Belowground Carbohydrate Reserves of Mature Southern Pines Reflect Seedling Strategy to Evolutionary History of Disturbance
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site Description
2.2. Experimental Design
2.3. Statistical Analysis
3. Results
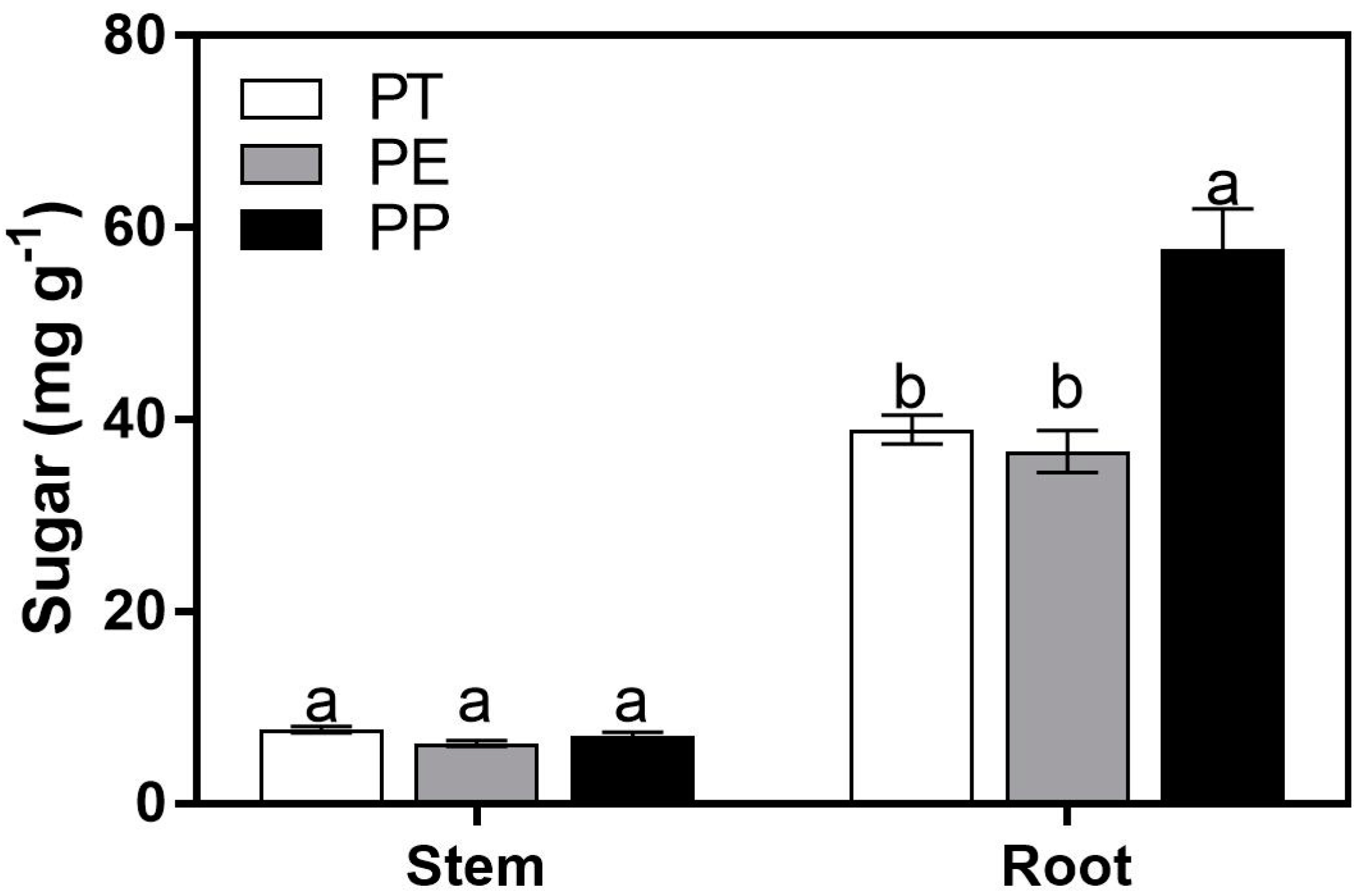
3.1. Sugar Concentrations
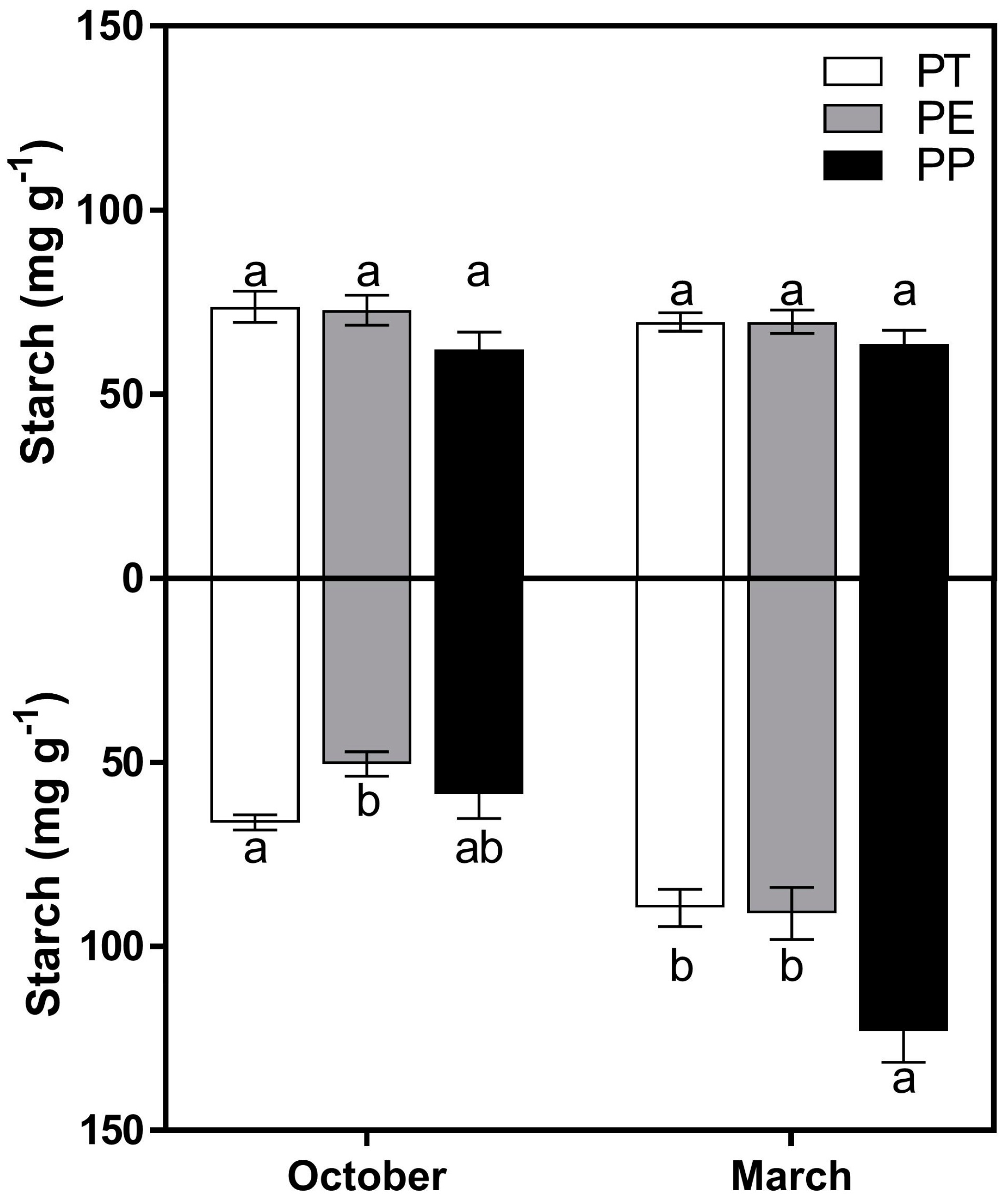
3.2. Starch Concentrations
3.3. TNC Concentrations
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Aubrey, D.P.; Mortazavi, B.; O’Brien, J.J.; McGee, J.D.; Hendricks, J.J.; Kuehn, K.A.; Teskey, R.O.; Mitchell, R.J. Influence of repeated canopy scorching on soil CO2 efflux. For. Ecol. Manag. 2012, 282, 142–148. [Google Scholar] [CrossRef]
- Guo, D.L.; Mitchell, R.J.; Hendricks, J.J. Fine root branch orders respond differentially to carbon source-sink manipulations in a longleaf pine forest. Oecologia 2004, 140, 450–457. [Google Scholar] [CrossRef] [PubMed]
- Aubrey, D.P.; Teskey, R.O. Stored root carbohydrates can maintain root respiration for extended periods. New Phytol. 2018, 218, 142–152. [Google Scholar] [CrossRef] [PubMed]
- Minelli, A. A principle of developmental inertia. In Epigenetics: Linking Genotype and Phenotype in Development and Evolution; University of California Press: Oakland, CA, USA, 2011; pp. 116–133. [Google Scholar]
- Minelli, A. Animal development, an open-ended segment of life. Biol. Theory 2011, 6, 4–15. [Google Scholar] [CrossRef]
- Farjon, A. A Natural History of Conifers; Timber Press: Portland, OR, USA, 2008. [Google Scholar]
- Stanturf, J.A.; Wade, D.D.; Waldrop, T.A.; Kennard, D.K.; Achtemeier, G.L. Fire in southern forest landscapes. In Southern Forest Resource Assessment; Gen. Tech. Rep. SRS-53; Wear, D.M., Greis, J., Eds.; US Dept. Agric., Forest Service, Southern Research Station: Asheville, NC, USA, 2002; Chapter 25; pp. 607–630. [Google Scholar]
- Platt, W.J.; Glitzenstein, J.S.; Streng, D.R. Evaluating Pyrogenicity and Its Effects on Vegetation in Longleaf Pine Savannas. In Proceedings of the Tall Timbers Fire Ecology Conference, Tallahassee, FL, USA, 30 May–2 June 1991; pp. 143–161. [Google Scholar]
- Landers, J.L.; Wade, D. Disturbance Influences on Pine Traits in the Southeastern United States. In Proceedings of the Tall Timbers Fire Ecology Conference, Tallahassee, FL, USA, 30 May–2 June 1991; Tall Timbers Research Station: Tallahassee, FL, USA, 1991; pp. 61–98. [Google Scholar]
- Hare, R.C. Contribution of bark to fire resistance of southern trees. J. For. 1965, 63, 248–251. [Google Scholar]
- Guérard, N.; Maillard, P.; Bréchet, C.; Lieutier, F.; Dreyer, E. Do trees use reserve or newly assimilated carbon for their defense reactions? A 13C labeling approach with young scots pines inoculated with a bark-beetle-associated fungus (Ophiostoma brunneo ciliatum). Ann. For. Sci. 2007, 64, 601–608. [Google Scholar] [CrossRef]
- Keeling, C.I.; Bohlmann, J. Genes, enzymes and chemicals of terpenoid diversity in the constitutive and induced defence of conifers against insects and pathogens. New Phytol. 2006, 170, 657–675. [Google Scholar] [CrossRef] [PubMed]
- Warren, J.M.; Allen, H.L.; Booker, F.L. Mineral nutrition, resin flow and phloem phytochemistry in loblolly pine. Tree Physiol. 1999, 19, 655–663. [Google Scholar] [CrossRef] [PubMed]
- Friedenberg, N.A.; Whited, B.M.; Slone, D.H.; Martinson, S.J.; Ayres, M.P. Differential impacts of the southern pine beetle, Dendroctonus frontalis, on Pinus palustris and Pinus taeda. Can. J. For. Res. 2007, 37, 1427–1437. [Google Scholar] [CrossRef]
- Hodges, J.D.; Elam, W.W.; Watson, W.F.; Nebeker, T.E. Oleoresin characteristics and susceptibility of four southern pines to southern pine beetle (coleoptera: Scolytidae) attacks. Can. Entomol. 1979, 111, 889–896. [Google Scholar] [CrossRef]
- Keeley, J.E.; Zedler, P.H. Evolution of life histories in pinus. In Ecology and Biogeography of Pinus; Cambridge University Press: Cambridge, UK, 2000; p. 219. [Google Scholar]
- Outcalt, K.W. The longleaf pine ecosystem of the south. Nat. Plants J. 2000, 1, 42–53. [Google Scholar] [CrossRef]
- O’Brien, J.J.; Hiers, J.K.; Callaham, M.A., Jr.; Mitchell, R.J.; Jack, S.B. Interactions among overstory structure, seedling life-history traits, and fire in frequently burned neotropical pine forests. AMBIO: J. Hum. Environ. 2008, 37, 542–547. [Google Scholar] [CrossRef]
- Pile, L.S.; Wang, G.G.; Knapp, B.O.; Liu, G.; Yu, D. Comparing morphology and physiology of southeastern us pinus seedlings: Implications for adaptation to surface fire regimes. Ann. For. Sci. 2017, 74, 68. [Google Scholar] [CrossRef]
- Dietze, M.C.; Sala, A.; Carbone, M.S.; Czimczik, C.I.; Mantooth, J.A.; Richardson, A.D.; Vargas, R. Nonstructural carbon in woody plants. Annu. Rev. Plant Biol. 2014, 65, 667–687. [Google Scholar] [CrossRef] [PubMed]
- Buysse, J.; Merckx, R. An improved colorimetric method to quantify sugar content of plant tissue. J. Exp. Bot. 1993, 44, 1627–1629. [Google Scholar] [CrossRef]
- Wahlenberg, W.G. Longleaf Pine: Its Use, Ecology, Regeneration, Protection, Growth, and Management; C.L. Pack Forestry Foundation and USDA Forest Service: Washington, DC, USA, 1946. [Google Scholar]
- De Ronde, C. The resistance of Pinus species to fire damage. S. Afr. For. J. 1982, 122, 22–27. [Google Scholar]
- McCune, B. Ecological diversity in North American pines. Am. J. Bot. 1988, 75, 353–368. [Google Scholar] [CrossRef]
- He, T.; Pausas, J.G.; Belcher, C.M.; Schwilk, D.W.; Lamont, B.B. Fire-adapted traits of pinus arose in the fiery cretaceous. New Phytol. 2012, 194, 751–759. [Google Scholar] [CrossRef] [PubMed]
- Keeley, J.E. Ecology and evolution of pine life histories. Ann. For. Sci. 2012, 69, 445–453. [Google Scholar] [CrossRef]
- Keeley, J.E.; Pausas, J.G.; Rundel, P.W.; Bond, W.J.; Bradstock, R.A. Fire as an evolutionary pressure shaping plant traits. Trends Plant Sci. 2011, 16, 406–411. [Google Scholar] [CrossRef] [PubMed]
- Pausas, J.G. Evolutionary fire ecology: Lessons learned from pines. Trends Plant Sci. 2015, 20, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Pausas, J.G.; Keeley, J.E. A burning story: The role of fire in the history of life. Bioscience 2009, 59, 593–601. [Google Scholar] [CrossRef]
- Van Lear, D.; Harlow, R. Fire in the eastern United States: Influence on wildlife habitat. In Proceedings: Role of Fire for Nongame Wildlife Management and Community Restoration: Traditional Uses and New Directions; Gen. Tech. Rep. NE-288; Ford, W.M., Russell, K.R., Moorman, C.E., Eds.; US Dept. of Agriculture, Forest Service, Northeastern Research Station: Newtown Square, PA, USA, 2002. [Google Scholar]
- Mitchell, R.J.; Hiers, J.K.; O’Brien, J.; Starr, G. Ecological forestry in the southeast: Understanding the ecology of fuels. J. For. 2009, 107, 391–397. [Google Scholar]
- Fonda, R.W. Burning characteristics of needles from eight pine species. For. Sci. 2001, 47, 390–396. [Google Scholar]
- Sousa, W.P. The role of disturbance in natural communities. Annu. Rev. Ecol. Syst. 1984, 15, 353–391. [Google Scholar] [CrossRef]
- White, P.S. Pattern, process, and natural disturbance in vegetation. Bot. Rev. 1979, 45, 229–299. [Google Scholar] [CrossRef]
- Lytle, D.A. Disturbance regimes and life-history evolution. Am. Nat. 2001, 157, 525–536. [Google Scholar] [PubMed]
- Lahr, E.C.; Sala, A. Species, elevation, and diameter affect whitebark pine and lodgepole pine stored resources in the sapwood and phloem: Implications for bark beetle outbreaks. Can. J. For. Res. 2014, 44, 1312–1319. [Google Scholar] [CrossRef]
- Lombardero, M.; Ayres, M.P.; Lorio, P.L., Jr.; Ruel, J.J. Environmental effects on constitutive and inducible resin defences of Pinus taeda. Ecol. Lett. 2000, 3, 329–339. [Google Scholar] [CrossRef]
- Christiansen, E.; Waring, R.H.; Berryman, A.A. Resistance of conifers to bark beetle attack: Searching for general relationships. For. Ecol. Manag. 1987, 22, 89–106. [Google Scholar] [CrossRef]
- Christiansen, E.; Ericsson, A. Starch reserves in Picea abies in relation to defence reaction against a bark beetle transmitted blue-stain fungus, Ceratocystis polonica. Can. J. For. Res. 1986, 16, 78–83. [Google Scholar] [CrossRef]
- Berryman, A.A. Resistance of conifers to invasion by bark beetle-fungus associations. Bioscience 1972, 22, 598–602. [Google Scholar] [CrossRef]
- Paine, T.; Raffa, K.; Harrington, T. Interactions among scolytid bark beetles, their associated fungi, and live host conifers. Annu. Rev. Entomol. 1997, 42, 179–206. [Google Scholar] [CrossRef] [PubMed]
- Raffa, K.F.; Phillips, T.W.; Salom, S.M. Strategies and mechanisms of host colonization by bark beetles. In Beetle-Pathogen Interactions in Conifer Forests; Academic Press: London, UK, 1993; pp. 102–128. [Google Scholar]
- Berryman, A.A. Theoretical explanation of mountain pine beetle dynamics in lodgepole pine forests. Environ. Entomol. 1976, 5, 1225–1233. [Google Scholar] [CrossRef]
- Billings, R. Southern pine beetle: Impact in wilderness and non-wilderness forests. Tex. For. 1994, 35, 16–17. [Google Scholar]
- Raffa, K.F.; Aukema, B.H.; Bentz, B.J.; Carroll, A.L.; Hicke, J.A.; Turner, M.G.; Romme, W.H. Cross-scale drivers of natural disturbances prone to anthropogenic amplification: The dynamics of bark beetle eruptions. AIBS Bull. 2008, 58, 501–517. [Google Scholar] [CrossRef]
- Outcalt, K.W. Lightning, fire and longleaf pine: Using natural disturbance to guide management. For. Ecol. Manag. 2008, 255, 3351–3359. [Google Scholar] [CrossRef]
- Johnsen, K.; Maier, C.; Sanchez, F.; Anderson, P.; Butnor, J.; Waring, R.; Linder, S. Physiological girdling of pine trees via phloem chilling: Proof of concept. Plant Cell Environ. 2007, 30, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Vilalta, J.; Sala, A.; Asensio, D.; Galiano, L.; Hoch, G.; Palacio, S.; Piper, F.I.; Lloret, F. Dynamics of non-structural carbohydrates in terrestrial plants: A global synthesis. Ecol. Monogr. 2016, 86, 495–516. [Google Scholar] [CrossRef]
- Gibson, M.D.; McMillin, C.W.; Shoulders, E. Above- and Below-Ground Biomass of Four Species of Southern Pine Growing on Three Sites in Louisiana; USDA Forest Service Final Report FS-SO-3201–59; USDA, Forest Service, Southern Forest Experiment Station: Pineville, LA, USA, 1985. [Google Scholar]
- Hoch, G.; Richter, A.; Korner, C. Non-structural carbon compounds in temperate forest trees. Plant Cell Environ. 2003, 26, 1067–1081. [Google Scholar] [CrossRef]
- Terziev, N.; Boutelje, J.; Larsson, K. Seasonal fluctuations of low-molecular-weight sugars, starch and nitrogen in sapwood of pinus sylvestris L. Scand. J. For. Res. 1997, 12, 216–224. [Google Scholar] [CrossRef]
- Mei, L.; Xiong, Y.; Gu, J.; Wang, Z.; Guo, D. Whole-tree dynamics of non-structural carbohydrate and nitrogen pools across different seasons and in response to girdling in two temperate trees. Oecologia 2015, 177, 333–344. [Google Scholar] [CrossRef] [PubMed]
- Iwasa, Y.; Kubo, T. Optimal size of storage for recovery after unpredictable disturbances. Evol. Ecol. 1997, 11, 41–65. [Google Scholar] [CrossRef]
- Schutz, A.E.N.; Bond, W.J.; Cramer, M.D. Juggling carbon: Allocation patterns of a dominant tree in a fire-prone savanna. Oecologia 2009, 160, 235–246. [Google Scholar] [CrossRef] [PubMed]
- Clarke, P.J.; Knox, K.J.E. Trade-offs in resource allocation that favour resprouting affect the competitive ability of woody seedlings in grassy communities. J. Ecol. 2009, 97, 1374–1382. [Google Scholar] [CrossRef]
- Bowen, B.J.; Pate, J.S. The significance of root starch in post-fire shoot recovery of the resprouter Stirlingia latifolia r. Br. (proteaceae). Ann. Bot. 1993, 72, 7–16. [Google Scholar] [CrossRef]
- Clarke, P.J.; Lawes, M.J.; Midgley, J.J.; Lamont, B.B.; Ojeda, F.; Burrows, G.E.; Enright, N.J.; Knox, K.J.E. Resprouting as a key functional trait: How buds, protection and resources drive persistence after fire. New Phytol. 2013, 197, 19–35. [Google Scholar] [CrossRef] [PubMed]



| Species | Month | Stem | Root | ||||
|---|---|---|---|---|---|---|---|
| Sugar (mg g−1) | Starch (mg g−1) | TNC (mg g−1) | Sugar (mg g−1) | Starch (mg g−1) | TNC (mg g−1) | ||
| Pinus taeda | October | 7.4(0.4) | 73.8(4.3) | 81.2(4.4) | 41.3(1.8) | 66.3(2.1) | 107.7(2.8) |
| March | 8.0(0.5) | 69.7(2.5) | 77.7(2.8) | 36.7(2.1) | 89.5(5.1) | 126.1(5.3) | |
| Pinus elliottii | October | 5.7(0.5) | 72.9(4.0) | 78.7(4.4) | 39.0(3.3) | 50.4(3.3) | 89.5 (4.1) |
| March | 6.7(0.3) | 69.7(3.2) | 76.4(3.5) | 34.3(2.8) | 91.0(7.1) | 125.4(7.5) | |
| Pinus palustris | October | 6.1(0.5) | 62.2(4.7) | 68.4(4.6) | 63.7(5.8) | 58.6(6. 6) | 122.3(10.8) |
| March | 8.0(0.3) | 63.7(3.7) | 71.7(3.7) | 51.9(5.8) | 123.5(8.4) | 175.3(12.2) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mims, J.T.; O’Brien, J.J.; Aubrey, D.P. Belowground Carbohydrate Reserves of Mature Southern Pines Reflect Seedling Strategy to Evolutionary History of Disturbance. Forests 2018, 9, 653. https://doi.org/10.3390/f9100653
Mims JT, O’Brien JJ, Aubrey DP. Belowground Carbohydrate Reserves of Mature Southern Pines Reflect Seedling Strategy to Evolutionary History of Disturbance. Forests. 2018; 9(10):653. https://doi.org/10.3390/f9100653
Chicago/Turabian StyleMims, Joshua T., Joseph J. O’Brien, and Doug P. Aubrey. 2018. "Belowground Carbohydrate Reserves of Mature Southern Pines Reflect Seedling Strategy to Evolutionary History of Disturbance" Forests 9, no. 10: 653. https://doi.org/10.3390/f9100653
APA StyleMims, J. T., O’Brien, J. J., & Aubrey, D. P. (2018). Belowground Carbohydrate Reserves of Mature Southern Pines Reflect Seedling Strategy to Evolutionary History of Disturbance. Forests, 9(10), 653. https://doi.org/10.3390/f9100653





