Changes in Soil Arthropod Abundance and Community Structure across a Poplar Plantation Chronosequence in Reclaimed Coastal Saline Soil
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Experimental and Sampling Design
2.3. Soil Arthropod Sampling and Extraction
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Grgič, T.; Kos, I. Influence of forest development phase on centipede diversity in managed beech forests in Slovenia. Biodivers. Conserv. 2005, 14, 1841–1862. [Google Scholar] [CrossRef]
- Cakir, M.; Makineci, E. Humus characteristics and seasonal changes of soil arthropod communities in a natural sessile oak (Quercus petraea L.) stand and adjacent Austrian pine (Pinus nigra Arnold) plantation. Environ. Monit. Assess. 2013, 185, 8943–8955. [Google Scholar] [CrossRef] [PubMed]
- Cortet, J.; Vauflery, A.G.; Poinsotbalaguer, N.; Gomot, L.; Texier, C.; Cluzeau, D. The use of invertebrate soil fauna in monitoring pollutant effects. Eur. J. Soil Biol. 1999, 35, 115–134. [Google Scholar] [CrossRef]
- Wardle, D.A.; Bardgett, R.D.; Klironomos, J.N.; Setala, H.; van der Putten, W.H.; Wall, D.H. Ecological linkages between aboveground and belowground biota. Science 2004, 304, 1629–1633. [Google Scholar] [CrossRef] [PubMed]
- Mace, G.M.; Norris, K.; Fitter, A.H. Biodiversity and ecosystem services: A multilayered relationship. Trends Ecol. Evol. 2012, 27, 19–26. [Google Scholar] [CrossRef] [PubMed]
- De Deyn, G.B.; Van der Putten, W.H. Linking aboveground and belowground diversity. Trends Ecol. Evol. 2005, 20, 625–633. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.; Coleman, D.C.; Hendrix, P.F.; Crossley, D.A. Responses of trophic groups of soil nematodes to residue application under conventional tillage and no-till regimes. Soil Biol. Biochem. 2000, 32, 1731–1741. [Google Scholar] [CrossRef]
- Siemann, E. Experimental tests of effects of plant productivity and diversity on grassland arthropod diversity. Ecology 1998, 79, 2057–2070. [Google Scholar] [CrossRef]
- Hansen, R.A. Effect of habitat complexity and composition on a diverse litter microarthropod assemblage. Ecology 2000, 81, 1120–1132. [Google Scholar] [CrossRef]
- Callaham, M.A.; Richter, D.D.; Coleman, D.C.; Hofmockel, M. Long-term land-use effects on soil invertebrate communities in Southern Piedmont soils, USA. Eur. J. Soil Biol. 2006, 42, S150–S156. [Google Scholar] [CrossRef]
- De Deyn, G.B.; Raaijmakers, C.E.; Zoomer, H.R.; Berg, M.P.; de Ruiter, P.C.; Verhoef, H.A.; Bezemer, T.M.; van der Putten, W.H. Soil invertebrate fauna enhances grassland succession and diversity. Nature 2003, 422, 711–713. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Pu, L.; Zhu, M.; Zhang, J.; Li, P.; Dai, X.; Xu, Y.; Liu, L. Evolution of soil properties following reclamation in coastal areas: A review. Geoderma 2014, 226–227, 130–139. [Google Scholar] [CrossRef]
- Sun, Y.; Li, X.; Mander, Ü.; He, Y.; Jia, Y.; Ma, Z.; Guo, W.; Xin, Z. Effect of reclamation time and land use on soil properties in Changjiang River Estuary, China. Chin. Geogr. Sci. 2011, 21, 403–416. [Google Scholar] [CrossRef]
- Brais, S.; Camiré, C.; Bergeron, Y.; Parécd, D. Changes in nutrient availability and forest floor characteristics in relation to stand age and forest composition in the southern part of the boreal forest of northwestern Quebec. For. Ecol. Manag. 1995, 76, 181–189. [Google Scholar] [CrossRef]
- Binkley, D.; Valentine, D.W.; Wells, C.G.; Valentine, U. An empirical analysis of the factors contributing to 20-year decrease in soil pH in an old-field plantation of loblolly pine. Biogeochemistry 1989, 8, 39–54. [Google Scholar] [CrossRef]
- Ge, Z.; Fang, S.; Chen, H.; Zhu, R.; Peng, S.; Ruan, H. Soil aggregation and organic carbon dynamics in poplar plantations. Forests 2018, 9, 508. [Google Scholar] [CrossRef]
- Wang, S.; Chen, H.Y.H.; Tan, Y.; Fan, H.; Ruan, H. Fertilizer regime impacts on abundance and diversity of soil fauna across a poplar plantation chronosequence in coastal Eastern China. Sci. Rep. 2016, 6, 20816. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.Y.H.; Biswas, S.R.; Sobey, T.M.; Brassard, B.W.; Bartels, S.F.; Mori, A. Reclamation strategies for mined forest soils and overstorey drive understorey vegetation. J. Appl. Ecol. 2018, 55, 926–936. [Google Scholar] [CrossRef]
- Jucevica, E.; Melecis, V. Global warming affect Collembola community: A long-term study. Pedobiologia 2006, 50, 177–184. [Google Scholar] [CrossRef]
- Liu, R.; Zhu, F.; Song, N.; Yang, X.; Chai, Y. Seasonal distribution and diversity of ground arthropods in microhabitats following a shrub plantation age sequence in desertified steppe. PLoS ONE 2013, 8, e77962. [Google Scholar] [CrossRef]
- Basset, Y.; Cizek, L.; Cuenoud, P.; Didham, R.K.; Novotny, V.; Odegaard, F.; Roslin, T.; Tishechkin, A.K.; Schmidl, J.; Winchester, N.N.; et al. Arthropod distribution in a tropical rainforest: Tackling a four dimensional puzzle. PLoS ONE 2015, 10, e0144110. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Liu, X.; Liu, S.; Wang, J.; Wang, Y. Composition and spatio-temporal variation of soil microarthropods in the biodiversity hotspot of northern Hengduan Mountains, China. Eur. J. Soil Biol. 2014, 62, 30–38. [Google Scholar] [CrossRef]
- Eisenbeis, G.; Wichard, W. Atlas on the Biology of Soil Arthropods; Springer Science & Business Media: New York, NY, USA, 2012. [Google Scholar]
- Salmon, S.; Artuso, N.; Frizzera, L.; Zampedri, R. Relationships between soil fauna communities and humus forms: Response to forest dynamics and solar radiation. Soil Biol. Biochem. 2008, 40, 1707–1715. [Google Scholar] [CrossRef]
- Salmon, S.; Mantel, J.; Frizzera, L.; Zanella, A. Changes in humus forms and soil animal communities in two developmental phases of Norway spruce on an acidic substrate. For. Ecol. Manag. 2006, 237, 47–56. [Google Scholar] [CrossRef]
- Zaitsev, A. Oribatid mite diversity and community dynamics in a spruce chronosequence. Soil Biol. Biochem. 2002, 34, 1919–1927. [Google Scholar] [CrossRef]
- Bokhorst, S.; Wardle, D.A.; Nilsson, M.-C.; Gundale, M.J. Impact of understory mosses and dwarf shrubs on soil micro-arthropods in a boreal forest chronosequence. Plant Soil 2014, 379, 121–133. [Google Scholar] [CrossRef]
- Miller, H.G. Forest fertilization: Some guiding concepts. Forestry 1981, 54, 157–167. [Google Scholar] [CrossRef]
- Scheu, S.; Albers, D.; Alphei, J.; Buryn, R.; Klages, U.; Migge, S.; Platner, C.; Salamon, J.-A. The soil fauna community in pure and mixed stands of beech and spruce of different age: Trophic structure and structuring forces. Oikos 2003, 101, 225–238. [Google Scholar] [CrossRef]
- Magura, T.; Bogyó, D.; Mizser, S.; Nagy, D.D.; Tóthmérész, B. Recovery of ground-dwelling assemblages during reforestation with native oak depends on the mobility and feeding habits of the species. For. Ecol. Manag. 2015, 339, 117–126. [Google Scholar] [CrossRef]
- Scheu, S. Plants and generalist predators as links between the below-ground and above-ground system. Basic Appl. Ecol. 2001, 2, 3–13. [Google Scholar] [CrossRef]
- Seastedt, T.R. The role of microarthropods in decomposition and mineralization processes. Ann. Rev. Entomol. 1984, 29, 25–46. [Google Scholar] [CrossRef]
- Vasconcellos, R.L.F.; Segat, J.C.; Bonfima, J.A.; Baretta, D.; Cardoso, E.J.B.N. Soil macrofauna as an indicator of soil quality in an undisturbed riparian forest and recovering sites of different ages. Eur. J. Soil Biol. 2013, 58, 105–112. [Google Scholar] [CrossRef]
- Kardol, P.; Reynolds, W.N.; Norby, R.J.; Classen, A.T. Climate change effects on soil microarthropod abundance and community structure. Appl. Soil Ecol. 2011, 47, 37–44. [Google Scholar] [CrossRef]
- Wiwatwitaya, D.; Takeda, H. Seasonal changes in soil arthropod abundance in the dry evergreen forest of north-east Thailand, with special reference to collembolan communities. Ecol. Res. 2004, 20, 59–70. [Google Scholar] [CrossRef]
- Lindberg, N.; Bengtsson, J. Population responses of oribatid mites and collembolans after drought. Appl. Soil Ecol. 2005, 28, 163–174. [Google Scholar] [CrossRef]
- Pflug, A.; Wolters, V. Influence of drought and litter age on Collembola communities. Eur. J. Soil Biol. 2001, 37, 305–308. [Google Scholar] [CrossRef]
- Sartori, F.; Lal, R.; Ebinger, M.H.; Eaton, J.A. Changes in soil carbon and nutrient pools along a chronosequence of poplar plantations in the Columbia Plateau, Oregon, USA. Agric. Ecosyst. Environ. 2007, 122, 325–339. [Google Scholar] [CrossRef]
- Wang, H.; Xu, Y.; Zhang, J.; Zhang, Y.; Yang, X. Study on soil improvement effect of the Populus tomentos pure forest of saline land on the muddy sea-coast in northern Jiangsu Province. Sci. Soil Water Conserv. 2013, 11, 65–68. (In Chinese) [Google Scholar]
- Mueller-Dumbois, D.; Ellenberg, H. Aims and Methods of Vegetation Ecology; John Wiley & Sons: Toronto, ON, Canada, 1974. [Google Scholar]
- Decaëns, T.; Dutoitb, T.; Alardb, D.; Lavelle, P. Factors influencing soil macrofaunal communities in post-pastoral successions of westem France. Appl. Soil Ecol. 1998, 9, 361–367. [Google Scholar] [CrossRef]
- Yin, W.Y. Pictorial Keys to Soil Animals of China; Science Press: Beijing, China, 1998. (In Chinese) [Google Scholar]
- Gkisakis, V.D.; Kollaros, D.; Bàrberi, P.; Livieratos, I.C.; Kabourakis, E.M. Soil arthropod diversity in organic, integrated, and conventional olive orchards and different agroecological zones in Crete, Greece. Agroecol. Sustain. Food Syst. 2015, 39, 276–294. [Google Scholar] [CrossRef]
- Cotes, B.; Campos, M.; Pascual, F.; García, P.A.; Ruano, F. Comparing taxonomic levels of epigeal insects under different farming systems in Andalusian olive agroecosystems. Appl. Soil Ecol. 2010, 44, 228–236. [Google Scholar] [CrossRef]
- Biaggini, M.; Consorti, R.; Dapporto, L.; Dellacasa, M.; Paggetti, E.; Corti, C. The taxonomic level order as a possible tool for rapid assessment of arthropod diversity in agricultural landscapes. Agric. Ecosyst. Environ. 2007, 122, 183–191. [Google Scholar] [CrossRef]
- Salamon, J.-A.; Wissuwa, J.; Moder, K.; Frank, T. Effects of Medicago sativa, Taraxacum officinale and Bromus sterilis on the density and diversity of Collembola in grassy arable fallows of different ages. Pedobiologia 2011, 54, 63–70. [Google Scholar] [CrossRef]
- Chauvat, M.; Zaitsev, A.S.; Wolters, V. Successional changes of Collembola and soil microbiota during forest rotation. Oecologia 2003, 137, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Chao, A.; Gotelli, N.J.; Hsieh, T.C.; Sander, E.L.; Ma, K.H.; Colwell, R.K.; Ellison, A.M. Rarefaction and extrapolation with Hill numbers: A framework for sampling and estimation in species diversity studies. Ecol. Monogr. 2014, 84, 45–67. [Google Scholar] [CrossRef]
- Colwell, R.K.; Chao, A.; Gotelli, N.J.; Lin, S.Y.; Mao, C.X.; Chazdon, R.L.; Longino, J.T. Models and estimators linking individual-based and sample-based rarefaction, extrapolation and comparison of assemblages. J. Plant Ecol. 2012, 5, 3–21. [Google Scholar] [CrossRef]
- Hsieh, T.C.; Ma, K.H.; Chao, A.; McInerny, G. iNEXT: An R package for rarefaction and extrapolation of species diversity (Hill numbers). Methods Ecol. Evol. 2016, 7, 1451–1456. [Google Scholar] [CrossRef]
- Bates, D.; Bolker, B.; Walker, S.; Christensen, R.H.B.; Singmann, H.; Dai, B.; Grothendieck, G. lme4: Linear Mixed-Effects Models Using Eigen and S4. R Package; Version 1.1-13. Available online: https://cran.r-project.org/web/packages/lme4/index.html (accessed on 1 September 2016).
- Canty, A.; Ripley, B. Package ‘boot’, Version 1.3-19; Available online: https://cran.r-project.org/web/packages/boot/index.html (accessed on 11 February 2017).
- Wickham, H.; Chang, W. ggplot2: Create Elegant Data Visualisations Using the Grammar of Graphics; Version 2.2.1; Springer: New York, NY, USA, 2016. [Google Scholar]
- Anderson, M.J. A new method for non-parametric multivariate analysis of variance. Austral Ecol. 2001, 26, 32–46. [Google Scholar]
- Anderson, M.J.; Crist, T.O.; Chase, J.M.; Vellend, M.; Inouye, B.D.; Freestone, A.L.; Sanders, N.J.; Cornell, H.V.; Comita, L.S.; Davies, K.F.; et al. Navigating the multiple meanings of beta diversity: A roadmap for the practicing ecologist. Ecol. Lett. 2011, 14, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O′Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. vegan: Community Ecology Package. Available online: https://cran.r-project.org/web/packages/vegan/index.html (accessed on 7 April 2017).
- De Caceres, M.; Jansen, F. Package ‘Indicspecies’. Available online: https://cran.r-project.org/web/packages/indicspecies/indicspecies.pdf (accessed on 30 August 2016).
- Miggea, S.; Maraun, M.; Scheu, S.; Schaefer, M. The oribatid mite community (Acarina) of pure and mixed stands of beech (Fagus sylvatica) and spruce (Picea abies) of different age. Appl. Soil Ecol. 1998, 9, 115–121. [Google Scholar] [CrossRef]
- Berg, M.P.; Hemerik, L. Secondary succession of terrestrial isopod, centipede, and millipede communities in grasslands under restoration. Biol. Fertil. Soils 2004, 40, 163–170. [Google Scholar] [CrossRef]
- Wu, P.; Liu, S.; Liu, X. Composition and spatio-temporal changes of soil macroinvertebrates in the biodiversity hotspot of northern Hengduanshan Mountains, China. Plant Soil 2012, 357, 321–338. [Google Scholar] [CrossRef]
- Sanders, N.J.; Gotelli, N.J.; Wittman, S.E.; Ratchford, J.S.; Ellison, A.M.; Jules, E.S. Assembly rules of ground-foraging ant assemblages are contingent on disturbance, habitat and spatial scale. J. Biogeogr. 2007, 34, 1632–1641. [Google Scholar] [CrossRef]
- Letourneau, D.K.; Dyer, L.A.; Burslem, D.; Pinard, M.; Hartley, S. Multi-trophic interactions and biodiversity: Beetles, ants, caterpillars, and plants. In Biotic Interactions in the Tropics: Their Role in the Maintenance of Species Diversity; Cambridge University Press: Cambridge, UK, 2005; pp. 366–385. [Google Scholar]
- Connell, J.H. Diversity in tropical rain forests and coral reefs—High diversity of trees and corals is maintained only in a non-equilibrium state. Science 1978, 199, 1302–1310. [Google Scholar] [CrossRef] [PubMed]
- Peña-Peña, K.; Irmler, U. Moisture seasonality, soil fauna, litter quality and land use as drivers of decomposition in Cerrado soils in SE-Mato Grosso, Brazil. Appl. Soil Ecol. 2016, 107, 124–133. [Google Scholar] [CrossRef]
- González, G.; Seastedt, T.R. Comparison of the abundance and composition of litter fauna in tropical and subalpine forests. Pedobiologia 2000, 44, 545–555. [Google Scholar] [CrossRef]
- Wang, S.; Tan, Y.; Fan, H.; Ruan, H.; Zheng, A. Responses of soil microarthropods to inorganic and organic fertilizers in a poplar plantation in a coastal area of eastern China. Appl. Soil Ecol. 2015, 89, 69–75. [Google Scholar] [CrossRef]
- Berthe, S.C.F.; Derocles, S.A.P.; Lunt, D.H.; Kimball, B.A.; Evans, D.M. Simulated climate-warming increases Coleoptera activity-densities and reduces community diversity in a cereal crop. Agric. Ecosyst. Environ. 2015, 210, 11–14. [Google Scholar] [CrossRef]
- Bokhorst, S.; Huiskes, A.H.; Convey, P.; Van Bodegom, P.M.; Aerts, R. Climate change effects on soil arthropod communities from the Falkland Islands and the Maritime Antarctic. Soil Biol. Biochem. 2008, 40, 1547–1556. [Google Scholar] [CrossRef]
- Frampton, G.K.; Brink, P.J.V.D.; Gould, P.J.L. Effects of spring drought and irrigation on farmland and arthropods in southern Britain. J. Appl. Ecol. 2000, 37, 865–883. [Google Scholar] [CrossRef]
- Kaneda, S.; Kaneko, N. Influence of Collembola on nitrogen mineralization varies with soil moisture content. Soil Sci. Plant Nutr. 2011, 57, 40–49. [Google Scholar] [CrossRef]
- Chikoski, J.M.; Ferguson, S.H.; Meyer, L. Effects of water addition on soil arthropods and soil characteristics in a precipitation-limited environment. Acta Oecol. 2006, 30, 203–211. [Google Scholar] [CrossRef]
- Salamon, J.-A.; Scheu, S.; Schaefer, M. The Collembola community of pure and mixed stands of beech (Fagus sylvatica) and spruce (Picea abies) of different age. Pedobiologia 2008, 51, 385–396. [Google Scholar] [CrossRef]
- Rotheray, T.D.; Boddy, L.; Jones, T.H. Collembola foraging responses to interacting fungi. Ecol. Entomol. 2009, 34, 125–132. [Google Scholar] [CrossRef]
- Jonas, J.L.; Wilson, G.W.T.; White, P.M.; Joern, A. Consumption of mycorrhizal and saprophytic fungi by Collembola in grassland soils. Soil Biol. Biochem. 2007, 39, 2594–2602. [Google Scholar] [CrossRef]
- Chen, B.R.; Wise, D.H. Bottom-up limitation of predaceous arthropods in a detritus-based terrestrial food web. Ecology 1999, 80, 761–772. [Google Scholar] [CrossRef]
- Lewis, J.G.E. The Biology of Centipedes; Cambridge University Press: Cambridge, UK, 1981. [Google Scholar]
- Gao, M.; Taylor, M.K.; Callaham, M.A. Trophic dynamics in a simple experimental ecosystem: Interactions among centipedes, Collembola and introduced earthworms. Soil Biol. Biochem. 2017, 115, 66–72. [Google Scholar] [CrossRef]
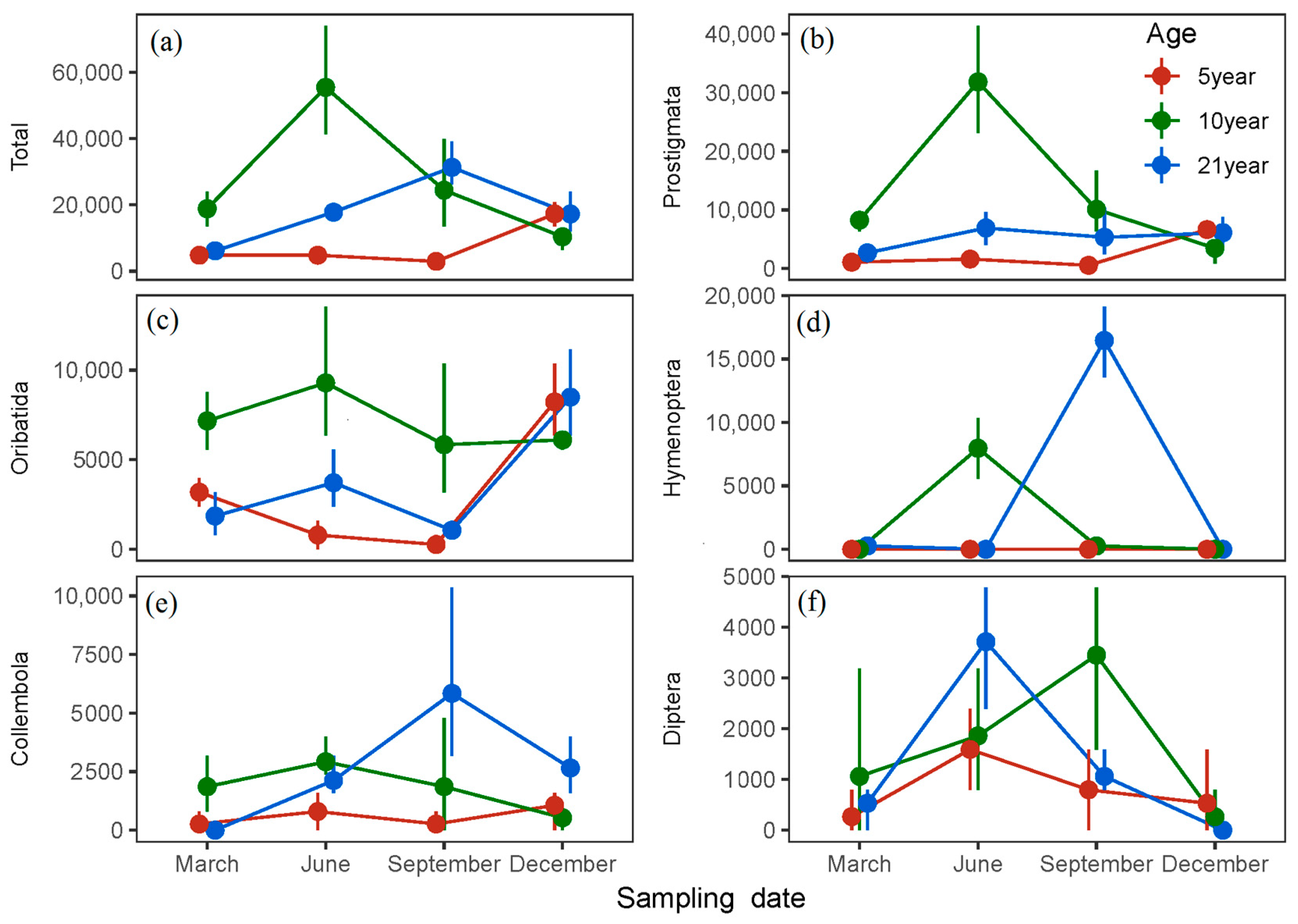
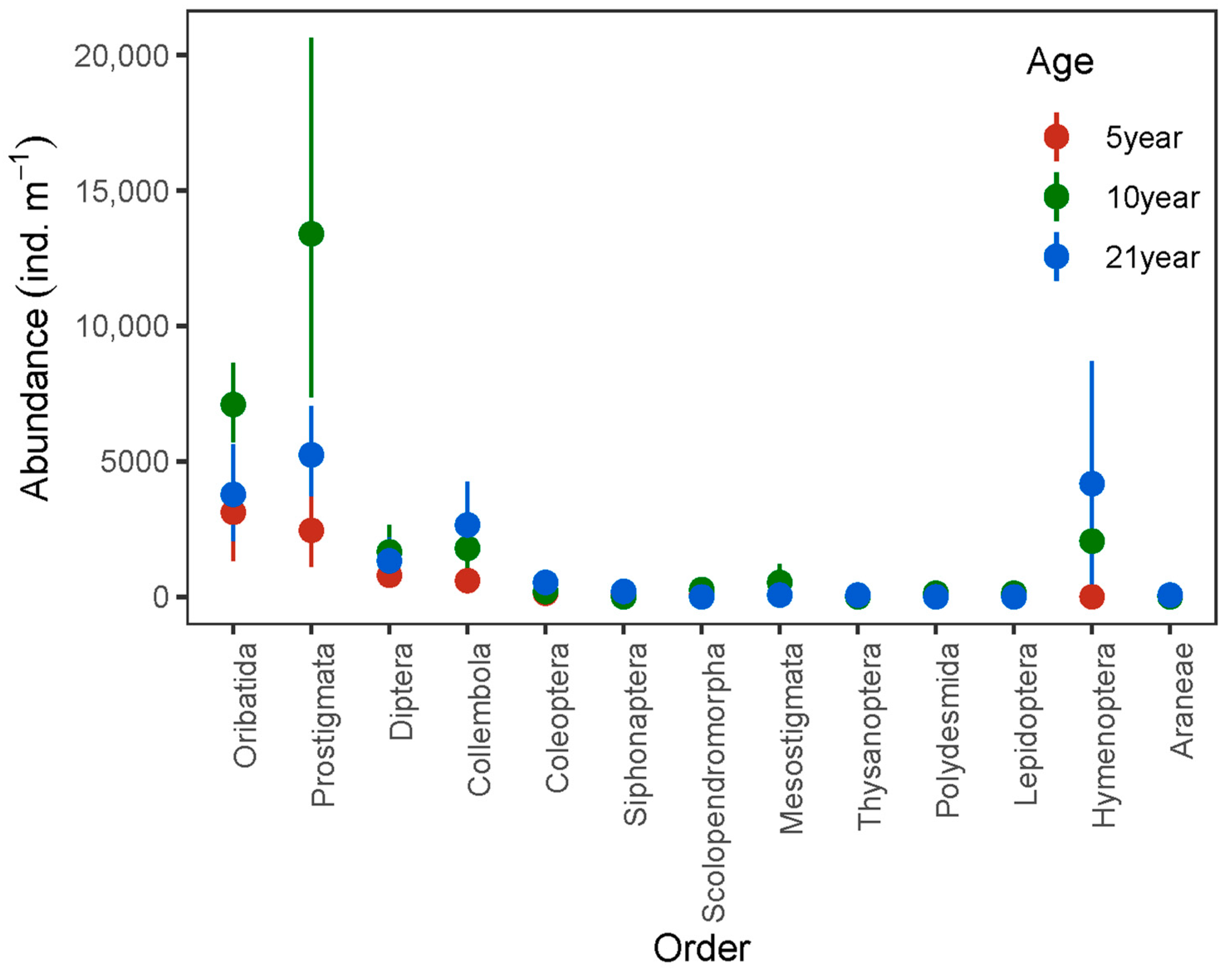
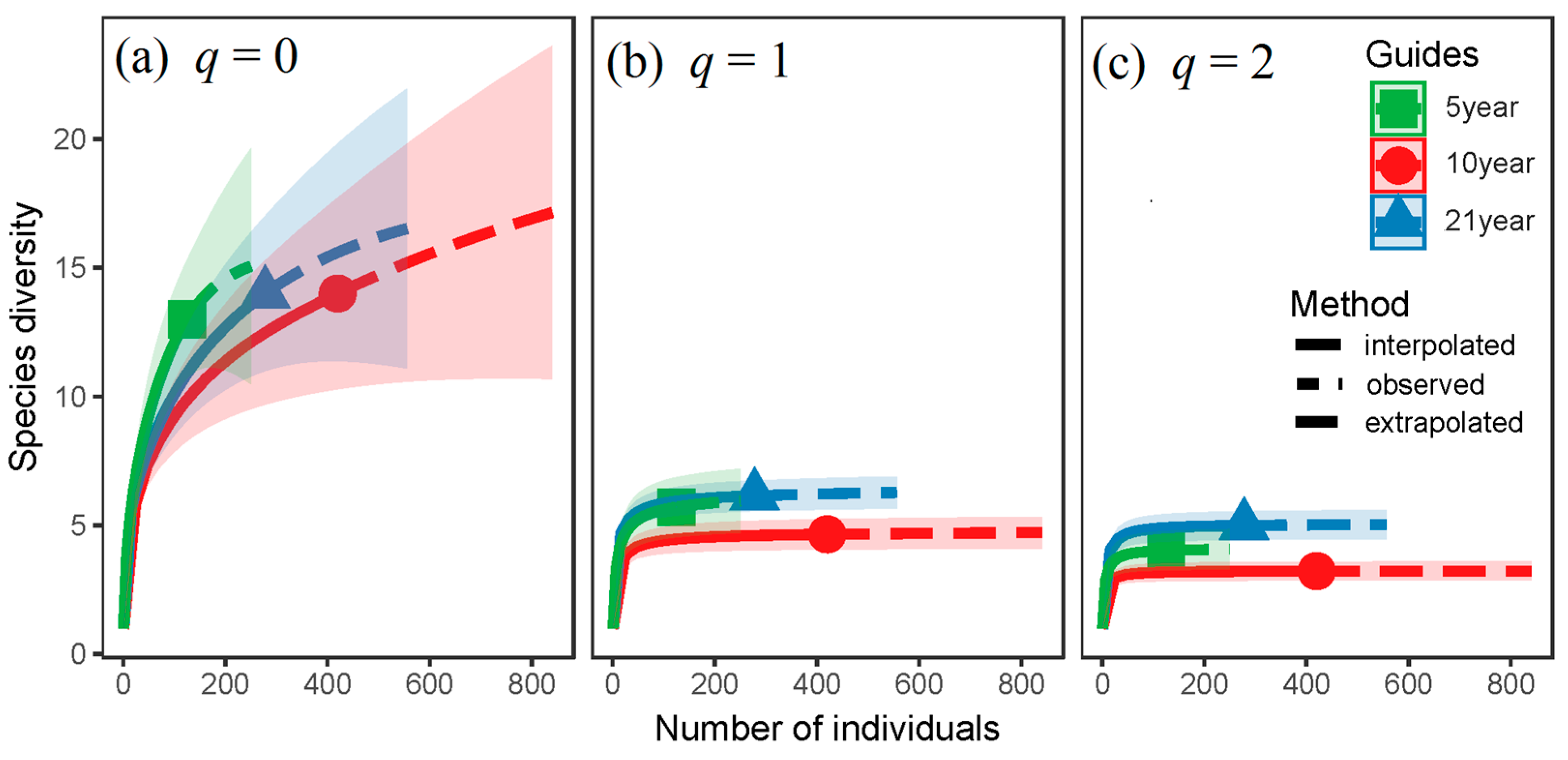
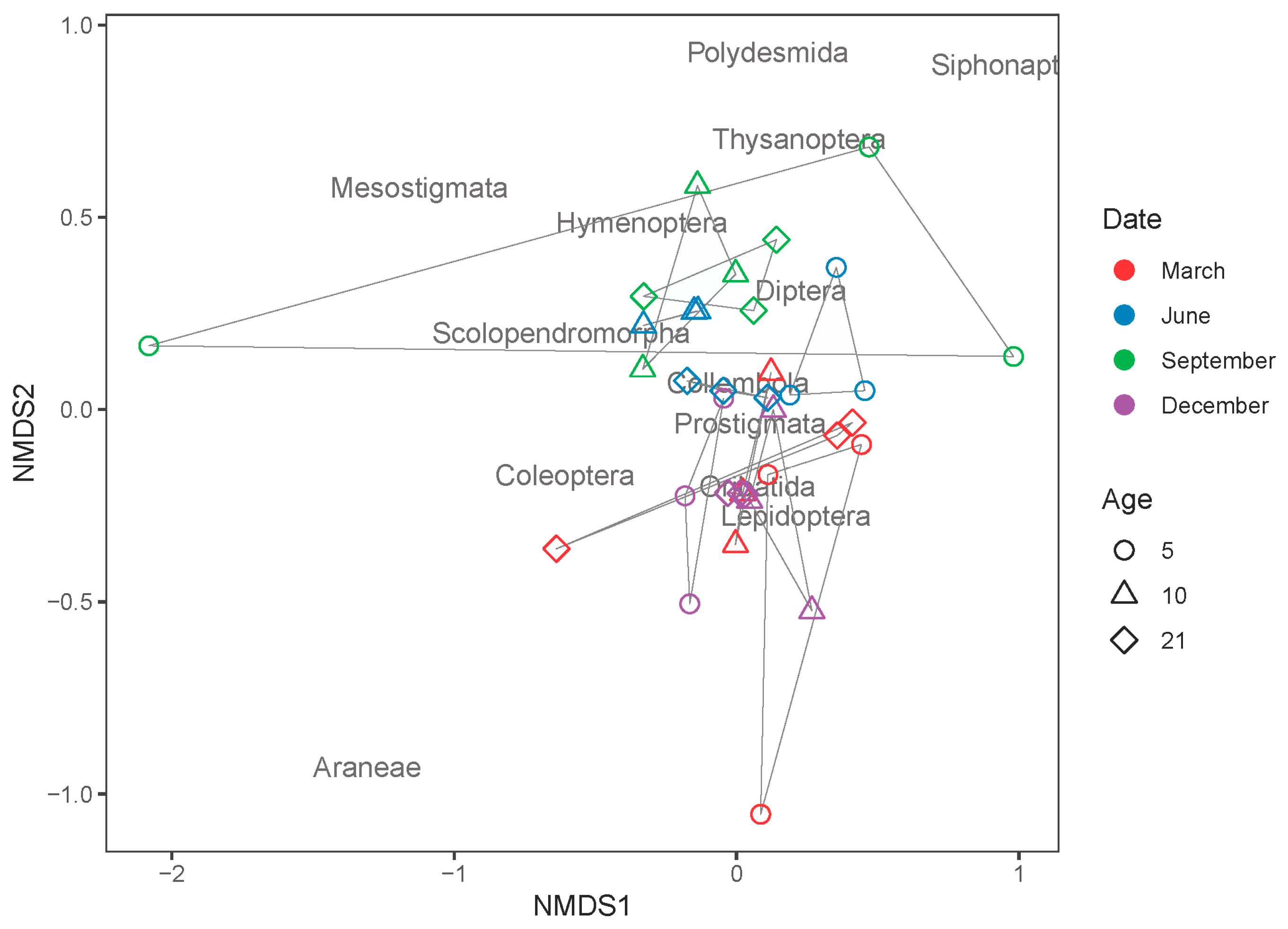
| Parameters | 5 Year Old | 10 Year Old | 21 Year Old | |
|---|---|---|---|---|
| Total abundance | 7433.3 ± 1823.4 a | 27,271.8 ± 5837.8 b | 18,113.7 ± 2935.7 ab | |
| Prostigmata | 2454.9 ± 762.0 a | 13,402.3 ± 3569.8 b | 5241.5 ± 856.9 a | |
| Oribatida | 3118.4 ± 992.0 a | 7099.3 ± 810.9 b | 3781.8 ± 955.8 a | |
| Mesostigmata | 66.3 ± 66.3 | 530.8 ± 315.0 | 66.3 ± 66.3 | |
| Collembola | 597.1 ± 199.0 a | 1791.4 ± 450.4 ab | 2653.9 ± 816.0 b | |
| Epedaphic | 265.4 ± 113.2 | 663.5 ± 165.0 | 1194.3 ± 443.7 | |
| Hemiedaphic | 199.0 ± 142.9 | 597.1 ± 221.9 | 729.8 ± 206.9 | |
| Euedaphic | 133.0 ± 89.5 | 530.8 ± 149.7 | 729.8 ± 267.6 | |
| Diptera | 796.5 ± 240.0 | 1659.4 ± 494.8 | 1327.0 ± 436.1 | |
| Coleoptera | 133.4 ± 89.4 | 199.0 ± 142.9 | 531.1 ± 246.8 | |
| Hymenoptera | 0 | 2056.8 ± 1071.1 | 4179.9 ± 2165.2 | |
| Araneae | 0 | 0 | 66.4 ± 66.3 | |
| Scolopendromorpha | 66.7 ± 66.3 | 256.7 ± 149.6 | 0 | |
| Polydesmida | 0.67 ± 0.45 | 133.4 ± 89.9 | 0.33 ± 0.33 | |
| Thysanoptera | 66.3 ± 66.3 | 0.33 ± 0.33 | 66.3 ± 66.3 | |
| Siphonaptera | 132.7 ± 89.4 | 0.33 ± 0.33 | 199.0 ± 199.0 | |
| Lepidoptera | 0.33 ± 0.33 | 133.0 ± 89.4 | 0 |
| Source | df | Sum Squares | F | R2 | p |
|---|---|---|---|---|---|
| A | 2 | 0.26 | 2.05 | 0.08 | 0.04 |
| D | 3 | 1.01 | 5.27 | 0.29 | 0.001 |
| A × D | 6 | 0.69 | 1.79 | 0.19 | 0.015 |
| Residuals | 24 | 1.53 | 0.44 |
| Stand Age (Years) | Date | Indicator | Indicator Value | Specificity | Sensitivity | p |
|---|---|---|---|---|---|---|
| 10 | June | Scolopendromorpha | 0.800 | 1.00 | 0.894 | 0.005 |
| 10 | June | Prostigmata | 0.377 | 1.00 | 0.614 | 0.001 |
| 21 | September | Collembola | 0.290 | 1.00 | 0.538 | 0.035 |
| Dimension | Characteristics | 5 Year Old | 10 Year Old | 21 Year Old |
|---|---|---|---|---|
| Aboveground | DBH (cm) | 16.50 ± 1.63 c | 26.04 ± 0.46 b | 32.77 ± 1.48 a |
| Plant species richness | 19.0 ± 0.58 ab | 23.0 ± 1.52 a | 17.3 ± 3.79 b | |
| Plant coverage (%) | 75.37 ± 3.87 a | 91.1 ± 2.74 b | 77.5 ± 4.22 a | |
| 0–10 cm soil | Moisture (%) | 19.74 ± 0.79 b | 24.88 ± 0.36 a | 23.61 ± 1.43 a |
| pH | 8.25 ± 0.12 | 8.09 ± 0.11 | 8.03 ± 0.09 | |
| N (g kg−1) | 1.69 ± 0.04 b | 2.10 ± 0.06 a | 2.24 ± 0.02 a | |
| C (g kg−1) | 17.05 ± 0.23 | 17.25 ± 0.30 | 18.26 ± 0.35 | |
| 10–20 cm soil | Moisture (%) | 19.25 ± 0.2 b | 24.6 ± 0.27 a | 23.59 ± 1.11 a |
| pH | 8.35 ± 0.06 | 8.31 ± 0.13 | 8.29 ± 0.13 | |
| N (g kg−1) | 1.66 ± 0.02 b | 1.93 ± 0.05 a | 1.97 ± 0.07 a | |
| C (g kg−1) | 16.07 ± 0.23 | 16.27 ± 0.30 | 16.09 ± 0.35 |
| Parameters | Plant Species Richness | Plant Coverage | Moisture | pH | C | N |
|---|---|---|---|---|---|---|
| Total abundance | 0.11 | 0.10 | 0.55 * | −0.37 | 0.51 * | 0.75 *** |
| Prostigmata | 0.24 | 0.31 | 0.59 ** | −0.18 | 0.08 | 0.47 * |
| Oribatida | 0.39 | 0.54 * | 0.52 * | −0.02 | 0.04 | 0.33 |
| Hymenoptera | −0.38 | −0.15 | 0.22 | −0.38 | 0.71 ** | 0.63 ** |
| Collembola | −0.39 | −0.29 | 0.28 | −0.40 | 0.41 | 0.56 * |
| Diptera | 0.29 | 0.33 | 0.34 | 0.23 | −0.53 * | −0.1 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Chen, H.Y.H.; Song, Q.; Liao, J.; Xu, Z.; Huang, S.; Ruan, H. Changes in Soil Arthropod Abundance and Community Structure across a Poplar Plantation Chronosequence in Reclaimed Coastal Saline Soil. Forests 2018, 9, 644. https://doi.org/10.3390/f9100644
Li Y, Chen HYH, Song Q, Liao J, Xu Z, Huang S, Ruan H. Changes in Soil Arthropod Abundance and Community Structure across a Poplar Plantation Chronosequence in Reclaimed Coastal Saline Soil. Forests. 2018; 9(10):644. https://doi.org/10.3390/f9100644
Chicago/Turabian StyleLi, Yuanyuan, Han Y. H. Chen, Qianyun Song, Jiahui Liao, Ziqian Xu, Shide Huang, and Honghua Ruan. 2018. "Changes in Soil Arthropod Abundance and Community Structure across a Poplar Plantation Chronosequence in Reclaimed Coastal Saline Soil" Forests 9, no. 10: 644. https://doi.org/10.3390/f9100644
APA StyleLi, Y., Chen, H. Y. H., Song, Q., Liao, J., Xu, Z., Huang, S., & Ruan, H. (2018). Changes in Soil Arthropod Abundance and Community Structure across a Poplar Plantation Chronosequence in Reclaimed Coastal Saline Soil. Forests, 9(10), 644. https://doi.org/10.3390/f9100644





