Allelopathy of Wild Mushrooms—An Important Factor for Assessing Forest Ecosystems in Japan
Abstract
1. Introduction
2. Materials and Methods
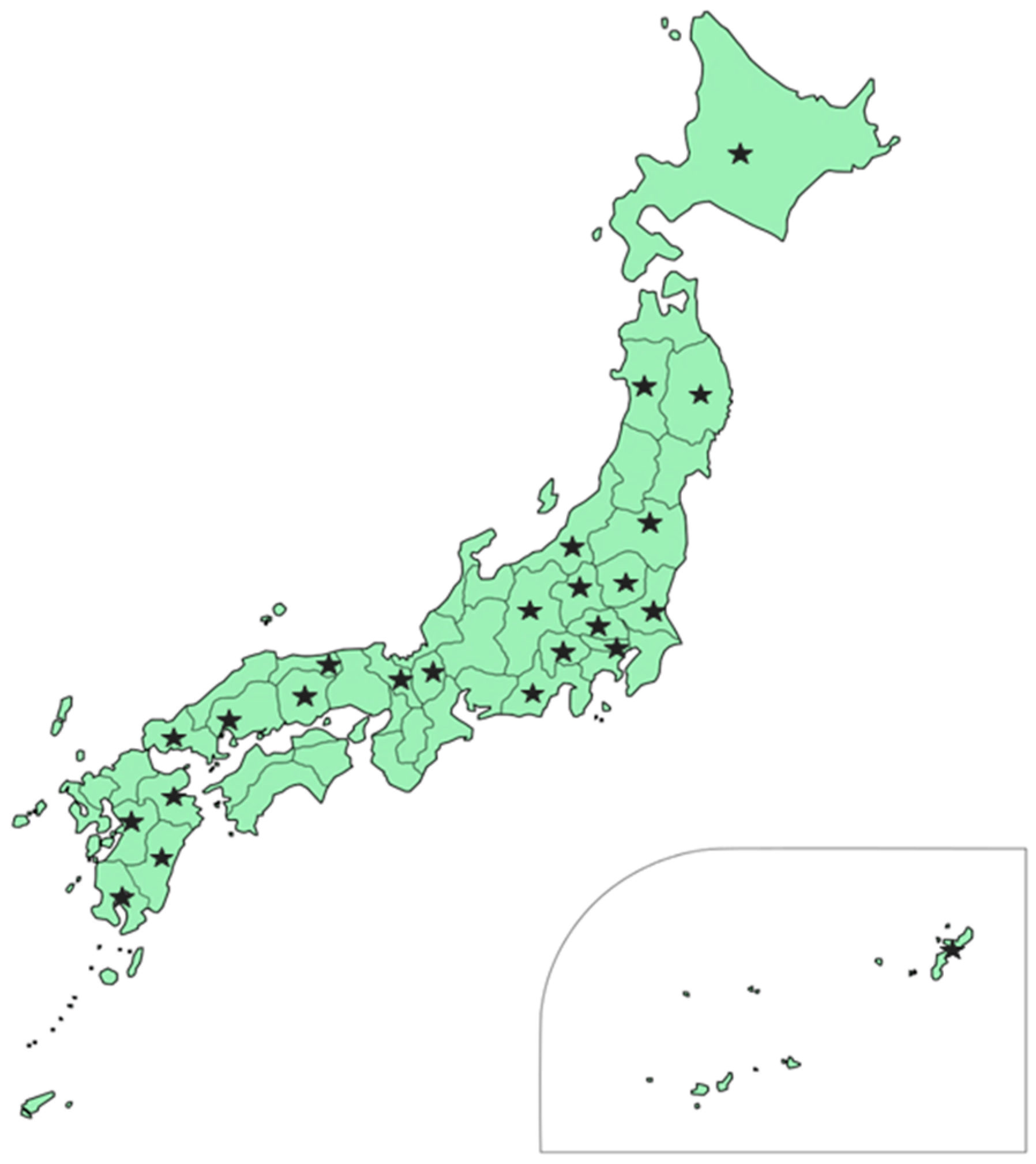
2.1. Fungal Collection
2.2. Bioassay of Allelopathic Activity of Mushroom Fruiting Bodies Using the Modified Sandwich Method
2.3. Statistics
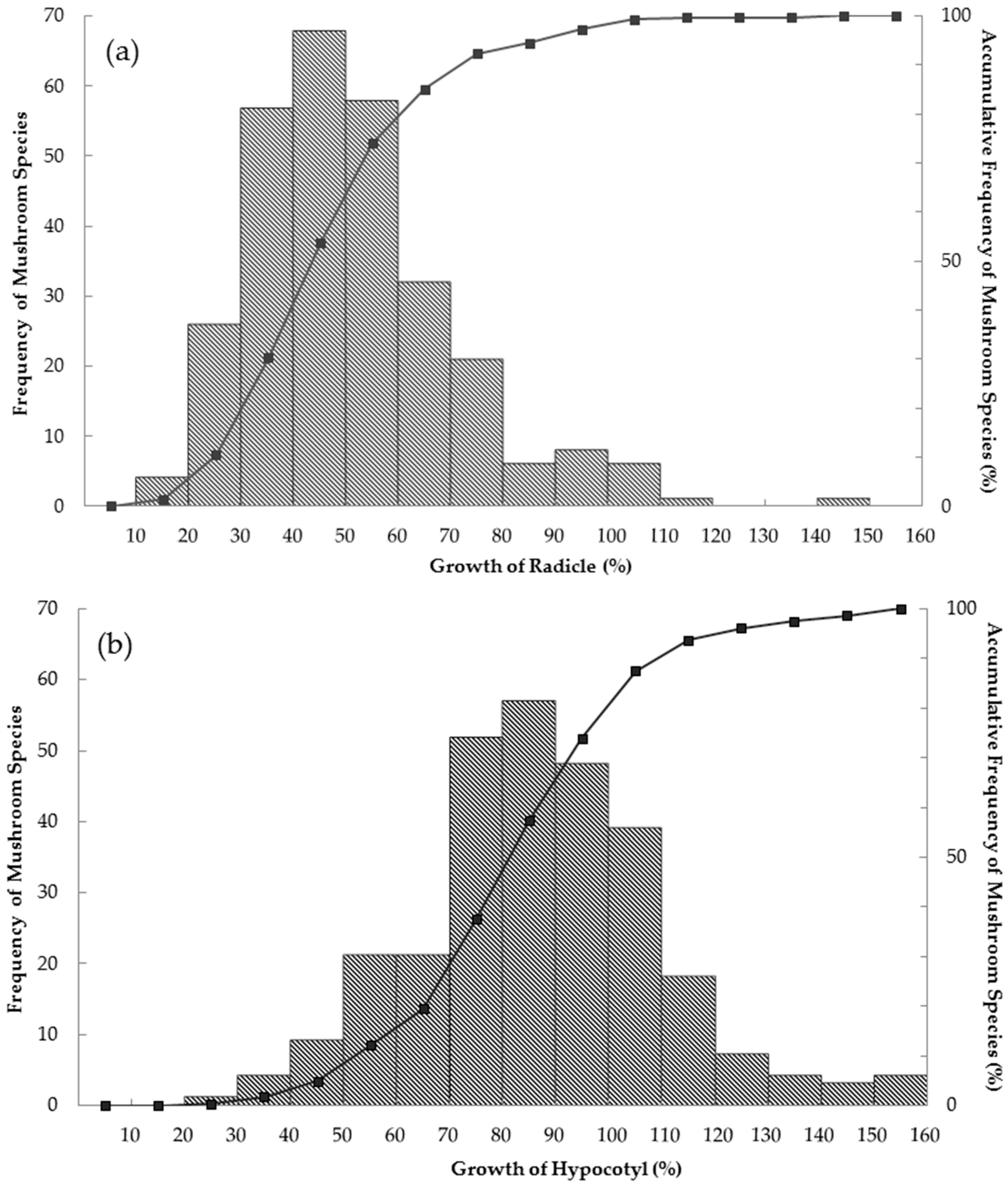
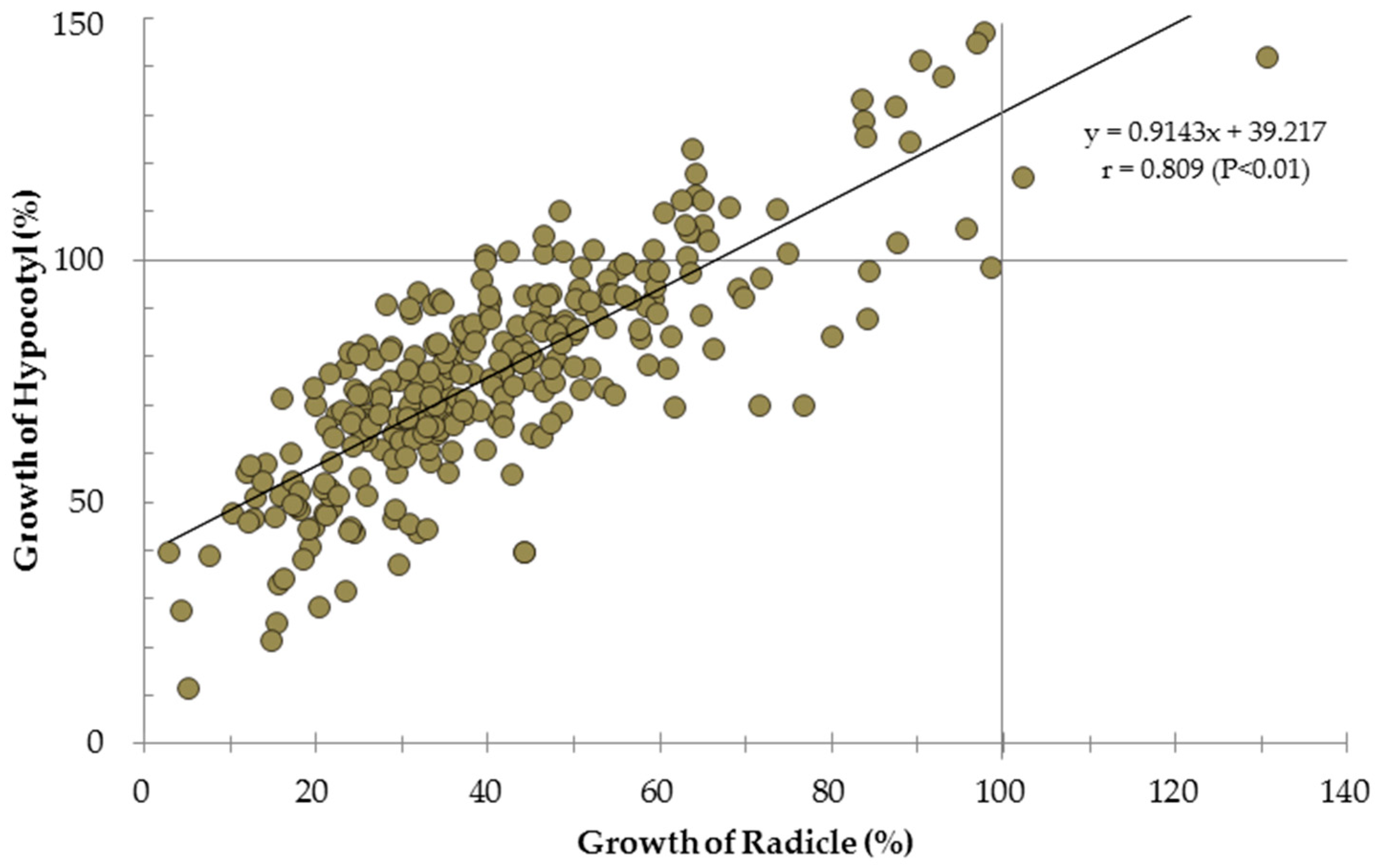
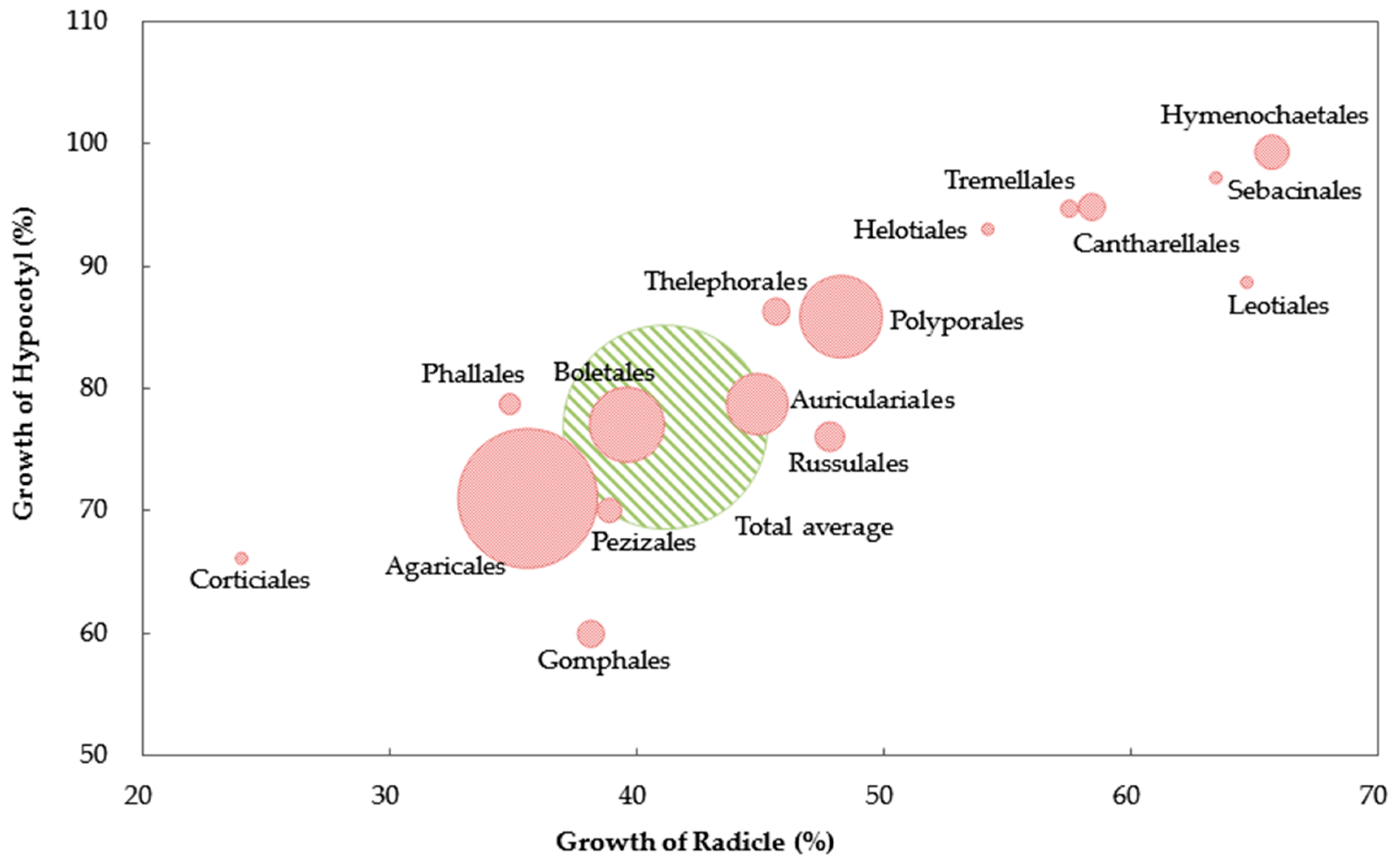
3. Results
4. Discussions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Reigosa, M.J.; González, L. Forest ecosystems and allelopathy. In Allelopathy: A Physiological Process with Ecological Implications; Reigosa, M., Pedrol, N., González, L., Eds.; Springer: Dordrecht, The Netherlands, 2006; pp. 451–463. [Google Scholar]
- Blanco, J.A. The representation of allelopathy in ecosystem-level forest models. Ecol. Model. 2007, 209, 65–77. [Google Scholar] [CrossRef]
- Kumar, A.S.; Bais, H.P. Allelopathy and exotic plant invasion. In Plant Communication from an Ecological Perspective; Baluška, F., Ninkovic, V., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 61–74. [Google Scholar]
- Williamson, G.B. Allelopathy, Koch’s postulates, and the neck riddle. In Perspectives on Plant Competition; Grace, J.B., Tilman, D., Eds.; Academic press, Inc.: San Diego, CA, USA; pp. 143–162.
- Choi, J.H.; Abe, N.; Tanaka, H.; Fushimi, K.; Nishina, Y.; Morita, A.; Kiriiwa, Y.; Motohashi, R.; Hashizume, D.; Koshino, H.; et al. Plant-growth regulator, imidazole-4-carboxamide, produced by the fairy ring forming fungus Lepista sordida. J. Agric. Food Chem. 2010, 58, 9956–9959. [Google Scholar] [CrossRef] [PubMed]
- Tobina, H.; Choi, J.H.; Asai, T.; Kiriiwa, Y.; Asakawa, T.; Kan, T.; Morita, A.; Kawagishi, H. 2-Azahypoxanthine and imidazole-4-carboxamide produced by the fairy-ring-forming fungus increase wheat yield. Field Crop. Res. 2014, 162, 6–11. [Google Scholar] [CrossRef]
- Badri, D.V.; Weir, T.L.; Van der Lelie, D.; Vivanco, J.M. Rhizosphere chemical dialogues: Plant-microbe interactions. Curr. Opin. Biotechol. 2009, 20, 642–650. [Google Scholar] [CrossRef]
- Biemelt, S.; Sonnewald, U. Plant-microbe interactions to probe regulation of plant carbon metabolism. J. Plant. Physiol. 2006, 163, 307–318. [Google Scholar] [CrossRef] [PubMed]
- Dangl, J.; Jones, J.D. Plant-microbe interactions affairs of the plant: Colonization, intolerance, exploitation and co-operation in plant-microbe interactions. Curr. Opin. Plant Biol. 1998, 1, 285–287. [Google Scholar] [CrossRef]
- Jonasson, S.; Castro, J.; Michelsen, A. Interactions between plants, litter and microbes in cycling of nitrogen and phosphorus in the arctic. Soil Biol. Biochem. 2006, 38, 526–532. [Google Scholar] [CrossRef]
- Brown, R.T. Influence of naturally occurring compounds on germination and growth of jack pine. Ecology 1967, 48, 542–546. [Google Scholar] [CrossRef]
- Wardle, D.A.; Karban, R.; Callaway, R.M. The ecosystem and evolutionary contexts of allelopathy. Trends Ecol. Evolut. 2011, 26, 655–662. [Google Scholar] [CrossRef]
- Wurzbacher, C.M.; Bärlocher, F.; Grossart, H.P. Fungi in lake ecosystems. Aquat. Microb. Ecol. 2010, 59, 125–149. [Google Scholar] [CrossRef]
- Fujii, Y.; Shibuya, T.; Nakatani, K.; Itani, T.; Hiradate, S.; Parvez, M.M. Assessment method for allelopathic effect from leaf litter leachates. Weed Biol. Manag. 2004, 4, 19–23. [Google Scholar] [CrossRef]
- Miller, R.M.; Fitzsimons, M.S. Fungal Growth in Soil. In Architecture and Biology of Soils: Life in Inner Space; Ritz, K., Young, I., Eds.; CABI: London, UK, 2011; p. 150. [Google Scholar]
- Araya, H. Allelochemicals of Mushrooms. In Recent Agrochemicals and Technical Papers (in Japanese); CMC publishing: Tokyo, Japan, 2017; pp. 568–577. [Google Scholar]
- Araya, H. Allelopathy of Mushrooms. In New Developments in Allelopathy Research; Price, J.E., Ed.; Nova Publisher: New York, NY, USA, 2015; pp. 1–14. [Google Scholar]
- Fujii, Y.; Pariasca, D.; Shibuya, T.; Yasuda, T.; Kahn, B.; Waller, G.R. Plant-box method: A specific biosassay to evaluate allelopathy through root exudates. In Allelopathy: New Concepts and Methodology; Fujii, Y., Hiradate, S., Eds.; Science Publisher: Enfield, NH, USA, 2007; pp. 39–56. [Google Scholar]
- Fujii, Y. Screening of allelopathic candidates by new specific discrimination, and assessment methods for allelopathy, and the identification of L-DOPA as the allelopathic substance from the most promising velvetbean (Mucuna pruriens). Bull. Natl. Inst. Agro-Environ. 1994, 10, 115–218. [Google Scholar]
- Fujii, Y.; Shibuya, T.; Yasuda, T. L-3,4-Dihydroxyphenylalanine as an allelochemical candidate from Mucuna pruriens (L.) DC. var. utilis. Agr. Biol. Chem. 1991, 55, 617–618. [Google Scholar] [CrossRef]
- Hiradate, S.; Morita, S.; Sugie, H.; Fujii, Y.; Harada, J. Phytotoxic cis-cinnamoyl glucosides from Spiraea thunbergii. Phytochemistry 2004, 65, 731–739. [Google Scholar] [CrossRef] [PubMed]
- Hirai, N.; Sakashita, S.I.; Sano, T.; Inoue, T.; Ohigashi, H.; Premasthira, C.U.; Asakawa, Y.; Harada, J.; Fujii, Y. Allelochemicals of the tropical weed Sphenoclea zeylanica. Phytochemistry 2000, 55, 131–140. [Google Scholar] [CrossRef]
- Iqbal, Z.; Nasir, H.; Hiradate, S.; Fujii, Y. Plant growth inhibitory activity of Lycoris radiata Herb. and the possible involvement of lycorine as an allelochemical. Weed Biol. Manag. 2006, 6, 221–227. [Google Scholar] [CrossRef]
- Kamo, T.; Hiradate, S.; Fujii, Y. First isolation of natural cyanamide as a possible allelochemical from hairy vetch Vicia villosa. J. Chem. Ecol. 2003, 29, 275–283. [Google Scholar] [CrossRef]
- Nakano, H.; Fujii, Y.; Yamada, K.; Kosemura, S.; Yamamura, S.; Hasegawa, K.; Suzuki, T. Isolation and identification of plant growth inhibitors as candidate(s) for allelopathic substance(s), from aqueous leachate from mesquite (Prosopis juliflora (Sw.) DC.) leaves. Plant Growth Regul. 2002, 37, 113–117. [Google Scholar] [CrossRef]
- Takemura, T.; Kamo, T.; Ismil, R.; Bakar, B.; Wasano, N.; Hiradate, S.; Fujii, Y. Plant growth inhibitor from the Malaysian medicinal plant Goniothalamus andersonii and related species. Nat. Prod. Commun. 2012, 7, 1197–1198. [Google Scholar]
- Araya, H. Allelopathic activities in litters of mushrooms. In New Discoveries in Agrochemicals; Clark, J.M., Ohkawa, H., Eds.; American Chemical Society: Washington, DC, USA, 2005; pp. 63–72. [Google Scholar]
- Fujii, Y.; Matsuyama, M.; Hiradate, S.; Shimozawa, H. Dish pack method: A new bioassay for volatile allelopathy. In Proceedings of the 4th World Congress on Allelopathy, Establishing the Scientific Base, Fourth World Congress on Allelopathy, Wagga Wagga, New South Wales, Australia, 21–26 August 2005; pp. 493–497. [Google Scholar]
- Fujii, Y.; Parvez, S.S.; Parvez, M.; Ohmae, Y.; Iida, O. Screening of 239 medicinal plant species for allelopathic activity using the sandwich method. Weed Biol. Manag. 2003, 3, 233–241. [Google Scholar] [CrossRef]
- Araya, H. Fruiting bodies of mushrooms as allelopathic plants. In Allelopathy: New Concepts and Methodology; Fujii, Y., Hiradate, S., Eds.; Science Publisher: Enfield, NH, USA, 2007; pp. 341–352. [Google Scholar]
- Morikawa, C.I.O.; Miyaura, R.; Tapiay-Figueroa, M.D.L.; Rengifo-Salgado, E.L.; Fujii, Y. Screening of 170 Peruvian plant species for allelopathic activity by using the sandwich method. Weed Biol. Manag. 2012, 12, 1–11. [Google Scholar] [CrossRef]
- Sari, M.; Prange, A.; Lelley, J.I.; Hambitzer, R. Screening of beta-glucan contents in commercially cultivated and wild growing mushrooms. Food Chem. 2017, 216, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Spiteller, P.; Rüth, M.; Von Nussbaum, F.; Steglich, W. Detection of a 2,3-Aminomutase in the mushroom Cortinarius violaceus. Angew. Chem. 2000, 39, 2754–2756. [Google Scholar] [CrossRef]
- Ikeda, M.; Naganuma, Y.; Ohta, K.; Sassa, T.; Miura, Y. Isolation and identification of a plant-growth inhibitor, azetidine-2-carboxylic acid, from Clavaria miyabeana S. Ito and its occurrence in the family Clavariaceae. J. Agr. Chem. Soc. Jpn. 1977, 51, 519–522. [Google Scholar]
- Sitta, N.; Floriani, M. Nationalization and globalization trends in the wild mushroom commerce of Italy with emphasis on porcini (Boletus edulis and allied Species), Econ. Bot. 2008, 62, 307. [Google Scholar] [CrossRef]
- Clericuzio, M.; Tabasso, S.; Garbarino, J.A.; Piovano, M.; Cardile, V.; Russo, A.; Vidari, G. Non-Phenolic dicinnamamides from Pholiota Spumosa: Isolation, synthesis and antitumour activity. Eur. J. Org. Chem. 2007, 70, 137–139. [Google Scholar] [CrossRef]
- Barua, B.S.; Ohya, T.; Suzuki, A.; Fujimoto, H. Screening of the wild mushrooms producing antioxidants, Part-1. In Abstracts of Papers Presented at the Meeting of the Mycological Society of Japan, Proceedings of the 50th Anniversary of Annual Meeting for the Mycological Society of Japan; The Mycological Society of Japan: Tokyo, Japan, 2006. [Google Scholar] [CrossRef]
- Luo, H.; Liu, Y.; Fang, L.; Li, X.; Tang, N.; Zhang, K. Coprinus comatus damages nematode cuticles mechanically with spiny balls and produces potent toxins to immobilize nematodes. Appl. Environ. Microbiol. 2007, 73, 3916–3923. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, H.; Toida, T.; Sakai, S.; Amakura, Y.; Kondo, K.; Sugita-Konishi, Y.; Maitani, T. Determination of cyanide and thiocyanate in Sugihiratake mushroom using HPLC method with fluorometric detection. J. Health Sci. 2006, 52, 73–77. [Google Scholar] [CrossRef]
- Wakimoto, T.; Asakawa, T.; Akahoshi, S.; Suzuki, T.; Nagai, K.; Kawagishi, H.; Kan, T. Proof of the existence of an unstable amino acid: Pleurocybellaziridine in Pleurocybella porrigens. Angew. Chem. 2011, 123, 1200–1202. [Google Scholar] [CrossRef]
- Kawagishi, H.; Miyazawa, T.; Kume, H.; Arimoto, Y.; Inakuma, T. Aldehyde dehydrogenase inhibitors from the mushroom Clitocybe clavipes. J. Nat. Prod. 2002, 65, 1712–1714. [Google Scholar] [CrossRef] [PubMed]
- Edwards, P.J. Effects of the fairy ring fungus Agaricus arvensis on nutrient availability in grassland. New Phytol. 1988, 110, 377–381. [Google Scholar] [CrossRef]
- Iwafuchi, Y.; Morita, T.; Kobayashi, H.; Kasuga, K.; Ito, K.; Nakagawa, O.; Kunisada, K.; Miyazaki, S.; Kamimura, A. Delayed onset acute renal failure associated with Amanita pseudoporphyria hongo ingestion. Internal. Med. 2003, 42, 78–81. [Google Scholar] [CrossRef]
- Endo, Y.; Minowa, A.; Kanamori, R.; Araya, H. A rare α-pyrone from bitter tooth mushroom, Sarcodon scabrosus (Fr.) Karst. Biochem. Syst. Ecol. 2012, 44, 286–288. [Google Scholar] [CrossRef]
- Hirota, M.; Morimura, K.; Shibata, H. Anti-inflammatory compounds from the bitter mushroom, Sarcodon scabrosus. Biosci. Biotechnol. Biochem. 2002, 66, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.G.; Kim, J.W.; Ryoo, I.J.; Kim, J.P.; Kim, Y.H.; Yoo, I.D. Boletunones A and B, highly functionalized novel sesquiterpenes from Boletus calopus. Org. Lett. 2004, 6, 823–826. [Google Scholar] [CrossRef] [PubMed]
- Sontag, B.; Arnold, N.; Steglich, W.; Anke, T. Montadial A, a cytotoxic metabolite from Bondarzewia montana. J. Nat. Prod. 1999, 62, 1425–1426. [Google Scholar] [CrossRef]
- Chung, K.S.; Lee, J.S.; Jo, M.J.; Lee, I.S. Studies on the hemolytic activities of Korean wild mushrooms (ii)-screening of 22 mushrooms including Laccaria vinaceoavellanea for their hemolytic activities, Korean J. Mycol. 2000, 28, 123–125. [Google Scholar]
- Ohta, T.; Matsuda, M.; Kobayashi, T.; Nagao, S.; Nozoe, S. Laccarin, A new alkaloid from the mushroom, Laccaria vinaceoavellanea. Heterocycles 1996, 43, 685–690. [Google Scholar] [CrossRef]
- Teichert, A.; Lübken, T.; Schmidt, J.; Kuhnt, C.; Huth, M.; Porzel, A.; Wessjohann, L.; Arnold, N. Determination of β-carboline alkaloids in fruiting bodies of Hygrophorus spp. by liquid chromatography/electrospray ionisation tandem mass spectrometry. Phytochem. Anal. 2008, 19, 335–341. [Google Scholar] [CrossRef]
- Michels, K.; Heinke, R.; Schöne, P.; Kuipers, O.P.; Arnold, N.; Wessjohann, L.A. A fluorescence-based bioassay for antibacterials and its application in screening natural product extracts. J. Antibiot. 2015, 68, 734. [Google Scholar] [CrossRef]
- Elmastas, M.; Isildak, O.; Turkekul, I.; Temur, N. Determination of antioxidant activity and antioxidant compounds in wild edible mushrooms. J. Food Compos. Anal. 2007, 20, 337–345. [Google Scholar] [CrossRef]
- Arnone, A.; Cardillo, R.; Di Modugno, V.; Nasini, G. Secondary mould metabolites. Part 29. Isolation and structure elucidation of candicansol, 3-epi-illudol and 1-O-acetyl-3-epi-illudol, novel sesquiterpenoids from Clitocybe candicans, and absolute configuration of 3-epi-illudol. J. Chem. Soc. Perkin Trans. 1 1989, 0, 1995–2000. [Google Scholar] [CrossRef]
- Chen, J.T.; Su, H.J.; Huang, J.W. Isolation and identification of secondary metabolites of Clitocybe nuda responsible for inhibition of zoospore germination of Phytophthora capsici. J. Agric. Food. Chem. 2012, 60, 7341–7344. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.Y.; Yang, X.L.; Fang, L.Z.; Zhang, L.; Dong, Z.J.; Liu, J.K. Purpuracolide: A new alliacane sesquiterpene from the basidiomycete Gomphus purpuraceus. J. Chem. Sci. 2008, 63, 1012–1014. [Google Scholar] [CrossRef]
- Donnelly, D.M.; Konishi, T.; Dunne, O.; Cremin, P. Sesquiterpene aryl esters from Armillaria tabescens. Phytochemistry 1997, 44, 1473–1478. [Google Scholar] [CrossRef]
- Herath, H.B.; Jacob, M.; Wilson, A.D.; Abbas, H.K.; Nanayakkara, N.D. New secondary metabolites from bioactive extracts of the fungus Armillaria tabescens. Nat. Prod. Res. 2013, 27, 1562–1568. [Google Scholar] [CrossRef]
- Mihail, J.D. Bioluminescence patterns among North American Armillaria species. Fungal Biol. 2015, 119, 528–537. [Google Scholar] [CrossRef]
- Kim, K.H.; Noh, H.J.; Choi, S.U.; Park, K.M.; Seok, S.J.; Lee, K.R. Lactarane sesquiterpenoids from Lactarius subvellereus and their cytotoxicity. Bioorg. Med. Chem. Lett. 2010, 20, 5385–5388. [Google Scholar] [CrossRef]
- Kerrigan, R.W. Agaricus subrufescens, a cultivated edible and medicinal mushroom, and its synonyms. Mycologia 2005, 97, 12–24. [Google Scholar] [CrossRef]
- Breene, W.M. Nutritional and medicinal value of specialty mushrooms. J. Food. Protect. 1990, 53, 883–894. [Google Scholar] [CrossRef]
- Han, J.J.; Bao, L.; He, L.W.; Zhang, X.Q.; Yang, X.L.; Li, S.J.; Yao, Y.J.; Liu, H.W. Phaeolschidins A-E, five hispidin derivatives with antioxidant activity from the fruiting body of Phaeolus schweinitzii collected in the Tibetan Plateau. J. Nat. Prod. 2013, 76, 1448–1453. [Google Scholar] [CrossRef] [PubMed]
- Smolskaitė, L.; Slapšytė, G.; Mierauskienė, J.; Dedonytė, V.; Venskutonis, P.R. Antioxidant and genotoxic properties of hispidin isolated from Phaeolus schweinitzii mushroom. Int. J. Med. Mushrooms 2017, 19, 967–980. [Google Scholar] [CrossRef] [PubMed]
- Vaskovsky, V.E.; Khotimchenko, S.V.; Boolugh, E.M. Distribution of diacylglycerotrimethylhomoserine and phosphatidylcholine in mushrooms. Phytochemistry 1998, 47, 755–760. [Google Scholar] [CrossRef]
- Veau, B.; Guillot, J.; Damez, M.; Dusser, M.; Konska, G.; Botton, B. Purification and characterization of an anti-(A+B) specific lectin from the mushroom Hygrophorus hypothejus. Biochim. Biophys. Acta: Gen. Subj. 1999, 1428, 39–44. [Google Scholar] [CrossRef]
- Tsuchihashi, H.; Yadomae, T.; Miyazaki, T. Isolation and characterization of an endo-β-D-glucuronidase from the fungus Kobayasia nipponica. J. Biochem. 1984, 96, 1799–1805. [Google Scholar] [CrossRef] [PubMed]
- Mihail, J.D.; Bruhn, J.N. Dynamics of bioluminescence by Armillaria gallica, A. mellea and A. tabescens. Mycologia 2007, 99, 341–350. [Google Scholar] [CrossRef]
- Valášková, V.; De Boer, W.; Gunnewiek, P.J.K.; Pospíšek, M.; Baldrian, P. Phylogenetic composition and properties of bacteria coexisting with the fungus Hypholoma fasciculare in decaying wood. ISME 2009, 3, 1218. [Google Scholar] [CrossRef]
- Jeon, S.M.; Wang, E.J.; Ka, K.H. Growth Characteristics and Extracellular Enzyme Activities of Pholiota spp. Mycol. Rep. Proc. 2015, 27, 106. [Google Scholar]
- Komatsu, N.; Sakai, S.; Saito, G.; Kikumoto, S.; Kimura, K.; Taito Co Ltd.; Kaken Kagaku KK. Treatment of Bacterial Infections with Glucan Compositions. U.S. Patent 3,943,247, 9 March 1976. [Google Scholar]
- Keishi, H.; Fuyuki, S.; Naganori, O.; Saori, T.; Kazuyuki, H. Stimulative effects of (22E,24R)-ergosta-7,22-diene-3β,5α,6β-triol from fruiting bodies of Tricholoma auratum, on a mouse osteoblastic cell line, MC3T3-E1, on a mouse osteoblastic cell line, mc3t3-e1. Biol. Pharm. Bull. 2002, 25, 1040–1044. [Google Scholar] [CrossRef]
- Appiah, K.S.; Li, Z.; Zeng, R.S.; Luo, S.; Oikawa, Y.; Fujii, Y. Determination of allelopathic potentials in plant species in Sino-Japanese floristic region by sandwich method and dish pack method. IJBAS 2015, 4, 381. [Google Scholar] [CrossRef]
- Möbius, N.; Hertweck, C. Fungal phytotoxins as mediators of virulence. Curr. Opin. Plant Biol. 2009, 12, 390–398. [Google Scholar] [CrossRef] [PubMed]
- Pedras, M.S.C.; Chumala, P.B.; Jin, W.; Islam, M.S.; Hauck, D.W. The phytopathogenic fungus Alternaria brassicicola: Phytotoxin production and phytoalexin elicitation. Phytochemistry 2009, 70, 394–402. [Google Scholar] [CrossRef] [PubMed]
- Hongo, K.; Ikeda, Y. New Version of Mushroom’s Encyclopedia; Hashimoto Shinpo-do: Tokyo, Japan, 2013; ISBN 978-4-89379-158-0. (In Japanese) [Google Scholar]
- Imazeki, R.; Otani, Y.; Hongo, T.; Izawa, M. Fungi of Japan; Yama-kei: Tokyo, Japan, 2011; ISBN 978-4-635-09044-5. (In Japanese) [Google Scholar]
- Thies, W.G.; Russell, K.W. Controlling root rots in coniferous forests of Northwestern North America. In Proceedings of the Sixth International Conference on Root and Butt Rots of Forest Trees, Melbourne, Victoria and Gympie, Queensland, Australia, 25–31 August 1983; pp. 379–386. [Google Scholar]
- Kobori, H.; Sekiya, A.; Suzuki, T.; Choi, J.H.; Hirai, H.; Kawagishi, H. Bioactive sesquiterpene aryl esters from the culture broth of Armillaria sp. J. Nat. Prod. 2014, 78, 163–167. [Google Scholar] [CrossRef] [PubMed]
- Gilbertson, R.L.; Ryvarden, L. North American Polypores, Volume 2; Fungiflora: Oslo, Norway, 1987; pp. 437–885. [Google Scholar]
- Leuschner, C.; Backes, K.; Hertel, D.; Schipka, F.; Schmitt, U.; Terborg, O.; Runge, M. Drought responses at leaf, stem and fine root levels of competitive Fagus sylvatica L. and Quercus petraea (Matt.) Liebl. trees in dry and wet years. For. Ecol. Manag. 2001, 149, 33–46. [Google Scholar] [CrossRef]
- Munir, A.T.; Tawaha, A.R.M. Inhibitory effects of aqueous extracts of black mustard on germination and growth of lentil. Pak. J. Agron. 2002, 1, 28–30. [Google Scholar] [CrossRef]
| Orders | Family | Species | Prop. | Pthg. | R. Grw. | H. Grw. | Criteria | Bio. Act. & Bi. Com. |
|---|---|---|---|---|---|---|---|---|
| Agaricales | Mycenaceae | Xeromphalina tenuipes | Sapro. | No | 5.1 | 11.4 | ****** | NR |
| Agaricales | Cortinariaceae | Cortinarius violaceus | Myco. | No | 4.3 | 27.4 | ***** | Cysteine protease inhibitor, (R)-β-dopa, β-glucans [32,33] |
| Agaricales | Clavariaceae | Clavaria miyabeana | Myco./Sapro. | NR | 7.6 | 38.8 | **** | L-azetidine-2-carboxylic acid [34] |
| Agaricales | Lyophyllaceae | Calocybe gambosa | Sapro. | Yes | 2.8 | 39.8 | **** | Fairy ring [35] |
| Agaricales | Entolomataceae | Entoloma clypeatum | Myco. | No | 20.3 | 28.4 | **** | NR |
| Agaricales | Strophariaceae | Pholiota spumosa | Sapro. | No | 15.4 | 25.1 | **** | (R)-2-hydroxyputrescine dicinnamamide [36] |
| Agaricales | Tricholomataceae | Leucopaxillus septentrionalis | Sapro. | No | 14.8 | 21.4 | **** | Antibiotic activity (clitocine), antioxidant activity [37] |
| Boletales | Boletaceae | Heimiella japonica | Myco. | No | 10.2 | 47.7 | *** | NR |
| Agaricales | Coprinaceae | Coprinus comatus | Sapro. | No | 15.6 | 33.2 | *** | Fucogalactan, antimicrobial activity, nematophagous [38] |
| Agaricales | Entolomataceae | Entoloma abortivum | Para./Sapro. | No | 19.4 | 40.7 | *** | NR |
| Agaricales | Entolomataceae | Entoloma sarcopum | Myco. | No | 18.5 | 38.0 | *** | NR |
| Agaricales | Marasmiaceae | Pleurocybella porrigens | Sapro. | No | 16.2 | 34.0 | *** | Aziridine, cyanide salt [39,40] |
| Agaricales | Tricholomataceae | Clitocybe clavipes | Sapro. | No | 23.5 | 31.5 | *** | Acetaldehyde dehydrogenase inhibitor [41] |
| Agaricales | Agaricaceae | Agaricus arvensis | Sapro. | No | 13.0 | 50.9 | ** | Fairy ring, sinapic acid [42] |
| Agaricales | Amanitaceae | Amanita pseudoporphyria | Myco. | No | 15.8 | 51.3 | ** | Nephrotoxin [43] |
| Agaricales | Amanitaceae | Amanita sinensis | Myco. | No | 17.3 | 54.2 | ** | NR |
| Thelephorales | Bankeraceae | Sarcodon scabrosus | Myco. | No | 12.2 | 45.6 | ** | Neosarcodonin, 4-Methoxy-6-phenyl-2H-pyran-2-one [44,45] |
| Boletales | Boletaceae | Boletellus floriformis | Myco. | No | 21.1 | 47.4 | ** | NR |
| Boletales | Suillaceae | Boletinus paluster | Myco. | No | 21.0 | 53.9 | ** | Xerocomic acid, variegatic acid, variegatorubin [46] |
| Boletales | Boletaceae | Boletus quercinus | Myco. | No | 13.8 | 54.4 | ** | NR |
| Boletales | Boletaceae | Xanthoconium affine | Myco. | No | 20.7 | 52.7 | ** | NR |
| Russulales | Bondarzewiaceae | Bondarzewia mesenterica | Sapro. | Yes | 17.3 | 49.3 | ** | Montadial A [47] |
| Agaricales | Hydnangiaceae | Laccaria vinaceoavellanea | Myco. | No | 12.8 | 46.4 | ** | Hemolytic toxins, laccarin (I) [48,49] |
| Agaricales | Hygrophoraceae | Hygrophorus erubescens var. capreolarius | Myco. | No | 19.7 | 44.8 | ** | Harmane and norharmane [50] |
| Agaricales | Hygrophoraceae | Hygrophorus pudorinus | Myco. | No | 19.2 | 44.5 | ** | Antibacterial activity [51] |
| Agaricales | Inocybaceae | Inocybe lutea | Myco. | No | 18.1 | 48.3 | ** | NR |
| Polyporales | Polyporaceae | Polyporus squamosus | Para./Sapro. | No | 17.7 | 49.0 | ** | Antioxidant and antimicrobial activity [52] |
| Agaricales | Tricholomataceae | Clitocybe candicans | Sapro. | No | 21.0 | 47.5 | ** | Candicansol, epi-illudol and 1-O-acetyl-3-epi-illudol [53] |
| Agaricales | Tricholomataceae | Clitocybe nuda | Sapro. | NR | 18.0 | 51.9 | ** | Antibacterial (2-methoxy-5- methyl-6-methoxy-methyl-p-benzoquinone, 6-hydroxy-2H-pyran-3-carbaldehyde and indole-3-carbaldehyde) [54] |
| Agaricales | Tricholomataceae | Tricholosporum porphyrophyllum | Sapro. | NR | 15.2 | 47.0 | ** | NR |
| Gomphales | Gomphaceae | Gomphus purpuraceus | Myco. | No | 29.6 | 37.1 | ** | Purpuracolide [55] |
| Gomphales | Gomphaceae | Ramaria fennica | Myco. | No | 44.3 | 39.5 | ** | NR |
| Agaricales | Physalacriaceae | Armillaria tabescens | Sapro. | Yes | 44.3 | 39.5 | ** | Bioluminescence,4-dehydro-14-hydroxy-dihydromelleolide,4-dehydro-dihydro-melleolide, 14-hydroxy-dihydromelleolide, 13-hydroxy-4-methoxy-melleolide and 5β,10α-dihydroxy-l-orsellinate-dihydromelleolide, emestrin-F, emestrin-G, 6-O-(4-O-methyl-β-d-glucopyranosyl)-8-hydroxy-2,7-dimethyl-4H-benzopyran-4-one, purpuracolide, and cephalosporolide-J [56,57,58] |
| Agaricales | Agaricaceae | Lanopila nipponica | NR | NR | 20.0 | 69.8 | * | NR |
| Agaricales | Agaricaceae | Lycoperdon pratense | Sapro. | No | 16.1 | 71.5 | * | NR |
| Auriculariales | Auriculariaceae | Exidia uvapassa | Sapro. | No | 21.1 | 65.7 | * | NR |
| Agaricales | Cortinariaceae | Cortinarius caperatus | Myco. | No | 19.7 | 73.7 | * | NR |
| Agaricales | Pluteaceae | Pluteus leoninus | Sapro. | No | 14.1 | 57.9 | * | NR |
| Polyporales | Polyporaceae | Microporus vernicipes | Sapro. | No | 12.3 | 57.6 | * | NR |
| Russulales | Russulaceae | Lactarius subvellereus | Myco. | No | 17.0 | 60.1 | * | Subvellerolactones [59] |
| Agaricales | Tricholomataceae | Lepista graveolens | Sapro. | No | 11.9 | 56.0 | * | NR |
| Agaricales | Agaricaceae | Agaricus subrutilescens | Sapro. | No | 21.8 | 48.6 | * | L-α-amino-γ-nitraminobutyric acid [60] |
| Auriculariales | Auriculariaceae | Auricularia minor | Sapro. | No | 29.1 | 48.3 | * | Antitumor activity [61] |
| Boletales | Boletaceae | Leccinum versipelle | Myco. | No | 21.6 | 51.3 | * | NR |
| Polyporales | Fomitopsidaceae | Phaeolus schweinitzii | Sapro. | Yes | 33.0 | 44.3 | * | Antitumor and radical-scavenging activity, Hispidin, pinillidine [62,63] |
| Agaricales | Hygrophoraceae | Hygrophorus speciosus | Myco. | No | 31.8 | 43.6 | * | NR |
| Agaricales | Hygrophoraceae | Hygrophorus hypothejus | Myco. | No | 24.4 | 43.7 | * | Anti-(A+B) blood type specific lectin, 1,2-diacylglycero-O-4’-(N,N,N-trimethyl) homoserine [64,65] |
| Phallales | Phallaceae | Kobayasia nipponica | Myco. | No | 22.7 | 51.2 | * | β-Glucuronidase [66] |
| Agaricales | Physalacriaceae | Armillaria gallica | Sapro. | No | 21.6 | 52.9 | * | Bioluminescence [67] |
| Russulales | Russulaceae | Russula neoemetica | Myco. | No | 23.9 | 44.1 | * | NR |
| Agaricales | Strophariaceae | Hypholoma fasciculare | Sapro. | No | 28.9 | 46.7 | * | Bactericidal effects [68] |
| Agaricales | Strophariaceae | Pholiota terrestris | Sapro. | No | 26.0 | 51.2 | * | Highest cellulase and laccase activities among Pholiota genius [69] |
| Agaricales | Tricholomataceae | Melanoleuca verrucipes | Sapro. | No | 24.0 | 44.6 | * | Glucans [70] |
| Agaricales | Tricholomataceae | Tricholoma auratum | Myco. | No | 30.9 | 45.4 | * | (22E,24R)-Ergosta-7,22-diene-3β,5α,6β-triol [71] |
| Mean (m) | 41.1 | 76.8 | ||||||
| Standard deviation (σ) | 19.7 | 22.3 | ||||||
| m−σ (*) | 21.4 | 54.5 | ||||||
| m−1.5σ (**) | 11.5 | 43.4 | ||||||
| m−2σ (***) | 1.69 | 32.2 | ||||||
| m−2.5σ (****) | 21.1 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Osivand, A.; Araya, H.; Appiah, K.S.; Mardani, H.; Ishizaki, T.; Fujii, Y. Allelopathy of Wild Mushrooms—An Important Factor for Assessing Forest Ecosystems in Japan. Forests 2018, 9, 773. https://doi.org/10.3390/f9120773
Osivand A, Araya H, Appiah KS, Mardani H, Ishizaki T, Fujii Y. Allelopathy of Wild Mushrooms—An Important Factor for Assessing Forest Ecosystems in Japan. Forests. 2018; 9(12):773. https://doi.org/10.3390/f9120773
Chicago/Turabian StyleOsivand, Asma, Hiroshi Araya, Kwame S. Appiah, Hossein Mardani, Takayuki Ishizaki, and Yoshiharu Fujii. 2018. "Allelopathy of Wild Mushrooms—An Important Factor for Assessing Forest Ecosystems in Japan" Forests 9, no. 12: 773. https://doi.org/10.3390/f9120773
APA StyleOsivand, A., Araya, H., Appiah, K. S., Mardani, H., Ishizaki, T., & Fujii, Y. (2018). Allelopathy of Wild Mushrooms—An Important Factor for Assessing Forest Ecosystems in Japan. Forests, 9(12), 773. https://doi.org/10.3390/f9120773







