Spatial Pattern and Competitive Relationships of Moso Bamboo in a Native Subtropical Rainforest Community
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site and Data Collection
2.2. Data Analysis
2.2.1. Community Structure and Quantitative Characteristics
2.2.2. Pair-Correlation Functions
2.2.3. Marked Correlation Functions and Marked Variograms
3. Results
3.1. Stand Structure and Composition
3.2. Competition Regular Spatial Distribution Patterns of Tree Species
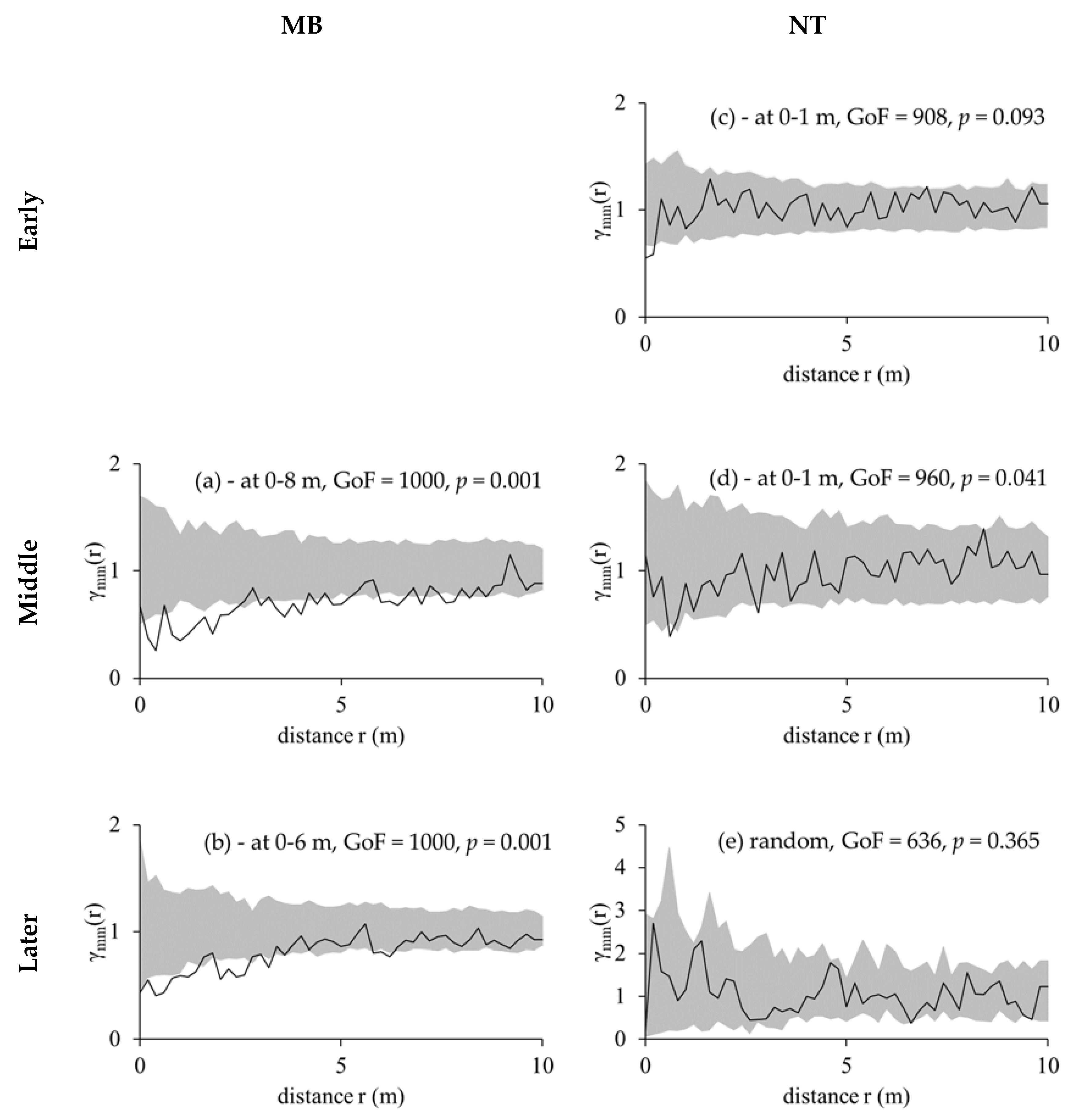
3.3. Competition Cause Size Correlation and Autocorrelation
4. Discussion
4.1. Different Space Regulation Mechanisms in Moso Bamboo and Native Forest
4.2. Bamboo Expands through Small Clonal Patches
4.3. The Size Equality and Competition in Nearby Bamboo
4.4. Management of Moso Bamboo in Forests
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Steffen, W.; Sanderson, R.A.; Tyson, P.D.; Jäger, J.; Matson, P.; Moore, B., III; Oldfield, F.; Richardson, K.; Schellnhuber, H.; Turner, B., II; et al. Global Change and the Earth System: A Planet under Pressure; Heidelberg: Berlin, Germany, 2004. [Google Scholar]
- Newbold, T.; Hudson, L.N.; Phillips, H.R.P.; Hill, S.L.L.; Contu, S.; Lysenko, I.; Blandon, A.; Butchart, S.H.M.; Booth, H.L.; Day, J.; et al. A global model of the response of tropical and sub-tropical forest biodiversity to anthropogenic pressures. Proc. R. Soc. London Ser. B: Biol. Sci. 2014, 281, 20141371. [Google Scholar] [CrossRef] [PubMed]
- Da, L.J.; Kang, M.M.; Song, K.; Shang, K.K.; Yang, Y.C.; Xia, A.M.; Qi, Y.F. Altitudinal zonation of human-disturbed vegetation on Mt. Tianmu, eastern China. Ecol. Res. 2009, 24, 1287–1299. [Google Scholar] [CrossRef]
- Feng, G.; Svenning, J.C.; Mi, X.; Jia, Q.; Rao, M.; Ren, H.; Bebber, D.P.; Ma, K. Anthropogenic disturbance shapes phylogenetic and functional tree community structure in a subtropical forest. For. Ecol. Manag. 2014, 313, 188–198. [Google Scholar] [CrossRef]
- Ahrends, A.; Hollingsworth, P.M.; Ziegler, A.D.; Fox, J.M.; Chen, H.; Su, Y.; Xu, J. Current trends of rubber plantation expansion may threaten biodiversity and livelihoods. Glob. Environ. Chang. 2015, 34, 48–58. [Google Scholar] [CrossRef]
- Gaveau, D.L.A.; Sheil, D.; Salim, M.A.; Arjasakusuma, S.; Ancrenaz, M.; Pacheco, P.; Meijaard, E. Rapid conversions and avoided deforestation: Examining four decades of industrial plantation expansion in borneo. Sci. Rep. 2016, 6, 32017. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, S.; Nakagoshi, N. Expansion of bamboo forests caused by reduced bamboo-shoot harvest under different natural and artificial conditions. Ecol. Res. 2008, 23, 641–647. [Google Scholar] [CrossRef]
- Kudo, G.; Amagai, Y.; Hoshino, B.; Kaneko, M. Invasion of dwarf bamboo into alpine snow-meadows in northern japan: Pattern of expansion and impact on species diversity. Ecol. Evol. 2011, 1, 85–96. [Google Scholar] [CrossRef]
- Raf, L.; Rother, D.C.; Muler, A.E.; Lepsch, I.F.; Rodrigues, R.R. Bamboo overabundance alters forest structure and dynamics in the atlantic forest hotspot. Biol. Conserv. 2012, 147, 32–39. [Google Scholar]
- Xu, Q.F.; Liang, C.F.; Chen, J.H.; Li, Y.C.; Qin, H.; Fuhrmann, J.J. Retracted article: Running bamboo invasion in native and non-native regions worldwide. Biol. Invasions 2017, 19, 3459. [Google Scholar] [CrossRef]
- Lin, Y.T.; Whitman, W.B.; Coleman, D.C.; Jien, S.H.; Chiu, C.Y. Cedar and bamboo plantations alter structure and diversity of the soil bacterial community from a hardwood forest in subtropical mountain. Appl. Soil Ecol. 2017, 112, 28–33. [Google Scholar] [CrossRef]
- Shanmughavel, P.; Francis, K. Balance and turnover of nutrients in a bamboo plantation (bambusa bambos) of different ages. Biol. Fert. Soils 1997, 25, 69–74. [Google Scholar] [CrossRef]
- Gupta, A.; Kumar, A. Potential of bamboo in sustainable development. Asia Pac. Bus. Rev. 2008, 4, 100–107. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, X.; Zhang, Y.; Trevor, B.; He, X. Changes of carbon stocks in bamboo stands in China during 100 years. For. Ecol. Manag. 2009, 258, 1489–1496. [Google Scholar] [CrossRef]
- Shi, J.; Zhang, G.; Bai, K.; Tang, J. Effects of invasion of dendrocalamus membranaceus munro on community biomass and plant diversity. J. Plant Resour. Environ. 2001, 10, 34–37, (In Chinese with English abstract). [Google Scholar]
- Environmental Protection Bureau of Guizhou Province. Scientific Research Collection for Chishui Alsophila National Nature Reserve; Guizhou nationalities Publishing House: Guizhou, China, 1990; (In Chinese with English abstract).
- Lin, Y.T.; Tang, S.L.; Pai, C.W.; Whitman, W.B.; Coleman, D.C.; Chiu, C.Y. Changes in the soil bacterial communities in a cedar plantation invaded by moso bamboo. Microb. Ecol. 2014, 67, 421–429. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.X.; Wang, Z.L.; Zhou, G.M.; Du, Q.Z. Monitoring phyllostachys pubescens stands expansion in national nature reserve of mount tianmu by remote sensing. J. Zhejiang For. Coll. 2006, 23, 297–300, (In Chinese with English abstract). [Google Scholar]
- Zong, X.; Zhang, H.; Wang, X.; Li, Z.; Wu, H.; Liang, S.; Deng, H. Community characteristics and species diversity of alsophila spinulosa in chishui alsophila national nature reserve. Acta Bot. Boreal. Occident. Sin. 2016, 36, 1225–1232, (In Chinese with English abstract). [Google Scholar]
- Gratani, L.; Crescente, M.L.; Fabrini, G.; Digiulio, E. Growth pattern and photosynthetic activity of different bamboo species growing in the botanical garden of Rome. Flora 2008, 203, 77–84. [Google Scholar] [CrossRef]
- Nishikawa, R.; Murakami, T.; Yoshida, S.; Mitsuda, Y.; Nagashima, K.; Mizoue, N. Characteristic of temporal range shifts of bamboo stands according to adjacent landcover type. J. Jpn. For. Soc. 2005, 87, 402–409. [Google Scholar] [CrossRef]
- Okutomi, K.; Shinoda, S.; Fukuda, H. Causal analysis of the invasion of broad-leaved forest by bamboo in japan. J. Veg. Sci. 2010, 7, 723–728. [Google Scholar] [CrossRef]
- Akutsu, H.; Aizawa, M.; Matsue, K.; Ohkubo, T. Distribution and invasion of phyllostachys pubescens stands into neighboring forests in nasukarasuyama, tochigi prefecture. Bull. Utsunomiya Univ. For. 2012, 48, 139–151. [Google Scholar]
- Xu, Q.F.; Jiang, P.K.; Wu, J.S.; Zhou, G.M.; Shen, R.F.; Fuhrmann, J.J. Bamboo invasion of native broadleaf forest modified soil microbial communities and diversity. Biol. Invasions 2015, 17, 433–444. [Google Scholar] [CrossRef]
- Kudo, G.; Kawai, Y.; Amagai, Y.; Winkler, D.E. Degradation and recovery of an alpine plant community: Experimental removal of an encroaching dwarf bamboo. Alp. Bot. 2017, 127, 1–9. [Google Scholar] [CrossRef]
- Chang, E.H.; Chiu, C.Y. Changes in soil microbial community structure and activity in a cedar plantation invaded by moso bamboo. Appl. Soil Ecol. 2015, 91, 1–7. [Google Scholar] [CrossRef]
- Griscom, B.W.; Ashton, P.M.S. Bamboo control of forest succession: Guadua sarcocarpa in southeastern peru. For. Ecol. Manag. 2003, 175, 445–454. [Google Scholar] [CrossRef]
- Larpkern, P.; Moe, S.R.; Totland, Ø. Bamboo dominance reduces tree regeneration in a disturbed tropical forest. Oecologia 2011, 165, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Stoll, P.; Bergius, E. Pattern and process: Competition causes regular spacing of individuals within plant populations. J. Ecol. 2005, 93, 395–403. [Google Scholar] [CrossRef]
- Wiegand, T.; Moloney, K.A. Rings, circles, and null-models for point pattern analysis in ecology. Oikos 2004, 104, 209–229. [Google Scholar] [CrossRef]
- Wiegand, T.; Moloney, K.A. Handbook of Spatial Point Pattern Analysis in Ecology; CRC Press: Boca Raton, FL, USA, 2013. [Google Scholar]
- Svátek, M.; Matula, R. Fine-scale spatial patterns in oak sprouting and mortality in a newly restored coppice. For. Ecol. Manag. 2015, 348, 117–123. [Google Scholar] [CrossRef]
- Stoyan, D.; Penttinen, A. Recent applications of point process methods in forestry statistics. Stat. Sci. 2000, 15, 61–78. [Google Scholar]
- Illian, J.; Penttinen, A.; Stoyan, H.; Stoyan, D. Statistical Analysis and Modelling of Spatial Point Patterns; John Wiley and Sons: Chichester, UK, 2008. [Google Scholar]
- Getzin, S.; Dean, C.; He, F.; Trofymow, J.A.; Wiegand, K.; Wiegand, T. Spatial patterns and competition of tree species in a douglas-fir chronosequence on vancouver island. Ecography 2006, 29, 671–682. [Google Scholar] [CrossRef]
- Szmyt, J.; Tarasiuk, S. Species-specific spatial structure, species coexistence and mortality pattern in natural, uneven-aged Scots pine (Pinus sylvestris L.)-dominated forest. Eur. J. For. Res. 2017, 137, 1–16. [Google Scholar] [CrossRef]
- Pommerening, A.; Särkkä, A. What mark variograms tell about spatial plant interactions. Ecol. Model. 2013, 251, 64–72. [Google Scholar] [CrossRef]
- Kuehne, C.; Weiskittel, A.; Pommerening, A.; Wagner, R.G. Evaluation of 10-year temporal and spatial variability in structure and growth across contrasting commercial thinning treatments in spruce-fir forests of northern maine, USA. Ann. For. Sci. 2018, 75, 20. [Google Scholar] [CrossRef]
- Wiegand, T.; Raventós, J.; Mújica, E.; González, E.; Bonet, A. Spatio-temporal analysis of the effects of hurricane ivan on two contrasting epiphytic orchid species in guanahacabibes, Cuba. Biotropica 2013, 45, 441–449. [Google Scholar] [CrossRef]
- Getzin, S.; Wiegand, K.; Schumacher, J.; Gougeon, F.A. Scale-dependent competition at the stand level assessed from crown areas. For. Ecol. Manag. 2008, 255, 2478–2485. [Google Scholar] [CrossRef]
- Getzin, S.; Worbes, M.; Wiegand, T.; Wiegand, K. Size dominance regulates tree spacing more than competition within height classes in tropical Cameroon. J. Trop. Ecol. 2011, 27, 93–102. [Google Scholar] [CrossRef]
- Wang, H.; Wan, P.; Wang, Q.; Liu, L.; Zhang, G.; Hui, G. Prevalence of inter-tree competition and its role in shaping the community structure of a natural mongolian Scots Pine (Pinus sylvestris var. mongolica) forest. Forests 2017, 8, 84. [Google Scholar] [CrossRef]
- Weiner, J. Asymmetric competition in plant populations. Trends Ecol. Evol. 1990, 5, 360–364. [Google Scholar] [CrossRef]
- Suzuki, S.N.; Kachi, N.; Suzuki, J. Development of a local size hierarchy causes regular spacing of trees in an even-aged abies forest: Analyses using spatial autocorrelation and the mark correlation function. Ann. Bot. 2008, 102, 435–441. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.Y. Vegetation of China; Science Press: Beijing, China, 1980. (In Chinese) [Google Scholar]
- Silver, W.L.; Kueppers, L.M.; Lugo, A.E.; Ostertag, R.; Matzek, V. Carbon sequestration and plant community dynamics following reforestation of tropical pasture. Ecol. Appl. 2004, 14, 1115–1127. [Google Scholar] [CrossRef]
- Harms, E.K.; Wright, J.S.; Calderón, O.; HernaÂndez, A.; Herre, E.A. Pervasive density-dependent recruitment enhances seedling diversity in a tropical forest. Nature 2000, 404, 493–495. [Google Scholar] [CrossRef] [PubMed]
- Beckman, N.G.; Neuhauser, C.; Muller-Landau, H.C. The interacting effects of clumped seed dispersal and distance- and density-dependent mortality on seedling recruitment patterns. J. Ecol. 2012, 100, 862–873. [Google Scholar] [CrossRef]
- Comita, L.S.; Queenborough, S.A.; Murphy, S.J.; Eck, J.L.; Xu, K.; Krishnadas, M.; Beckman, N.; Zhu, Y. Testing predictions of the janzen-connell hypothesis: A meta-analysis of experimental evidence for distance- and density-dependent seed and seedling survival. J. Ecol. 2014, 102, 845–856. [Google Scholar] [CrossRef] [PubMed]
- Schmid, B.; Bazzaz, F.A. Clonal integration and population structure in perennials: Effects of severing rhizome connections. Ecology 1987, 68, 2016–2022. [Google Scholar] [CrossRef] [PubMed]
- Dimov, L.D.; Chambers, J.L.; Lockhart, B.R. Spatial Continuity of Tree Attributes in Bottomland Hardwood Forests in the Southeastern United States. For. Sci. 2005, 51, 532–540. [Google Scholar]
- Reed, D.D.; Burkhart, H.E. Spatial autocorrelation of individual tree characteristics in loblolly pine stands. For. Sci. 1985, 31, 575–587. [Google Scholar]
- Kroon, H.D.; Hara, T.; Kwant, R. Size hierarchies of shoots and clones in clonal herb monocultures: Do clonal and non-clonal plants compete differently? Oikos 1992, 63, 410–419. [Google Scholar] [CrossRef]
- Schwinning, S.; Weiner, J. Mechanisms determining the degree of size asymmetry in competition among plants. Oecologia 1998, 113, 447–455. [Google Scholar] [CrossRef]
- Li, R.; Werger, M.J.A.; Kroon, H.D.; During, H.J.; Zhong, Z.C. Interactions between shoot age structure, nutrient availability and physiological integration in the giant bamboo Phyllostachys pubescens. Plant Biol. 2000, 2, 437–446. [Google Scholar] [CrossRef]
- Saitoh, T.; Seiwa, K.; Nishiwaki, A. Importance of physiological integration of dwarf bamboo to persistence in forest understorey: A field experiment. J. Ecol. 2002, 90, 78–85. [Google Scholar] [CrossRef]
- Weiner, J. How competition for light and nutrients affects size variability in ipomoea tricolor populations. Ecology 1986, 67, 1425–1427. [Google Scholar] [CrossRef]
- Cai, L.; Zhang, R.L.; Li, C.F.; Ding, Y. A method to inhibit the expansion of Phyllostachys pubescens stands based on the analysis of underground rhizome. J. Northeast For. Uni. 2003, 31, 68–70, (In Chinese with English abstract). [Google Scholar]
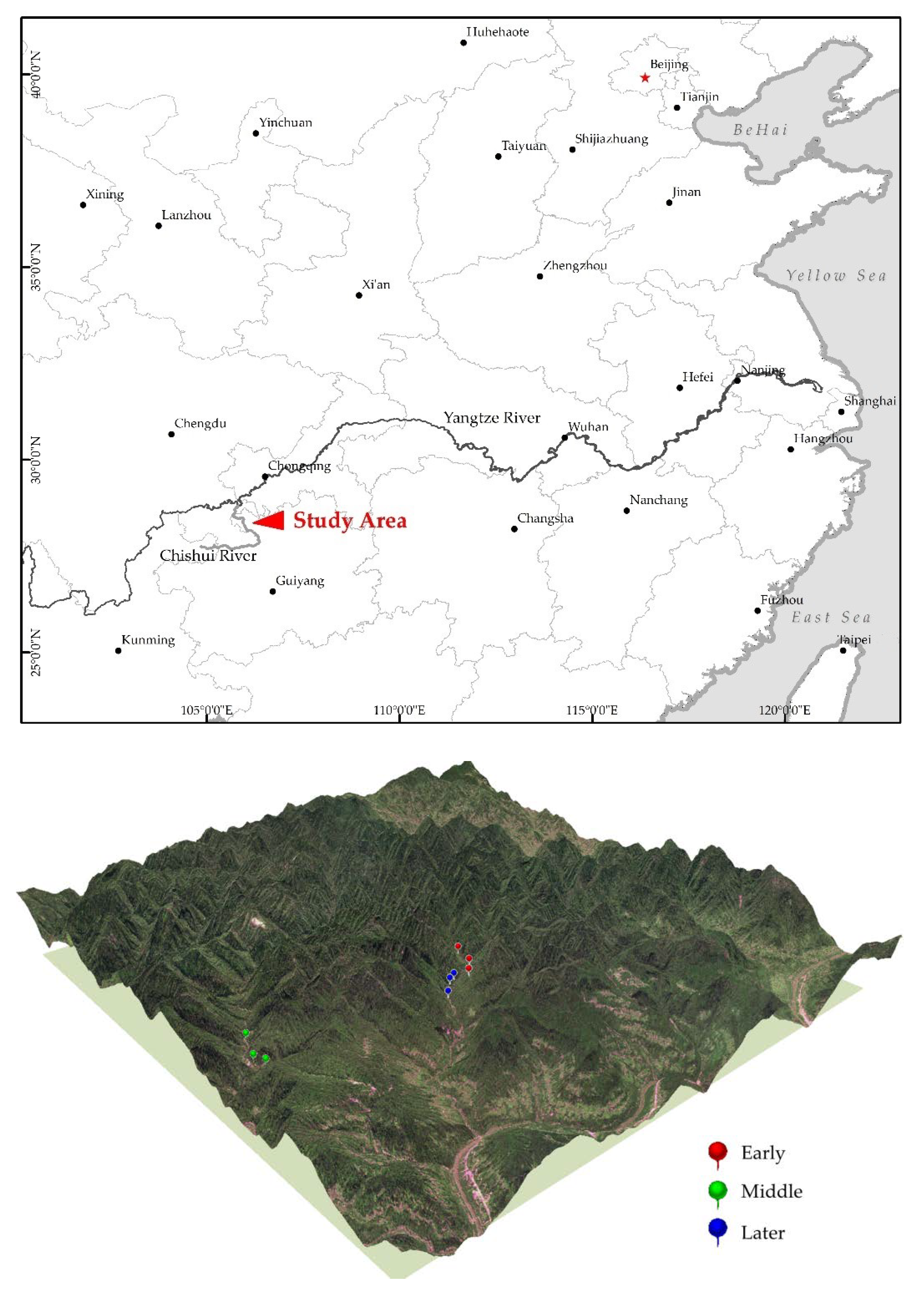
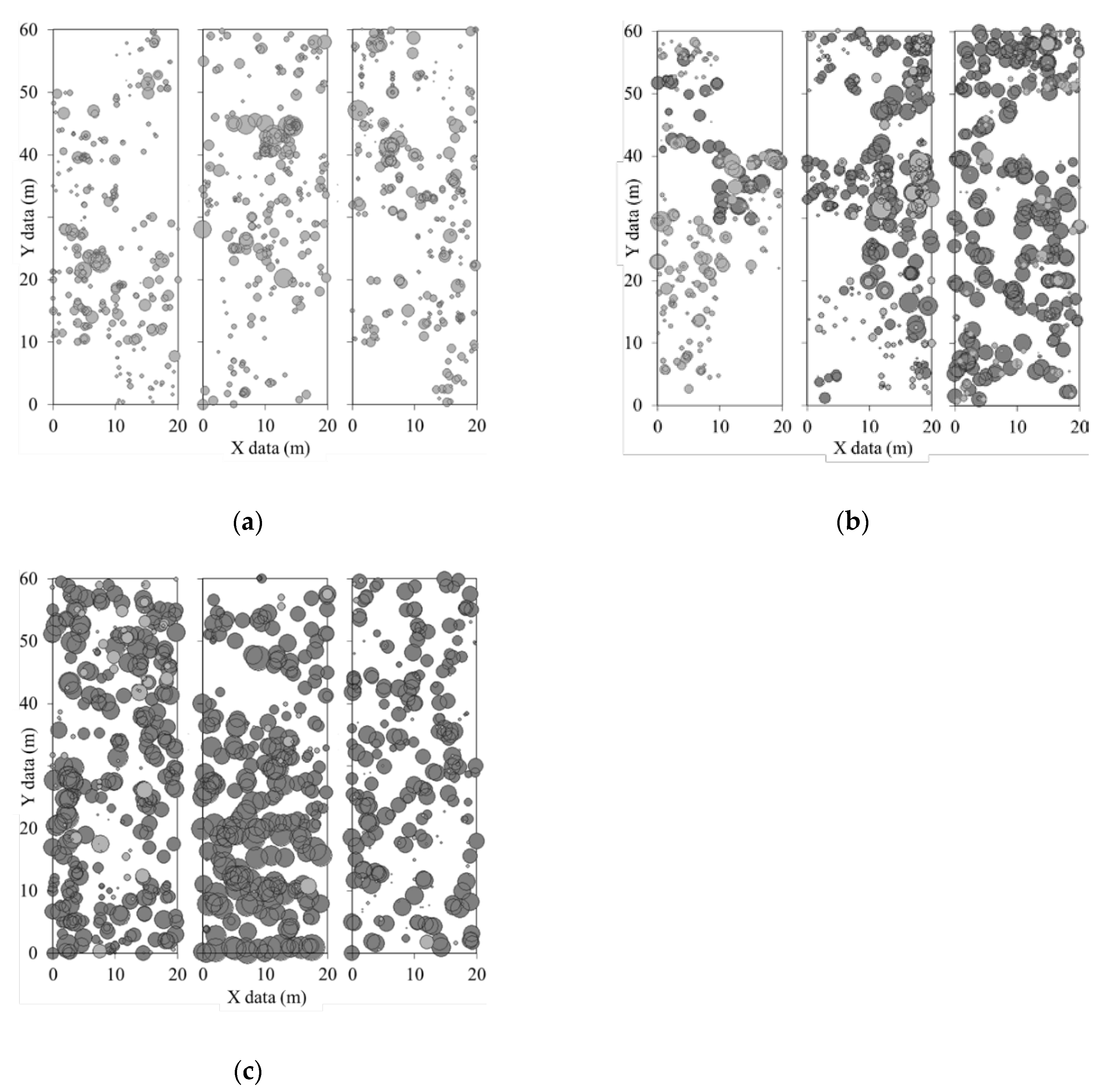
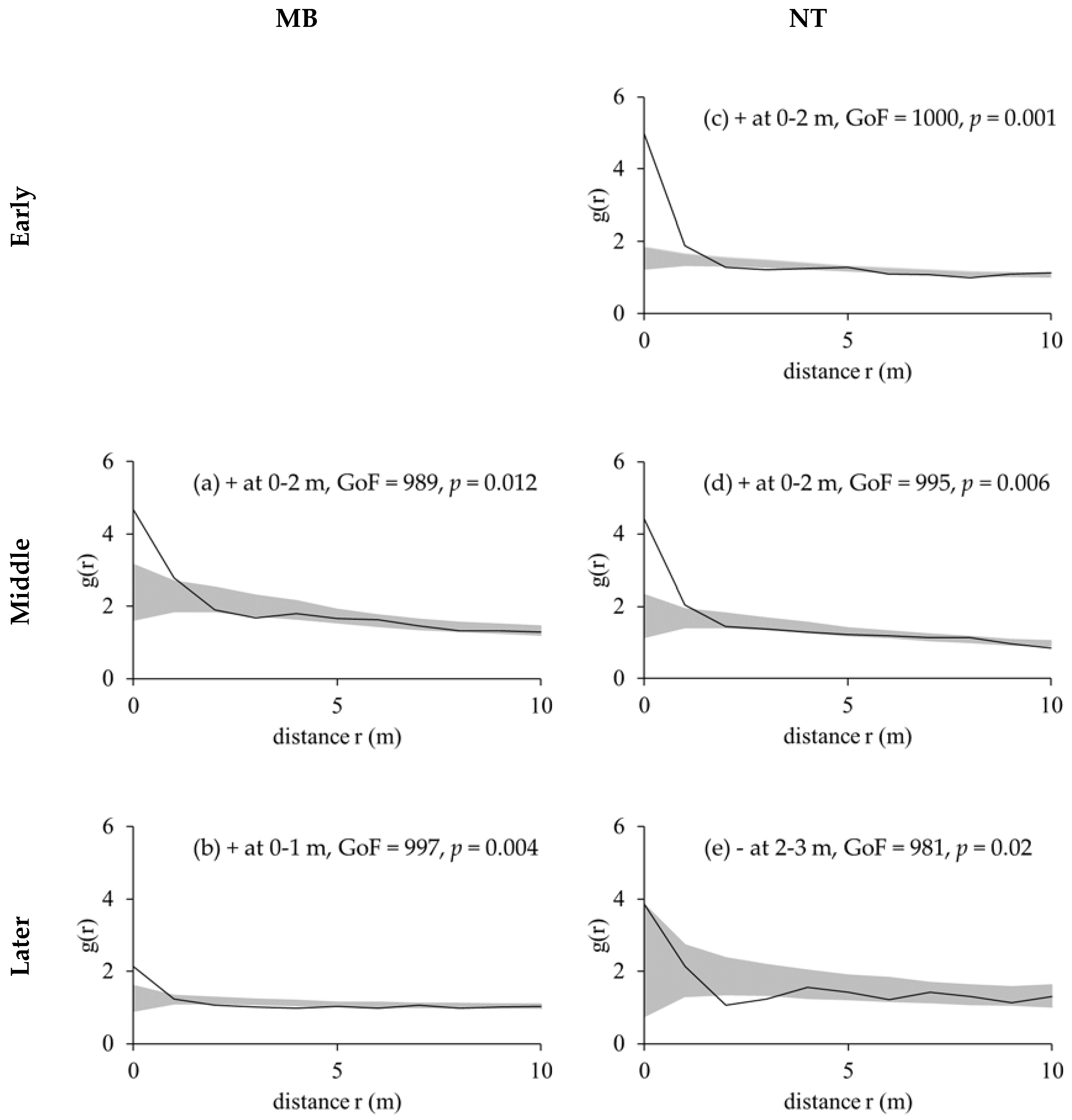
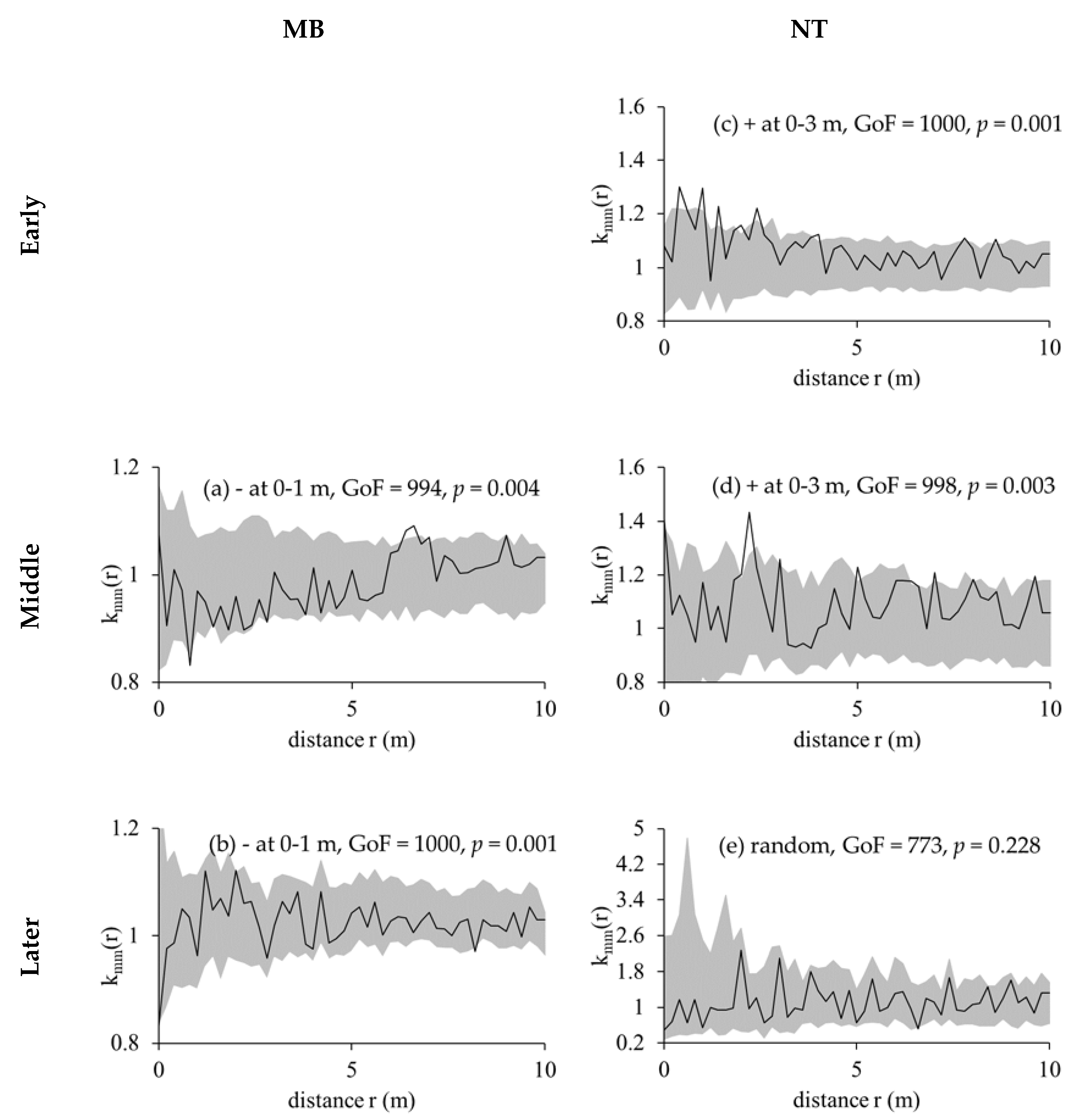
| Plot | Slope (°) | Mean Altitude (m) | Mean Density (Tree/ha) | Mean H (m) | Mean DBH (cm) | Dominant Species (% Stems) |
|---|---|---|---|---|---|---|
| Early | <18 | 536 ± 6 | 2478 ± 212 | 4.79 ± 0.43 | 7.80 ± 1.53 | Moso bamboo P. pubescens Mazel ex Houzeau de Lehaie (0) Native tree species M. balbisiana Colla (26.01) M. philippensis (Lam.) Muell.Arg. (15.36) L. chinensis (champ.) Benth. (10.09) |
| Middle | <22 | 545 ± 26 | 2775 ± 590 | 6.46 ± 0.64 | 8.92 ± 1.09 | Moso bamboo P. pubescens (47.25) Native tree species A. spinulosa (Wall. ex Hook.) R. M. Tryon (13.68) M. balbisiana (7.96) |
| Later | <24 | 562 ± 33 | 2450 ± 383 | 9.53 ± 1.56 | 9.42 ± 0.8 | Moso bamboo P. pubescens (79.82) Native tree species A. spinulosa (10.2) B. glomerulata (Blume) Regel (4.04) |
| Traits | Phases of Bamboo Expansionary | |||||
|---|---|---|---|---|---|---|
| Early | Middle | Later | ||||
| MB | NTs | MB | NTs | MB | NTs | |
| Tree Density (D)/(ha−1) | - | 2478 ± 212 | 1311 ± 706 | 1464 ± 143 | 1956 ± 286 | 494 ± 224 |
| Height/(m) | - | 4.79 ± 0.43 | 9.25 ± 0.26 | 4.16 ± 0.56 | 11 ± 1.04 | 3.4 ± 0.88 |
| DBH/(cm) | - | 7.8 ± 1.53 | 10.29 ± 1.02 | 7.66 ± 1.66 | 10.17 ± 0.25 | 6.77 ± 1.73 |
| Importance Value (IV) | - | 1 | 0.38 ± 0.14 | 0.62 ± 0.14 | 0.71 ± 0.08 | 0.29 ± 0.08 |
| Shannon-Weiner in plot | 2.46 ± 0.12 | 1.9 ± 0.24 | 0.89 ± 0.23 | |||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Xue, J. Spatial Pattern and Competitive Relationships of Moso Bamboo in a Native Subtropical Rainforest Community. Forests 2018, 9, 774. https://doi.org/10.3390/f9120774
Zhang H, Xue J. Spatial Pattern and Competitive Relationships of Moso Bamboo in a Native Subtropical Rainforest Community. Forests. 2018; 9(12):774. https://doi.org/10.3390/f9120774
Chicago/Turabian StyleZhang, Haonan, and Jianhui Xue. 2018. "Spatial Pattern and Competitive Relationships of Moso Bamboo in a Native Subtropical Rainforest Community" Forests 9, no. 12: 774. https://doi.org/10.3390/f9120774
APA StyleZhang, H., & Xue, J. (2018). Spatial Pattern and Competitive Relationships of Moso Bamboo in a Native Subtropical Rainforest Community. Forests, 9(12), 774. https://doi.org/10.3390/f9120774





