Variation in Tree Community Composition and Carbon Stock under Natural and Human Disturbances in Andean Forests, Peru
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Sites
2.2. Field Survey
2.3. Estimation of Biomass, CWD, and Total Carbon Stock
2.4. Analyses of Community Composition and Species Richness
3. Results
3.1. AGB, CWD, and Total Carbon Stock
3.2. Ordination of the Study Plots
3.3. Classification of Different Elevation Zones
3.4. Factors Affecting Community Composition
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Smith, P.; Bustamante, M.; Ahammad, H.; Clark, H.; Dong, H.; Elsiddig, E.A.; Haberl, H.; Harper, R.; House, J.; Jafari, M.; et al. Agriculture, Forestry and Other Land Use (AFOLU). In Climate Change 2014: Mitigation of Climate Change. Contribution of Working Group III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Edenhofer, O., Pichs-Madruga, R., Sokona, Y., Farahani, E., Kadner, S., Seyboth, K., Adler, A., Baum, I., Brunner, S., Eickemeier, P., et al., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2014; pp. 811–922. [Google Scholar]
- Strassburg, B.B.N.; Rodrigues, A.S.L.; Gusti, M.; Balmford, A.; Fritz, S.; Obersteiner, M.; Kerry Turner, R.; Brooks, T.M. Impacts of incentives to reduce emissions from deforestation on global species extinctions. Nat. Clim. Chang. 2012, 2, 350. [Google Scholar] [CrossRef]
- Barlow, J.; Lennox, G.D.; Ferreira, J.; Berenguer, E.; Lees, A.C.; Nally, R.M.; Thomson, J.R.; Ferraz, S.F.D.B.; Louzada, J.; Oliveira, V.H.F.; et al. Anthropogenic disturbance in tropical forests can double biodiversity loss from deforestation. Nature 2016, 535, 144. [Google Scholar] [CrossRef] [PubMed]
- United Nations Framework Convention on Climate Change. Report of the Conference of the Parties on Its Thirteenth Session; United Nations: New York, NY, USA, March 2008. [Google Scholar]
- United Nations Framework Convention on Climate Change. Report of the Conference of the Parties on Its Sixteenth Session; United Nations: New York, NY, USA, March 2011. [Google Scholar]
- Hirata, Y.; Takao, G.; Sato, T.; Toriyama, J. REDD-Plus Cookbook; REDD Research and Development Center, Forestry and Forest Products Research Institute: Tsukuba, Japan, 2012. [Google Scholar]
- Gardner, T.A.; Burgess, N.D.; Aguilar-Amuchastegui, N.; Barlow, J.; Berenguer, E.; Clements, T.; Danielsen, F.; Ferreira, J.; Foden, W.; Kapos, V.; et al. A framework for integrating biodiversity concerns into national REDD+ programmes. Biol. Conserv. 2012, 154, 61–71. [Google Scholar] [CrossRef]
- Kitayama, K. Co-Benefits of Sustainable Forestry: Ecological Studies of a Certified Bornean Rain Forest; Springer: Tokyo, Japan, 2013. [Google Scholar]
- Pereira, H.M.; Ferrier, S.; Walters, M.; Geller, G.N.; Jongman, R.H.; Scholes, R.J.; Bruford, M.W.; Brummitt, N.; Butchart, S.H.; Cardoso, A.C.; et al. Essential biodiversity variables. Science 2013, 339, 277–278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Imai, N.; Tanaka, A.; Samejima, H.; Sugau, J.B.; Pereira, J.T.; Titin, J.; Kurniawan, Y.; Kitayama, K. Tree community composition as an indicator in biodiversity monitoring of REDD+. For. Ecol. Manag. 2014, 313, 169–179. [Google Scholar] [CrossRef]
- Ioki, K.; Tsuyuki, S.; Hirata, Y.; Phua, M.H.; Wong, W.V.C.; Ling, Z.Y.; Johari, S.A.; Korom, A.; James, D.; Saito, H.; et al. Evaluation of the similarity in tree community composition in a tropical rainforest using airborne lidar data. Remote Sens. Environ. 2016, 173, 304–313. [Google Scholar] [CrossRef]
- Aoyagi, R.; Imai, N.; Fujiki, S.; Sugau, J.B.; Pereira, J.T.; Kitayama, K. The mixing ratio of tree functional groups as a new index for biodiversity monitoring in Bornean production forests. For. Ecol. Manag. 2017, 403, 27–43. [Google Scholar] [CrossRef]
- Laurance, W.F.; Goosem, M.; Laurance, S.G.W. Impacts of roads and linear clearings on tropical forests. Trends Ecol. Evol. 2009, 24, 659–669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Armenteras, D.; Retana, J. Dynamics, patterns and causes of fires in northwestern Amazonia. PLoS ONE 2012, 7, e35288. [Google Scholar] [CrossRef] [PubMed]
- Toivonen, J.M.; Kessler, M.; Ruokolainen, K.; Hertel, D. Accessibility predicts structural variation of Andean Polylepis forests. Biodivers. Conserv. 2011, 20, 1789–1802. [Google Scholar] [CrossRef]
- Toivonen, J.M.; Gonzales-Inca, C.A.; Bader, M.Y.; Ruokolainen, K.; Kessler, M. Elevational shifts in the topographic position of Polylepis forest stands in the Andes of southern Peru. Forests 2018, 9, 7. [Google Scholar] [CrossRef]
- Ministerio del Ambiente (MINAM); Ministerio de Agricultura y Riego (MINAGRI). Memoria Descriptiva del Mapa de Ecozonas: Proyecto Inventario Nacional Forestal y Manejo Forestal Sostenible del Perú ante el Cambio Climático; MINAM and MINAGRI: Lima, Peru, 2014. (In Spanish)
- Pickettet, S.T.A.; White, P.S. The Ecology of Natural Disturbance and Patch Dynamics; Academic Press: San Diego, CA, USA, 1985. [Google Scholar]
- Ito, S. Forest formation and disturbance regime. In Forest Ecology; Masaki, T., Aiba, S., Eds.; Kyoritsu Shuppan: Tokyo, Japan, 2011; pp. 38–54. (In Japanese) [Google Scholar]
- Barlow, J.; Peres, C.A. Ecological responses of Amazonian forests to El Niño-induced surface fires. In Tropical Forests and Global Atmospheric Change; Malhi, Y., Phillips, O.L., Eds.; Oxford University Press: Oxford, UK, 2005. [Google Scholar]
- Román-Cuesta, R.M.; Salinas, N.; Asbjornsen, H.; Oliveras, I.; Huaman, V.; Gutierrez, Y.; Puelles, L.; Kala, J.; Yabar, D.; Rojas, M.; et al. Implications of fires on carbon budgets in Andean cloud montane forest: The importance of peat soils and tree resprouting. For. Ecol. Manag. 2011, 261, 1987–1997. [Google Scholar] [CrossRef]
- Climate-Data.org. Climate: Cusco. Available online: https://en.climate-data.org/region/1060/ (accessed on 4 January 2018).
- Sato, T.; Miyamoto, K. REDD-Plus Cookbook Annex. Research Manual Vol. 1 Ground-Based Inventory; REDD Research and Development Center, Forestry and Forest Products Research Institute: Tsukuba, Japan, 2016. [Google Scholar]
- Chazdon, R.L.; Chao, A.; Colwell, R.K.; Lin, S.Y.; Norden, N.; Letcher, S.G.; Clark, D.B.; Finegan, B.; Arroyo, J.P. A novel statistical method for classifying habitat generalists and specialists. Ecology 2011, 92, 1332–1343. [Google Scholar] [CrossRef] [PubMed]
- Ministerio de Agricultura y Riego (MINAGRI); Ministerio del Ambiente (MINAM). Manual de Campo–Ecozonas Costa y Sierra–Inventario Nacional Forestal: Proyecto Inventario Nacional Forestal y Manejo Forestal Sostenible ante el Cambio Climático en el Perú; MINAGRI and MINAM: Lima, Peru, 2015. (In Spanish)
- Chao, K.-J.; Phillips, O.L.; Baker, T.R. Wood density and stocks of coarse woody debris in a northwestern Amazonian landscape. Can. J. For. Res. 2008, 38, 795–805. [Google Scholar] [CrossRef]
- Chave, J.; Réjou-Méchain, M.; Búrquez, A.; Chidumayo, E.; Colgan, M.S.; Delitti, W.B.; Duque, A.; Eid, T.; Fearnside, P.M.; Goodman, R.C.; et al. Improved allometric models to estimate the aboveground biomass of tropical trees. Glob. Chang. Biol. 2014, 20, 3177–3190. [Google Scholar] [CrossRef] [PubMed]
- Zanne, A.E.; Lopez-Gonzalez, G.; Coomes, D.A.; Ilic, J.; Jansen, S.; Lewis, S.L.; Miller, R.B.; Swenson, N.G.; Wiemann, M.C.; Chave, J. Data from: Towards a Worldwide Wood Economics Spectrum. Dryad Data Repository. 2009. Available online: https://doi.org/10.5061/dryad.234 (accessed on 6 June 2017).
- Reyes, G.; Brown, S.; Chapman, J.; Lugo, A.E. Wood Densities of Tropical Tree Species; United States Department of Agriculture, Forestry Service, Southern Forest Experimental Station: New Orleans, LA, USA, 1992. [Google Scholar]
- Mokany, K.; Raison, R.J.; Prokushkin, A.S. Critical analysis of root: Shoot ratios in terrestrial biomes. Glob. Chang. Biol. 2006, 12, 84–96. [Google Scholar] [CrossRef]
- Aalde, H.; Gonzalez, P.; Gytarsky, M.; Krug, T.; Kurz, WA.; Ogle, S.; Raison, J.; Schoene, D.; Ravindranath, N.H.; Elhassan, N.G.; et al. Chapter 4: Forest Land. In 2006 IPCC Guidelines for National Greenhouse Gas Inventories, Prepared by the National Greenhouse Gas Inventories Programme, Volume 4 Agriculture, Forestry and Other Land Use; Eggleston, H.S., Buendia, L., Miwa, K., Ngara, T., Tanabe, K., Eds.; Institute for Global Environmental Strategies (IGES): Hayama, Japan, 2006; pp. 4.1–4.83. [Google Scholar]
- Chao, A.; Chazdon, R.L.; Colwell, R.K.; Shen, T.J. A new statistical approach for assessing similarity of species composition with incidence and abundance data. Ecol. Lett. 2005, 8, 148–159. [Google Scholar] [CrossRef]
- Doi, H.; Okamura, H. Similarity indices, ordination, and community analysis tests using the software R. Jpn. J. Ecol. 2011, 61, 3–20. (In Japanese) [Google Scholar]
- Damgaard, C.; Weiner, J. Describing inequality in plant size or fecundity. Ecology 2000, 81, 1139–1142. [Google Scholar] [CrossRef]
- Hakkenberg, C.R.; Song, C.; Peet, R.K.; White, P.S. Forest structure as a predictor of tree species diversity in the North Carolina piedmont. J. Veg. Sci. 2016, 27, 1151–1163. [Google Scholar] [CrossRef]
- Wang, Y.; Naumann, U.; Wright, S.T.; Warton, D.I. mvabund—An R package for model-based analysis of multivariate abundance data. Methods Ecol. Evol. 2012, 3, 471–474. [Google Scholar] [CrossRef]
- Warton, D.I.; Wright, S.T.; Wang, Y. Distance-based multivariate analyses confound location and dispersion effects. Methods Ecol. Evol. 2012, 3, 89–101. [Google Scholar] [CrossRef]
- Gotelli, N.J.; Colwell, R.K. Quantifying biodiversity: Procedures and pitfalls in the measurement and comparison of species richness. Ecol. Lett. 2001, 4, 379–391. [Google Scholar] [CrossRef]
- Plourde, B.T.; Boukili, V.K.; Chazdon, R.L. Radial changes in wood specific gravity of tropical trees: Inter- and intraspecific variation during secondary succession. Funct. Ecol. 2015, 29, 111–120. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018; Available online: http://www.R-project.org/ (accessed on 2 April 2018).
- Moser, G.; Leuschner, C.; Hertel, D.; Graefe, S.; Soethe, N.; Iost, S. Elevation effects on the carbon budget of tropical mountain forests (S Ecuador): The role of the belowground compartment. Glob. Chang. Biol. 2011, 17, 2211–2226. [Google Scholar] [CrossRef]
- Wilcke, W.; Yasin, S.; Abramowski, U.; Valarezo, C.; Zech, W. Nutrient storage and turnover in organic layers under tropical montane rain forest in Ecuador. Eur. J. Soil Sci. 2002, 53, 15–27. [Google Scholar] [CrossRef]
- Gibbon, A.; Silman, M.R.; Malhi, Y.; Fisher, J.B.; Meir, P.; Zimmermann, M.; Dargie, G.C.; Farfan, W.R.; Garcia, K.C. Ecosystem carbon storage across the grassland-forest transition in the high Andes of Manu national park, Peru. Ecosystems 2010, 13, 1097–1111. [Google Scholar] [CrossRef]
- Baker, T.R.; Honorio Coronado, E.N.; Phillips, O.L.; Martin, J.; van der Heijden, G.M.F.; Garcia, M.; Silva Espejo, J. Low stocks of coarse woody debris in a southwest Amazonian forest. Oecologia 2007, 152, 495–504. [Google Scholar] [CrossRef] [PubMed]
- Clark, K.E.; West, A.J.; Hilton, R.G.; Asner, G.P.; Quesada, C.A.; Silman, M.R.; Saatchi, S.S.; Farfan-Rios, W.; Martin, R.E.; Horwath, A.B.; et al. Storm-triggered landslides in the Peruvian Andes and implications for topography, carbon cycles, and biodiversity. Earth Surf. Dyn. 2016, 4, 47–70. [Google Scholar] [CrossRef] [Green Version]
- Barber, C.P.; Cochrane, M.A.; Souza, C.M.; Laurance, W.F. Roads, deforestation, and the mitigating effect of protected areas in the Amazon. Biol. Conserv. 2014, 177, 203–209. [Google Scholar] [CrossRef]
- Kleinschroth, F.; Healey, J.R. Impacts of logging roads on tropical forests. Biotropica 2017, 49, 620–635. [Google Scholar] [CrossRef]
- De Valenca, A.W.; Vanek, S.J.; Meza, K.; Ccanto, R.; Olivera, E.; Scurrah, M.; Lantinga, E.A.; Fonte, S.J. Land use as a driver of soil fertility and biodiversity across an agricultural landscape in the central Peruvian Andes. Ecol. Appl. 2017, 27, 1138–1154. [Google Scholar] [CrossRef] [PubMed]
- Mitchard, E.T.A.; Feldpausch, T.R.; Brienen, R.J.W.; Lopez-Gonzalez, G.; Monteagudo, A.; Baker, T.R.; Lewis, S.L.; Lloyd, J.; Quesada, C.A.; Gloor, M.; et al. Markedly divergent estimates of Amazon forest carbon density from ground plots and satellites. Glob. Ecol. Biogeogr. 2014, 23, 935–946. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goetz, S.J.; Hansen, M.; Houghton, R.A.; Walker, W.; Laporte, N.; Busch, J. Measurement and monitoring needs, capabilities and potential for addressing reduced emissions from deforestation and forest degradation under REDD+. Environ. Res. Lett. 2015, 10. [Google Scholar] [CrossRef]
- Lutz, D.A.; Powell, R.L.; Silman, M.R. Four decades of Andean timberline migration and implications for biodiversity loss with climate change. PLoS ONE 2013, 8, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Girardin, C.A.J.; Malhi, Y.; Aragao, L.; Mamani, M.; Huasco, W.H.; Durand, L.; Feeley, K.J.; Rapp, J.; Silva-Espejo, J.E.; Silman, M.; et al. Net primary productivity allocation and cycling of carbon along a tropical forest elevational transect in the Peruvian Andes. Glob. Chang. Biol. 2010, 16, 3176–3192. [Google Scholar] [CrossRef] [Green Version]
- Baccini, A.; Goetz, S.J.; Walker, W.S.; Laporte, N.T.; Sun, M.; Sulla-Menashe, D.; Hackler, J.; Beck, P.S.A.; Dubayah, R.; Friedl, M.A.; et al. Estimated carbon dioxide emissions from tropical deforestation improved by carbon-density maps. Nat. Clim. Chang. 2012, 2, 182–185. [Google Scholar] [CrossRef]
- Ioki, K.; Tsuyuki, S.; Hirata, Y.; Phua, M.-H.; Wong, W.V.C.; Ling, Z.-Y.; Saito, H.; Takao, G. Estimating above-ground biomass of tropical rainforest of different degradation levels in northern Borneo using airborne lidar. For. Ecol. Manag. 2014, 328, 335–341. [Google Scholar] [CrossRef]
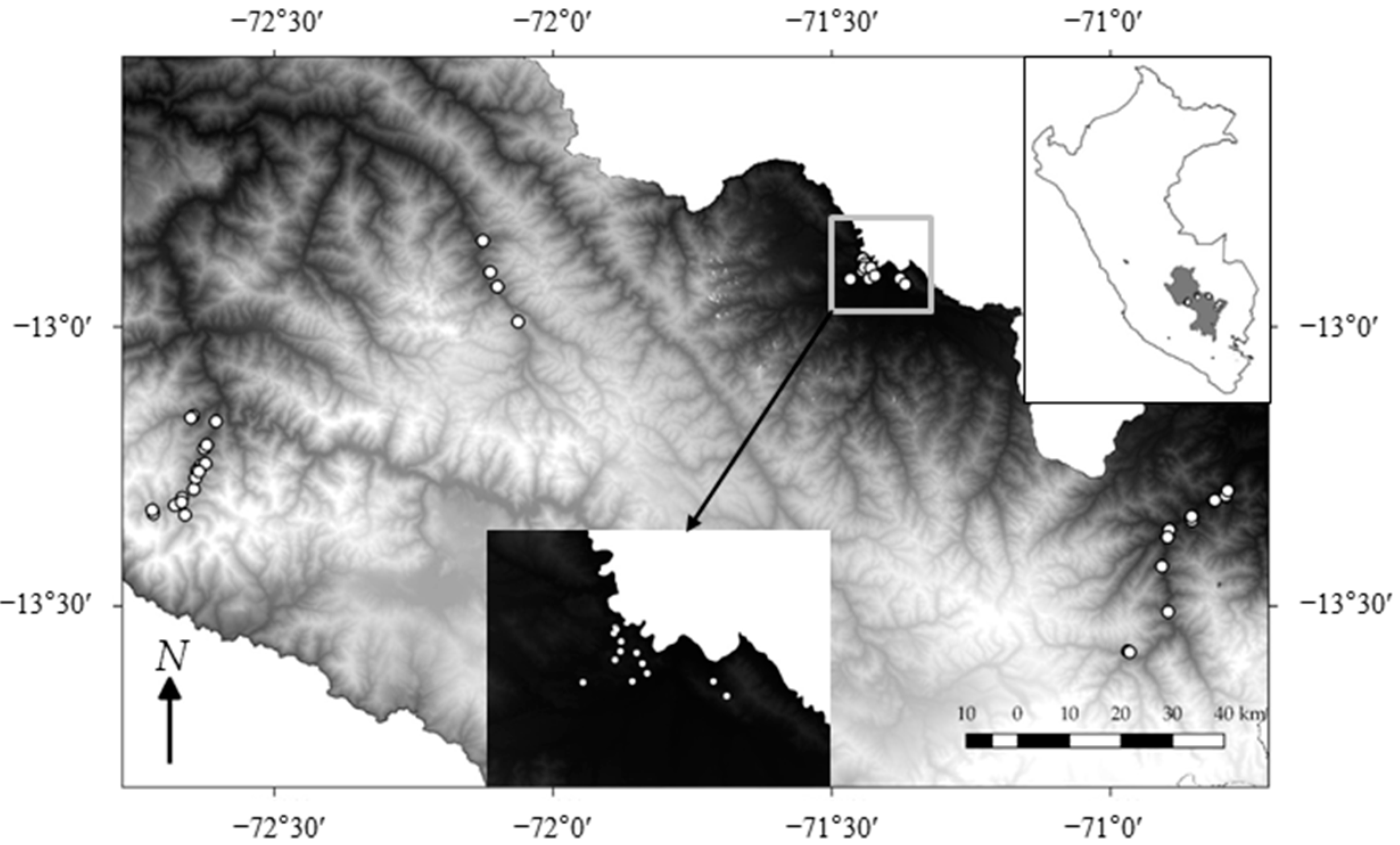
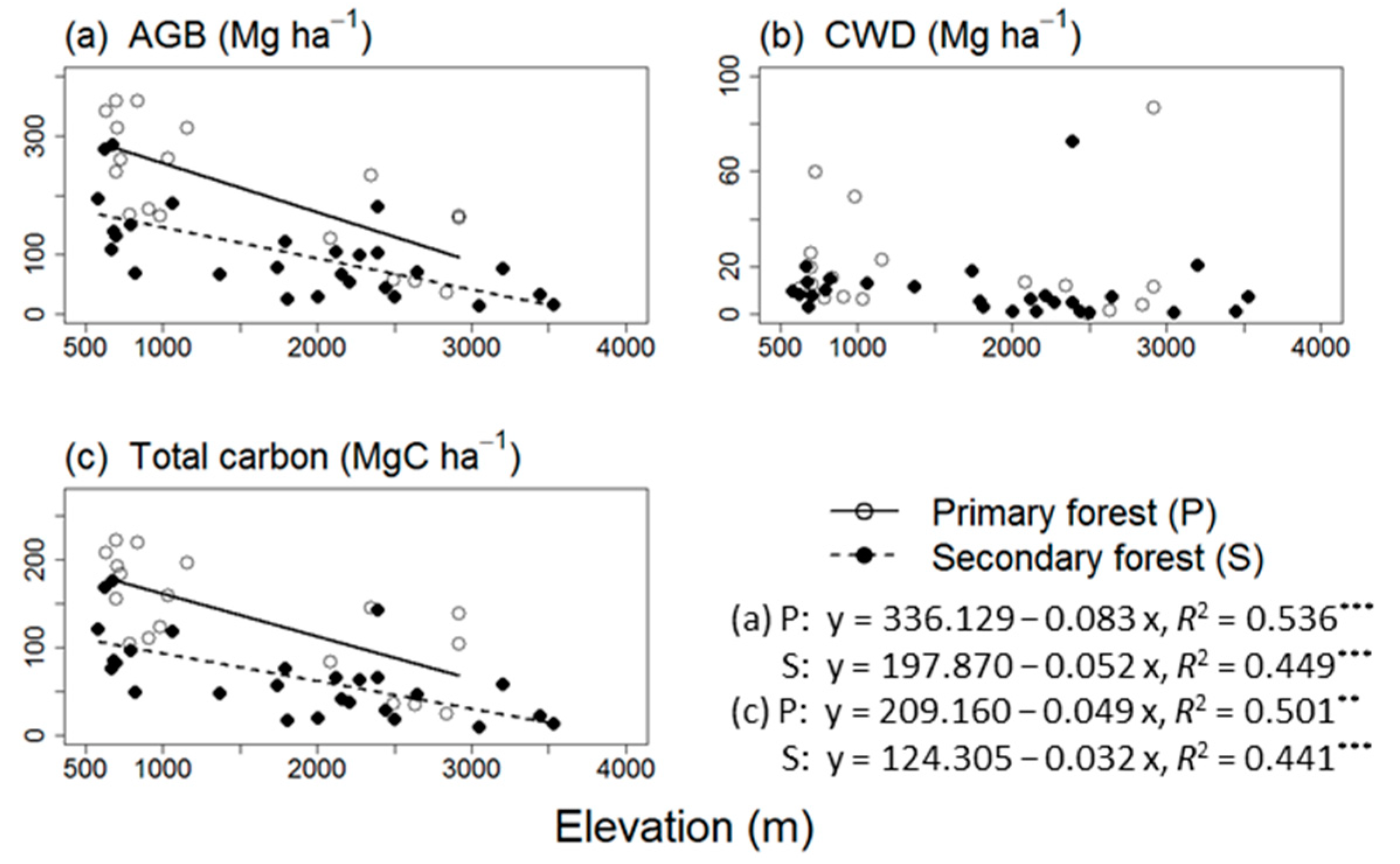
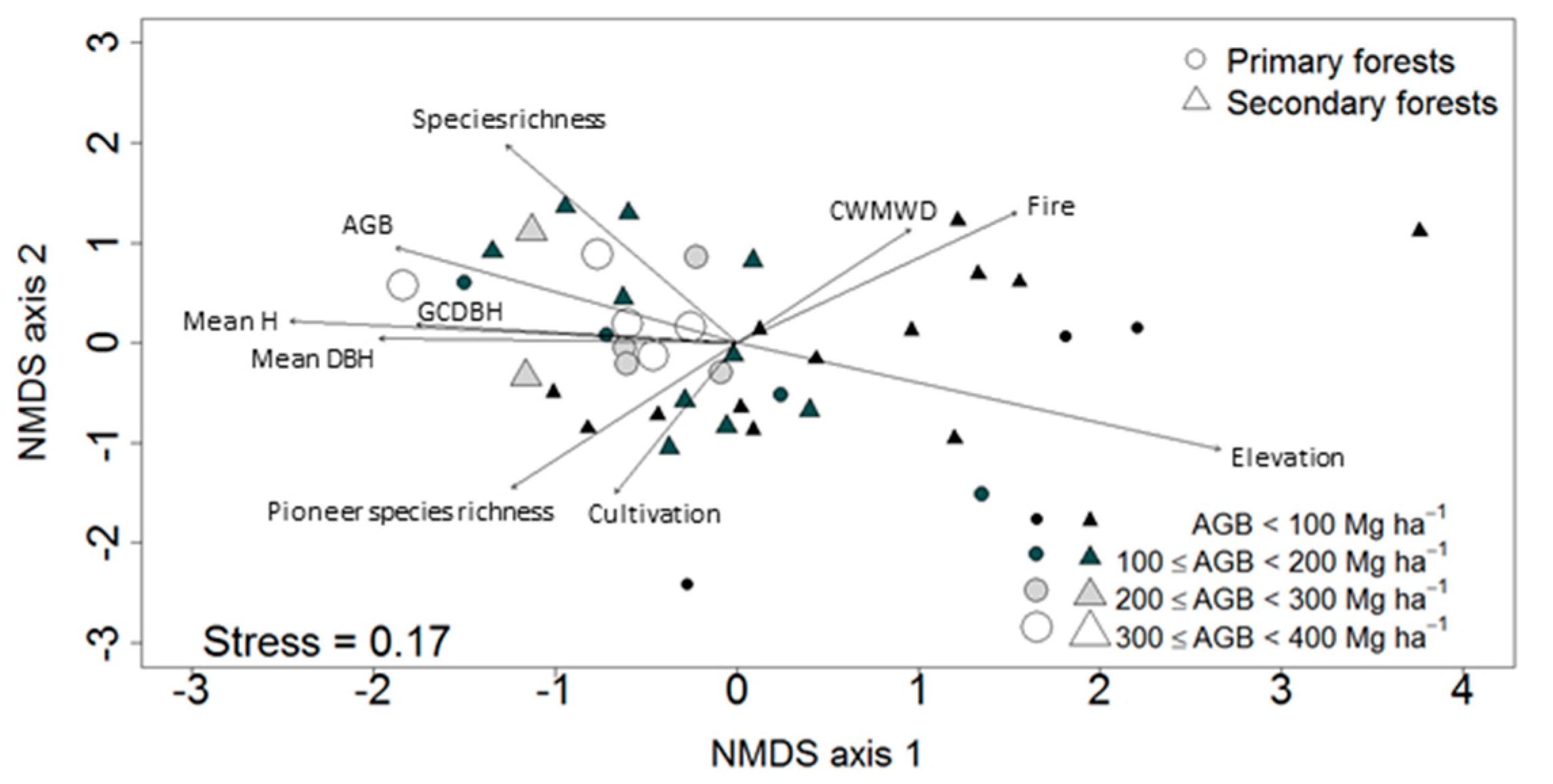
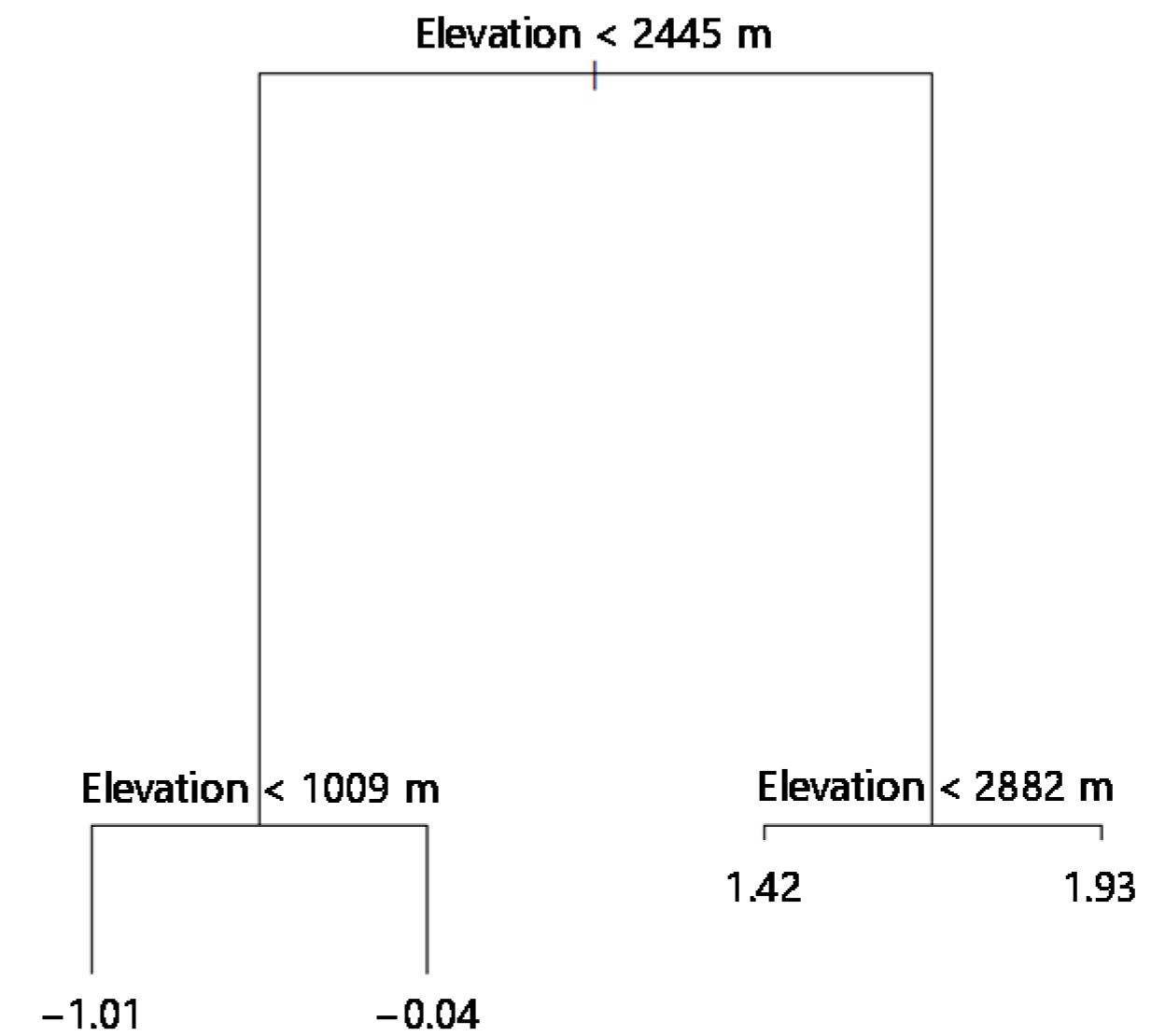
| Variables | Whole Elevation Range (584–3535 m) n = 41 (df 39 vs. 38, 32) a | ||
|---|---|---|---|
| LR b | AIC c | ΔAIC d | |
| Elevation (m) | — | 6174 | — |
| + Cultivation | 266.0 * | 6266 | 92 |
| + Erosion | 248.7 * | 6284 | 109 |
| + Fire | 125.8 | 6407 | 232 |
| + Infrastructure | 250.1 * | 6282 | 108 |
| + Logging | 234.1 | 6298 | 124 |
| + Wind | 159.9 | 6373 | 198 |
| + Topography | 907.6 * | 7773 | 1598 |
| Variables | Low Elevation (<1000 m) n = 16 (df 14 vs. 13, 9) a | Mid Elevation (1000–2400 m) n = 15 (df 13 vs. 12, 10) a | High Elevation (≥2400m) n = 10 (df 8 vs. 7, 5) a | ||||||
|---|---|---|---|---|---|---|---|---|---|
| LR | AIC | ΔAIC | LR | AIC | ΔAIC | LR | AIC | ΔAIC | |
| Elevation (m) | — | 2730 | — | — | 2085 | — | — | 1009 | — |
| + Cultivation | 115.7 | 2840 | 110 | 87.4 | 2148 | 63 | 48.4 | 1059 | 50 |
| + Erosion | 233.0 ** | 2723 | −7 | 115.5 † | 2120 | 34 | 21.8 | 1085 | 76 |
| + Fire | NA | NA | NA | NA | NA | NA | 81.1 | 1026 | 17 |
| + Infrastructure | 133.0 † | 2823 | 93 | 69.2 | 2166 | 81 | 133.6 * | 973 | −36 |
| + Logging | 108.6 | 2847 | 117 | 96.5 | 2139 | 54 | 87.7 | 1019 | 10 |
| + Wind | 83.8 | 2872 | 142 | 126.4 † | 2109 | 24 | NA | NA | NA |
| + Topography | 587.6 ** | 3497 | 768 | 250.9 * | 2585 | 499 | 223.8 † | 1275 | 266 |
| Variables b | Whole Elevation Range (584–3535 m) n = 41 (df 39 vs. 38) a | ||
|---|---|---|---|
| LR | AIC | ΔAIC | |
| Elevation (m) | — | 6174 | — |
| + Forest type (primary/secondary) | 322.4 ** | 6210 | 36 |
| + BA (m ha−1) | 257.5 * | 6275 | 101 |
| + AGB (Mg ha−1) | 274.5 † | 6258 | 83 |
| + BGB (Mg ha−1) | 273.1 † | 6259 | 85 |
| + Total CWD (Mg ha−1) | 224.6 | 6308 | 133 |
| + Total carbon (MgC ha−1) | 279.9 * | 6252 | 78 |
| + CWM wood density (g cm−3) | 272.1 * | 6260 | 86 |
| + Mean DBH (cm) | 187.2 | 6345 | 170 |
| + CWM DBH (cm) | 173.4 | 6359 | 185 |
| + Max. DBH (cm) | 233.1 † | 6299 | 125 |
| + Mean tree height (m) | 341.1 ** | 6191 | 17 |
| + CWM tree height (m) | 186.2 | 6346 | 172 |
| + Max. tree height (m) | 329.8 ** | 6203 | 28 |
| + Species richness (all species) c | 307.9 ** | 6219 | 44 |
| + Species richness (pioneers) c | 356.5 ** | 6176 | 1 |
| + Species richness (late-successional) c | 294.5 * | 6238 | 64 |
| + Gini coefficient (DBH basis) | 230.1 | 6302 | 128 |
| + Gini coefficient (Tree height basis) | 180.9 | 6352 | 177 |
| Variables b | Low Elevation (<1000 m) n = 16 (df 14 vs. 13) a | Mid Elevation (1000–2400 m) n = 15 (df 13 vs. 12) a | High Elevation (≥2400m) n = 10 (df 8 vs. 7) a | ||||||
|---|---|---|---|---|---|---|---|---|---|
| LR | AIC | ΔAIC | LR | AIC | ΔAIC | LR | AIC | ΔAIC | |
| Elevation (m) | — | 2730 | — | — | 2085 | — | — | 1009 | — |
| + Forest type (primary/secondary) | 174.9 † | 2781 | 51 | 125.4 † | 2110 | 25 | 73.3 | 1034 | 25 |
| + BA (m ha−1) | 167.0 † | 2789 | 59 | 183.8 * | 2052 | −34 | 90.0 | 1017 | 8 |
| + AGB (Mg ha−1) | 160.2 | 2796 | 66 | 206.1 * | 2029 | −56 | 41.3 | 1066 | 57 |
| + BGB (Mg ha−1) | 160.3 | 2795 | 66 | 203.2 * | 2032 | −53 | 41.5 | 1065 | 56 |
| + Total CWD (Mg ha−1) | 125.3 | 2830 | 101 | 194.9 * | 2040 | −45 | 50.2 | 1057 | 48 |
| + Total carbon (MgC ha−1) | 162.4 | 2793 | 64 | 200.7 * | 2035 | −51 | 41.9 | 1065 | 56 |
| + CWM wood density (g cm−3) | 140.9 | 2815 | 85 | 185.7 * | 2050 | −36 | 108.4 | 998 | −10 |
| + Mean DBH (cm) | 131.5 | 2824 | 94 | 256.5 ** | 1979 | −107 | 52.9 | 1054 | 45 |
| + CWM DBH (cm) | 129.2 | 2827 | 97 | 212.6 ** | 2023 | −63 | 53.4 | 1053 | 45 |
| + Max. DBH (cm) | 149.8 † | 2806 | 76 | 205.7 ** | 2030 | −56 | 33.6 | 1073 | 64 |
| + Mean tree height (m) | 243.6 ** | 2713 | −17 | 251.7 ** | 1984 | −102 | 111.6 | 995 | −14 |
| + CWM tree height (m) | 133.6 | 2822 | 92 | 155.5 † | 2080 | −6 | 105.0 | 1002 | −7 |
| + Max. tree height (m) | 166.9 † | 2789 | 59 | 282.9 *** | 1952 | −133 | 101.4 * | 1005 | −3 |
| + Species richness (all species) c | 184.7 * | 2771 | 41 | 190.5 * | 2045 | −41 | 188.4 ** | 919 | −90 |
| + Species richness (pioneers) c | 247.0 ** | 2709 | −21 | 140.6 | 2095 | 9 | 155.1 * | 952 | −57 |
| + Species richness (late-successional) c | 240.9 ** | 2715 | −15 | 108.4 | 2127 | 42 | 108.7 | 998 | −11 |
| + Gini coefficient (DBH basis) | 135.4 | 2820 | 91 | 207.8 ** | 2028 | −58 | 83.5 | 1023 | 15 |
| + Gini coefficient (Tree height basis) | 141.6 | 2814 | 81 | 180.7 † | 2055 | −31 | 86.4 | 1020 | 12 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miyamoto, K.; Sato, T.; Arana Olivos, E.A.; Clostre Orellana, G.; Rohner Stornaiuolo, C.M. Variation in Tree Community Composition and Carbon Stock under Natural and Human Disturbances in Andean Forests, Peru. Forests 2018, 9, 390. https://doi.org/10.3390/f9070390
Miyamoto K, Sato T, Arana Olivos EA, Clostre Orellana G, Rohner Stornaiuolo CM. Variation in Tree Community Composition and Carbon Stock under Natural and Human Disturbances in Andean Forests, Peru. Forests. 2018; 9(7):390. https://doi.org/10.3390/f9070390
Chicago/Turabian StyleMiyamoto, Kazuki, Tamotsu Sato, Edgar Alexs Arana Olivos, Gabriel Clostre Orellana, and Christian Marcel Rohner Stornaiuolo. 2018. "Variation in Tree Community Composition and Carbon Stock under Natural and Human Disturbances in Andean Forests, Peru" Forests 9, no. 7: 390. https://doi.org/10.3390/f9070390
APA StyleMiyamoto, K., Sato, T., Arana Olivos, E. A., Clostre Orellana, G., & Rohner Stornaiuolo, C. M. (2018). Variation in Tree Community Composition and Carbon Stock under Natural and Human Disturbances in Andean Forests, Peru. Forests, 9(7), 390. https://doi.org/10.3390/f9070390




