Distribution Changes of Phosphorus in Soil–Plant Systems of Larch Plantations across the Chronosequence
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Description and Experimental Design
2.2. Plant Sampling and Analysis
2.3. Soil Sampling and Analysis
2.4. Statistical Analysis
3. Results
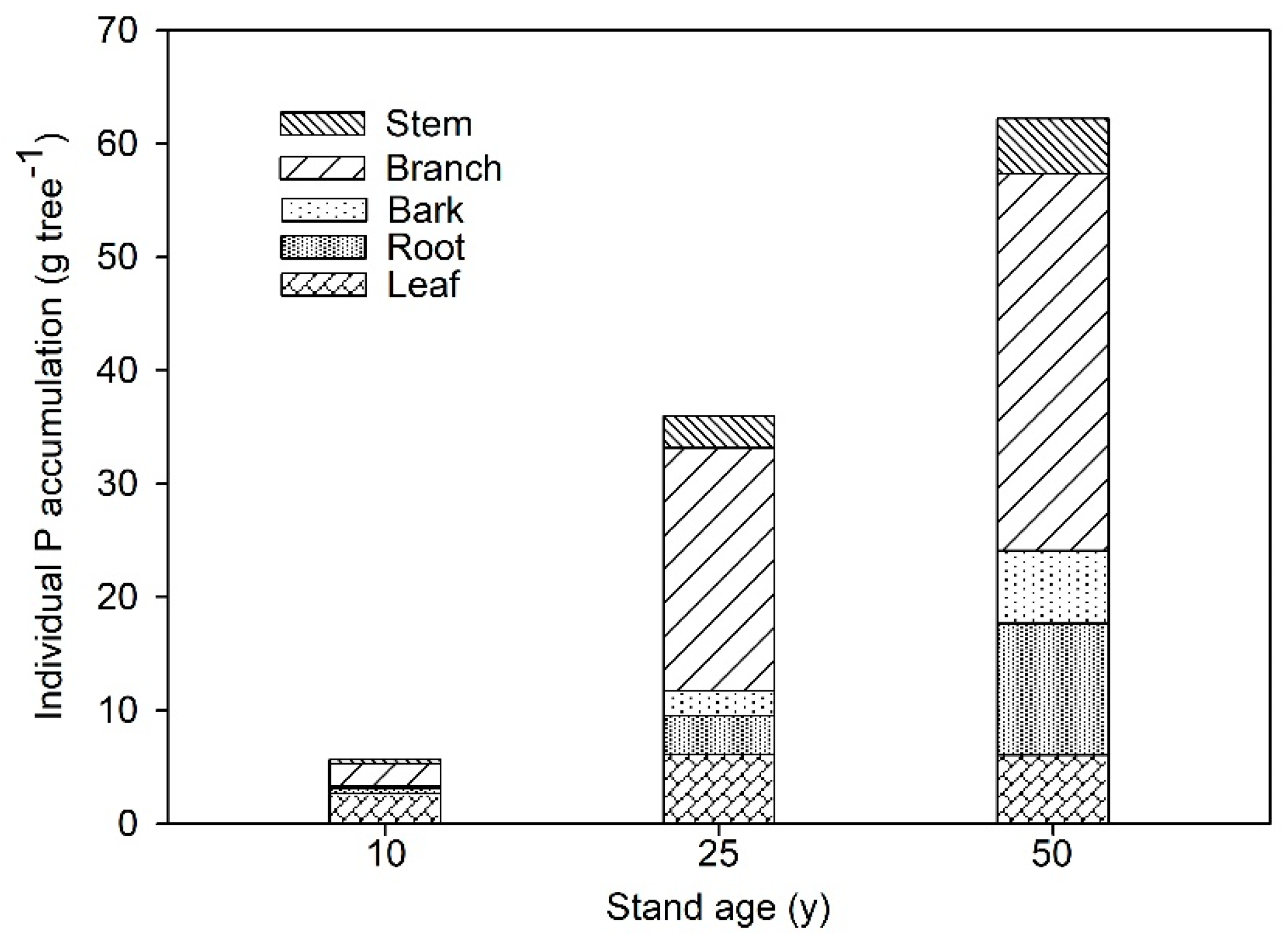
3.1. P Distribution Changes in Tree Components with Stand Age on Larch Plantations
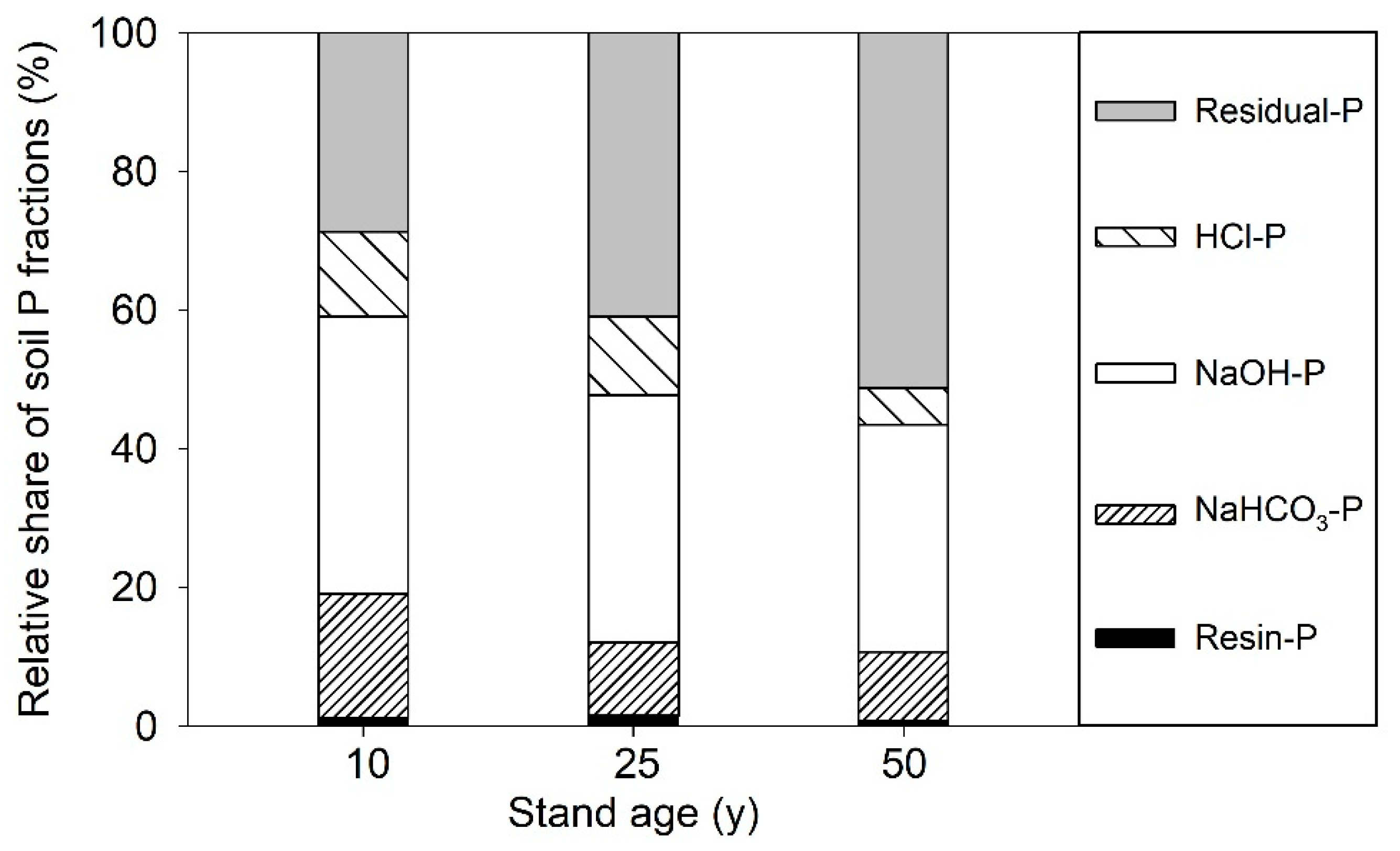
3.2. Variation of Soil P Fractions with Stand Age of Larch Plantations
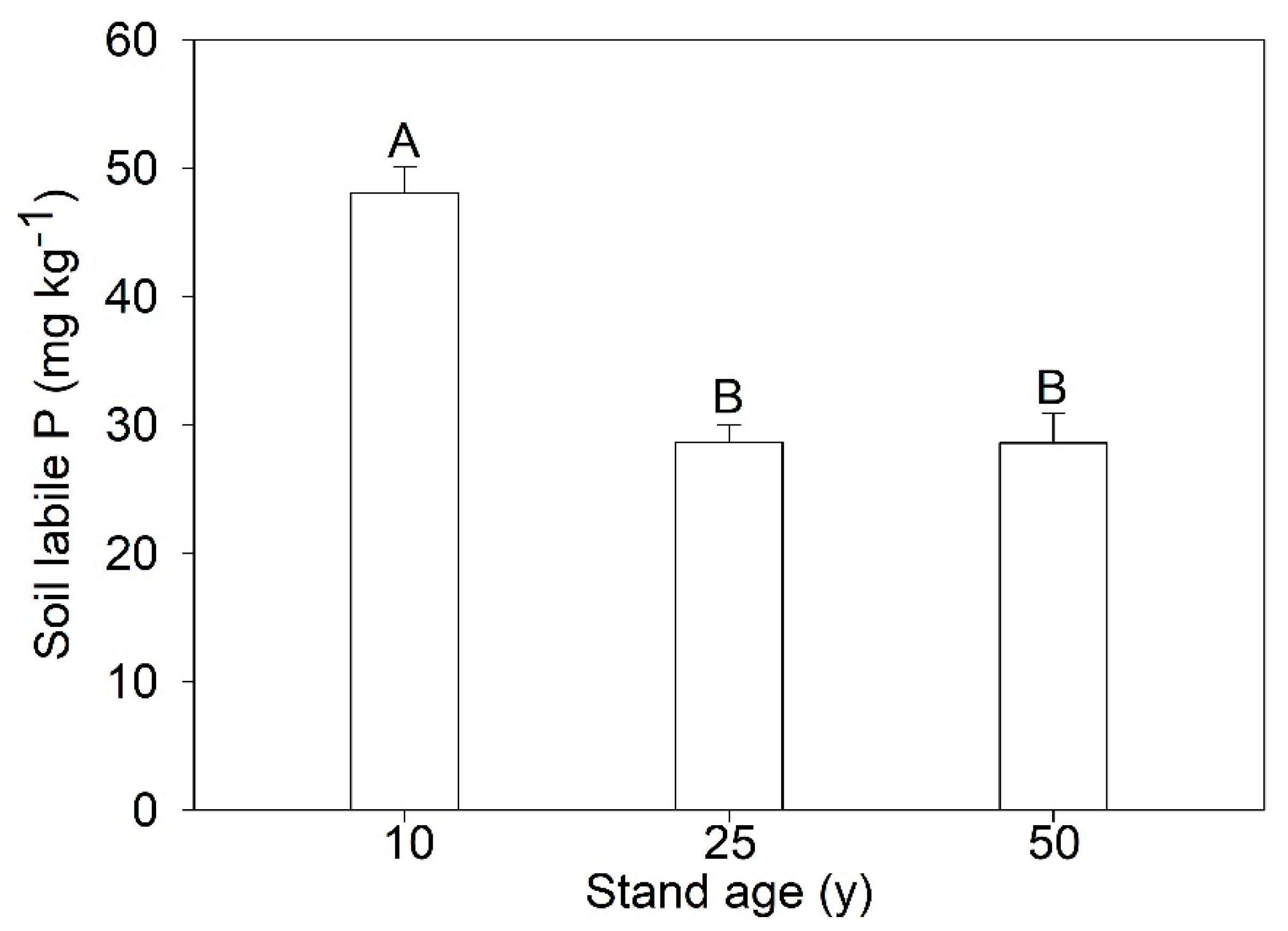
3.3. Stock of Soil Labile P and Larch P
4. Discussion
4.1. Variations of Larch N:P Ratio, PRE, P Concentration, and P Accumulation across the Larch-Plantation Chronosequence
4.2. Variations of Soil P Fractions across the Larch-Plantation Chronosequence
4.3. Stock of Larch P and Soil Labile P
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhu, J.J.; Liu, Z.G.; Wang, H.X.; Yan, Q.L.; Fang, H.Y.; Hu, L.L.; Yu, L.Z. Effects of site preparation on emergence and early establishment of Laix olgensis in montane regions of northeast China. New For. 2008, 36, 247–260. [Google Scholar] [CrossRef]
- Yang, K.; Zhu, J.J.; Yan, Q.L.; Sun, J.O. Changes in soil P chemistry as affected by conversion of nature secondary forests to larch plantations. For. Ecol. Manag. 2010, 260, 422–428. [Google Scholar] [CrossRef]
- Yang, K.; Shi, W.; Zhu, J.J. The impact of secondary forests conversion into larch plantations on soil chemical and microbiological properties. Plant Soil 2013, 368, 535–546. [Google Scholar] [CrossRef]
- Yan, T.; Zhu, J.J.; Yang, K.; Yu, L.Z.; Zhang, J.X. Nutrient removal under different harvesting scenarios for larch plantations in northeast China: Implications for nutrient conservation and management. For. Ecol. Manag. 2017, 400, 150–158. [Google Scholar] [CrossRef]
- Zheng, X.F.; Yuan, J.; Zhang, T.; Hao, F.; Jose, S.; Zhang, S.X. Soil Degradation and the decline of available nitrogen and phosphorus in soils of the main forest types in the Qinling Mountains of China. Forests 2017, 8, 460. [Google Scholar] [CrossRef]
- Chen, L.X.; Zhang, C.; Duan, W.B. Temporal variations in phosphorus fractions and phosphatase activities in rhizosphere and bulk soil during the development of Larix olgensis plantations. J. Plant Nutr. Soil Sci. 2016, 179, 67–77. [Google Scholar] [CrossRef]
- Yan, T.; Lü, X.T.; Zhu, J.J.; Yang, K.; Yu, L.Z.; Gao, T. Changes in nitrogen and phosphorus cycling suggest a transition to phosphorus limitation with the stand development of larch plantations. Plant Soil 2018, 422, 385–396. [Google Scholar] [CrossRef]
- Slazak, A.; Freese, D.; Matos, E.S.; Hüttl, R.F. Soil organic phosphorus fraction in pine-oak forest stands in Northeastern Germany. Geoderma 2010, 158, 156–162. [Google Scholar] [CrossRef]
- Galván-Tejada, N.C.; Peña-Ramírez, V.; Mora-Palomino, L.; Siebe, C. Soil P fractions in a volcanic soil chronosequence of Central Mexico and their relationship to foliar P in pine trees. J. Plant Nutr. Soil Sci. 2014, 177, 792–802. [Google Scholar] [CrossRef]
- Shiau, Y.J.; Pai, C.W.; Tsai, J.W.; Liu, W.C.; Yam, R.W.; Chang, S.C.; Tang, S.L.; Chiu, C.Y. Characterization of Phosphorus in a Toposequence of Subtropical Perhumid Forest Soils Facing a Subalpine Lake. Forests 2018, 9, 294. [Google Scholar] [CrossRef]
- Hedley, M.J.; Stewart, W.B.; Chauhan, B.S. Changes in inorganic and organic soil phosphorus fractions induced by cultivation practices and by laboratory incubations. Soil Sci. 1982, 46, 970–976. [Google Scholar] [CrossRef]
- Cross, A.F.; Schesinger, W.H. A literature review and evaluation of the Hedley fractionation: Applications to the biogeochemical cycle of soil phosphorus in natural ecosystems. Geoderma 1995, 64, 197–214. [Google Scholar] [CrossRef]
- Zhang, K.; Su, Y.Z.; Yang, R. Biomass and nutrient allocation strategies in a desert ecosystem in the Hexi Corridor, northwest China. J. Plant Res. 2017, 130, 699–708. [Google Scholar] [CrossRef] [PubMed]
- Müller, I.; Schmid, B.; Weiner, J. The effect of nutrient availability on biomass allocation patterns in 27 species of herbaceous plants. Perspect. Plant Ecol. Evol. Syst. 2000, 3, 115–127. [Google Scholar] [CrossRef]
- Glynn, C.; Herms, D.A.; Egawa, M.; Hansen, R.; Mattson, W.J. Effects of nutrient availability on biomass allocation as well as constitutive and rapid induced herbivore resistance in poplar. Oikios 2003, 101, 385–397. [Google Scholar] [CrossRef]
- Bargaza, A.; Noyceb, G.L.; Fulthorpeb, R.; Carlssona, G.; Furzeb, J.R.; Jensena, E.S.; Dhiba, D.; Isaacb, M.E. Species interactions enhance root allocation, microbial diversity and P acquisition in intercropped wheat and soybean under P deficiency. Appl. Soil Ecol. 2017, 120, 179–188. [Google Scholar] [CrossRef]
- Yang, K.; Zhu, J.J. The effects of N and P additions on soil microbial properties in paired stands of temperate secondary forests and adjacent larch plantations in Northeast China. Soil Biol. Biochem. 2015, 90, 80–86. [Google Scholar] [CrossRef]
- Vergutz, L.; Manzoni, S.; Porporato, A.; Novais, R.F.; Jackson, R.B. Global resorption efficiencies and concentrations of carbon and nutrients in leaves of terrestrial plants. Ecol. Monogr. 2012, 82, 205–220. [Google Scholar] [CrossRef]
- Murphy, J.; Riley, J.P. A modified single solution method for determination of phosphate in natural waters. Anal. Chim. Acta 1962, 27, 31–36. [Google Scholar] [CrossRef]
- Tiessen, H.; Moir, J.O. Characterization of available P by sequential extraction. In Oil Sampling and Methods of Analysis, 2nd ed.; Carter, M., Gregorich, E.G., Eds.; CRC Press: Boca Raton, FL, USA, 2008; pp. 293–305. ISBN 9781420005271. [Google Scholar]
- Ohno, T.; Zibilske, L.M. Determination of low concentrations of phosphorus in soil extracts using malachite green. Soil Sci. Soc. Am. J. 1991, 46, 892–895. [Google Scholar] [CrossRef]
- Koerselman, W.; Meuleman, A.F.M. The vegetation N:P a new tool to detect the nature of nutrient limitation. J. Appl. Ecol. 1996, 33, 1441–1450. [Google Scholar] [CrossRef]
- Kobe, R.K.; Lepczyk, C.A.; Iyer, M. Resorption efficiency decreases with increasing green leaf nutrients in a global data set. Ecology 2005, 86, 2780–2792. [Google Scholar] [CrossRef]
- Gholz, R.F.; Fisher, R.F.; Prichett, W.L. Nutrient Dynamics in Slash Pine Plantation Ecosystems. Ecology 1986, 66, 647–659. [Google Scholar] [CrossRef]
- Hayes, P.; Turner, B.L.; Lambers, H.; Laliberté, E. Foliar nutrient concentrations and resorption efficiency in plants of contrasting nutrient-acquisition strategies along a 2-million-yeardune chronosequence. J. Ecol. 2014, 102, 396–410. [Google Scholar] [CrossRef]
- Viera, M.; Schumacher, M.V.; Bonacina, D.M.; Ramos, L.O.O.; Rodríguez-Soalleiro, R. Biomass and nutrient allocation to aboveground components in fertilized Eucalyptus saligna and E. urograndis plantations. New For. 2017, 48, 445–462. [Google Scholar] [CrossRef]
- Carlyle, J.C.; Malcom, D.C. Larch litter and nitrogen availability in mixed larch spruce stands. 1. Nutrient withdrawal, redistribution, and leaching loss from larch foliage at senescence. Can. J. For. Res. 1986, 16, 321–326. [Google Scholar] [CrossRef]
- Kramer-Walter, K.R.W.; Laughlin, D.C. Root nutrient concentration and biomass allocation are more plastic than morphological traits in response to nutrient limitation. Plant Soil 2017, 416, 539–550. [Google Scholar] [CrossRef]
- Wu, P.F.; Wang, G.Y.; Farooq, T.H.; Li, Q.; Zou, X.H. Low phosphorus and competition affect Chinese fir cutting growth and root organic acid content: Does neighboring root activity aggravate P nutrient deficiency? J. Soils Sediments 2017, 17, 2775–2785. [Google Scholar] [CrossRef]
- Sardans, J.; Peñuelas, J. Tree growth changes with climate and forest type are associated with relative allocation of nutrients, especially phosphorus, to leaves and wood. Glob. Ecol. Biogeogr. 2013, 22, 494–507. [Google Scholar] [CrossRef]
- Ewe, S.M.L.; Sternberg, L.S.L. Growth and gas exchange response of Brazilian pepper (Schinus terebinthifolius) and native South Florida species to salinity. Trees 2005, 19, 119–128. [Google Scholar] [CrossRef]
- Peichl, M.; Arain, M.A. Above- and belowground ecosystem biomass and carbon pools in an age-sequence of temperate pine plantation forests. Agric. For. Meteorol. 2006, 140, 51–63. [Google Scholar] [CrossRef]
- Hansen, J.C.; Cade-Menun, B.J.; Strawn, D.G. Phosphorus speciation in manure-amended alkaline soils. J. Environ. Qual. 2004, 33, 1521–1527. [Google Scholar] [CrossRef] [PubMed]
- Frossard, E.; Condron, L.M.; Oberson, A.; Sinaj, S.; Fardeau, J.C. Processes governing phosphorus availability in temperate soils. J. Environ. Qual. 2000, 29, 15–23. [Google Scholar] [CrossRef]
- Cumming, J.R.; Weinstein, L.H. Utilization of AlPO4 as a phosphorus source by ectomycorrhizal Pinus rigida Mill. seedlings. New Phytol. 1990, 116, 99–106. [Google Scholar] [CrossRef]
- Turner, B.L.; Condron, L.M.; Richardson, S.J.; Peltzer, D.A.; Allison, V.J. Soil organic phosphorus transformations during pedogenesis. Ecosystems 2007, 10, 1166–1181. [Google Scholar] [CrossRef]
- Frizano, J.; Johnson, A.H.; Vann, D.R.; Scatena, F.N. Soil phosphorus fractionation during forest development on landslide scars in the Luquillo Mountains, Puerto Rico. Biotropica 2002, 34, 17–26. [Google Scholar] [CrossRef]
- Walker, T.W.; Syers, J.K. The fate of phosphorus during pedogenesis. Geoderma 1976, 15, 1–19. [Google Scholar] [CrossRef]
- Aerts, R. Nutrient resorption from senescing leaves of perennials: Are there general patterns? J. Ecol. 1996, 84, 597–608. [Google Scholar] [CrossRef]
- Brant, A.N.; Chen, H.Y.H. Patterns and mechanisms of nutrient resorption in plants. Crit. Rev. Plant Sci. 2015, 34, 471–486. [Google Scholar] [CrossRef]
| Stand Properties | Stand Age (Years) | ||
|---|---|---|---|
| 10 | 25 | 50 | |
| Elevation (m) | 525 (3) | 620 (3) | 650 (5) |
| Slope (°) | 13 (0.7) | 15 (0.9) | 17 (0.6) |
| Density (trees ha−1) | 3688 (320) | 1966 (220) | 960 (130) |
| DBH (cm) | 6.15 (0.1) | 15.25 (0.2) | 25.08 (0.4) |
| Tree height (m) | 7.20 (0.3) | 18.24 (0.1) | 24.38 (0.6) |
| Soil type | Typical brown forest soil | Typical brown forest soil | Typical brown forest soil |
| Soil bulk density (g cm−3) | 1.41 (0.05) | 1.13 (0.07) | 1.23 (0.06) |
| SOC (%) | 2.57 (0.24) | 3.00 (0.09) | 3.14 (0.39) |
| Soil total Fe (%) | 2.47 (0.13) | 2.88 (0.11) | 2.32 (0.07) |
| Soil total Al (%) | 6.33 (0.56) | 6.34 (0.31) | 5.98 (0.54) |
| Soil pH | 5.98 (0.09) | 5.79 (0.11) | 5.84 (0.06) |
| Tree Component | Phosphorus (P) Concentration (g kg−1) | ||
|---|---|---|---|
| 10-Year-Old | 25-Year-Old | 50-Year-Old | |
| Green leaf | 1.8 (0.04) A | 1.6 (0.02) B | 1.0 (0.01) C |
| Root | 0.3 (0.01) B | 0.3 (0.01) B | 0.5 (0.01) A |
| Bark | 0.2 (0.02) A | 0.2 (0.01) A | 0.2 (0.03) A |
| Branch | 0.5 (0.02) C | 0.8 (0.01) A | 0.7 (0.05) B |
| Stem | 0.1 (0.01) A | 0.1 (0.01) A | 0.1 (0.01) A |
| Senesced leaf | 1.3 (0.02) A | 0.9 (0.01) B | 0.2 (0.01) C |
| N:P Ratio | |||
| Green Leaf | 13.9 (0.22) C | 15.4 (0.56) B | 18.8 (0.42) A |
| Root | 23.3 (0.32) A | 15.8 (0.40) B | 11.0 (0.58) C |
| Bark | 12.5 (0.24) A | 12.9 (0.12) A | 12.7 (0.36) A |
| Branch | 7.5 (0.47) A | 4.8 (0.36) C | 6.1 (0.67) B |
| Stem | 18.2 (0.46) A | 20.0 (0.84) A | 18.8 (0.64) A |
| Senesced leaf | 6.3 (0.21) A | 8.1 (0.34) B | 32.6 (1.21) C |
| Tree Component | Biomass (kg tree−1) | ||
|---|---|---|---|
| 10-Year-Old | 25-Year-Old | 50-Year-Old | |
| Leaf | 1.50 | 3.86 | 5.87 |
| Root | 1.69 | 13.84 | 52.77 |
| Bark | 2.00 | 21.47 | 33.23 |
| Branch | 0.76 | 10.85 | 35.80 |
| Stem | 3.67 | 69.77 | 244.71 |
| Total tree | 9.62 | 119.79 | 372.38 |
| Stand Age (years) | Resin-P (mg kg−1) | NaHCO3-P (mg kg−1) | NaOH-P (mg kg−1) | HCl-P (mg kg−1) | Residual-P (mg kg−1) | ||
|---|---|---|---|---|---|---|---|
| NaHCO3-Pi | NaHCO3-Po | NaOH-Pi | NaOH-Po | ||||
| 10 | 4.3 | 19.8 | 24.0 | 29.3 | 125.8 | 47.2 | 153.4 |
| (0.06) A | (0.23) A | (1.88) A | (0.65) B | (3.97) A | (0.82) A | (7.27) A | |
| 25 | 3.7 | 11.8 | 13.7 | 34.9 | 52.1 | 27.7 | 108.9 |
| (0.18) B | (0.81) B | (1.35) B | (1.43) A | (6.33) C | (0.45) B | (5.45) B | |
| 50 | 1.9 | 5.2 | 21.5 | 14.9 | 74.3 | 14.4 | 145.1 |
| (0.08) C | (0.30) C | (1.21) A | (1.52) C | (8.38) B | (0.67) C | (5.90) A | |
| Stand Age (years) | Soil Labile P Stock (kg ha−1) | Larch P Stock (kg ha−1) | Green Leaf P Stock (kg ha−1) | Senesced Leaf P Stock (kg ha−1) | PRE (%) |
|---|---|---|---|---|---|
| 10 | 135.61 | 15.87 | 9.79 | 5.42 | 44.60 |
| 25 | 66.15 | 50.15 | 11.91 | 5.01 | 58.01 |
| 50 | 70.53 | 65.07 | 5.47 | 0.93 | 83.71 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, F.; Chen, X.; Huang, B.; Chi, G. Distribution Changes of Phosphorus in Soil–Plant Systems of Larch Plantations across the Chronosequence. Forests 2018, 9, 563. https://doi.org/10.3390/f9090563
Zeng F, Chen X, Huang B, Chi G. Distribution Changes of Phosphorus in Soil–Plant Systems of Larch Plantations across the Chronosequence. Forests. 2018; 9(9):563. https://doi.org/10.3390/f9090563
Chicago/Turabian StyleZeng, Fanpeng, Xin Chen, Bin Huang, and Guangyu Chi. 2018. "Distribution Changes of Phosphorus in Soil–Plant Systems of Larch Plantations across the Chronosequence" Forests 9, no. 9: 563. https://doi.org/10.3390/f9090563
APA StyleZeng, F., Chen, X., Huang, B., & Chi, G. (2018). Distribution Changes of Phosphorus in Soil–Plant Systems of Larch Plantations across the Chronosequence. Forests, 9(9), 563. https://doi.org/10.3390/f9090563




