Abstract
Resprouting is an important functional trait for determining community dynamics and the persistence of individuals and populations. However, community-wide research on resprouting has primarily focused on severely damaged trees. We investigated resprouting from trees in a range of undisturbed natural forests along an elevational gradient in central Japan and analyzed the data at inter- and intraspecific levels. First, we formulated interspecific relationships among resprout production, parent stem growth, multi-stemmedness, and dominance in forest communities using a structural equation model (SEM). Second, we analyzed intraspecific variation in the resprout number per stem for nine resprouting species using a hierarchical Bayesian method. We found that resprout production and parent stem growth were negatively correlated; resprouting resulted in multi-stemmed adult forms, and species with multi-stemmed forms tended to be less dominant in undisturbed forest communities. We observed various intraspecific resprouting responses to parental and environmental factors. For example, soil temperature had generally positive effects for most species, whereas dbh appeared to have only weak effects on a few species. Our SEM summarized well the direct and indirect relationships of species’ traits, including resprout production, in the undisturbed forests. The observed intraspecific patterns in the resprouting responses can serve as a starting point for understanding species’ traits within this context.
1. Introduction
Although less attention has traditionally been paid to resprouting of woody species than to seedlings as a mode of regeneration, resprouting is an important demographic process influencing various aspects of the community dynamics and evolutionary processes of trees (e.g., [1,2,3,4,5,6,7,8]; the term “resprouting” is defined as young shoots/branches emerging from the trunk bases of already-established tree individuals, including those from both damaged and undamaged individuals in this study). Indeed, many woody species living in various types of forests and woodlands do resprout when damaged (e.g., [3,5,9,10,11]). Resprouting helps damaged individuals survive, regrow, and persist in the habitat (e.g., [12,13,14,15]). As a natural consequence of the persistence of individuals, resprouting influences population dynamics [16]. Previous models have indicated that resprouting of component species can have significant effects on community dynamics [2,4,17]. The growing appreciation of the importance of tree resprouting has led to the concept of the “persistence niche” of species [4].
Varying extents of resprout production have been observed among species coexisting in forest or woodland communities, ranging from obligate resprouters to obligate reseeders [1,3,6,8,12]. Interspecific variation in resprouting has been characterized according to the type of frequency distribution of species based on the extent of resprout production. The distribution tends to be a continuum in communities with less intense disturbances such as tropical and temperate forests where the major disturbance agent is wind, whereas it tends to be dichotomous, with distinct groups of resprouters and non-resprouters in communities with intense disturbances such as fire [7]. The coexistence of species with various sprouting abilities suggests that trade-offs in resource allocation to different tree parts (stems, reproductive organs, storage in roots and stems for future resprouting, and export to growing resprouts) or functions (growth, tolerance, persistence, colonization, and regeneration) underlie the variation [2,4,6,18,19,20,21,22,23]. The most important trade-off for understanding variation in resprouting may be that between resource allocation to parent stem growth versus resprouting. This trade-off can be detected via negative interspecific correlations between the growth rate of parental stems and the resprouting frequency [4,6,22]. Various extents of resprout production of species can have several direct or indirect effects on their growth form and dominance in forest communities. For example, individuals of frequently resprouting species tend to be multi-stemmed, i.e., the above-ground part of individuals consists of multiple stems ramifying from single root systems [18,19,20]. Because multi-stemmed individuals must allocate growth to multiple stems, multi-stemmedness may reduce height growth [6,18,19]. Moreover, multi-stemmed growth forms can decrease the structural stability of above-ground parts of trees, thereby reducing their growth [18,24]. Consequently, species with many multi-stemmed individuals are likely to be less successful in forest communities with strong competition for light. Resprouting species are often predicted to be non-dominant in productive forest communities with high canopies, high tree densities, and less intense disturbances [18,19,24]. However, complicated interspecific relationships among resprout production, parent stem growth, multi-stemmedness, and dominance in undisturbed forest communities have not yet been explained.
Substantial variation in resprouting has also been observed among individuals within each species. The factors that produce variation in resprout number within single species can be categorized into two groups: factors influencing resprout production and factors causing mortality of existing resprouts. Previous studies have revealed that the production of resprouts within a single resprouting species is influenced by various factors including parent stem size (e.g., [1,3,10,11,25,26,27]), light intensity [25,28,29,30,31], site productivity/soil nutrient availability [5,27,32], and severity of damage to parent stems [1,33,34]. The resprouting responses to such factors are thought to be primarily caused by phenotypic plasticity; hence, intraspecific variation in resprouting responses is an essential issue for achieving a better understanding of species’ characteristics (e.g., [5,31]). One well-known factor that causes mortality of existing resprouts is herbivory by large mammals (e.g., deer). Browsing by large mammalian herbivores can have substantial impacts on the regeneration processes of woody species via seedlings and resprouts (e.g., [35,36,37]). Therefore, the evaluation of browsing effects on resprouting should not be neglected for forest communities under observable herbivory pressure by large mammals.
Despite increasing interest in resprouting in woody species, most research has focused on resprouting of severely damaged individuals (often accompanied by the total loss of stems and leaves by severe disturbances such as fire, clipping, and strong winds) and the role of resprouting in stand restoration after disturbances (e.g., [1,3,7,10,12,13,14,15,31,38]). In fact, research on resprouting of undamaged individuals is rather scarce [29,39,40,41,42,43,44]. However, many species also resprout without observable damage [1,39,40]. Resprouts from undamaged individuals, often referred to as the “sprout bank” [1,28,29,41], can help to maintain individuals by replacing stems [42] or filling gaps immediately after they are created [1]. Resprouting of undamaged individuals may cause adults to form multi-stems [23]. However, most research on resprouting of undamaged individuals has focused on only a few target species, making it difficult to evaluate interspecific variation and to examine underlying trade-offs. Therefore, community-wide analyses of resprouting of individuals in undisturbed forests where most individuals are undamaged are required.
In this study, we measured resprouts of tree individuals in 56 plots each 10 × 10-m in size (half of which were within deer exclosures, and the other half outside exclosures) established in a range of undisturbed natural forests along an elevational gradient in central Japan. We also obtained data on parental and environmental factors (parent diameter at breast height (dbh), parent dbh growth, soil temperature, soil nitrogen concentration, soil pH, slope inclination, total stand basal area, cumulative basal area of stems larger than focal stems, and presence/absence of deer exclosures) as variables potentially influencing resprouting. Using the above data, we analyzed inter- and intraspecific variation in resprouting from trees in these undisturbed forests. In the analyses of interspecific variation, we posed the following questions: (1-1) Does the interspecific variation in resprout production in undisturbed forests, expressed as the ratio of resprouting individuals and the mean number of resprouts per individual, form a continuum or a dichotomy with distinct groups of resprouters and non-resprouters? (1-2) How are species-specific resprout production of individuals, parent stem growth, multi-stemmedness of adult trees, and relative dominance mutually dependent? To address question 1-2, we used structural equation modeling (SEM). In the analyses of intraspecific variation, we posed the following question: (2-1) How do parental and environmental factors affect resprout number per stem within each resprouting species? To formulate the effect of parental and environmental factors on resprout number per stem, we used hierarchical Bayesian analysis with a zero-inflated Poisson distribution.
2. Materials and Methods
2.1. Study Area
This study was conducted in natural forests along an elevational gradient ranging from 900 to 1851 m above sea level in the University of Tokyo Chichibu forest (UTCF), central Japan (Figure 1). Stand structure and species composition varied depending on both the elevation (i.e., from cool-temperate deciduous broad-leaved forests at lower elevations to sub-alpine evergreen coniferous forests at higher elevations) and past artificial disturbance history (i.e., from old-growth forests to secondary-growth forests). Climatic factors also varied along the elevational gradient. The estimated annual mean temperatures in the study area ranged from 4.5 to 9.5 °C, annual mean precipitation from 1495.8 to 1593.8 mm, and mean annual maximum depth of snow from 24 to 33 cm [45]. The topography of the research area was characterized by relatively steep slopes, with a mean (±SD) of 29.5° ± 8.1° in the study plots (Figure 1). Bedrock primarily consisted of sandstone and mudstone throughout the study area [46]. The soil type in most of the study area was Cambisols, with Humo-Ferric Podzols found at higher elevations and Andosols in some limited areas [47]. The major agent of natural disturbance in the cool-temperate forests in Japan is strong winds (e.g., typhoons) [48]. However, clear evidence for recent major natural disturbances could not be found at the research sites or in the forest around the research sites [49]. Artificial disturbances (i.e., cutting) have occurred at some lower elevations of the study area, but the study sites have not been artificially disturbed for at least the past 45 years. Bark stripping by sika deer (Cervus nippon Temminck) has become another possible source of mortality for adult trees over the last decade [50].
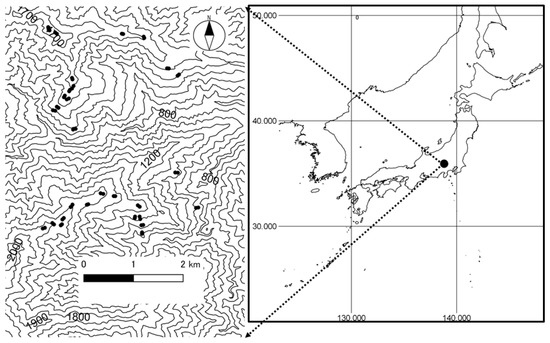
Figure 1.
Locations of plots in the University of Tokyo Chichibu forest (35°55′ N, 138°50′ E), Chichibu City, Saitama Prefecture, central Japan. In the topographic map (left), solid circles represent plots, and the contour interval is 100 m.
2.2. Data Collection
Data collection was conducted at 30 research sites in the above-mentioned natural forests as a part of a broader research project in which we are monitoring ecosystem dynamics, biodiversity, and biological interactions in forests under browsing pressure by sika deer.
Between September 2012 (after the cessation of radial growth) and June 2013 (before the onset of radial growth), we established two 30 × 30-m plots at each site (60 plots in total): one inside a deer exclosure and the other with no deer exclosure. We marked all tree stems in the plots larger than 3.18 cm in diameter at breast height (dbh), identified them to species, recorded whether they were damaged in their crowns or bark, and measured dbh (first census). We also recorded whether tree individuals had multiple stems with dbh >3.18 cm. From June to November 2015, we conducted measurements at 28 sites (56 plots) for the present study (second census). Two sites were excluded from the study due to labor constraints. We recorded the resprouting status of the stems (>3.18 cm in dbh in the first census; hereafter, target stems) in the center 10 × 10-m subplot in each plot. We re-measured the dbh of all target stems. We also recorded the number and lengths of resprouts on the target stems. For practical reasons, measurements of resprouts were only conducted for resprouts with stem lengths longer than 15 cm that were emerging from the parent stem surface within 30 cm from the ground. Between September 2016 and May 2017, we re-measured the dbh of all target stems (third census), and we also recorded whether target stems had survived during the period between the first and third census and whether they had incurred damaged in their crowns or bark. The total number of observed target (parent) stems was 853.
The extent of damage to target stems was generally low. We found that only 2 of the 853 target stems were damaged in their crown (dieback) in the first census. During the period from the first to third census, injuries to bark occurred in 7.1% of stems in the plots without deer exclosures, whereas injuries occurred in only 1.1% of stems in plots inside deer exclosures; this difference was likely due to bark stripping by deer. However, the bark stripping was not severe, as it did not result in any perceivable mortality for adult trees; the annual mortality of stems (by any mortality agent) did not differ significantly between plots inside deer exclosures (2.06% year−1) and plots without deer exclosures (2.23% year−1; p = 0.291 using the likelihood ratio test for logistic regression).
To examine possible effects of environmental factors on resprouting, we measured soil temperature, soil nitrogen content, soil pH, and slope inclination. From 23 June to 17 September 2015, soil temperatures were monitored at the center of each plot at a depth of 10 cm from the soil surface; mean soil temperatures during this period were calculated for the plots. Soil nitrogen content and pH were measured at five regularly spaced points in each plot at a depth of 5–10 cm from the top soil in 2014 [51], and mean values for plots were calculated. Slope inclination was calculated from the three-dimensional points on the forest floor in the plots during October–November 2011 using an aerial LiDAR survey (a joint project between UTCF and Suntory Natural Water Sanctuary).
2.3. Data Analyses
Variation in resprouting behavior was analyzed at inter- and intraspecific levels. In the analyses of interspecific variation, we chose as the targets of the analyses 30 species with more than six individuals whose resprouting status was recorded. In the analyses of intraspecific variation, we chose 9 resprouting species with more than nine parent stems (Acer micranthum Sieb. et Zucc., Acer rufinerve Sieb. et Zucc., Acer ukurunduense Trautv. et C. A. Mey., Clethra barbinervis Sieb. et Zucc., Fagus japonica Maxim., Fraxinus lanuginosa Koidz., Meliosma myriantha Sieb. et Zucc., Quercus crispula Blume var. crispula, and Sorbus commixta Hedl. var. rufoferruginea). We deemed a species to be sprouting if more than 20% of the stems of a species had resprouts.
2.3.1. Interspecific Variation in Resprouting
We quantified a species’ resprout production using two indices: the resprouting ratio (the ratio of the number of resprouting individuals to the total number of individuals) and the mean number of resprouts per individual. We then visually determined whether the distributions of these variables across species were a continuum or could be clearly classified into dichotomous groups (i.e., resprouters and non-resprouters).
2.3.2. Relationships among Resprout Production, Parent Stem Growth, Multi-Stemmedness, and Dominance in Forest Communities
We quantitatively described potentially complicated relationships among resprout production, parent stem growth, multi-stemmedness, and dominance in forest communities using structural equation modeling (SEM) [52]. SEM is a multivariate statistical methodology used to analyze a network of relationships between observed and latent variables. We introduced four latent variables (resprout production, parent stem growth, multi-stemmedness, and dominance in forest communities) into the SEM, and we assumed that they were represented by one to three observed variables (indicators): resprout production was represented by the resprouting ratio, mean resprout number, and mean resprout length; parent stem growth was represented by mean absolute dbh growth rate and mean relative dbh growth rate; multi-stemmedness was represented by the multi-stemmed ratio (the ratio of the number of multi-stemmed individuals to the total number of individuals) and the mean number of stems per individual; and dominance in forest communities was represented by species’ mean relative basal area (Figure 2). Mean dbh growth rates (both absolute and relative) were calculated using the dbh measurements from the first and second censuses. The species’ mean relative basal area was calculated using the data obtained in the first census. Inferring such latent variables from observed variables is more reasonable than arbitrarily choosing one observed variable as a factor (e.g., choosing only the resprouting ratio for resprout production). In terms of the relationships among latent variables, we hypothesized that (1) resprout production and parent stem growth are correlated because of the trade-off in biomass allocation to resprout production and parent stem growth (Figure 2) [4,6,22]; (2) resprout production affects multi-stemmedness [18,19,23]; (3) multi-stemmedness affects parent stem growth [6,18,19]; and (4) both parent stem growth and multi-stemmedness determine the species’ dominance in forest communities [18,19,24]. Prior to the analysis, all observed variables were log-transformed [with formulas log(x), or log(x + 0.01) if the original data included zero] and standardized. We fitted the hypothetical model (Figure 2) to the data using the maximum likelihood method with the ‘lavaan’ (ver. 0.6-3) package [53] in R software ver. 3.4.3 [54]. We began model construction with the initial model depicted in Figure 2 and improved the model using modification indices calculated using the function modification Indices ().
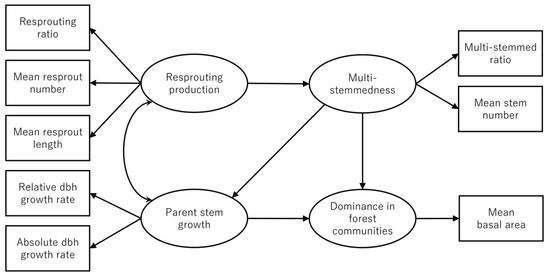
Figure 2.
Hypothetical relationships among resprout production, parent stem growth, multi-stemmedness, and dominance in forest communities.
2.3.3. Effects of Parental and Environmental Factors on Resprout Number per Stem within Species
We analyzed the number of resprouts per parent stem (N) of the nine resprouting species using the hierarchical Bayesian method with the zero-inflated Poisson distribution (ZIP) [55]. We assumed that N followed ZIP, because the observed frequencies of N = 0 were much higher than expected by the Poisson distribution. The probability that the number of resprouts (Nij) emerging from the ith parent stem belonging to the jth species following ZIP will take a particular non-negative integer value n is given by (Equation (1))
where λij and pij denote the mean of the Poisson distribution and the probability of inflated zeros, respectively. The expected number of Nij can be calculated by (1 − pij) λij. To relate Nij to parental and environmental factors, we assumed
where Dij denotes the dbh of the ith parent stem belonging to the jth species; Gij is the dbh growth rate of the parent stem; Tsij is mean soil temperature; Nsij is soil nitrogen concentration; Psij is soil pH; Sij is slope inclination; Btij is total basal area of the stand; Blij is cumulative basal area of stems larger than the focal parent stem; Eij is a binary variable expressing the presence/absence of deer exclosures; and aj0–aj9, bj0–bj9 are species-specific parameters. Total basal area was included in the model as a surrogate for light intensity at the forest floor (the larger the basal area was, the darker the forest floor was assumed to be). The cumulative basal area of larger stems was included in the model to represent the one-sided competition effect of larger stems on the focal parent stems [56,57,58,59].
To estimate the species-specific parameters (aj0–aj9, bj0–bj9), we used the hierarchical Bayesian method where species-specific parameters (e.g., aj0, j = 1–9) were assumed to follow a normal distribution with hyper-species parameters: a mean (e.g., μa0) and a standard deviation (e.g., σa0). We assumed that these hyper-species parameters (μa0–μa9, μb0–μb9, σa0–σa9, σb0–σb9) had non-informative priors.
The posterior distributions of all parameters were determined by the Markov chain Monte Carlo (MCMC) method using Stan software (ver. 2.17.0) [60] from R [54] with library RStan (ver. 2.17.3) [61]. We ran four independent MCMC chains. In most cases, we recorded 1200 samples after a burn-in of 600 for each chain, but in cases where we could not obtain satisfactory convergence levels, we increased the sampling numbers up to 7200. The chains were thinned every four runs to reduce autocorrelation. To attain rapid convergence of MCMC samplings, all explanatory variables in the model were standardized during samplings but converted to the original scales when output. For each parameter, we checked the convergence of the MCMC chains using an value [62]. When all values were <1.1, we deemed the sampling from the posterior distribution to have reached convergence.
To obtain a model with better predictive accuracy by excluding unimportant predictors, we conducted model selection using widely applicable information criterion (WAIC) values [63]. To calculate WAIC, we used the function ‘waic’ in R [64].
Quantifying the strength of the effect of each explanatory variable in the above model on the expected resprout number is difficult because a variable exerts effects through two paths (through pij and λi). Therefore, we calculated the range width (i.e., maximum–minimum) of the expected resprout number within the observed range of a focal explanatory variable to quantify the strength of the effect of each explanatory variable for each species. When calculating the expected resprout number, we used the best-supported model and mean values of explanatory variables excluding the focal variable. When Eij was included in the non-focal explanatory variables, we used one for the value of Eij, which implied that the stems were inside the deer exclosures.
3. Results
3.1. Interspecific Variation of Resprouting
Of the 30 species with more than six individuals whose resprouting status were recorded, 17 species had at least one individual with resprouts, while 13 species had no resprouting individuals (Table S1). The resprouting ratio (the ratio of the number of resprouting individuals to the total number of individuals) varied substantially among species from 0.00 to 0.86 (Figure 3; Table S1). The distribution of the resprouting ratio formed a continuum, with a peak in the smallest resprouting ratio class. The mean number of resprouts per individual had a very similar distribution, which was continuous with a peak in the smallest class (Figure 3).
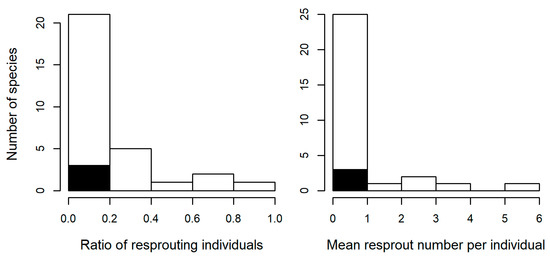
Figure 3.
Frequency distribution of species (open: hardwood, closed: conifers) in terms of resprouting.
3.2. Relationships among Resprout Production, Parent Stem Growth, Multi-Stemmedness, and Dominance in Forest Communities
Based on the results of the modification indices, we added a correlation between the relative dbh growth rate and the absolute dbh growth rate to the initial model (Figure 2) to ultimately reach a final model (Figure 4). The addition of this correlation substantially improved the fit of the final model (χ2 = 17.563; df = 14; p = 0.227; AIC = 486.374) compared to the initial model (χ2 = 24.296; df = 15; p = 0.06; AIC = 491.107).
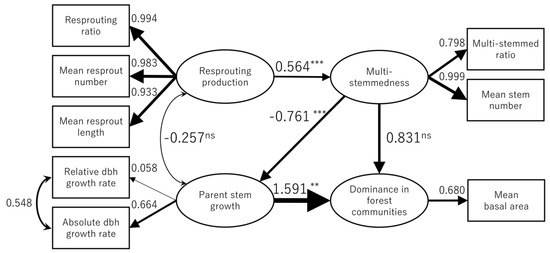
Figure 4.
Final structural equation model summarizing relationships among resprout production, parent stem growth, multi-stemmedness, and dominance in forest communities. (ns: not signifcant; **: p < 0.01; ***: p < 0.001).
The estimated standardized coefficients of the SEM are presented in Figure 4. Among the eight indicators, relative dbh growth rate had a very low standardized coefficient, indicating that this variable was not a good indicator of parent individual growth. Resprout production and parent stem growth had a weak negative direct correlation (−0.257). However, another indirect path existed between resprout production and parent stem growth through multi-stemmedness. After the effect through this path was added, the total correlation between resprout production and parent stem growth increased to −0.686 (= −0.257 + 0.564 × (−0.761)). Resprout production had a positive effect on multi-stemmedness. Multi-stemmedness had a negative effect on parent stem growth, whereas it had a positive effect on dominance in forest communities. Parent stem growth had a strong positive effect on dominance in forest communities. Although the direct effect of multi-stemmedness on dominance in forest communities was positive (0.831), the total effect, including an indirect effect through parent stem growth, was negative (0.831 + (−0.761) × 1.591 = −0.380). Similarly, the total effect of resprout production on dominance in forest communities was negative (0.564 × 0.831 + 0.564 × (−0.761) × 1.591 = −0.214).
3.3. Effects of Parental and Environmental Factors on Resprout Number Per Stem within Species
The model including dbh of parent stems, dbh growth rate, soil temperature, soil nitrogen concentration, soil pH, slope inclination, cumulative basal area of larger stems, and the presence/absence of deer exclosures was selected as the best-supported model predicting resprout number per stem for nine resprouting species (Table 1, see Table S2 for parameter estimates). The total basal area was not included in the best-supported model, indicating that this factor was not important in predicting resprout numbers. Although the explanatory variables were linearly combined without any interaction terms in the model (Equations (2) and (3)), the relationships between the expected resprout number and each of the explanatory variables (Figures S1–S7) were not always simple because the model contained two link functions (logit and log), and the expected number was calculated by (1 − pij) × λij.

Table 1.
Strength of response in expected resprout number to changes in explanatory variables. Red and blue font indicate that the expected resprout number exhibits an increasing or decreasing relationship with the focal explanatory variable, respectively. Green font indicates that the expected resprout number reaches a maximum or minimum at an intermediate value of the focal explanatory variable. See text for the detailed method of calculation.
Almost consistent but not necessarily strong effects of deer exclosures on resprout number were observed (Table 1; Figures S1–S7); parent stems generally had more resprouts if they were located inside deer exclosures compared to if they were outside exclosures. The strength of the effect varied depending on species. The difference in the expected resprout number between parent stems inside and outside deer exclosures was smallest for Acer micranthum (−0.03) and highest for Clethra barbinervis (3.19).
Because the expected resprout number was generally low for parent stems outside deer exclosures, which made the relationships between the expected resprout number and explanatory variables less clear (dotted lines in Figures S1–S7), the following descriptions of the effects of the selected explanatory variables are primarily based on patterns observed inside deer exclosures (solid lines in Figures S1–S7).
The dbh of parent stems had weak effects on resprout number, judging from the narrow range in the expected resprout number compared to the effects of other selected variables (Table 1, Figure S1). The responses of the expected resprout number to parent stem dbh varied depending on species.
The effect of dbh growth on the resprout number was negative (Table 1, Figure S2). The effect of dbh growth was generally not strong, but a relatively strong negative effect of dbh growth was observed for Clethra barbinervis.
Mean soil temperature had generally strong positive effects on resprout number within the observed temperature ranges (Table 1, Figure S3). One exception occurred in Clethra barbinervis, for which mean soil temperature had a negative effect.
Soil nitrogen and soil pH had weak effects on resprout number (Table 1, Figures S4 and S5). The responses of the expected resprout number to these factors varied depending on species.
4. Discussion
Studies of resprouting in undisturbed forests, where most observed individuals are undamaged, have been relatively scarce, and most available studies have only been conducted on a few species [1,29,40,41,42,43,44]. Therefore, comparisons in the following discussion are made mainly between the current study and previous studies on resprouting from severely damaged trees or previous studies on epicormic shoots developing from trunks. Resprouting shoots emerge from the same location (i.e., the trunk base) in both undamaged and damaged trees; however, these trees differ in physiological conditions (e.g., hormones) and sources of resources for resprouting (e.g., resources stored in underground and aboveground parts vs. currently produced and/or absorbed resources) [65]. On the other hand, resprouts emerging from trunk bases and epicormic shoots emerging from trunks generally share the same ontogenetic process and differ only in the location from which new shoots emerge and the ecological roles they play [5,65].
4.1. Interspecific Variation in Resprouting
Although the observed distributions of the two indices used to evaluate the qualitative and quantitative aspects of resprout production (the ratio of the number of resprouting individuals and the mean resprout number per individual) had distinct peaks in the lowest class of the indices, both distributions exhibited wide ranges. These wide ranges indicate that resprout production from individuals in undisturbed forests does not interspecifically converge upon similar values. The coexistence of species with various degrees of resprout production from severely damaged individuals has also been reported for various forest/woodland communities (e.g., [1,3,6,8]). The distributions observed in the present study showed similar community-wide variation in resprout production for individuals in undisturbed forests as well.
The distributions of indices for resprout production did not exhibit two peaks, indicating that the observed species did not separate into dichotomous groups of resprouters vs. non-resprouters. This finding is consistent with the general pattern observed by Vesk and Westoby [7] in that the distribution of resprout production tends to be a continuum in communities with less intense disturbances (e.g., wind) such as tropical and temperate forests, whereas it tends to be dichotomous, with distinct groups of resprouters and non-resprouters, in communities with intense disturbances (e.g., fire) [7,66]. The distributions observed in this study may be those observed at one end of a gradient in disturbance severity, as the data were obtained primarily from undamaged individuals in undisturbed forests.
4.2. Relationships among Resprout Production, Parent Stem Growth, Multi-Stemmedness, and Dominance in Forest Communities
A considerable number of previous studies on resprouting from severely damaged individuals in various types of forests have documented significant negative interspecific relationships between the parent growth rate and resprout production, which have been expressed as contrasts between slow-growing resprouters and fast-growing reseeders [3,5,9,18,19,20,21]. Similarly, the SEM results indicated a negative total correlation (−0.686) between resprout production and parent stem growth. Furthermore, the SEM showed that the negative total correlation consisted of a relatively weak direct correlation and a moderately strong indirect effect of resprout production on parent stem growth through multi-stemmedness. This result indicates that two different causal relationships or mechanisms underlie the apparent bivariate correlation between resprout production and parent stem growth. The direct negative correlation between these factors may be due to the trade-off in the allocation of growth materials to resprout production versus parent stem growth. The existence of this trade-off seems logical because the same types of materials are used for both processes. However, the trade-off can be less important [9,26,67], particularly for large vigorous trees, because the allocation to stem growth can be very large compared to allocation to resprout production. This may be one reason for the weak direct correlation found in the present study. The indirect effect of resprout production on parent stem growth through multi-stemmedness has rarely been examined quantitatively, although it has often been discussed in previous studies [3,5,18,19,20,21]. The present study demonstrated that this pathway is considerably strong in undisturbed forest communities.
Although multi-stemmedness has previously been assumed to function as a surrogate for resprout production of undamaged trees [23], multi-stemmedness (i.e., the presence of multiple large stems in single trees) is not an inevitable result of resprouting (i.e., the development of small young shoots from stem bases) from undamaged trees, as several processes can influence the extent of multi-stemmedness. These processes include growth and mortality of resprouts, which are affected by the support from and competition with parent stems. Therefore, young resprouts currently observed on undamaged trees will not necessarily become large stems to form multi-stemmed individuals, although a statistically significant effect of resprout production on multi-stemmedness was observed in this study. Additionally, current multi-stemmedness may be a consequence of past resprouting that occurred after damage to the parent stems. Multi-stemmedness resulting from resprouting after damage during severe disturbances has been well documented (e.g., [24,39]).
The SEM results presented here indicate that the extent of multi-stemmedness affected a species’ dominance in forest communities through two pathways with opposite effects: a positive direct effect and a negative indirect effect through parent stem growth. Multi-stemmedness may have a positive effect on species’ dominance because of the large numbers of growing stems associated with multi-stemmedness. On the other hand, multi-stemmedness reduced parent stem growth and had a negative indirect effect on dominance. The negative effect of multi-stemmedness on parent stem growth may be due to the reduced allocation of resources to multiple stems within single individuals [6,18,19]. Reduced structural stability of multi-stemmed individuals may play a role in reducing the species’ dominance [24]. The total effect of multi-stemmedness, i.e., the summation of the strong negative indirect effect and the relatively weak positive effect, was negative in this study.
Because resprout production had a positive effect on multi-stemmedness, the former also had a negative effect on species’ dominance through two pathways, each with a positive or negative effect. Similar negative relationships between the extent of resprout production and species’ dominance have been reported for various forests or woodlands with high productivity, high canopy, and consequently, strong competition among individual trees [18,19,21], as in the present study forest. However, if the relative strengths of the two pathways vary depending on the ecological context, which influences resprouting behavior and species dominance, various (from negative to positive) relationships can exist between species’ resprout production and their dominance. For example, the most dominant species in a montane forest in Costa Rica had the highest resprouting ratio [68], whereas species abundance was not related to resprout production in a Malaysian rain forest [69].
4.3. Effects of Parental and Environmental Factors on Resprout Number Per Stem Within Species
The presence of deer exclosures exerted the consistent (but not necessarily strong) positive effect on the resprout number per stem within each species (Table 1, Figures S1–S7), indicating that browsing pressure by sika deer on resprouts was considerable in the study forests. This result is not surprising given that deer density in the region, including in the study forests, has increased to a level at which deer browsing has substantially reduced understory vegetation [50], and large mammalian herbivores utilize not only seedlings/saplings but also resprouts [36,44,70,71,72]. The strength of the effect of deer exclosures varied among species (Table 1), suggesting that deer exhibited preferences for certain tree species [36,70]. Because our measurements were conducted only 2 years after the establishment of deer exclosures, the strength of the effect of deer exclosures on resprout number will likely increase as the effect accumulates over time. Therefore, browsing pressure on resprouts can have substantial effects on community dynamics and ecosystem functions [36,71,72].
Parent stem size is probably the most frequently examined factor that can affect tree resprouting within single species (e.g., [1,3,5,8,10,11,25,26,27,29,32,34,41,67,73]). As in most previous studies, an effect of dbh on resprout number was detected in this study for at least some species, but the strength and direction of the effect were variable (i.e., species specific) (Table 1, Figure S1). This variation in the relationship between resprout production and parent stem size may be due to the existence of multiple mechanisms. The mechanisms that have been proposed so far include (1) larger parent stems have more resource reserves that can be allocated to resprout production [25,34]; (2) larger individuals are likely to be damaged in their upper crowns by strong winds, which stimulates resprout production [29]; and (3) larger trees can avoid browsing herbivores and lethal heat from low-intensity fire, and thus lose their ability to produce resprouts [8]. These multiple mechanisms may result in non-linear (i.e., convex) relationships between resprout production and parent stem size, where resprout production initially increases with parent stem size and then decreases after reaching a maximum at an intermediate stem size [5,26]. In this case, the range of parent stem size used for observations or experiments can affect the results. The choice of the variables to express the extent of resprout production (e.g., the probability of resprout production, resprout number, or resprout size) can also affect the results. Additionally, the effect of dbh was relatively weak compared to those of environmental factors, implying that important factors influencing resprouting activity have been overlooked, whereas the effects of easily measured factors (i.e., parental factors) have often been the primary focus. Future research on tree resprouting should include both parental and environmental factors in the analyses, similar to several studies on tree growth and mortality (e.g., [74,75]).
Dbh growth had negative effects on resprouting (Table 1, Figure S2), indicating that the trade-off between resprouting of new shoots and radial growth may be operating within individual species. A similar trade-off has been observed in studies of epicormic shoots [76,77,78]. Again, the relatively weak effects of dbh growth compared to those of environmental factors may imply that important factors influencing resprouting activity have been overlooked.
Of the environmental factors considered in this study, soil temperature generally exerted relatively strong positive effects on resprout number (Table 1; Figure S3). Several studies have investigated the effects of temperature during fires on resprouting (e.g., [79,80]), but to our knowledge, this is the first report on the significant effect of temperature on resprouting under conditions without disturbances. The positive effect of soil temperature on resprout production makes intuitive sense, given that the growth period of trees at the study sites is limited not by high temperature or low precipitation but instead by low temperature.
Although several previous studies have reported significant effects of site productivity or soil nutrient availability on resprouting [27,32], only weak effects of soil properties were detected in this study (Table 1; Figures S4 and S5). This discrepancy may be due to the difference in the condition of parent stems; parent stems were severely damaged by cutting or fire in the above-cited studies, whereas most parent stems were not damaged in the present study. Another possible reason is that the best-supported model in this study included dbh growth as an explanatory variable, which can mask the effect of site productivity or soil properties.
Although resprouting has been considered an important functional species trait in terms of allowing individuals to persist on unstable substrates such as steep slopes [14,81], the resprouting response of individuals to slope inclination has not been explicitly examined. In the present study, a negative effect of slope inclination was observed more frequently (in five of nine species; Table 1; Figure S6) than a positive effect (in one species). This disparity may be due to the fact that the target species of the current analysis did not include species adapted to unstable substrates [14] or to frequently disturbed habitats [41].
Various directions and strengths of the effect of cumulative basal area of larger stems (a surrogate for one-sided competition on parent stems) (Table 1; Figure S7) may have been due to slightly different strategies of resprouting. Species that positively respond to increasing cumulative basal area (e.g., Fraxinus lanuginose) produce more resprouts when stems are suppressed by larger competitors. The resprouts produced in such situation can be an insurance against the forthcoming death of the parent stems. On the other hand, species that negatively respond to increasing cumulative basal area (e.g., Meliosma myriantha) produce more resprouts when stems are competitively advantageous. Such stems are often large and vigorous, and have large resource reserves that can be allocated to resprout production. The resprouts produced in such situation can form a resprout bank, which can help to maintain individuals by replacing main stems when they are severely damaged by disturbances (e.g., strong wind).
5. Conclusions
The present study demonstrated that both inter- and intraspecific variation in the resprouting of individuals was substantial in undisturbed forests. Similar levels of inter- and intraspecific variation in resprouting have been observed for severely damaged individuals (e.g., [1,3,6,7,12,15,38]). The interspecific variation observed here appeared to occur at one end of the spectrum from a continuous distribution of resprouting production in communities with less intense disturbances to a dichotomous distribution of resprouters and non-resprouters, which is often observed in communities with intense disturbances. Our SEM summarized well the direct and indirect relationships of species’ traits including resprout production, parent stem growth, multi-stemmedness, and relative dominance in the undisturbed forests. Because studies of the resprouting of individuals in undisturbed forests are relatively scarce, additional data are necessary before general conclusions can be drawn for this process. In particular, more research effort should focus on the effects of environmental factors. Future studies should also evaluate the significance of this process in the context of forest community dynamics and the evolution of species traits.
Supplementary Materials
The following are available online at http://www.mdpi.com/1999-4907/9/11/672/s1, Figure S1: Relationship between resprout number per stem and dbh of parent stem for nine species, Figure S2: Relationship between resprout number per stem and dbh growth rate of parent stem for nine species, Figure S3: Relationship between resprout number per stem and mean soil temperature for nine species, Figure S4: Relationship between resprout number per stem and soil nitrogen concentration for nine species, Figure S5: Relationship between resprout number per stem and soil pH for nine species, Figure S6: Relationship between resprout number per stem and slope inclination for nine species, Figure S7: Relationship between resprout number per stem and cumulative basal area of stems larger than focal stems for nine species, Table S1: Summary of species traits concerning resprouting production and multi-stemmedness in natural forests along an elevational gradient in the University of Tokyo Chichibu forest, Chichibu city, Saitama prefecture, central Japan, Table S2: Posterior means and 95% Bayesian credible intervals for parameters in the best-supported model predicting resprout number per stem.
Author Contributions
Conceptualization, K.U.; Formal analysis, K.U. and M.K.; Funding acquisition, K.U. and T.H.; Investigation, K.U., M.K., N.S. and T.H.; Writing-original draft, K.U. and M.K.; Writing-review & editing, K.U., M.K., N.S. and T.H.
Funding
This research was supported by the joint project between the University of Tokyo Chichibu Forest and Suntory Natural Water Sanctuary, the Asahi Glass Foundation and a JSPS Grant-in-Aid for Scientific Research (no. 17K07834).
Acknowledgments
We are grateful to staff of the University of Tokyo Chichibu Forest and students of Faculty of Horticulture, Chiba University for helping on fieldwork.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bellingham, P.J.; Tanner, E.V.J.; Healey, J.R. Sprouting of trees in Jamaican montane forests, after a hurricane. J. Ecol. 1994, 82, 747–758. [Google Scholar] [CrossRef]
- Bellingham, P.J.; Sparrow, A.D. Resprouting as a life history strategy in woody plant communities. OIKOS 2000, 89, 409–416. [Google Scholar] [CrossRef]
- Paciorek, C.J.; Condit, R.; Hubbell, S.P.; Foster, R.B. The demographics of resprouting in tree and shrub species of a moist tropical forest. J. Ecol. 2000, 88, 765–777. [Google Scholar] [CrossRef]
- Bond, W.J.; Midgley, J.J. Ecology of sprouting in woody plants: The persistence niche. Trends Ecol. Evol. 2001, 16, 45–51. [Google Scholar] [CrossRef]
- Del Tredici, P. Sprouting in temperate trees: A morphological and ecological review. Bot. Rev. 2001, 67, 121–140. [Google Scholar] [CrossRef]
- Bond, W.J.; Midgley, J.J. The evolutionary ecology of sprouting in woody plants. Int. J. Plant Sci. 2003, 164, S103–S114. [Google Scholar] [CrossRef]
- Vesk, P.A.; Westoby, M. Sprouting ability across diverse disturbances and vegetation types worldwide. J. Ecol. 2004, 92, 310–320. [Google Scholar] [CrossRef]
- Vesk, P.A. Plant size and resprouting ability: Trading tolerance and avoidance of damage? J. Ecol. 2006, 94, 1027–1034. [Google Scholar] [CrossRef]
- Bell, D.T. Ecological response syndromes in the flora of southwestern Western Australia: Fire resprouters versus reseeders. Bot. Rev. 2001, 67, 417–440. [Google Scholar] [CrossRef]
- Wu, L.; Shinzato, T.; Chen, C.; Aramoto, M. Sprouting characteristics of a subtropical evergreen broad-leaved forest following clear-cutting in Okinawa, Japan. New For. 2008, 36, 239–246. [Google Scholar] [CrossRef]
- Leonardsson, J.; Götmark, F. Differential survival and growth of stumps in 14 woody species after conservation thinning in mixed oak-rich temperate forests. Eur. J. For. Res. 2014, 134, 199–209. [Google Scholar] [CrossRef]
- Putz, F.E.; Nicholas, V.L.B. Sprouting of broken trees on Barro Colorado Island, Panama. Ecology 1989, 70, 508–512. [Google Scholar] [CrossRef]
- Vesk, P.A.; Westoby, M. Sprouting by plants: The effects of modular organization. Funct. Ecol. 2004, 18, 939–945. [Google Scholar] [CrossRef]
- Sakai, A.; Ohsawa, T.; Ohsawa, M. Adaptive significance of sprouting of Euptelea polyandra, a deciduous tree growing on steep slopes with shallow soil. J. Plant Res. 1995, 108, 377–386. [Google Scholar] [CrossRef]
- Poorter, L.; Kitajima, K.; Mercado, P.; Chubiña, J.; Melgar, I.; Prins, H.H.T. Resprouting as a persistence strategy of tropical forest trees: Relations with carbohydrate storage and shade tolerance. Ecology 2010, 91, 2613–2627. [Google Scholar] [CrossRef] [PubMed]
- Hara, M.; Kanno, H.; Hirabuki, Y.; Takehara, A. Population dynamics of four understorey shrub species in beech forest. J. Veg. Sci. 2004, 15, 475–484. [Google Scholar] [CrossRef]
- Paul, C.; Madhur, A. Effects of disturbance frequency, species traits and resprouting on directional succession in an individual-based model of forest dynamics. J. Ecol. 2009, 97, 1028–1036. [Google Scholar]
- Midgley, J.J. Why the world’s vegetation is not totally dominated by resprouting plants; because resprouters are shorter than reseeders. Ecography 1996, 19, 92–95. [Google Scholar] [CrossRef]
- Kruger, L.M.; Midgley, J.J.; Cowling, R.M. Resprouters vs. reseeders in South African forest trees; a model based on forest canopy height. Funct. Ecol. 1997, 11, 101–105. [Google Scholar] [CrossRef]
- Clarke, P.J.; Knox, K.J.E. Trade-offs in resource allocation that favour resprouting affect the competitive ability of woody seedlings in grassy communities. J. Ecol. 2009, 97, 1374–1382. [Google Scholar] [CrossRef]
- Clarke, P.J.; Lawes, M.J.; Midgley, J.J. Resprouting as a key functional trait in woody plants—Challenges to developing new organizing principles. New Phytol. 2010, 188, 651–654. [Google Scholar] [CrossRef] [PubMed]
- Clarke, P.J.; Lawes, M.J.; Midgley, J.J.; Lamont, B.B.; Ojeda, F.; Burrows, G.E.; Enright, N.J.; Knox, K.J.E. Resprouting as a key functional trait: How buds, protection and resources drive persistence after fire. New Phytol. 2013, 197, 19–35. [Google Scholar] [CrossRef] [PubMed]
- Shibata, R.; Kurokawa, H.; Shibata, M.; Tanaka, H.; Iida, S.; Masaki, T.; Nakashizuka, T. Relationships between resprouting ability, species traits and resource allocation patterns in woody species in a temperate forest. Funct. Ecol. 2016, 1205–1215. [Google Scholar] [CrossRef]
- Bellingham, P.J.; Sparrow, A.D. Multi-stemmed trees in montane rain forests: Their frequency and demography in relation to elevation, soil nutrients and disturbance. J. Ecol. 2009, 97, 472–483. [Google Scholar] [CrossRef]
- Sonoyama, N.; Watanabe, N.; Watanabe, O.; Niwa, S.; Kubota, Y. Ecological significance of sprouting traits of cool-temperate tree species in a northern mixed forest. Jpn. J. Ecol. 1997, 47, 21–29. [Google Scholar]
- Shibata, R.; Shibata, M.; Tanaka, H.; Iida, S.; Masaki, T.; Hatta, F.; Kurokawa, H.; Nakashizuka, T. Interspecific variation in the size-dependent resprouting ability of temperate woody species and its adaptive significance. J. Ecol. 2014, 102, 209–220. [Google Scholar] [CrossRef]
- Keyser, T.L.; Loftis, D.L. Stump sprouting of 19 upland hardwood species 1 year following initiation of a shelterwood with reserves silvicultural system in the southern Appalachian Mountains, USA. New For. 2015, 46, 449–464. [Google Scholar] [CrossRef]
- Ohkubo, T.; Tanimoto, T.; Peters, R. Response of Japanese beech (Fagus japonica maxim.) sprouts to canopy gaps. Plant Ecol. 1996, 124, 1–8. [Google Scholar] [CrossRef]
- Miura, M.; Yamamoto, S.-I. Structure and dynamics of a Castanopsis cuspidata var. Sieboldii population in an old-growth, evergreen, broad-leaved forest: The importance of sprout regeneration. Ecol. Res. 2003, 18, 115–129. [Google Scholar] [CrossRef]
- Kabeya, D.; Sakai, A.; Matsui, K.; Sakai, S. Resprouting ability of Quercus crispula seedlings depends on the vegetation cover of their microhabitats. J. Plant Res. 2003, 116, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Keyser, T.L.; Zarnoch, S.J. Stump sprout dynamics in response to reductions in stand density for nine upland hardwood species in the southern Appalachian Mountains. For. Ecol. Manag. 2014, 319, 29–35. [Google Scholar] [CrossRef]
- Weigel, D.R.; Peng, C.-Y.J. Predicting stump sprouting and competitive success of five oak species in southern Indiana. Can. J. For. Res. 2002, 32, 703–712. [Google Scholar] [CrossRef]
- Basnet, K. Recovery of a tropical rain forest after hurricane damage. Vegetatio 1993, 109, 1–4. [Google Scholar] [CrossRef]
- Masaka, K.; Ohno, Y.; Yamada, K. Fire tolerance and fire-related sprouting characteristics of two cool-temperate broad-leaved tree species. Ann. Bot. 2000, 85, 137–142. [Google Scholar] [CrossRef]
- Takatsuki, S. Effects of sika deer on vegetation in Japan: A review. Biol. Conserv. 2009, 142, 1922–1929. [Google Scholar] [CrossRef]
- Forrester, J.A.; Lorimer, C.G.; Dyer, J.H.; Gower, S.T.; Mladenoff, D.J. Response of tree regeneration to experimental gap creation and deer herbivory in north temperate forests. For. Ecol. Manag. 2014, 329, 137–147. [Google Scholar] [CrossRef]
- Habeck, C.W.; Schultz, A.K. Community-level impacts of white-tailed deer on understorey plants in North American forests: A meta-analysis. AoB Plants 2015, 7. [Google Scholar] [CrossRef] [PubMed]
- Raquel, R.B.N. Sprouting after uprooting of canopy trees in the Atlantic rain forest of Brazil. Biotropica 1995, 27, 448–454. [Google Scholar]
- Ito, S.; Gyokusen, K. Analysis of the multi-stem clump structure of Litsea japonica juss. Growing in a coastal dwarf forest. Ecol. Res. 1996, 11, 17–22. [Google Scholar] [CrossRef]
- Nanami, S.; Kawaguchi, H.; Tateno, R.; Li, C.; Katagiri, S. Sprouting traits and population structure of co-occurring Castanopsis species in an evergreen broad-leaved forest in southern China. Ecol. Res. 2004, 19, 341–348. [Google Scholar] [CrossRef]
- Kubo, M.; Sakio, H.; Shimano, K.; Ohno, K. Age structure and dynamics of Cercidiphyllum japonicum sprouts based on growth ring analysis. For. Ecol. Manag. 2005, 213, 253–260. [Google Scholar] [CrossRef]
- Fujiki, D.; Kikuzawa, K. Stem turnover strategy of multiple-stemmed woody plants. Ecol. Res. 2006, 21, 380–386. [Google Scholar] [CrossRef]
- Saeki, I. Juvenile sprouting ability of the endangered maple, Acer pycnanthum. Landsc. Ecol. Eng. 2010, 6, 1–9. [Google Scholar] [CrossRef]
- Tanentzap, A.J.; Mountford, E.P.; Cooke, A.S.; Coomes, D.A. The more stems the merrier: Advantages of multi-stemmed architecture for the demography of understorey trees in a temperate broadleaf woodland. J. Ecol. 2012, 100, 171–183. [Google Scholar] [CrossRef]
- Japan Meteorological Agency. Available online: http://www.data.jma.go.jp/obd/stats/etrn/view/atlas.html (accessed on 24 October 2018).
- Geological Survey of Japan, AIST. Seamless Digital Geological Map of Japan 1:200,000. May 12, 2007 Version. Research Information Database db084; Geological Survey of Japan, National Institute of Advanced Industrial Science and Technology: Tsukuba, Japan, 2007.
- Economic Planning Agency. Fundamental Land Classification Survey: Geomorphology, Subsurface Geology and Soil; Economic Planning Agency: Tokyo, Japan, 1973.
- Nakashizuka, T.; Yamamoto, S.-I. Natural disturbance and stability of forest community. Jpn. J. Ecol. 1987, 37, 19–30. [Google Scholar]
- Sawada, H.; Ohkubo, T.; Kaji, M.; Oomura, K. Spatial distribution and topographic dependence of vegetatiion types and tree populations of natural forests in the Chichibu Mountains, central Japan. J. Jpn. For. Soc. 2005, 87, 293–303. [Google Scholar] [CrossRef]
- Sakio, H.; Kubo, M.; Kawanishi, M.; Higa, M. Effects of deer feeding on forest floor vegetation in the Chichibu Mountains, Japan. J. Jpn. Soc. Reveg. Technol. 2013, 39, 226–231. [Google Scholar] [CrossRef]
- Shigyo, N.; Umeki, K.; Ohashi, H.; Kawada, K.; Hirao, T. Phylogenetic constraints to soil properties determine elevational diversity gradients of forest understory vegetation. Plant Ecol. 2017, 218, 821–834. [Google Scholar] [CrossRef]
- Grace, J.B. Structural Equation Modeling and Natural Systems; Cambridge University Press: Cambridge, UK, 2006. [Google Scholar]
- Rosseels, Y. Package Lavaan. Available online: http://cran.r-project.org/web/packages/lavaan/lavaan.pdf (accessed on 24 October 2018).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2017; Available online: https://www.R-project.org/ (accessed on 24 October 2018).
- Lambert, D. Zero-inflated Poisson regression, with an application to defects in manufacturing. Technometrics 1992, 34, 1–14. [Google Scholar] [CrossRef]
- Kohyama, T. Simulation of the structural development of warm-temperate rain forest stands. Ann. Bot. 1989, 63, 625–634. [Google Scholar] [CrossRef]
- Wykoff, W.R. A basal area increment model for individual conifers in the northern Rocky Mountains. For. Sci. 1990, 36, 1077–1104. [Google Scholar]
- Yokozawa, M.; Hara, T. A canopy photosynthesis model for the dynamics of size structure and self-thinning in plant populations. Ann. Bot. 1992, 70, 305–316. [Google Scholar] [CrossRef]
- Umeki, K. Growth characteristics of six tree species on Hokkaido Island, northern Japan. Ecol. Res. 2001, 16, 435–450. [Google Scholar] [CrossRef]
- Carpenter, B.; Gelman, A.; Hoffman, M.D.; Lee, D.; Goodrich, B.; Betancourt, M.; Brubaker, M.; Guo, J.; Li, P.; Riddell, A. Stan: A Probabilistic Programming Language. J. Stat. Softw. 2017, 76. [Google Scholar] [CrossRef]
- Stan Development Team. Rstan: The R Interface to Stan. Available online: http://mc-stan.org/ (accessed on 24 October 2018).
- Gelman, A.; Carlin, J.B.; Stern, H.S.; Dunson, D.B.; Vehtari, A.; Rubin, D.B. Bayesian Data Analysis, 3rd ed.; Chapman and Hall: London, UK, 2013; Volume 178. [Google Scholar]
- Watanabe, S. Asymptotic equivalence of bayes cross validation and widely applicable information criterion in singular learning theory. J. Mach. Learn. Res. 2010, 11, 3571–3594. [Google Scholar]
- Vehtari, A.; Gelman, A. Waic and Cross-Validation in Stan. Available online: http://www.stat.columbia.edu/~gelman/research/unpublished/waic_stan.pdf (accessed on 24 October 2018).
- Meier, A.R.; Saunders, M.R.; Michler, C.H. Epicormic buds in trees: A review of bud establishment, development and dormancy release. Tree Physiol. 2012, 32, 565–584. [Google Scholar] [CrossRef] [PubMed]
- Vesk, P.A.; Warton, D.I.; Westoby, M. Sprouting by semi-arid plants: Testing a dichotomy and predictive traits. Oikos 2004, 107, 72–89. [Google Scholar] [CrossRef]
- Chong, C.; Edwards, W.; Waycott, M. Differences in resprouting ability are not related to seed size or seedling growth in four riparian woody species. J. Ecol. 2007, 95, 840–850. [Google Scholar] [CrossRef]
- Matelson, T.J.; Nadkarni, N.M.; Solano, R. Tree damage and annual mortality in a montane forest in Monteverde, Costa Rica. Biotropica 1995, 27, 441–447. [Google Scholar] [CrossRef]
- Ickes, K.; Dewalt, S.J.; Thomas, S.C. Resprouting of woody saplings following stem snap by wild pigs in a Malaysian rain forest. J. Ecol. 2003, 91, 222–233. [Google Scholar] [CrossRef]
- Cutini, A.; Bongi, P.; Chianucci, F.; Pagon, N.; Grignolio, S.; Amorini, E.; Apollonio, M. Roe deer (Capreolus capreolus L.) browsing effects and use of chestnut and turkey oak coppiced areas. Ann. For. Sci. 2011, 68, 667–674. [Google Scholar] [CrossRef]
- Seager, S.T.; Eisenberg, C.; St. Clair, S.B. Patterns and consequences of ungulate herbivory on aspen in western North America. For. Ecol. Manag. 2013, 299, 81–90. [Google Scholar] [CrossRef]
- Chianucci, F.; Mattioli, L.; Amorini, E.; Giannini, T.; Marcon, A.; Chirichella, R.; Apollonio, M.; Cutini, A. Early and long-term impacts of browsing by roe deer in oak coppiced woods along a gradient of population density. Ann. Silvic. Res. 2015, 39, 32–36. [Google Scholar]
- Gould, P.J.; Fei, S.; Steiner, K.C. Modeling sprout-origin oak regeneration in the central Appalachians. Can. J. For. Res. 2007, 37, 170–177. [Google Scholar] [CrossRef]
- Umeki, K. Tree mortality of five major species on Hokkaido Island, northern Japan. Ecol. Res. 2002, 17, 575–589. [Google Scholar] [CrossRef]
- Manso, R.; Morneau, F.; Ningre, F.; Fortin, M. Effect of climate and intra- and inter-specific competition on diameter increment in beech and oak stands. Forestry 2015, 88, 540–551. [Google Scholar] [CrossRef]
- Nicolini, E.; Chanson, B.; Bonne, F. Stem growth and epicormic branch formation in understorey beech trees (Fagus sylvatica L.). Ann. Bot. 2001, 87, 737–750. [Google Scholar] [CrossRef]
- Nicolini, E.; Caraglio, Y.; Pélissier, R.; Leroy, C.; Roggy, J.C. Epicormic branches: A growth indicator for the tropical forest tree, Dicorynia guianensis Amshoff (Caesalpiniaceae). Ann. Bot. 2003, 92, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Colin, F.; Sanjines, A.; Fortin, M.; Bontemps, J.-D.; Nicolini, E. Fagus sylvatica trunk epicormics in relation to primary and secondary growth. Ann. Bot. 2012, 110, 995–1005. [Google Scholar] [CrossRef] [PubMed]
- Franklin, S.B.; Robertson, P.A.; Fralish, J.S. Small-scale fire temperature patterns in upland Quercus communites. J. Appl. Ecol. 1997, 34, 613–630. [Google Scholar] [CrossRef]
- Drewa, P.B.; Platt, W.J.; Moser, E.B. Fire effects on resprouting of shrubs in headwaters of Southeastern longleaf pine savannas. Ecology 2002, 83, 755–767. [Google Scholar] [CrossRef]
- Tang, C.Q.; Ohsawa, M. Tertiary relic deciduous forests on a humid subtropical mountain, Mt. Emei, Sichuan, China. Folia Geobot. 2002, 37, 93–106. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).