Consequences of a Reduced Number of Plant Functional Types for the Simulation of Forest Productivity
Abstract
1. Introduction
- How does the number of PFTs in forest models affect the predictions of aboveground biomass, basal area, forest productivity (GPP, NPP) and carbon sequestration (NEE)?
- What is the influence of pioneer species on the simulation of forest productivity during the early successional phase in tropical forests?
2. Materials and Methods
2.1. Study Site
2.2. Overview of the FORMIND Forest Model
2.3. Species Grouping into Plant Functional Types
2.4. Model Parameterization Versions
2.5. Simulation Experiments
3. Results
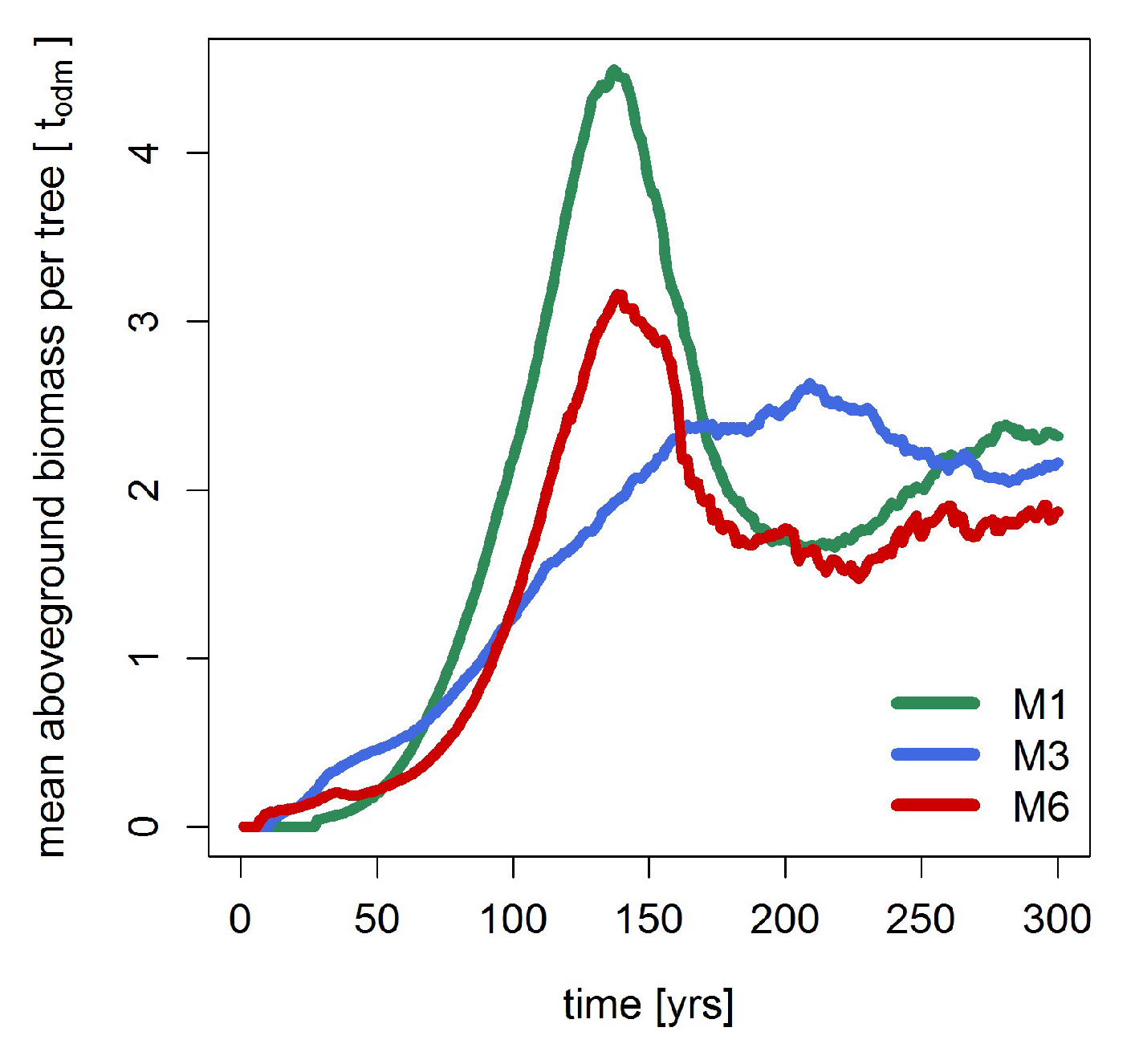
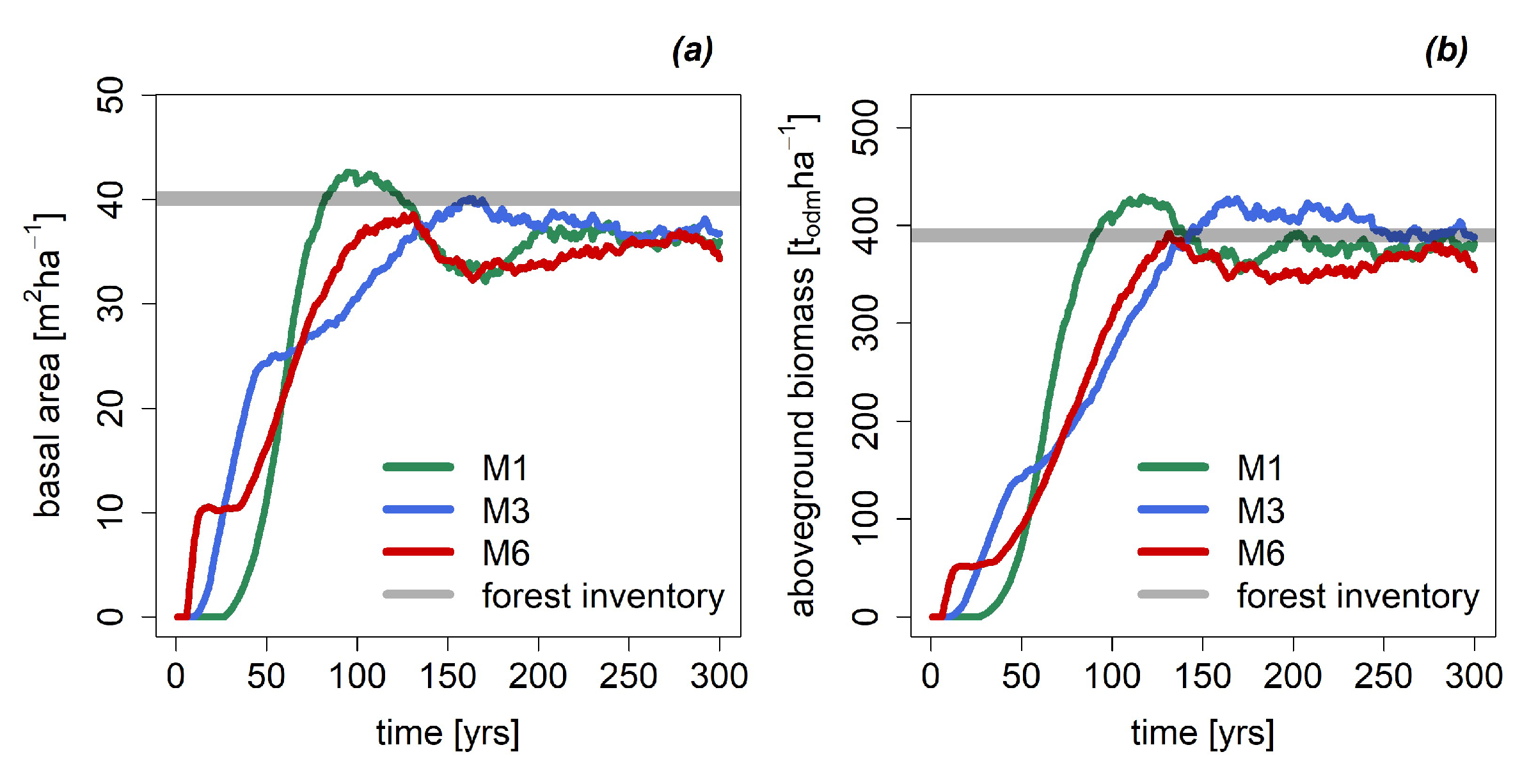
3.1. Basal Area and Biomass
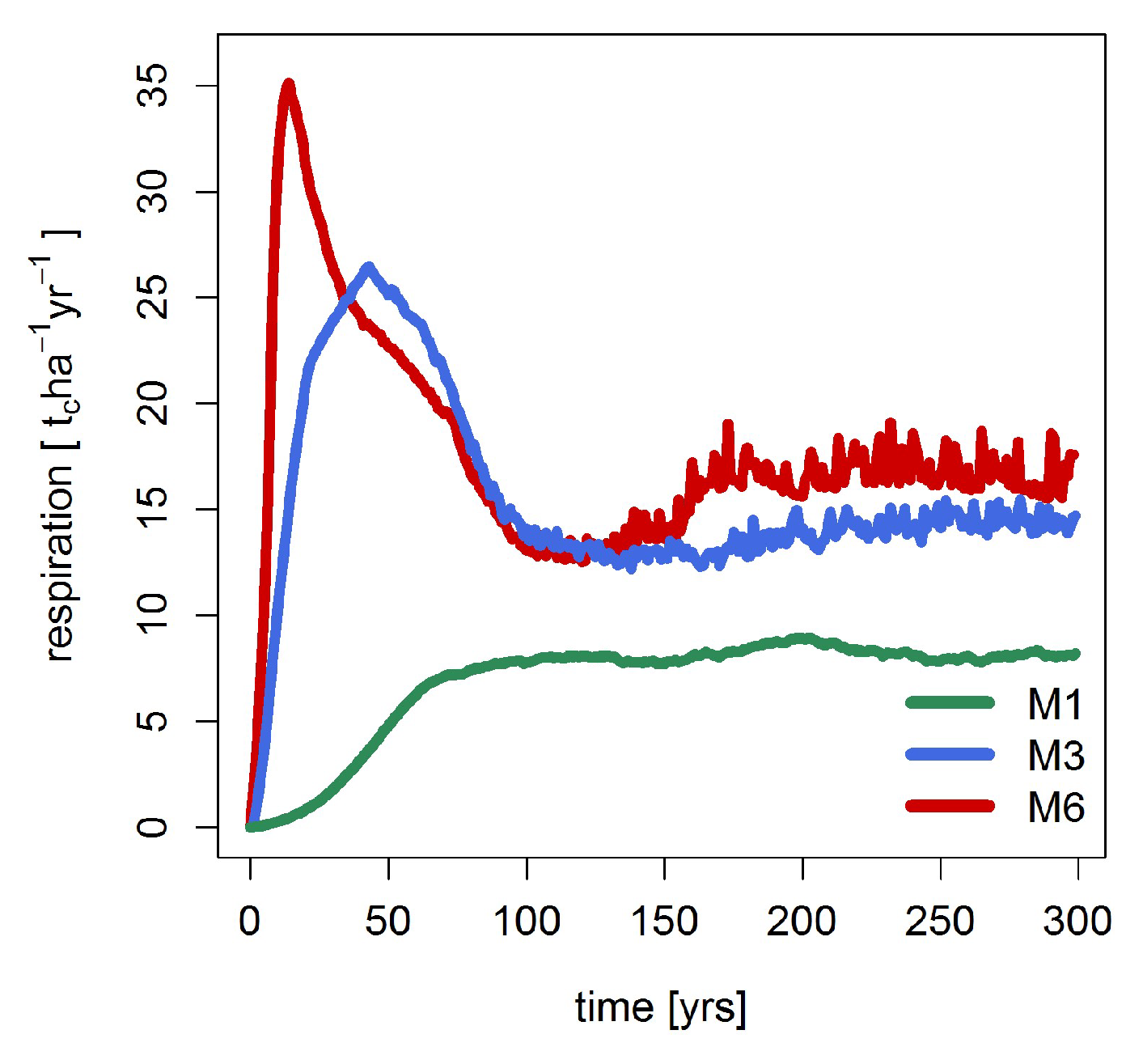
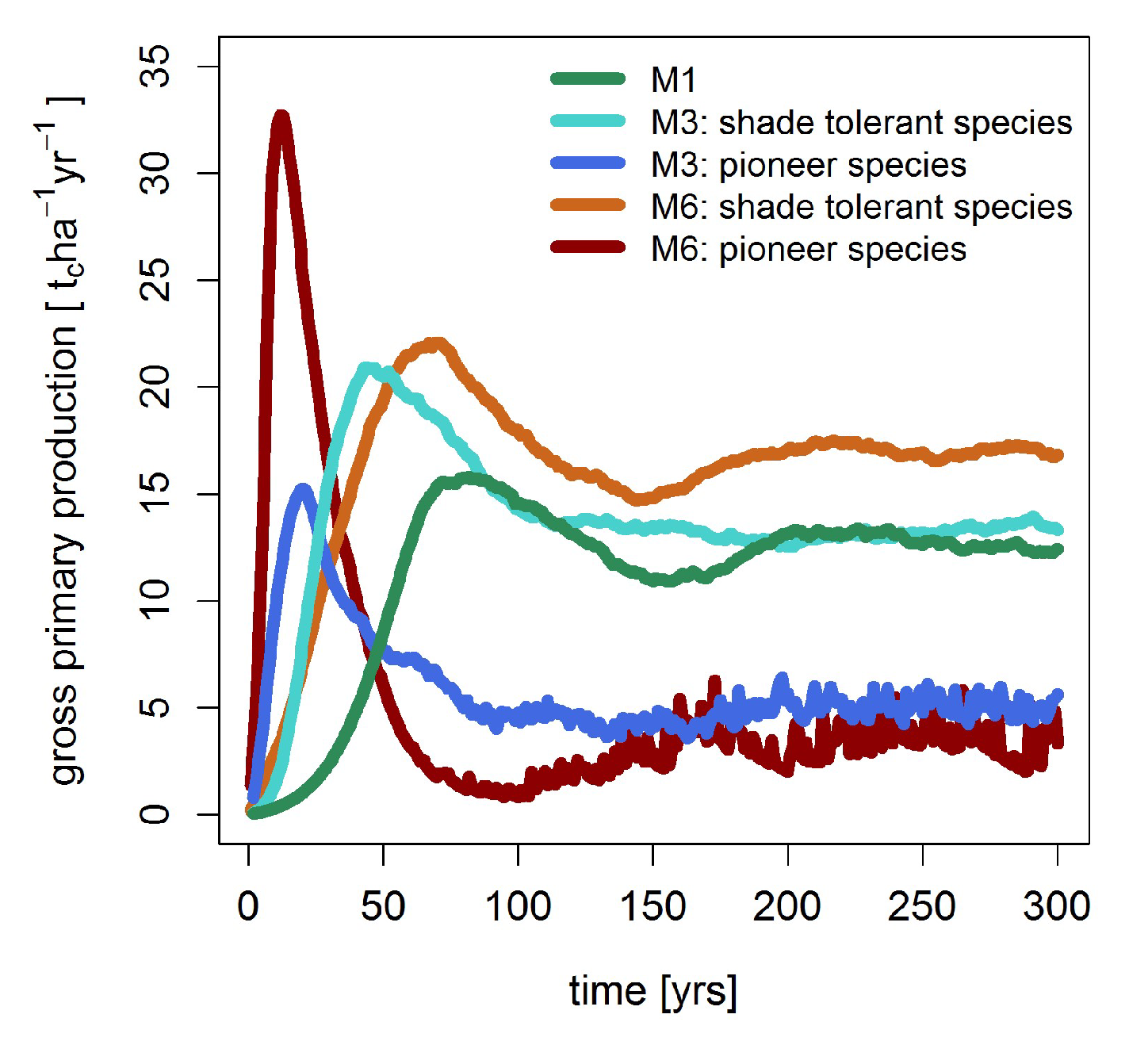
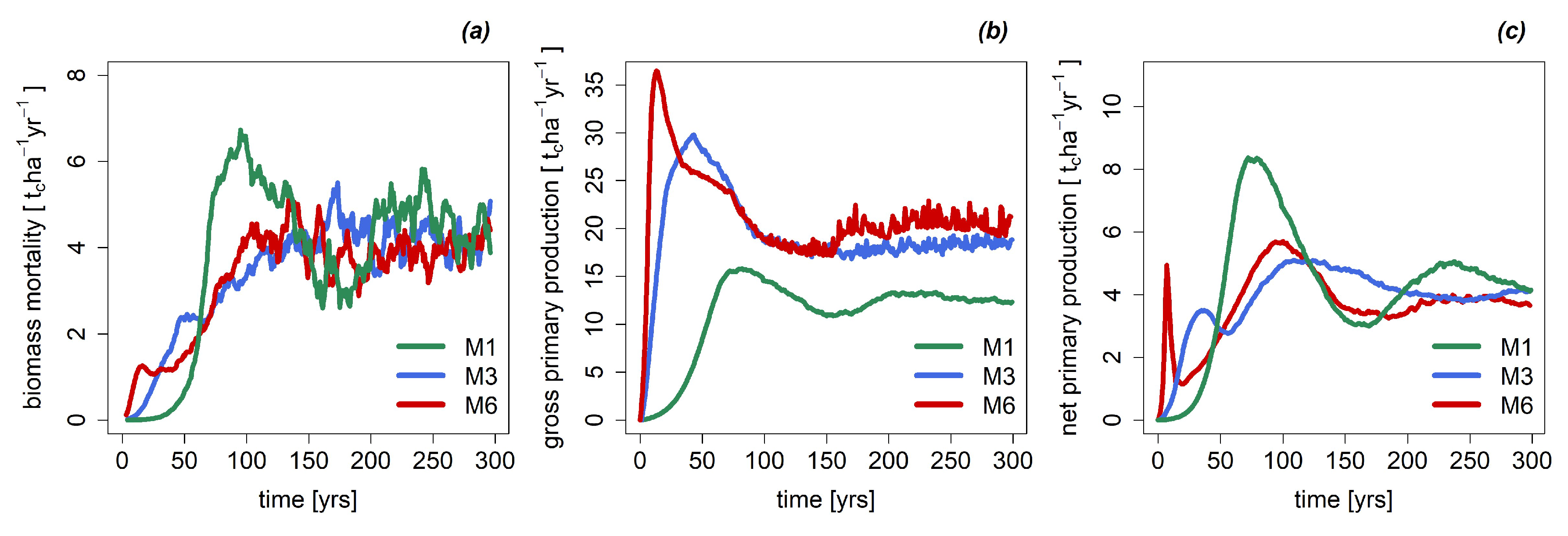
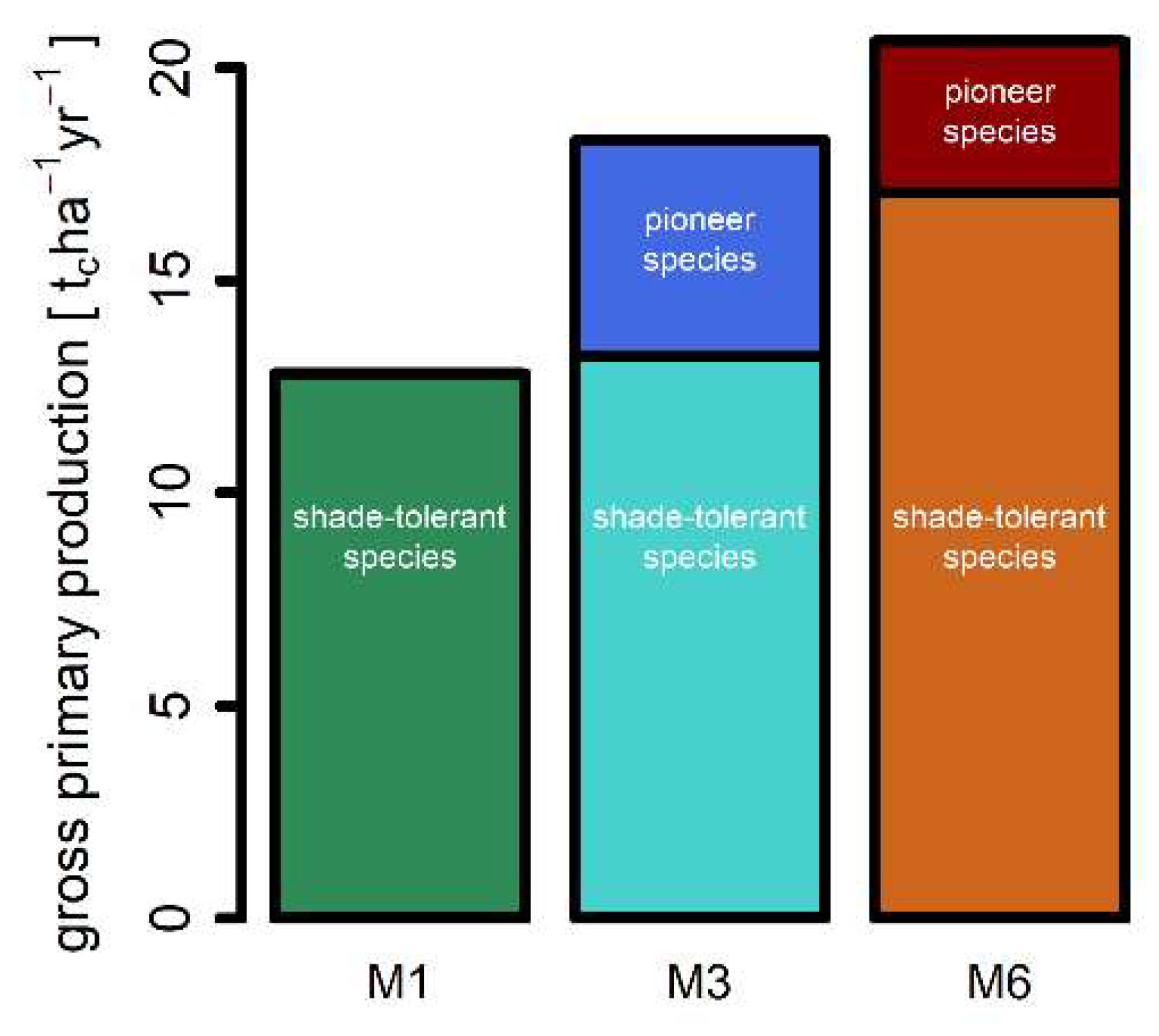
3.2. Mortality, Productivity and Respiration
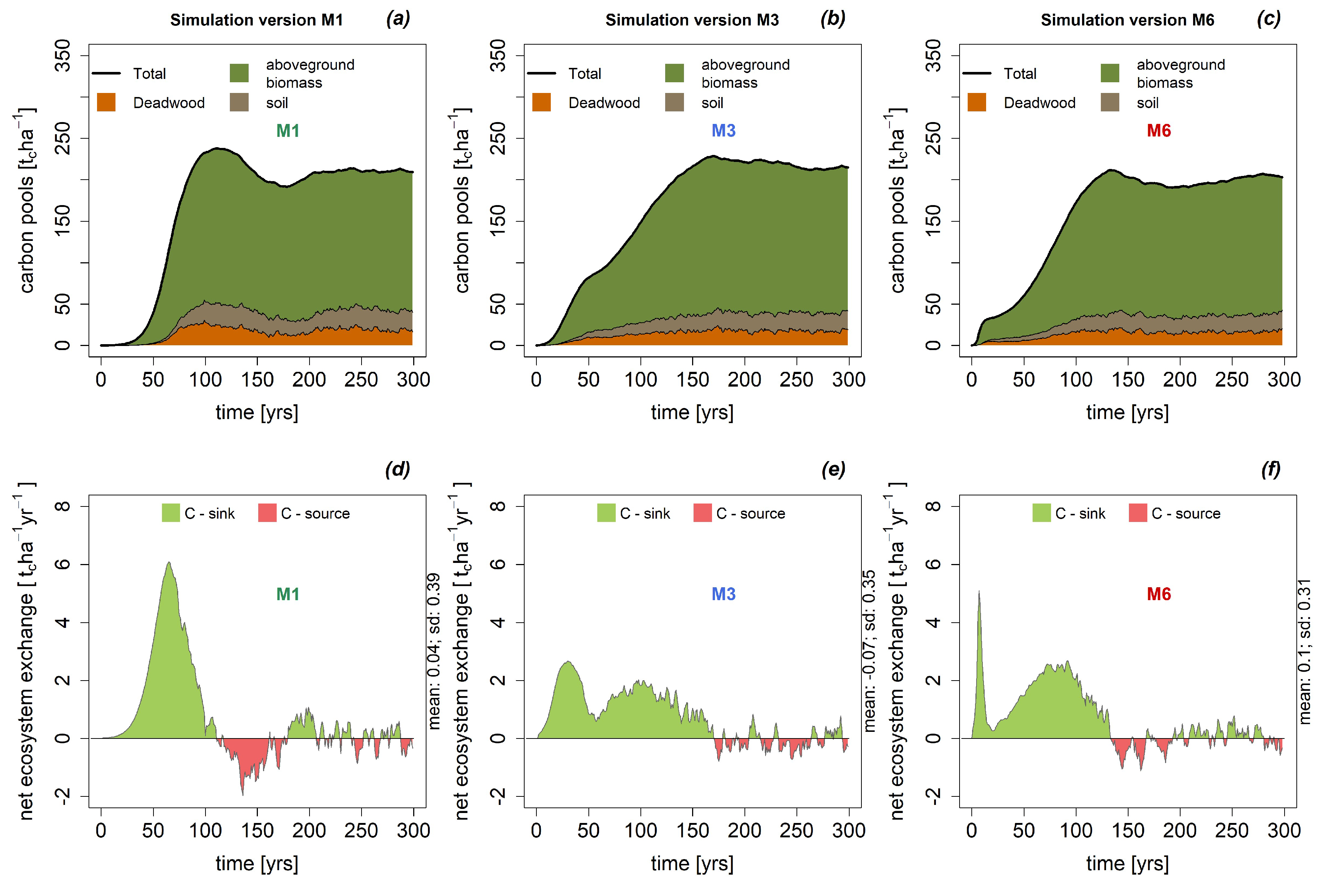
3.3. Carbon Stocks and Carbon Fluxes
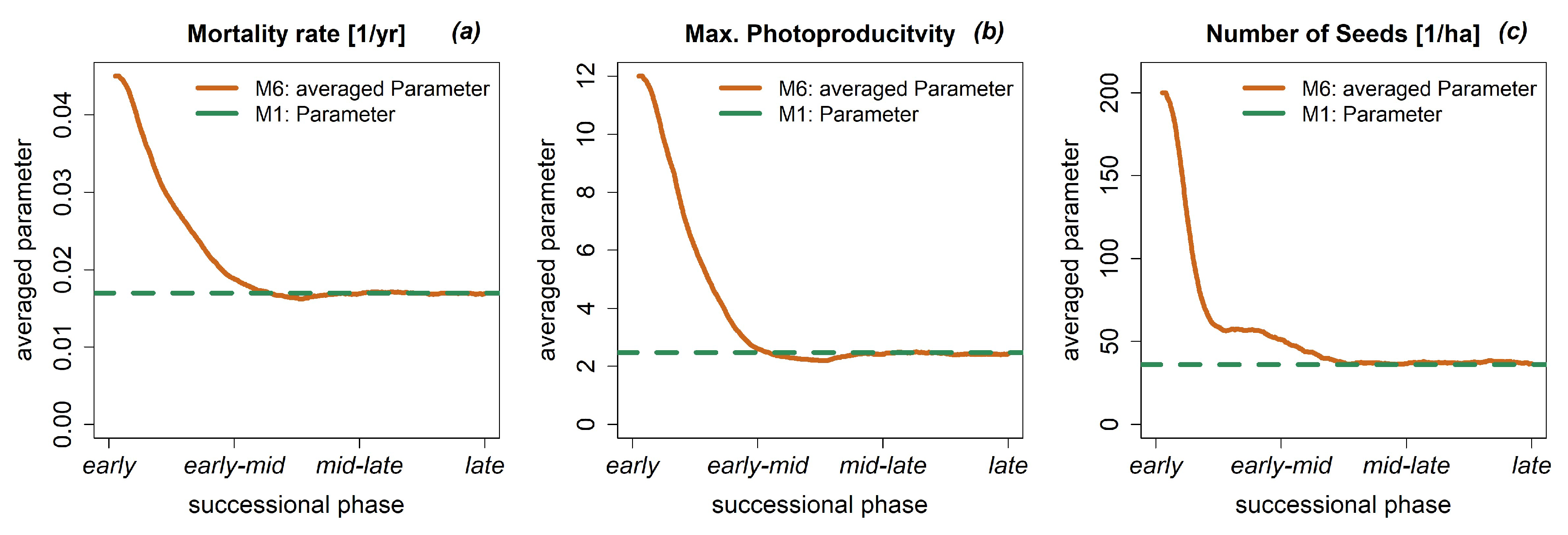
3.4. Dynamic Model Parameters
4. Discussion
4.1. The Influence of Species Grouping on Forest Dynamic Simulations
4.2. Functional Diversity and Forest Structure in DGVMs
4.3. Challenges of the PFT Approach in Forest Models
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
Appendix A.1.: Model Description and Parameter Values
| Parameter | Unit | Value | References | |
|---|---|---|---|---|
| General | tend | year | 300 | technical parameter |
| ty | year | 1 | technical parameter | |
| Aarea | ha | 9 | technical parameter | |
| Apatch | m2 | 400 | technical parameter | |
| MaxGrp | 1,3,6 | technical parameter | ||
| Δh | m | 0.5 | technical parameter | |
| Carbon Cycle | AET | mm year−1 | 1300 | [30] |
| tSslow -> A | year−1 | 1/750 | [11] | |
| tSfast -> A | year−1 | 1/15 | [11] | |
| Photo-synthesis | I0 | μmolphoton m−2 s−1 | 870 | [30] |
| k | 0.7 | [30,52] | ||
| lday | h | 12 | [30] | |
| ϕact | d | 360 | [30] | |
| Geometry | cl0 | 0.30 | [30,31,53] | |
| cd0 | 13.75 | field data | ||
| cd1 | 0.68 | field data | ||
| σ | 0.70 | [30,31,54] | ||
| f0 | 0.34 | [30,31] | ||
| f1 | −0.18 | [30,31] | ||
| l0 | 3.17 | [30,31] | ||
| l1 | 0.10 | [30,31] | ||
| Others | ffall | 0.4 | [30,55] | |
| Others | rg | 0.25 | [30,56] |
| Parameter | Unit | Plant Functional Type (PFT) | Ref. | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | ||||
| Geometry | Hmax | m | 56 | 33 | 33 | 28 | 16 | 16 | field data |
| h0 | 45.28 | 30.66 | 36.56 | 30.93 | 20.82 | 47.55 | field data | ||
| h1 | 0.57 | 0.41 | 0.44 | 0.43 | 0.34 | 0.6 | field data | ||
| ρ | tODM/m3 | 0.55 | 0.54 | 0.41 | 0.4 | 0.52 | 0.47 | field data | |
| Recruitment | Nseed | ha−1 year−1 | 30 | 156 | 21 | 300 | 2 | 200 | [30] |
| Iseed | 0.03 | 0.01 | 0.05 | 0.20 | 0.03 | 0.20 | [30,57] | ||
| Dmin | m | 0.02 | [30] | ||||||
| Mortality | MB | year−1 | 0.015 | 0.03 | 0.029 | 0.04 | 0.021 | 0.045 | [30] |
| Photo-synthesis | pmax | μmolCO2 μmolphoton−1 | 2.0 | 3.1 | 6.8 | 11.0 | 7.0 | 12.0 | [30,31,58,59] |
| α | μmolCO2 m−2 s−1 | 0.36 | 0.28 | 0.23 | 0.20 | 0.30 | 0.20 | [30,31,58,59] | |
| Growth | ∆D max | m year−1 | 0.012 | 0.012 | 0.019 | 0.029 | 0.011 | 0.029 | [30] |
| D ∆D max | % | 0.33 | 0.34 | 0.23 | 0.60 | 0.33 | 0.60 | [30] | |
| Parameter | Unit | Version M3 | Version M1 | |||
|---|---|---|---|---|---|---|
| I (1,2,5) | II (3) | III (4,6) | I (1–6) | |||
| Geometry | Hmax | m | 55.2 | 33 | 27.1 | 53.2 |
| h0 | 44.79 | 36.56 | 32.14 | 44.049 | ||
| h1 | 0.56 | 0.44 | 0.44 | 0.554 | ||
| ρ | tODM/m3 | 0.54 | 0.41 | 0.41 | 0.537 | |
| Recruitment | Nseed | ha−1 year−1 | 34 | 21 | 293 | 33 |
| Iseed | 0.029 | 0.05 | 0.20 | 0.032 | ||
| Dmin | m | 0.02 | 0.02 | |||
| Mortality | MB | year−1 | 0.0154 | 0.029 | 0.0404 | 0.017 |
| Photo-synthesis | pmax | μmolCO2 μmolphoton−1 | 2.05 | 6.80 | 11.07 | 2.479 |
| α | μmolCO2 m−2 s−1 | 0.357 | 0.230 | 0.200 | 0.346 | |
| Growth | ∆D max | m year−1 | 0.012 | 0.019 | 0.029 | 0.013 |
| D ∆D max | % | 0.3303 | 0.23 | 0.60 | 0.323 | |
Appendix A.2.: Simulation of the Forest Dynamics with FORMIND
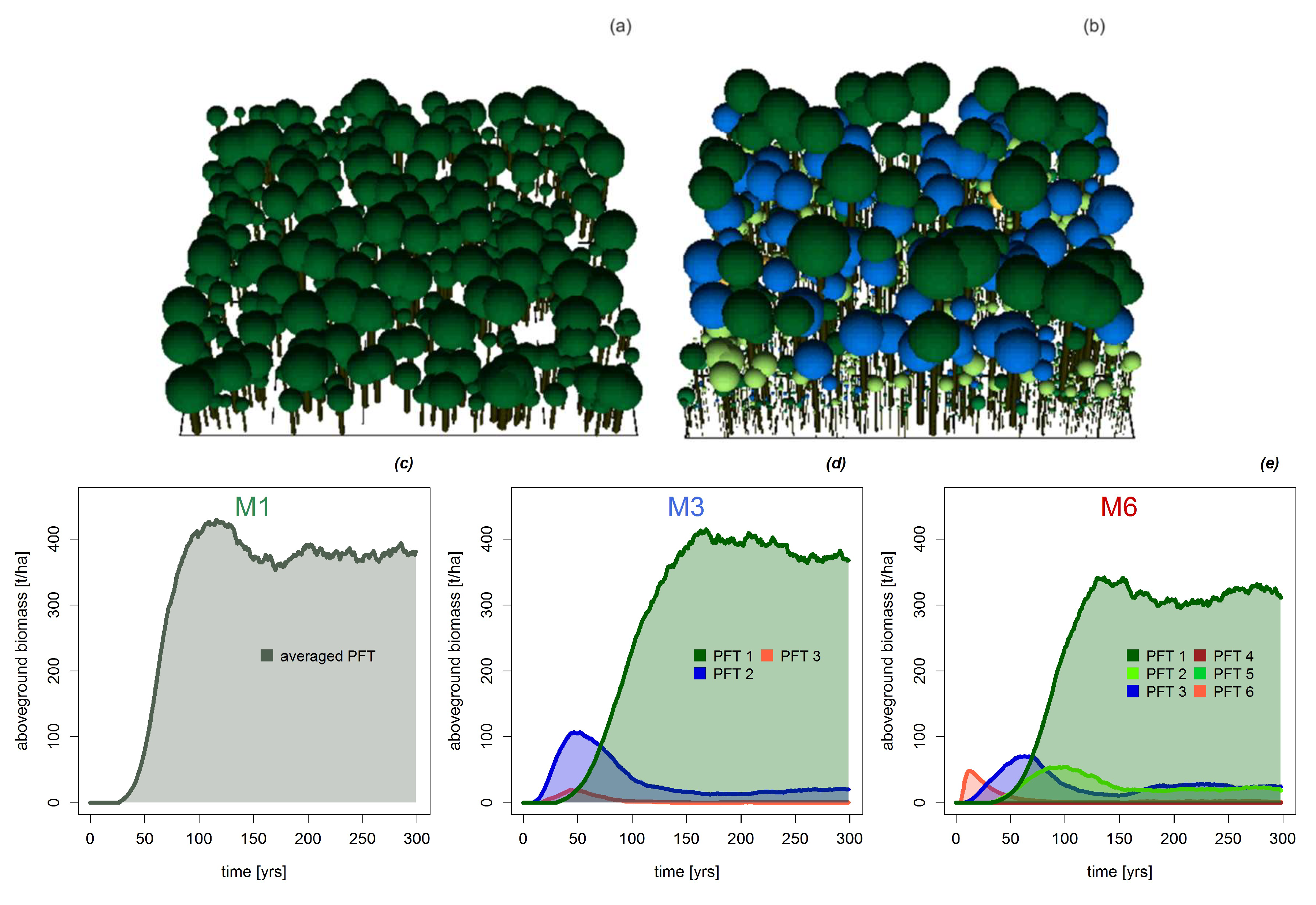
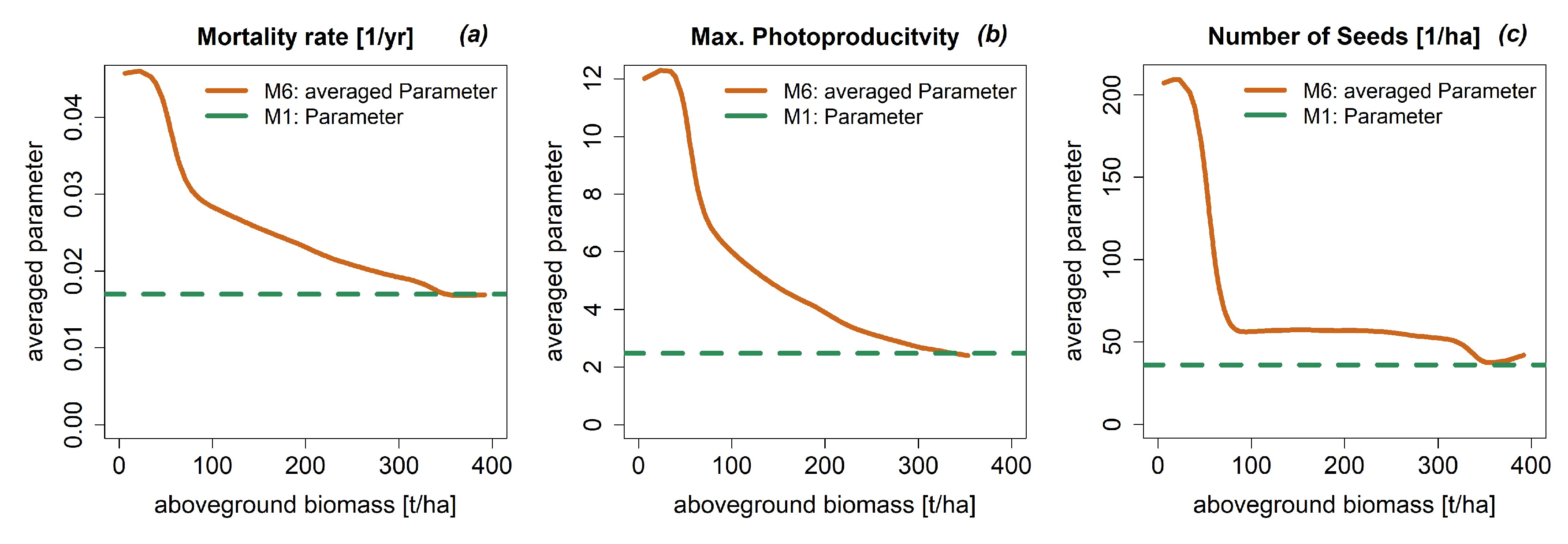
Appendix A.3.: Testing Model Simulations with Field Data
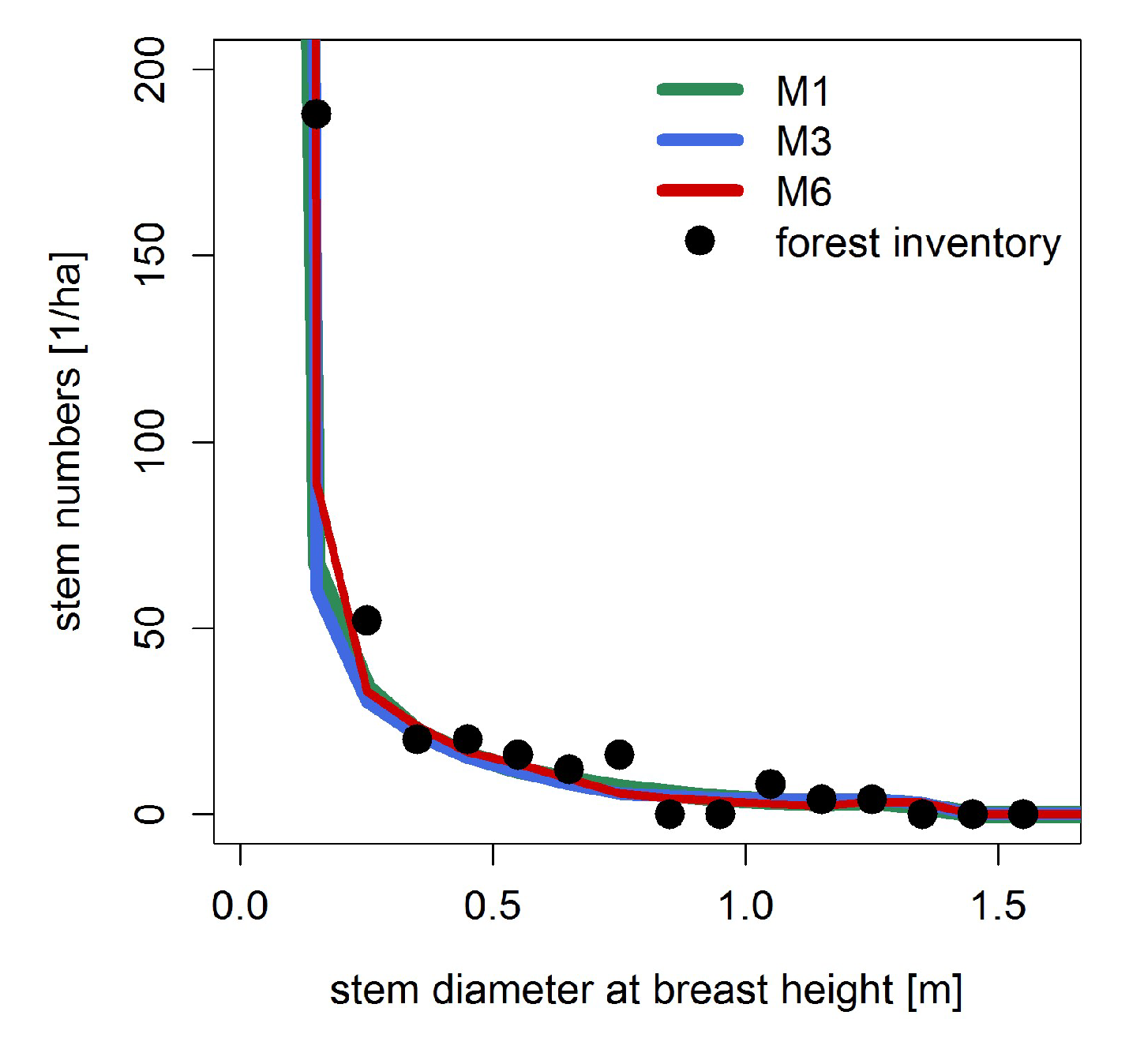
References
- Bonan, G.B. Forests and climate change: Forcings, feedbacks, and the climate benefits of forests. Science 2008, 320, 1444–1449. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.D.; Birdsey, R.A.; Fang, J.Y.; Houghton, R.; Kauppi, P.E.; Kurz, W.A.; Phillips, O.L.; Shvidenko, A.; Lewis, S.L.; Canadell, J.G.; et al. A large and persistent carbon sink in the world's forests. Science 2011, 333, 988–993. [Google Scholar] [CrossRef] [PubMed]
- Pimm, S.L.; Raven, P. Biodiversity - extinction by numbers. Nature 2000, 403, 843–845. [Google Scholar] [CrossRef] [PubMed]
- Slik, J.W.F.; Arroyo-Rodríguez, V.; Aiba, S.-I.; Alvarez-Loayza, P.; Alves, L.F.; Ashton, P.; Balvanera, P.; Bastian, M.L.; Bellingham, P.J.; van den Berg, E.; et al. An estimate of the number of tropical tree species. Proc. Natl. Acad. Sci. USA 2015, 112, 7472–7477. [Google Scholar] [CrossRef] [PubMed]
- ter Steege, H.; Pitman, N.C.A.; Sabatier, D.; Baraloto, C.; Salomão, R.P.; Guevara, J.E.; Phillips, O.L.; Castilho, C.V.; Magnusson, W.E.; Molino, J.-F.; et al. Hyperdominance in the amazonian tree flora. Science 2013, 342. [Google Scholar] [CrossRef] [PubMed]
- Snell, R.S.; Huth, A.; Nabel, J.E.M.S.; Bocedi, G.; Travis, J.M.J.; Gravel, D.; Bugmann, H.; Gutiérrez, A.G.; Hickler, T.; Higgins, S.I.; et al. Using dynamic vegetation models to simulate plant range shifts. Ecography 2014, 37, 1184–1197. [Google Scholar] [CrossRef]
- Friend, A.D.; Shugart, H.H.; Running, S.W. A physiology-based model of forest dynamics. Ecology 1993, 74, 792–797. [Google Scholar] [CrossRef]
- Rammig, A.; Jupp, T.; Thonicke, K.; Tietjen, B.; Heinke, J.; Ostberg, S.; Lucht, W.; Cramer, W.; Cox, P. Estimating the risk of amazonian forest dieback. New Phytol. 2010, 187, 694–706. [Google Scholar] [CrossRef] [PubMed]
- Friend, A.D.; Arneth, A.; Kiang, N.Y.; Lomas, M.; Ogee, J.; Rodenbeckk, C.; Running, S.W.; Santaren, J.D.; Sitch, S.; Viovy, N.; et al. Fluxnet and modelling the global carbon cycle. Glob. Chang. Biol. 2007, 13, 610–633. [Google Scholar] [CrossRef]
- Verbeeck, H.; Peylin, P.; Bacour, C.; Bonal, D.; Steppe, K.; Ciais, P. Seasonal patterns of co2 fluxes in amazon forests: Fusion of eddy covariance data and the orchidee model. J. Geophys. Res. Biogeosci. 2011, 116, G02018. [Google Scholar] [CrossRef]
- Sato, H.; Itoh, A.; Kohyama, T. Seib-dgvm: A new dynamic global vegetation model using a spatially explicit individual-based approach. Ecol. Model. 2007, 200, 279–307. [Google Scholar] [CrossRef]
- Köhler, P.; Ditzer, T.; Huth, A. Concepts for the aggregation of tropical tree species into functional types and the application on sabah's dipterocarp lowland rain forests. J. Trop. Ecol 2000, 16, 591–602. [Google Scholar] [CrossRef]
- Smith, T.M.; Shugart, H.H. Plant Funct. Types; Cambridge University Press: Cambridge, UK, 1997. [Google Scholar]
- Gourlet-Fleury, S.; Blanc, L.; Picard, N.; Sist, P.; Dick, J.; Nasi, R.; Swaine, M.D.; Forni, E. Grouping species for predicting mixed tropical forest dynamics: Looking for a strategy. Ann. For. Sci. 2005, 62, 785–796. [Google Scholar] [CrossRef]
- Picard, N.; Köhler, P.; Mortier, F.; Gourlet-Fleury, S. A comparison of five classifications of species into functional groups in tropical forests of french guiana. Ecol. Complex. 2012, 11, 75–83. [Google Scholar] [CrossRef]
- Vanclay, J.K.; Skovsgaard, J.P. Evaluating forest growth models. Ecol. Model. 1997, 98, 1–12. [Google Scholar] [CrossRef]
- Kazmierczak, M.; Wiegand, T.; Huth, A. A neutral vs. Non-neutral parametrizations of a physiological forest gap model. Ecol. Model. 2014, 288, 94–102. [Google Scholar] [CrossRef]
- Lavorel, S.; D¡az, S.; Cornelissen, J.H.C.; Garnier, E.; Harrison, S.P.; McIntyre, S.; Pausas, J.G.; P‚rez-Harguindeguy, N.; Roumet, C.; Urcelay, C. Plant functional types: Are we getting any closer to the holy grail. In Terrestrial Ecosystems in a Changing World; Canadell, J.G., Pataki, D., Pitelka, L.F., Eds.; Springer: Berlin, Germany, 2007. [Google Scholar]
- Poulter, B.; Ciais, P.; Hodson, E.; Lischke, H.; Maignan, F.; Plummer, S.; Zimmermann, N.E. Plant functional type mapping for earth system models. Geosci. Model Dev. 2011, 4, 993–1010. [Google Scholar] [CrossRef]
- Sitch, S.; Smith, B.; Prentice, I.C.; Arneth, A.; Bondeau, A.; Cramer, W.; Kaplan, J.O.; Levis, S.; Lucht, W.; Sykes, M.T.; et al. Evaluation of ecosystem dynamics, plant geography and terrestrial carbon cycling in the lpj dynamic global vegetation model. Glob. Chang. Biol. 2003, 9, 161–185. [Google Scholar] [CrossRef]
- Medvigy, D.; Wofsy, S.C.; Munger, J.W.; Hollinger, D.Y.; Moorcroft, P.R. Mechanistic scaling of ecosystem function and dynamics in space and time: Ecosystem demography model version 2. J. Geophys. Res. Biogeosci. 2009, 114, G01002. [Google Scholar] [CrossRef]
- Kim, Y.; Knox, R.G.; Longo, M.; Medvigy, D.; Hutyra, L.R.; Pyle, E.H.; Wofsy, S.C.; Bras, R.L.; Moorcroft, P.R. Seasonal carbon dynamics and water fluxes in an a mazon rainforest. Glob. Chang. Biol. 2012, 18, 1322–1334. [Google Scholar] [CrossRef]
- Shugart, H.H. A Theory of Forest Dynamics; The Blackburn Press: Blackburn Road, Prince George, BC, Canada, 2003. [Google Scholar]
- Köhler, P.; Huth, A. The effects of tree species grouping in tropical rainforest modelling: Simulations with the individual-based model formind. Ecol. Model. 1998, 109, 301–321. [Google Scholar] [CrossRef]
- Fischer, R.; Bohn, F.; Dantas de Paula, M.; Dislich, C.; Groeneveld, J.; Gutiérrez, A.G.; Kazmierczak, M.; Knapp, N.; Lehmann, S.; Paulick, S.; et al. Lessons learned from applying a forest gap model to understand ecosystem and carbon dynamics of complex tropical forests. Ecol. Model. 2016, 326, 124–133. [Google Scholar] [CrossRef]
- Köhler, P.; Huth, A. Simulating growth dynamics in a south-east asian rainforest threatened by recruitment shortage and tree harvesting. Clim. Chang. 2004, 67, 95–117. [Google Scholar]
- Peters, M.K.; Hemp, A.; Appelhans, T.; Behler, C.; Classen, A.T.; Detsch, F.; Ensslin, A.; Ferger, S.W.; Frederiksen, S.B.; Gebert, F.; et al. Predictors of elevational biodiversity gradients change from single taxa to the multi-taxa community level. Nat. Commun. 2016, 7, art. 13736. [Google Scholar] [CrossRef] [PubMed]
- Rutten, G.; Ensslin, A.; Hemp, A.; Fischer, M. Forest structure and composition of previously selectively logged and non-logged montane forests at mt. Kilimanjaro. For. Ecol. Manag. 2015, 337, 61–66. [Google Scholar] [CrossRef]
- Ensslin, A.; Rutten, G.; Pommer, U.; Zimmermann, R.; Hemp, A.; Fischer, M. Effects of elevation and land use on the biomass of trees, shrubs and herbs at mount kilimanjaro. Ecosphere 2015, 6, 1–15. [Google Scholar] [CrossRef]
- Fischer, R.; Ensslin, A.; Rutten, G.; Fischer, M.; Schellenberger Costa, D.; Kleyer, M.; Hemp, A.; Paulick, S.; Huth, A. Simulating carbon stocks and fluxes of an african tropical montane forest with an individual-based forest model. PLoS ONE 2015, 10, e0123300. [Google Scholar] [CrossRef] [PubMed]
- Dislich, C.; Günter, S.; Homeier, J.; Schröder, B.; Huth, A. Simulating forest dynamics of a tropical montane forest in south ecuador. Erdkunde 2009, 63, 347–364. [Google Scholar] [CrossRef]
- Shugart, H.H.; Wang, B.; Fischer, R.; Ma, J.; Fang, J.; Yan, X.; Huth, A.; Armstrong, A.H. Gap models and their individual-based relatives in the assessment of the consequences of global change. Environ. Res. Lett. 2018, 13, 033001. [Google Scholar] [CrossRef]
- Rödig, E.; Huth, A.; Bohn, F.; Rebmann, C.; Cuntz, M. Estimating the carbon fluxes of forests with an individual-based forest model. For. Ecosyst. 2017, 4. [Google Scholar]
- Van Bodegom, P.M.; Douma, J.C.; Witte, J.P.M.; Ordoñez, J.C.; Bartholomeus, R.P.; Aerts, R. Going beyond limitations of plant functional types when predicting global ecosystem–atmosphere fluxes: Exploring the merits of traits-based approaches. Glob. Ecol. Biogeogr. 2012, 21, 625–636. [Google Scholar] [CrossRef]
- Poulter, B.; Aragão, L.; Heyder, U.; Gumpenberger, M.; Heinke, J.; Langerwisch, F.; Rammig, A.; Thonicke, K.; Cramer, W. Net biome production of the amazon basin in the 21st century. Glob. Chang. Biol. 2010, 16, 2062–2075. [Google Scholar] [CrossRef]
- Poorter, L.; Bongers, F.; Aide, T.M.; Zambrano, A.M.A.; Balvanera, P.; Becknell, J.M.; Boukili, V.; Brancalion, P.H.; Broadbent, E.N.; Chazdon, R.L. Biomass resilience of neotropical secondary forests. Nature 2016, 530, 211–214. [Google Scholar] [CrossRef] [PubMed]
- Le Quéré, C.; Andrew, R.M.; Canadell, J.G.; Sitch, S.; Korsbakken, J.I.; Peters, G.P.; Manning, A.C.; Boden, T.A.; Tans, P.P.; Houghton, R.A.; et al. Global carbon budget 2016. Earth Syst. Sci. Data 2016, 8, 605–649. [Google Scholar] [CrossRef]
- Hickler, T.; Vohland, K.; Feehan, J.; Miller, P.A.; Smith, B.; Costa, L.; Giesecke, T.; Fronzek, S.; Carter, T.R.; Cramer, W.; et al. Projecting the future distribution of european potential natural vegetation zones with a generalized, tree species-based dynamic vegetation model. Glob. Ecol. Biogeogr. 2012, 21, 50–63. [Google Scholar] [CrossRef]
- Naudts, K.; Ryder, J.; McGrath, M.J.; Otto, J.; Chen, Y.; Valade, A.; Bellasen, V.; Berhongaray, G.; Bönisch, G.; Campioli, M.; et al. A vertically discretised canopy description for orchidee (svn r2290) and the modifications to the energy, water and carbon fluxes. Geosci. Model Dev. Dis. 2014, 7, 8565–8647. [Google Scholar] [CrossRef]
- Hickler, T.; Smith, B.; Sykes, M.T.; Davis, M.B.; Sugita, S.; Walker, K. Using a generalized vegetation model to simulate vegetation dynamics in northeastern USA. Ecology 2004, 85, 519–530. [Google Scholar] [CrossRef]
- Sakschewski, B.; von Bloh, W.; Boit, A.; Poorter, L.; Pena-Claros, M.; Heinke, J.; Joshi, J.; Thonicke, K. Resilience of amazon forests emerges from plant trait diversity. Nat. Clim. Chang. 2016, 6, 1032–1036. [Google Scholar] [CrossRef]
- Kattge, J.; Díaz, S.; Lavorel, S.; Prentice, I.C.; Leadley, P.; Bönisch, G.; Garnier, E.; Westoby, M.; Reich, P.B.; Wright, I.J.; et al. Try a global database of plant traits. Glob. Chang. Biol. 2011, 17, 2905–2935. [Google Scholar] [CrossRef]
- Seidl, R.; Rammer, W.; Scheller, R.M.; Spies, T.A. An individual-based process model to simulate landscape-scale forest ecosystem dynamics. Ecol. Model. 2012, 231, 87–100. [Google Scholar] [CrossRef]
- Rödig, E.; Cuntz, M.; Heinke, J.; Rammig, A.; Huth, A. Spatial heterogeneity of biomass and forest structure of the amazon rain forest: Linking remote sensing, forest modelling and field inventory. Glob. Ecol. Biogeogr. 2017, 26, 1292–1302. [Google Scholar] [CrossRef]
- Seidl, R.; Rammer, W.; Blennow, K. Simulating wind disturbance impacts on forest landscapes: Tree-level heterogeneity matters. Environ. Model. Softw. 2014, 51, 1–11. [Google Scholar]
- Rödig, E.; Cuntz, M.; Rammig, A.; Fischer, R.; Taubert, F.; Huth, A. The importance of forest structure for carbon fluxes of the amazon rainforest. Environ. Res. Lett. 2018, 13, 054013. [Google Scholar] [CrossRef]
- Seidl, R.; Spies, T.A.; Rammer, W.; Steel, E.A.; Pabst, R.J.; Olsen, K. Multi-scale drivers of spatial variation in old-growth forest carbon density disentangled with lidar and an individual-based landscape model. Ecosystems 2012, 15, 1321–1335. [Google Scholar] [CrossRef]
- Guisan, A.; Thuiller, W. Predicting species distribution: Offering more than simple habitat models. Ecol. Lett. 2005, 8, 993–1009. [Google Scholar] [CrossRef]
- Jeltsch, F.; Moloney, K.A.; Schurr, F.M.; Kochy, M.; Schwager, M. The state of plant population modelling in light of environmental change. Perspec. Plant Ecol. Evol. Syst. 2008, 9, 171–189. [Google Scholar] [CrossRef]
- Picard, N.; Franc, A. Are ecological groups of species optimal for forest dynamics modelling? Ecol. Model. 2003, 163, 175–186. [Google Scholar] [CrossRef]
- Bugmann, H. Functional types of trees in temperate and boreal forests: Classification and testing. J. Veg. Sci. 1996, 7, 359–370. [Google Scholar] [CrossRef]
- Huth, A.; Ditzer, T. Simulation of the growth of a lowland dipterocarp rain forest with formix3. Ecol. Model. 2000, 134, 1–25. [Google Scholar] [CrossRef]
- Rüger, N.; Gutiérrez, A.G.; Kissling, W.D.; Armesto, J.J.; Huth, A. Ecological impacts of different harvesting scenarios for temperate evergreen rain forest in southern chile - a simulation experiment. For. Ecol. Manag. 2007, 252, 52–66. [Google Scholar] [CrossRef]
- Nenninger, A. Oberirdische biomasse ausgewählter baumarten eines tropischen bergregenwaldes in südecuador. Diploma Thesis, Technische Universität München, Munich, Germany, 2006. [Google Scholar]
- Brokaw, N.V.L. Gap-phase regeneration in a tropical forest. Ecology 1985, 66, 682–687. [Google Scholar] [CrossRef]
- Ryan, M.G. Effects of climate change on plant respiration. Ecol. Appl. 1991, 1, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Rüger, N. Dynamics and Sustainable Use of Species-Rich Moist Forests. A Process-Based Modelling Approach. Ph.D. thesis. 30 June 2006.
- Cai, Z.Q.; Rijkers, T.; Bongers, F. Photosynthetic acclimation to light changes in tropical monsoon forest woody species differing in adult stature. Tree Physiol. 2005, 25, 1023–1031. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Chen, Y.J.; Song, L.Y.; Liu, N.; Sun, L.L.; Peng, C.L. Utilization of lightflecks by seedlings of five dominant tree species of different subtropical forest successional stages under low-light growth conditions. Tree Physiol. 2012, 32, 545–553. [Google Scholar] [CrossRef] [PubMed]
| PFT | Maximum Height [m] | Light Class | Exemplary Tree Species | Biomass [t ha−1] |
|---|---|---|---|---|
| 1 | >33 | Shade tolerant | Strombosia scheffleri | 344.18 |
| 2 | 16 > 33 | Shade tolerant | Heinsenia diervilleoides | 10.20 |
| 3 | 16 > 33 | Intermediate | Ficus sur | 33.22 |
| 4 | 16 > 33 | Shade intolerant | Polyscias albersiana | 1.15 |
| 5 | <16 | Shade tolerant | Leptonychia usambarensis | 0.96 |
| 6 | <16 | Shade intolerant | Cyathea manniana | 0.09 |
| Model Parameterization Version | Number of PFTs | Averaging of Traits | |
|---|---|---|---|
| M6 | Grouping by light demands and height classes | 6 | No averaging. Original PFT grouping (Table 1) |
| M3 | Grouping only by light demands | 3 | Pioneers (PFT 4 + 6) Intermediates (PFT 3) Climax (PFT 1 + 2 + 5) |
| M1 | No grouping. Mean species approach | 1 | Mean species (PFT 1−6) |
| Parameter | S Seeds [1 ha−1] | M Mortality Rate [1 year−1] | Hmax Max. Height [m] | Pmax Max. Photo-Synthesis [µ molCO2 / (m2s)] | Gy Maximum Yearly Increment of DBH [m/year] | W Wood Density [t/m3] |
|---|---|---|---|---|---|---|
| M6 with 6 PFTs | ||||||
| PFT 1 | 30 | 0.015 | 56 | 2.0 | 0.012 | 0.55 |
| PFT 2 | 156 | 0.030 | 33 | 3.1 | 0.012 | 0.54 |
| PFT 3 | 21 | 0.029 | 33 | 6.8 | 0.019 | 0.41 |
| PFT 4 | 300 | 0.040 | 28 | 11.0 | 0.029 | 0.40 |
| PFT 5 | 2 | 0.021 | 16 | 7.0 | 0.011 | 0.52 |
| PFT 6 | 200 | 0.045 | 16 | 12.0 | 0.029 | 0.47 |
| M3 with 3 PFTs | ||||||
| Shade tolerant (PFT 1, 2, 5) | 34 | 0.0154 | 55.2 | 2.05 | 0.012 | 0.549 |
| Interm. tolerant (PFT 3) | 21 | 0.0290 | 33.0 | 6.8 | 0.019 | 0.410 |
| Shade intolerant (PFT 4, 6) | 293 | 0.0404 | 27.1 | 11.07 | 0.029 | 0.405 |
| M1 with 1 PFT | ||||||
| Mean PFT | 33 | 0.017 | 53.2 | 2.5 | 0.013 | 0.53 |
| Characteristic | M1 | M3 | M6 |
|---|---|---|---|
| Total number of PFT-dependent parameter values | 12 | 36 | 72 |
| Runtime (9 ha, 300 years) [seconds] | 30 | 110 | 240 |
| Basal Area [m2 ha−1] | 36.2 | 36.8 | 35.9 |
| Biomass [t ha−1] | 379 | 391 | 370 |
| Mortality [tc ha−1 year−1] | 4.7 | 4.2 | 4.0 |
| Gross primary production [tc ha−1 year−1] | 12.8 | 18.3 | 20.6 |
| Net primary production [tc ha−1 year−1] | 4.6 | 4.0 | 3.8 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fischer, R.; Rödig, E.; Huth, A. Consequences of a Reduced Number of Plant Functional Types for the Simulation of Forest Productivity. Forests 2018, 9, 460. https://doi.org/10.3390/f9080460
Fischer R, Rödig E, Huth A. Consequences of a Reduced Number of Plant Functional Types for the Simulation of Forest Productivity. Forests. 2018; 9(8):460. https://doi.org/10.3390/f9080460
Chicago/Turabian StyleFischer, Rico, Edna Rödig, and Andreas Huth. 2018. "Consequences of a Reduced Number of Plant Functional Types for the Simulation of Forest Productivity" Forests 9, no. 8: 460. https://doi.org/10.3390/f9080460
APA StyleFischer, R., Rödig, E., & Huth, A. (2018). Consequences of a Reduced Number of Plant Functional Types for the Simulation of Forest Productivity. Forests, 9(8), 460. https://doi.org/10.3390/f9080460





