukumoto, R.; Parks, R.W.; Ferrari, M.G.; et al. Requirement of the human T-cell leukemia virus p12 and p30 products for infectivity of human dendritic cells and macaques but not rabbits. Blood 2010, 116, 3809–3817. [Google Scholar] [CrossRef] [PubMed]
- Bangham, C.R.; Cook, L.B.; Melamed, A. HTLV-1 clonality in adult T-cell leukaemia and non-malignant HTLV-1 infection. Semin. Cancer Biol. 2014, 26, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, C.; Gotuzzo, E.; Vandamme, A.M.; Verdonck, K. Family aggregation of human T-lymphotropic virus 1-associated diseases: A systematic review. Front. Microbiol. 2016, 7, 1674. [Google Scholar] [CrossRef] [PubMed]
- Osame, M.; Janssen, R.; Kubota, H.; Nishitani, H.; Igata, A.; Nagataki, S.; Mori, M.; Goto, I.; Shimabukuro, H.; Khabbaz, R.; et al. Nationwide survey of HTLV-I-associated myelopathy in japan: Association with blood transfusion. Ann. Neurol. 1990, 28, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Tsukasaki, K.; Tobinai, K. Clinical trials and treatment of ATL. Leuk. Res. Treat. 2012, 2012, 101754. [Google Scholar] [CrossRef] [PubMed]
- Uchiyama, T.; Yodoi, J.; Sagawa, K.; Takatsuki, K.; Uchino, H. Adult T-cell leukemia: Clinical and hematologic features of 16 cases. Blood 1977, 50, 481–492. [Google Scholar] [CrossRef]
- Shimoyama, M. Diagnostic criteria and classification of clinical subtypes of adult T-cell leukaemia-lymphoma. A report from the lymphoma study group (1984–1987). Br. J. Haematol. 1991, 79, 428–437. [Google Scholar] [CrossRef] [PubMed]
- Katsuya, H.; Ishitsuka, K.; Utsunomiya, A.; Hanada, S.; Eto, T.; Moriuchi, Y.; Saburi, Y.; Miyahara, M.; Sueoka, E.; Uike, N.; et al. Treatment and survival among 1594 patients with ATL. Blood 2015, 126, 2570–2577. [Google Scholar] [CrossRef] [PubMed]
- Mehta-Shah, N.; Ratner, L.; Horwitz, S.M. Adult T-cell leukemia/lymphoma. J. Oncol. Pract. 2017, 13, 487–492. [Google Scholar] [CrossRef] [PubMed]
- Dahmoush, L.; Hijazi, Y.; Barnes, E.; Stetler-Stevenson, M.; Abati, A. Adult t-cell leukemia/lymphoma: A cytopathologic, immunocytochemical, and flow cytometric study. Cancer 2002, 96, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Kagdi, H.H.; Demontis, M.A.; Fields, P.A.; Ramos, J.C.; Bangham, C.R.; Taylor, G.P. Risk stratification of adult T-cell leukemia/lymphoma using immunophenotyping. Cancer Med. 2017, 6, 298–309. [Google Scholar] [CrossRef] [PubMed]
- Yoshie, O.; Fujisawa, R.; Nakayama, T.; Harasawa, H.; Tago, H.; Izawa, D.; Hieshima, K.; Tatsumi, Y.; Matsushima, K.; Hasegawa, H.; et al. Frequent expression of CCR4 in adult T-cell leukemia and human T-cell leukemia virus type 1-transformed T cells. Blood 2002, 99, 1505–1511. [Google Scholar] [CrossRef] [PubMed]
- Nakahata, S.; Morishita, K. CADM1/TSLC1 is a novel cell surface marker for adult T-cell leukemia/lymphoma. J. Clin. Exp. Hematop. 2012, 52, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, S.; Nakano, K.; Watanabe, E.; Ishigaki, T.; Ohno, N.; Yuji, K.; Oyaizu, N.; Asanuma, S.; Yamagishi, M.; Yamochi, T.; et al. CADM1 expression and stepwise downregulation of CD7 are closely associated with clonal expansion of HTLV-I-infected cells in adult T-cell leukemia/lymphoma. Clin. Cancer Res. 2014, 20, 2851–2861. [Google Scholar] [CrossRef] [PubMed]
- Afonso, P.V.; Zamborlini, A.; Saib, A.; Mahieux, R. Centrosome and retroviruses: The dangerous liaisons. Retrovirology 2007, 4, 27. [Google Scholar] [CrossRef] [PubMed]
- Cook, L.; Melamed, A.; Yaguchi, H.; Bangham, C.R. The impact of HTLV-1 on the cellular genome. Curr. Opin. Virol. 2017, 26, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Kogure, Y.; Kataoka, K. Genetic alterations in adult T-cell leukemia/lymphoma. Cancer Sci. 2017, 108, 1719–1725. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T. Adult T-cell leukemia: Molecular basis for clonal expansion and transformation of HTLV-1-infected T cells. Blood 2017, 129, 1071–1081. [Google Scholar] [CrossRef] [PubMed]
- Currer, R.; Van Duyne, R.; Jaworski, E.; Guendel, I.; Sampey, G.; Das, R.; Narayanan, A.; Kashanchi, F. HTLV Tax: A fascinating multifunctional co-regulator of viral and cellular pathways. Front. Microbiol. 2012, 3, 406. [Google Scholar] [CrossRef] [PubMed]
- Journo, C.; Douceron, E.; Mahieux, R. HTLV gene regulation: Because size matters, transcription is not enough. Future Microbiol. 2009, 4, 425–440. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, K.; Shibata, H.; Fujisawa, J.I.; Inoue, H.; Hakura, A.; Tsukahara, T.; Fujii, M. Human T-cell leukemia virus type 1 Tax protein transforms rat fibroblasts via two distinct pathways. J. Virol. 1997, 71, 4445–4451. [Google Scholar] [PubMed]
- Tanaka, A.; Takahashi, C.; Yamaoka, S.; Nosaka, T.; Maki, M.; Hatanaka, M. Oncogenic transformation by the tax gene of human T-cell leukemia virus type I in vitro. Proc. Natl. Acad. Sci. USA 1990, 87, 1071–1075. [Google Scholar] [CrossRef] [PubMed]
- Shirinian, M.; Kambris, Z.; Hamadeh, L.; Grabbe, C.; Journo, C.; Mahieux, R.; Bazarbachi, A. A transgenic drosophila melanogaster model to study human T-lymphotropic virus oncoprotein Tax-1-driven transformation in vivo. J. Virol. 2015, 89, 8092–8095. [Google Scholar] [CrossRef] [PubMed]
- Niewiesk, S. Animals models of human t cell leukemia virus type I leukemogenesis. ILAR J. 2016, 57, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Bellon, M.; Baydoun, H.H.; Yao, Y.; Nicot, C. HTLV-I Tax-dependent and -independent events associated with immortalization of human primary T lymphocytes. Blood 2010, 115, 2441–2448. [Google Scholar] [CrossRef] [PubMed]
- Aboud, M.; Golde, D.W.; Bersch, N.; Rosenblatt, J.D.; Chen, I.S. A colony assay for in vitro transformation by human T cell leukemia viruses type I and type II. Blood 1987, 70, 432–436. [Google Scholar] [PubMed]
- Satou, Y.; Yasunaga, J.; Zhao, T.; Yoshida, M.; Miyazato, P.; Takai, K.; Shimizu, K.; Ohshima, K.; Green, P.L.; Ohkura, N.; et al. HTLV-1 bzip factor induces T-cell lymphoma and systemic inflammation in vivo. PLoS Pathog. 2011, 7, e1001274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takeda, S.; Maeda, M.; Morikawa, S.; Taniguchi, Y.; Yasunaga, J.; Nosaka, K.; Tanaka, Y.; Matsuoka, M. Genetic and epigenetic inactivation of Tax gene in adult T-cell leukemia cells. Int. J. Cancer 2004, 109, 559–567. [Google Scholar] [CrossRef] [PubMed]
- Koiwa, T.; Hamano-Usami, A.; Ishida, T.; Okayama, A.; Yamaguchi, K.; Kamihira, S.; Watanabe, T. 5′-long terminal repeat-selective cpg methylation of latent human T-cell leukemia virus type 1 provirus in vitro and in vivo. J. Virol. 2002, 76, 9389–9397. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, M.; Yasunaga, J.; Taniguchi, Y.; Tamiya, S.; Nakahata, T.; Matsuoka, M. Preferential selection of human T-cell leukemia virus type 1 provirus lacking the 5’ long terminal repeat during oncogenesis. J. Virol. 2007, 81, 5714–5723. [Google Scholar] [CrossRef] [PubMed]
- Satou, Y.; Yasunaga, J.; Yoshida, M.; Matsuoka, M. HTLV-I basic leucine zipper factor gene mrna supports proliferation of adult T cell leukemia cells. Proc. Natl. Acad. Sci. USA 2006, 103, 720–725. [Google Scholar] [CrossRef] [PubMed]
- Giam, C.Z.; Semmes, O.J. HTLV-1 infection and adult T-cell leukemia/lymphoma-a tale of two proteins: Tax and hbz. Viruses 2016, 8, 161. [Google Scholar] [CrossRef] [PubMed]
- Ma, G.; Yasunaga, J.; Matsuoka, M. Multifaceted functions and roles of HBZ in HTLV-1 pathogenesis. Retrovirology 2016, 13, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mesnard, J.M.; Barbeau, B.; Cesaire, R.; Peloponese, J.M. Roles of HTLV-1 basic zip factor (HBZ) in viral chronicity and leukemic transformation. Potential new therapeutic approaches to prevent and treat HTLV-1-related diseases. Viruses 2015, 7, 6490–6505. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto-Taguchi, N.; Satou, Y.; Miyazato, P.; Ohshima, K.; Nakagawa, M.; Katagiri, K.; Kinashi, T.; Matsuoka, M. HTLV-1 BZIP factor induces inflammation through labile foxp3 expression. PLoS Pathog. 2013, 9, e1003630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, L.; Liu, D.; Zhang, Y.; Zhang, H.; Cheng, H. Foxp3-dependent transformation of human primary CD4+ T lymphocytes by the retroviral protein Tax. Biochem. Biophys. Res. Commun. 2015, 466, 523–529. [Google Scholar] [CrossRef] [PubMed]
- Kannagi, M.; Hasegawa, A.; Takamori, A.; Kinpara, S.; Utsunomiya, A. The roles of acquired and innate immunity in human T-cell leukemia virus type 1-mediated diseases. Front. Microbiol. 2012, 3, 323. [Google Scholar] [CrossRef] [PubMed]
- Takamori, A.; Hasegawa, A.; Utsunomiya, A.; Maeda, Y.; Yamano, Y.; Masuda, M.; Shimizu, Y.; Tamai, Y.; Sasada, A.; Zeng, N.; et al. Functional impairment of Tax-specific but not cytomegalovirus-specific CD8+ T lymphocytes in a minor population of asymptomatic human T-cell leukemia virus type 1-carriers. Retrovirology 2011, 8, 100. [Google Scholar] [CrossRef] [PubMed]
- Mori, N.; Gill, P.S.; Mougdil, T.; Murakami, S.; Eto, S.; Prager, D. Interleukin-10 gene expression in adult T-cell leukemia. Blood 1996, 88, 1035–1045. [Google Scholar] [CrossRef] [PubMed]
- Niitsu, Y.; Urushizaki, Y.; Koshida, Y.; Terui, K.; Mahara, K.; Kohgo, Y.; Urushizaki, I. Expression of TGF-β gene in adult T cell leukemia. Blood 1988, 71, 263–266. [Google Scholar] [PubMed]
- Toulza, F.; Nosaka, K.; Tanaka, Y.; Schioppa, T.; Balkwill, F.; Taylor, G.P.; Bangham, C.R. Human T-lymphotropic virus type 1-induced cc chemokine ligand 22 maintains a high frequency of functional Foxp3+ regulatory t cells. J. Immunol. 2010, 185, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Bangham, C.R.; Toulza, F. Adult t cell leukemia/lymphoma: Foxp3(+) cells and the cell-mediated immune response to HTLV-1. Adv. Cancer Res. 2011, 111, 163–182. [Google Scholar] [PubMed]
- Kannagi, M.; Shida, H.; Igarashi, H.; Kuruma, K.; Murai, H.; Aono, Y.; Maruyama, I.; Osame, M.; Hattori, T.; Inoko, H.; et al. Target epitope in the tax protein of human T-cell leukemia virus type I recognized by class I major histocompatibility complex-restricted cytotoxic t cells. J. Virol. 1992, 66, 2928–2933. [Google Scholar] [PubMed]
- Tagaya, Y.; Gallo, R.C. The exceptional oncogenicity of HTLV-1. Front. Microbiol. 2017, 8, 1425. [Google Scholar] [CrossRef] [PubMed]
- Virgin, H.W.; Wherry, E.J.; Ahmed, R. Redefining chronic viral infection. Cell 2009, 138, 30–50. [Google Scholar] [CrossRef] [PubMed]
- Gessain, A.; Barin, F.; Vernant, J.C.; Gout, O.; Maurs, L.; Calender, A.; de The, G. Antibodies to human T-lymphotropic virus type-I in patients with tropical spastic paraparesis. Lancet 1985, 2, 407–410. [Google Scholar] [CrossRef]
- Osame, M.; Usuku, K.; Izumo, S.; Ijichi, N.; Amitani, H.; Igata, A.; Matsumoto, M.; Tara, M. HTLV-I associated myelopathy, a new clinical entity. Lancet 1986, 1, 1031–1032. [Google Scholar] [CrossRef]
- Kubota, R.; Soldan, S.S.; Martin, R.; Jacobson, S. Selected cytotoxic T lymphocytes with high specificity for HTLV-I in cerebrospinal fluid from a HAM/TSP patient. J. Neurovirol. 2002, 8, 53–57. [Google Scholar] [CrossRef] [PubMed]
- Nagai, M.; Yamano, Y.; Brennan, M.B.; Mora, C.A.; Jacobson, S. Increased HTLV-I proviral load and preferential expansion of HTLV-I tax-specific CD8+ T cells in cerebrospinal fluid from patients with HAM/TSP. Ann. Neurol. 2001, 50, 807–812. [Google Scholar] [CrossRef] [PubMed]
- Bangham, C.R.; Araujo, A.; Yamano, Y.; Taylor, G.P. HTLV-1-associated myelopathy/tropical spastic paraparesis. Nat. Rev. Dis. Primers 2015, 1, 15012. [Google Scholar] [CrossRef] [PubMed]
- Goon, P.K.; Hanon, E.; Igakura, T.; Tanaka, Y.; Weber, J.N.; Taylor, G.P.; Bangham, C.R. High frequencies of Th1-type CD4(+) T cells specific to HTLV-1 env and tax proteins in patients with HTLV-1-associated myelopathy/tropical spastic paraparesis. Blood 2002, 99, 3335–3341. [Google Scholar] [CrossRef] [PubMed]
- Yamano, Y.; Coler-Reilly, A. HTLV-1 induces a Th1-like state in CD4+CCR4+ T cells that produces an inflammatory positive feedback loop via astrocytes in HAM/TSP. J. Neuroimmunol. 2017, 304, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Tattermusch, S.; Skinner, J.A.; Chaussabel, D.; Banchereau, J.; Berry, M.P.; McNab, F.W.; O’Garra, A.; Taylor, G.P.; Bangham, C.R. Systems biology approaches reveal a specific interferon-inducible signature in HTLV-1 associated myelopathy. PLoS Pathog. 2012, 8, e1002480. [Google Scholar] [CrossRef] [PubMed]
- Goncalves, D.U.; Proietti, F.A.; Ribas, J.G.; Araujo, M.G.; Pinheiro, S.R.; Guedes, A.C.; Carneiro-Proietti, A.B. Epidemiology, treatment, and prevention of human T-cell leukemia virus type 1-associated diseases. Clin. Microbiol. Rev. 2010, 23, 577–589. [Google Scholar] [CrossRef] [PubMed]
- Kamoi, K.; Mochizuki, M. HTLV-1 uveitis. Front. Microbiol. 2012, 3, 270. [Google Scholar] [CrossRef] [PubMed]
- Hlela, C.; Bittencourt, A. Infective dermatitis associated with HTLV-1 mimics common eczemas in children and may be a prelude to severe systemic diseases. Dermatol. Clin. 2014, 32, 237–248. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, P.D.; Kachimarek, A.C.; Bittencourt, A.L. Early onset of HTLV-1 associated myelopathy/tropical spastic paraparesis (HAM/TSP) and adult T-cell leukemia/lymphoma (ATL): Systematic search and review. J. Trop. Pediatr. 2017. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, M.C.; Primo, J.; Bittencourt, A.; Siqueira, I.; de Fatima Oliveira, M.; Meyer, R.; Schriefer, A.; Santos, S.B.; Carvalho, E.M. Infective dermatitis has similar immunological features to human T lymphotropic virus-type 1-associated myelopathy/tropical spastic paraparesis. Clin. Exp. Immunol. 2009, 156, 455–462. [Google Scholar] [CrossRef] [PubMed]
- McKendall, R.R. Neurologic disease due to HTLV-1 infection. Handb. Clin. Neurol. 2014, 123, 507–530. [Google Scholar] [PubMed]
- Robert-Guroff, M.; Blayney, D.W.; Safai, B.; Lange, M.; Gelmann, E.P.; Gutterman, J.W.; Mansell, P.W.; Goedert, J.L.; Groopman, J.E.; Steigbigel, N.H.; et al. HTLV-I-specific antibody in aids patients and others at risk. Lancet 1984, 2, 128–131. [Google Scholar] [CrossRef]
- Beilke, M.A. Retroviral coinfections: HIV and HTLV: Taking stock of more than a quarter century of research. AIDS Res. Hum. Retrovir. 2012, 28, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Augusto, A.; Augusto, O.; Taquimo, A.; Nhachigule, C.; Siyawadya, N.; Tembe, N.; Bhatt, N.; Mbofana, F.; Gudo, E.S. First description of HTLV-1/2 seroprevalence in HIV-infected inmates in mozambique. J. Med. Virol. 2017, 89, 1498–1502. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, E.H.; Oliveira-Filho, A.B.; Souza, L.A.; da Silva, L.V.; Ishak, M.O.; Ishak, R.; Vallinoto, A.C. Human T-cell lymphotropic virus in patients infected with HIV-1: Molecular epidemiology and risk factors for transmission in piaui, northeastern Brazil. Curr. HIV Res. 2012, 10, 700–707. [Google Scholar] [CrossRef] [PubMed]
- Galetto, L.R.; Lunge, V.R.; Beria, J.U.; Tietzmann, D.C.; Stein, A.T.; Simon, D. Short communication: Prevalence and risk factors for human T cell lymphotropic virus infection in southern brazilian HIV-positive patients. AIDS Res. Hum. Retrovir. 2014, 30, 907–911. [Google Scholar] [CrossRef] [PubMed]
- Gudo, E.S.; Abreu, C.M.; Mussa, T.; Augusto Ado, R.; Otsuki, K.; Chambo, E.; Amade, N.; Tanuri, A.; Ferreira, O.C., Jr.; Jani, I.V.; et al. Serologic and molecular typing of human T-lymphotropic virus among blood donors in maputo city, mozambique. Transfusion 2009, 49, 1146–1150. [Google Scholar] [CrossRef] [PubMed]
- Kozlowski, A.G.; Matos, M.A.; Carneiro, M.A.; Lopes, C.L.; Teles, S.A.; Vicente, C.P.; Martins, R.M. Seroprevalence of htlv in a population of HIV1-infected patients in midwestern Brazil. Rev. Inst. Med. Trop. Sao Paulo 2016, 58, 80. [Google Scholar] [CrossRef] [PubMed]
- Nasir, I.A.; Ahmad, A.E.; Emeribe, A.U.; Shehu, M.S.; Medugu, J.T.; Babayo, A. Molecular detection and clinical implications of HTLV-1 infections among antiretroviral therapy-naive HIV-1-infected individuals in Abuja, Nigeria. Virology 2015, 6, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Opaleye, O.O.; Igboama, M.C.; Ojo, J.A.; Odewale, G. Seroprevalence of HIV, HBV, HCV, and HTLV among pregnant women in southwestern Nigeria. J. Immunoass. Immunochem. 2016, 37, 29–42. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.T.; Neves, E.S.; Grinsztejn, B.; de Melo Espindola, O.; Schor, D.; Araujo, A. Neurological manifestations of coinfection with HIV and human T-lymphotropic virus type 1. AIDS 2012, 26, 521–523. [Google Scholar] [CrossRef] [PubMed]
- Klase, Z.; Jeang, K.T. Reciprocal functional pseudotyping of HIV-1 and HTLV-1 viral genomes by the heterologous counterpart envelope proteins. Virology 2013, 443, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Spear, G.T.; Jiang, H.X.; Sullivan, B.L.; Gewurz, H.; Landay, A.L.; Lint, T.F. Direct binding of complement component c1q to human immunodeficiency virus (HIV) and human T lymphotrophic virus-I (HTLV-I) coinfected cells. AIDS Res. Hum. Retrovir. 1991, 7, 579–585. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.M.; Richman, D.D.; Little, S.J. HIV superinfection. J. Infect. Dis. 2005, 192, 438–444. [Google Scholar] [CrossRef] [PubMed]
- Cook, L.B.; Rowan, A.G.; Melamed, A.; Taylor, G.P.; Bangham, C.R. HTLV-1-infected T cells contain a single integrated provirus in natural infection. Blood 2012, 120, 3488–3490. [Google Scholar] [CrossRef] [PubMed]
- Leung, K.; Nabel, G.J. HTLV-1 transactivator induces interleukin-2 receptor expression through an NF-κB-like factor. Nature 1988, 333, 776–778. [Google Scholar] [CrossRef] [PubMed]
- Stroud, J.C.; Oltman, A.; Han, A.; Bates, D.L.; Chen, L. Structural basis of HIV-1 activation by NF-κB—A higher-order complex of p50: Rela bound to the HIV-1 LTR. J. Mol. Biol. 2009, 393, 98–112. [Google Scholar] [CrossRef] [PubMed]
- Geddes, V.E.V.; Jose, D.P.; Leal, F.E.; Nixon, D.F.; Tanuri, A.; Aguiar, R.S. HTLV-1 tax activates HIV-1 transcription in latency models. Virology 2017, 504, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Barrios, C.S.; Castillo, L.; Giam, C.Z.; Wu, L.; Beilke, M.A. Inhibition of HIV type 1 replication by human T lymphotropic virus types 1 and 2 tax proteins in vitro. AIDS Res. Hum. Retrovir. 2013, 29, 1061–1067. [Google Scholar] [CrossRef] [PubMed]
- Baba, M.; Imai, T.; Yoshida, T.; Yoshie, O. Constitutive expression of various chemokine genes in human T-cell lines infected with human T-cell leukemia virus type 1: Role of the viral transactivator tax. Int. J. Cancer 1996, 66, 124–129. [Google Scholar] [CrossRef]
- Cocchi, F.; DeVico, A.L.; Garzino-Demo, A.; Arya, S.K.; Gallo, R.C.; Lusso, P. Identification of rantes, mip-1 α, and mip-1 β as the major HIV-suppressive factors produced by CD8+ T cells. Science 1995, 270, 1811–1815. [Google Scholar] [CrossRef] [PubMed]
- Pilotti, E.; Bianchi, M.V.; De Maria, A.; Bozzano, F.; Romanelli, M.G.; Bertazzoni, U.; Casoli, C. HTLV-1/-2 and HIV-1 co-infections: Retroviral interference on host immune status. Front. Microbiol. 2013, 4, 372. [Google Scholar] [CrossRef] [PubMed]
- Pedroso, C.; Netto, E.M.; Weyll, N.; Brites, C. Coinfection by HIV-1 and human lymphotropic virus type 1 in Brazilian children is strongly associated with a shorter survival time. J. Acquir. Immune Defic. Syndr. 2011, 57, S208–S211. [Google Scholar] [CrossRef] [PubMed]
- Beilke, M.A.; Theall, K.P.; O′Brien, M.; Clayton, J.L.; Benjamin, S.M.; Winsor, E.L.; Kissinger, P.J. Clinical outcomes and disease progression among patients coinfected with HIV and human T lymphotropic virus types 1 and 2. Clin. Infect. Dis. 2004, 39, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Regis, C.; Oliveira, A.; Brites, C. Onset of opportunistic infections in patients co-infected by HTLV-1 and HIV-1, with high CD4+ cells count. Braz. J. Infect. Dis. 2009, 13, 311–313. [Google Scholar] [CrossRef] [PubMed]
- Shibata, D.; Brynes, R.K.; Rabinowitz, A.; Hanson, C.A.; Slovak, M.L.; Spira, T.J.; Gill, P. Human T-cell lymphotropic virus type I (HTLV-I)-associated adult T-cell leukemia-lymphoma in a patient infected with human immunodeficiency virus type 1 (HIV-1). Ann. Intern. Med. 1989, 111, 871–875. [Google Scholar] [CrossRef] [PubMed]
- Beilke, M.A.; Japa, S.; Moeller-Hadi, C.; Martin-Schild, S. Tropical spastic paraparesis/human T leukemia virus type 1-associated myelopathy in HIV type 1-coinfected patients. Clin. Infect. Dis. 2005, 41, e57–63. [Google Scholar] [CrossRef] [PubMed]
- Berger, J.R.; Raffanti, S.; Svenningsson, A.; McCarthy, M.; Snodgrass, S.; Resnick, L. The role of HTLV in HIV-1 neurologic disease. Neurology 1991, 41, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Casseb, J.; de Oliveira, A.C.; Vergara, M.P.; Montanheiro, P.; Bonasser, F.; Meilman Ferreira, C.; Smid, J.; Duarte, A.J. Presence of tropical spastic paraparesis/human T-cell lymphotropic virus type 1-associated myelopathy (TSP/HAM)-like among HIV-1-infected patients. J. Med. Virol. 2008, 80, 392–398. [Google Scholar] [CrossRef] [PubMed]
- Abrahao, M.H.; Lima, R.G.; Netto, E.; Brites, C. Short communication: Human lymphotropic virus type 1 coinfection modulates the synthesis of cytokines by peripheral blood mononuclear cells from HIV type 1-infected individuals. AIDS Res. Hum. Retrovir. 2012, 28, 806–808. [Google Scholar] [CrossRef] [PubMed]
- Oo, Z.; Barrios, C.S.; Castillo, L.; Beilke, M.A. High levels of cc-chemokine expression and downregulated levels of CCR5 during HIV-1/HTLV-1 and HIV-1/HTLV-2 coinfections. J. Med. Virol. 2015, 87, 790–797. [Google Scholar] [CrossRef] [PubMed]
- Uozumi, K. Treatment of adult T-cell leukemia. J. Clin. Exp. Hematop. 2010, 50, 9–25. [Google Scholar] [CrossRef] [PubMed]
- Yamada, Y.; Tomonaga, M.; Fukuda, H.; Hanada, S.; Utsunomiya, A.; Tara, M.; Sano, M.; Ikeda, S.; Takatsuki, K.; Kozuru, M.; et al. A new G-CSF-supported combination chemotherapy, lsg15, for adult T-cell leukaemia-lymphoma: Japan clinical oncology group study 9303. Br. J. Haematol. 2001, 113, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Tsukasaki, K.; Hermine, O.; Bazarbachi, A.; Ratner, L.; Ramos, J.C.; Harrington, W., Jr.; O′Mahony, D.; Janik, J.E.; Bittencourt, A.L.; Taylor, G.P.; et al. Definition, prognostic factors, treatment, and response criteria of adult t-cell leukemia-lymphoma: A proposal from an international consensus meeting. J. Clin. Oncol. 2009, 27, 453–459. [Google Scholar] [CrossRef] [PubMed]
- Ishida, T.; Hishizawa, M.; Kato, K.; Tanosaki, R.; Fukuda, T.; Taniguchi, S.; Eto, T.; Takatsuka, Y.; Miyazaki, Y.; Moriuchi, Y.; et al. Allogeneic hematopoietic stem cell transplantation for adult t-cell leukemia-lymphoma with special emphasis on preconditioning regimen: A nationwide retrospective study. Blood 2012, 120, 1734–1741. [Google Scholar] [CrossRef] [PubMed]
- Bazarbachi, A.; Hermine, O. Treatment with a combination of zidovudine and α-interferon in naive and pretreated adult T-cell leukemia/lymphoma patients. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 1996, 13, S186–S190. [Google Scholar] [CrossRef] [PubMed]
- Gill, P.S.; Harrington, W., Jr.; Kaplan, M.H.; Ribeiro, R.C.; Bennett, J.M.; Liebman, H.A.; Bernstein-Singer, M.; Espina, B.M.; Cabral, L.; Allen, S.; et al. Treatment of adult T-cell leukemia-lymphoma with a combination of interferon alfa and zidovudine. N. Engl. J. Med. 1995, 332, 1744–1748. [Google Scholar] [CrossRef] [PubMed]
- Bazarbachi, A.; Plumelle, Y.; Carlos Ramos, J.; Tortevoye, P.; Otrock, Z.; Taylor, G.; Gessain, A.; Harrington, W.; Panelatti, G.; Hermine, O. Meta-analysis on the use of zidovudine and interferon-alfa in adult T-cell leukemia/lymphoma showing improved survival in the leukemic subtypes. J. Clin. Oncol. 2010, 28, 4177–4183. [Google Scholar] [CrossRef] [PubMed]
- Cook, L.B.; Rowan, A.G.; Demontis, M.A.; Sagawe, S.; Gillet, N.A.; Melamed, A.; Greiller, C.; Witkover, A.; Bangham, C.R.M.; Taylor, G.P. Long-term clinical remission maintained after cessation of zidovudine and interferon-α therapy in chronic adult T-cell leukemia/lymphoma. Int. J. Hematol. 2017. [Google Scholar] [CrossRef] [PubMed]
- O′Mahony, D.; Morris, J.C.; Stetler-Stevenson, M.; Matthews, H.; Brown, M.R.; Fleisher, T.; Pittaluga, S.; Raffeld, M.; Albert, P.S.; Reitsma, D.; et al. EBV-related lymphoproliferative disease complicating therapy with the anti-CD2 monoclonal antibody, siplizumab, in patients with T-cell malignancies. Clin. Cancer Res. 2009, 15, 2514–2522. [Google Scholar] [CrossRef] [PubMed]
- Ceesay, M.M.; Matutes, E.; Taylor, G.P.; Fields, P.; Cavenagh, J.; Simpson, S.; Ho, A.; Devereux, S.; Mufti, G.J.; Pagliuca, A. Phase II study on combination therapy with chop-zenapax for HTLV-I associated adult T-cell leukaemia/lymphoma (ATLL). Leuk. Res. 2012, 36, 857–861. [Google Scholar] [CrossRef] [PubMed]
- Ishida, T.; Iida, S.; Akatsuka, Y.; Ishii, T.; Miyazaki, M.; Komatsu, H.; Inagaki, H.; Okada, N.; Fujita, T.; Shitara, K.; et al. The cc chemokine receptor 4 as a novel specific molecular target for immunotherapy in adult T-cell leukemia/lymphoma. Clin. Cancer Res. 2004, 10, 7529–7539. [Google Scholar] [CrossRef] [PubMed]
- Ishida, T.; Joh, T.; Uike, N.; Yamamoto, K.; Utsunomiya, A.; Yoshida, S.; Saburi, Y.; Miyamoto, T.; Takemoto, S.; Suzushima, H.; et al. Defucosylated anti-CCR4 monoclonal antibody (kw-0761) for relapsed adult T-cell leukemia-lymphoma: A multicenter phase II study. J. Clin. Oncol. 2012, 30, 837–842. [Google Scholar] [CrossRef] [PubMed]
- Ishida, T.; Jo, T.; Takemoto, S.; Suzushima, H.; Uozumi, K.; Yamamoto, K.; Uike, N.; Saburi, Y.; Nosaka, K.; Utsunomiya, A.; et al. Dose-intensified chemotherapy alone or in combination with mogamulizumab in newly diagnosed aggressive adult T-cell leukaemia-lymphoma: A randomized phase II study. Br. J. Haematol. 2015, 169, 672–682. [Google Scholar] [CrossRef] [PubMed]
- Ishida, T.; Utsunomiya, A.; Jo, T.; Yamamoto, K.; Kato, K.; Yoshida, S.; Takemoto, S.; Suzushima, H.; Kobayashi, Y.; Imaizumi, Y.; et al. Mogamulizumab for relapsed adult T-cell leukemia-lymphoma: Updated follow-up analysis of phase I and II studies. Cancer Sci. 2017, 108, 2022–2029. [Google Scholar] [CrossRef] [PubMed]
- Ishida, T.; Fujiwara, H.; Nosaka, K.; Taira, N.; Abe, Y.; Imaizumi, Y.; Moriuchi, Y.; Jo, T.; Ishizawa, K.; Tobinai, K.; et al. Multicenter phase II study of lenalidomide in relapsed or recurrent adult T-cell leukemia/lymphoma: ATLL-002. J. Clin. Oncol. 2016, 34, 4086–4093. [Google Scholar] [CrossRef] [PubMed]
- Ishitsuka, K.; Utsunomiya, A.; Katsuya, H.; Takeuchi, S.; Takatsuka, Y.; Hidaka, M.; Sakai, T.; Yoshimitsu, M.; Ishida, T.; Tamura, K. A phase II study of bortezomib in patients with relapsed or refractory aggressive adult T-cell leukemia/lymphoma. Cancer Sci. 2015, 106, 1219–1223. [Google Scholar] [CrossRef] [PubMed]
- Nakano, D.; Ishitsuka, K.; Ikeda, M.; Tsuchihashi, R.; Okawa, M.; Okabe, H.; Tamura, K.; Kinjo, J. Screening of promising chemotherapeutic candidates from plants against human adult T-cell leukemia/lymphoma (IV): Phenanthroindolizidine alkaloids from tylophora tanakae leaves. J. Nat. Med. 2015, 69, 397–401. [Google Scholar] [CrossRef] [PubMed]
- Pinto, M.T.; Slavov, S.N.; Valente, V.B.; Ubiali, E.M.; Covas, D.T.; Kashima, S. Evaluation of human T-lymphotropic virus prevalence/co-infection rates for a four-year period in a non-metropolitan blood center in southeast Brazil. Rev. Soc. Bras. Med. Trop. 2016, 49, 232–236. [Google Scholar] [CrossRef] [PubMed]
- Ishitsuka, K.; Kunami, N.; Katsuya, H.; Nogami, R.; Ishikawa, C.; Yotsumoto, F.; Tanji, H.; Mori, N.; Takeshita, M.; Miyamoto, S.; et al. Targeting Bcl-2 family proteins in adult T-cell leukemia/lymphoma: In vitro and in vivo effects of the novel Bcl-2 family inhibitor abt-737. Cancer Lett. 2012, 317, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Witzens-Harig, M.; Giaisi, M.; Kohler, R.; Krammer, P.H.; Li-Weber, M. HTLV-1-associated adult T cell leukemia is highly susceptible to navitoclax due to enhanced bax expression. Int. J. Cancer 2016, 138, 507–514. [Google Scholar] [CrossRef] [PubMed]
- Narita, T.; Ishida, T.; Ito, A.; Masaki, A.; Kinoshita, S.; Suzuki, S.; Takino, H.; Yoshida, T.; Ri, M.; Kusumoto, S.; et al. Cyclin-dependent kinase 9 is a novel specific molecular target in adult T-cell leukemia/lymphoma. Blood 2017, 130, 1114–1124. [Google Scholar] [CrossRef] [PubMed]
- Dassouki, Z.; Sahin, U.; El Hajj, H.; Jollivet, F.; Kfoury, Y.; Lallemand-Breitenbach, V.; Hermine, O.; de The, H.; Bazarbachi, A. Atl response to arsenic/interferon therapy is triggered by sumo/pml/rnf4-dependent tax degradation. Blood 2015, 125, 474–482. [Google Scholar] [CrossRef] [PubMed]
- Shimosaki, S.; Nakahata, S.; Ichikawa, T.; Kitanaka, A.; Kameda, T.; Hidaka, T.; Kubuki, Y.; Kurosawa, G.; Zhang, L.; Sudo, Y.; et al. Development of a complete human igg monoclonal antibody to transferrin receptor 1 targeted for adult T-cell leukemia/lymphoma. Biochem. Biophys. Res. Commun. 2017, 485, 144–151. [Google Scholar] [CrossRef] [PubMed]
- El Hajj, H.; Khalil, B.; Ghandour, B.; Nasr, R.; Shahine, S.; Ghantous, A.; Abdel-Samad, R.; Sinjab, A.; Hasegawa, H.; Jabbour, M.; et al. Preclinical efficacy of the synthetic retinoid st1926 for treating adult T-cell leukemia/lymphoma. Blood 2014, 124, 2072–2080. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, A.; Takeda, S.; Kariya, R.; Matsuda, K.; Urano, E.; Okada, S.; Komano, J. A novel therapeutic molecule against HTLV-1 infection targeting provirus. Leukemia 2013, 27, 1621–1627. [Google Scholar] [CrossRef] [PubMed]
- Coler-Reilly, A.L.G.; Sato, T.; Matsuzaki, T.; Nakagawa, M.; Niino, M.; Nagai, M.; Nakamura, T.; Takenouchi, N.; Araya, N.; Yagishita, N.; et al. Effectiveness of daily prednisolone to slow progression of human T-lymphotropic virus type 1-associated myelopathy/tropical spastic paraparesis: A multicenter retrospective cohort study. Neurotherapeutics 2017, 14, 1084–1094. [Google Scholar] [CrossRef] [PubMed]
- Martin, F.; Castro, H.; Gabriel, C.; Adonis, A.; Fedina, A.; Harrison, L.; Brodnicki, L.; Demontis, M.A.; Babiker, A.G.; Weber, J.N.; et al. Ciclosporin a proof of concept study in patients with active, progressive HTLV-1 associated myelopathy/tropical spastic paraparesis. PLoS Negl. Trop. Dis. 2012, 6, e1675. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shirabe, S.; Nakamura, T.; Tsujino, A.; Nishiura, Y.; Furuya, T.; Goto, H.; Suenaga, A.; Nakane, S.; Yoshimura, T.; Nagataki, S. Successful application of pentoxifylline in the treatment of HTLV-I associated myelopathy. J. Neurol. Sci. 1997, 151, 97–101. [Google Scholar] [CrossRef]
- Harrington, W.J., Jr.; Sheremata, W.A.; Snodgrass, S.R.; Emerson, S.; Phillips, S.; Berger, J.R. Tropical spastic paraparesis/HTLV-1-associated myelopathy (TSP/HAM): Treatment with an anabolic steroid danazol. AIDS Res. Hum. Retrovir. 1991, 7, 1031–1034. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, Y.; Kurohara, K.; Fujiyama, F.; Takashima, H.; Endo, C.; Matsui, M.; Neshige, R.; Kakigi, R. Systemic interferon-α in the treatment of HTLV-I-associated myelopathy. Acta Neurol. Scand. 1992, 86, 82–86. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki, K.; Kira, J.; Koyanagi, Y.; Kawano, Y.; Miyano-Kurosaki, N.; Nakamura, M.; Baba, E.; Suzuki, J.; Yamamoto, A.; Yamamoto, N.; et al. Long-term, high dose interferon-α treatment in HTLV-I-associated myelopathy/tropical spastic paraparesis: A combined clinical, virological and immunological study. J. Neurol. Sci. 1997, 147, 135–144. [Google Scholar] [CrossRef]
- Viana, G.M.; Silva, M.A.; Souza, V.L.; Lopes, N.B.; Silva, D.L.; Nascimento Mdo, D. Interferon β-1a treatment in HTLV-1-associated myelopathy/tropical spastic paraparesis: A case report. Rev. Inst. Med. Trop. Sao Paulo 2014, 56, 443–445. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, M.; Nakahara, K.; Maruyama, Y.; Kawabata, M.; Higuchi, I.; Kubota, H.; Izumo, S.; Arimura, K.; Osame, M. Therapeutic trials in 200 patients with HTLV-I-associated myelopathy/tropical spastic paraparesis. J. Neurovirol. 1996, 2, 345–355. [Google Scholar] [CrossRef] [PubMed]
- Olindo, S.; Belrose, G.; Gillet, N.; Rodriguez, S.; Boxus, M.; Verlaeten, O.; Asquith, B.; Bangham, C.; Signate, A.; Smadja, D.; et al. Safety of long-term treatment of HAM/TSP patients with valproic acid. Blood 2011, 118, 6306–6309. [Google Scholar] [CrossRef] [PubMed]
- Lezin, A.; Gillet, N.; Olindo, S.; Signate, A.; Grandvaux, N.; Verlaeten, O.; Belrose, G.; de Carvalho Bittencourt, M.; Hiscott, J.; Asquith, B.; et al. Histone deacetylase mediated transcriptional activation reduces proviral loads in HTLV-1 associated myelopathy/tropical spastic paraparesis patients. Blood 2007, 110, 3722–3728. [Google Scholar] [CrossRef] [PubMed]
- Boostani, R.; Vakili, R.; Hosseiny, S.S.; Shoeibi, A.; Fazeli, B.; Etemadi, M.M.; Sabet, F.; Valizade, N.; Rezaee, S.A. Triple therapy with prednisolone, pegylated interferon and sodium valproate improves clinical outcome and reduces human T-cell leukemia virus type 1 (HTLV-1) proviral load, tax and HBZ mRNA expression in patients with HTLV-1-associated myelopathy/tropical spastic paraparesis. Neurotherapeutics 2015, 12, 887–895. [Google Scholar] [PubMed]
- Yamauchi, J.; Coler-Reilly, A.; Sato, T.; Araya, N.; Yagishita, N.; Ando, H.; Kunitomo, Y.; Takahashi, K.; Tanaka, Y.; Shibagaki, Y.; et al. Mogamulizumab, an anti-CCR4 antibody, targets human T-lymphotropic virus type 1-infected CD8+ and CD4+ T cells to treat associated myelopathy. J. Infect. Dis. 2015, 211, 238–248. [Google Scholar] [CrossRef] [PubMed]
- Sa, K.N.; Macedo, M.C.; Andrade, R.P.; Mendes, S.D.; Martins, J.V.; Baptista, A.F. Physiotherapy for human T-lymphotropic virus 1-associated myelopathy: Review of the literature and future perspectives. J. Multidiscip. Healthc. 2015, 8, 117–125. [Google Scholar] [PubMed]
- Matsuoka, M. Human T-cell leukemia virus type I (HTLV-I) infection and the onset of adult T-cell leukemia (ATL). Retrovirology 2005, 2, 27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Satoh, M.; Toma, H.; Sato, Y.; Takara, M.; Shiroma, Y.; Kiyuna, S.; Hirayama, K. Reduced efficacy of treatment of strongyloidiasis in HTLV-I carriers related to enhanced expression of IFN-γ and TGF-β1. Clin. Exp. Immunol. 2002, 127, 354–359. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, E.M.; Da Fonseca Porto, A. Epidemiological and clinical interaction between HTLV-1 and strongyloides stercoralis. Parasite Immunol. 2004, 26, 487–497. [Google Scholar] [CrossRef] [PubMed]
- Gabet, A.S.; Mortreux, F.; Talarmin, A.; Plumelle, Y.; Leclercq, I.; Leroy, A.; Gessain, A.; Clity, E.; Joubert, M.; Wattel, E. High circulating proviral load with oligoclonal expansion of HTLV-1 bearing T cells in HTLV-1 carriers with strongyloidiasis. Oncogene 2000, 19, 4954–4960. [Google Scholar] [CrossRef] [PubMed]
- Gillet, N.A.; Cook, L.; Laydon, D.J.; Hlela, C.; Verdonck, K.; Alvarez, C.; Gotuzzo, E.; Clark, D.; Farre, L.; Bittencourt, A.; et al. Strongyloidiasis and infective dermatitis alter human T lymphotropic virus-1 clonality in vivo. PLoS Pathog. 2013, 9, e1003263. [Google Scholar] [CrossRef] [PubMed]
- Harrison, L.H.; Vaz, B.; Taveira, D.M.; Quinn, T.C.; Gibbs, C.J.; de Souza, S.H.; McArthur, J.C.; Schechter, M. Myelopathy among Brazilians coinfected with human T-cell lymphotropic virus type I and HIV. Neurology 1997, 48, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Pomier, C.; Rabaaoui, S.; Pouliquen, J.F.; Couppie, P.; El Guedj, M.; Nacher, M.; Lacoste, V.; Wattel, E.; Kazanji, M.; Mortreux, F. Antiretroviral therapy promotes an inflammatory-like pattern of human T-cell lymphotropic virus type 1 (HTLV-1) replication in human immunodeficiency virus type 1/HTLV-1 co-infected individuals. J. Gen. Virol. 2013, 94, 753–757. [Google Scholar] [CrossRef] [PubMed]
- Costin, J.M. Cytopathic mechanisms of HIV-1. Virol. J. 2007, 4, 100. [Google Scholar] [CrossRef] [PubMed]
- Shida, H.; Tochikura, T.; Sato, T.; Konno, T.; Hirayoshi, K.; Seki, M.; Ito, Y.; Hatanaka, M.; Hinuma, Y.; Sugimoto, M.; et al. Effect of the recombinant vaccinia viruses that express HTLV-I envelope gene on HTLV-I infection. EMBO J. 1987, 6, 3379–3384. [Google Scholar] [PubMed]
- Fujii, H.; Shimizu, M.; Miyagi, T.; Kunihiro, M.; Tanaka, R.; Takahashi, Y.; Tanaka, Y. A potential of an anti-HTLV-I GP46 neutralizing monoclonal antibody (lat-27) for passive immunization against both horizontal and mother-to-child vertical infection with human T cell leukemia virus type-I. Viruses 2016, 8, 41. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Takezaki, T.; Oki, T.; Kawakami, K.; Yashiki, S.; Fujiyoshi, T.; Usuku, K.; Mueller, N.; Osame, M.; Miyata, K.; et al. Inhibitory effect of maternal antibody on mother-to-child transmission of human T-lymphotropic virus type I. The mother-to-child transmission study group. Int. J. Cancer 1991, 49, 673–677. [Google Scholar] [CrossRef] [PubMed]
- Suehiro, Y.; Hasegawa, A.; Iino, T.; Sasada, A.; Watanabe, N.; Matsuoka, M.; Takamori, A.; Tanosaki, R.; Utsunomiya, A.; Choi, I.; et al. Clinical outcomes of a novel therapeutic vaccine with tax peptide-pulsed dendritic cells for adult T cell leukaemia/lymphoma in a pilot study. Br. J. Haematol. 2015, 169, 356–367. [Google Scholar] [CrossRef] [PubMed]
- Rowan, A.G.; Bangham, C.R. Is there a role for HTLV-1-specific CTL in adult T-cell leukemia/lymphoma? Leuk. Res. Treat. 2012, 2012, 391953. [Google Scholar] [CrossRef] [PubMed]
- Sugata, K.; Yasunaga, J.; Mitobe, Y.; Miura, M.; Miyazato, P.; Kohara, M.; Matsuoka, M. Protective effect of cytotoxic T lymphocytes targeting HTLV-1 BZIP factor. Blood 2015, 126, 1095–1105. [Google Scholar] [CrossRef] [PubMed]
- Willems, L.; Hasegawa, H.; Accolla, R.; Bangham, C.; Bazarbachi, A.; Bertazzoni, U.; Carneiro-Proietti, A.B.; Cheng, H.; Chieco-Bianchi, L.; Ciminale, V.; et al. Reducing the global burden of HTLV-1 infection: An agenda for research and action. Antivir. Res. 2017, 137, 41–48. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Futsch, N.; Mahieux, R.; Dutartre, H. HTLV-1, the Other Pathogenic Yet Neglected Human Retrovirus: From Transmission to Therapeutic Treatment. Viruses 2018, 10, 1. https://doi.org/10.3390/v10010001
Futsch N, Mahieux R, Dutartre H. HTLV-1, the Other Pathogenic Yet Neglected Human Retrovirus: From Transmission to Therapeutic Treatment. Viruses. 2018; 10(1):1. https://doi.org/10.3390/v10010001
Chicago/Turabian StyleFutsch, Nicolas, Renaud Mahieux, and Hélène Dutartre. 2018. "HTLV-1, the Other Pathogenic Yet Neglected Human Retrovirus: From Transmission to Therapeutic Treatment" Viruses 10, no. 1: 1. https://doi.org/10.3390/v10010001
APA StyleFutsch, N., Mahieux, R., & Dutartre, H. (2018). HTLV-1, the Other Pathogenic Yet Neglected Human Retrovirus: From Transmission to Therapeutic Treatment. Viruses, 10(1), 1. https://doi.org/10.3390/v10010001



